18 August 2020: Clinical Research
Effects of a Secondary Prevention Combination Therapy with beta-Blocker and Statin on Major Adverse Cardiovascular Events in Acute Coronary Syndrome Patients
Ling Zhu12ABCDEFG, Qianwei Cui1BCEF, Ying Liu3BCEF, Zhongwei Liu1BCE, Yong Zhang1BCF, Fuqiang Liu1ACDEFG, Junkui Wang1ACDEFG*DOI: 10.12659/MSM.925114
Med Sci Monit 2020; 26:e925114
Abstract
BACKGROUND: The efficacy of a beta-blocker or statin alone versus combination therapy is uncertain. We compared the effects of a combination of beta-blocker and statin with those of one-drug therapies with regard to the occurrence of a major adverse cardiovascular event (MACE) in patients with acute coronary syndrome (ACS).
MATERIAL AND METHODS: From 2011 to 2013, 636 ACS patients were included. Based on their risk category, enrolled subjects were assigned into 4 groups receiving consistent beta-blocker and/or statin treatment: no therapy group (n=139), with never use or inconsistent use beta-blocker and statin; beta-blocker monotherapy group (n=71); statin monotherapy group (n=149); and cotherapy group (n=277).
RESULTS: Men composed 66.8% of the cohort, which had a mean age of 60.42±9.83 years. Compared with the no therapy group, the statin monotherapy group and cotherapy group had a lower risk of MACE (statin monotherapy group: adjusted hazard ratio [HR] 0.35, 95% confidence interval [CI] 0.20-0.60, P<.001; cotherapy group: adjusted HR 0.16, 95% CI 0.09–0.28, P<.001). Subgroup analysis indicated that, compared with beta-blocker monotherapy and statin monotherapy, cotherapy significantly reduced the risks of MACE occurrences in ACS patients (beta-blocker monotherapy group: adjusted HR 0.28, 95% CI 0.13–0.59, P=.001; statin monotherapy group: adjusted HR 0.54, 95% CI 0.29–0.98, P=.044).
CONCLUSIONS: Beta-blocker and statin combination therapy lowered the risk of developing MACE in ACS patients.
Keywords: acute coronary syndrome, Adrenergic beta-Antagonists, Hydroxymethylglutaryl-CoA Reductase Inhibitors, Drug Therapy, Combination, secondary prevention
Background
The therapeutic effects of beta-blockers and statins have been well demonstrated in reducing major adverse cardiovascular events (MACEs) [1–9]. However, the efficacy of a beta-blocker or statin alone versus combination therapy is less well established. Although a few previous studies showed that combined therapy with statin and beta-blocker was correlated with reduced short-term (30 days to 1 year) MACE occurrence in patients with coronary arterial disease [10–13], the long-term effects were still not clear. Therefore, we conducted a cohort study to investigate the long-term effects of the monotherapies and the combined therapy in patients with acute coronary syndrome (ACS). Results from this investigation provide novel evidence supporting the use of combination therapy with beta-blocker and statin in the clinical treatment of ACS.
Material and Methods
STUDY POPULATION:
A retrospective and observational cohort methodology was used in this study. During January 2011 to December 2013, 729 ACS subjects treated in Shaanxi Provincial People’s Hospital were enrolled. Patients with incomplete data (21 patients), New York Heart Association (NYHA) cardiac functional class III or IV (10 patients), active infections (7 patient), immune system disease (6 patients), kidney disease (4 patients), and malignant tumor (2 patients) were excluded. Forty-three patients (6.3%) were excluded because they were lost to follow-up. Finally, 636 subjects were eventually included (Supplementary Figure 1).
Subjects were divided into 4 groups for beta-blocker and statin treatment based on their risk category: (1) no therapy group, which included never use and inconsistent use beta-blocker and statin; (2) beta-blocker monotherapy group, which was defined by consistent use of a beta-blocker and never use or inconsistent use of a statin; (3) statin monotherapy group, which was defined by consistent use of a statin and never use or inconsistent use of a beta-blocker; and (4) cotherapy group, which was defined by consistent and regular use of both a beta-blocker and a statin. For consistent use of a beta-blocker, patients were discharged with a beta-blocker and reported use in each interval. For never use of beta-blockers, patients were discharged without a beta-blocker and reported no use during the study interval. Inconsistent use of a beta-blocker meant that patients did not meet the criteria for either of the previous 2 patterns. For consistent use of a statin, patients were discharged with statin and reported use in each interval. For never use of a statin, patients were discharged without statin and reported no use during the study interval. Inconsistent use of a statin meant that patients did not meet the criteria for either of the previous 2 patterns.
CLINICAL DATA COLLECTION:
Collected medical data were entered and maintained in the network database (Likang Times Technology Co. Ltd, Beijing, China). Raw data checking was performed by using the double entry method. Data eventually entered the database when the values of the 2 entries were consistent. Otherwise, the error would be automatically tagged by the system and corrected by checking the raw data.
DEFINITIONS:
ACS was defined as high-risk unstable angina, non-ST-elevated myocardial infarction (MI), or ST-elevated MI, which were diagnosed by significant increases in serum creatine phosphokinase MB and troponin I. MACE endpoints included cardiovascular death, MI, ischemia-driven revascularization, progress to NYHA III or IV, and stroke. The definition of ischemia-driven revascularization was repeat percutaneous coronary intervention or coronary artery bypass grafting [14]. NYHA cardiac functional class III was defined as patients exhibiting obvious physical activity limitation due to cardiac diseases. Such patients are comfortable at rest, but even limited activity causes fatigue, palpitation, or dyspnea. NYHA functional class IV was defined as patients exhibiting an inability to carry on any physical activity without discomfort due to cardiac diseases. Symptoms are present even at rest or with minimal exertion. If any physical activity is undertaken, discomfort is increased [15].
STATISTICS:
The baseline characteristics among the 4 groups were analyzed by analysis of variance (ANOVA) for parametric variables, the Kruskal-Wallis test for nonparametric variables, and the chi-square test for categorical variables. Cumulative event curves of MACEs were derived from the Kaplan-Meier method and the log-rank test was used for comparison. The impact of combination therapy with beta-blocker and statin on MACEs was estimated with univariate and multivariate Cox proportional hazards regression models. Four regression models were used: Model 1, unadjusted; Model 2, adjusted for age, sex, smoking, and body mass index; Model 3, adjusted for age, sex, smoking, body mass index, diabetes, hypertension, old MI, and atrial fibrillation; and Model 4, adjusted for age, sex, smoking, body mass index, diabetes, hypertension, old MI, atrial fibrillation, always use of aspirin, use of clopidogrel at 1 year, always use of angiotensin converting enzyme inhibitors (ACEI) or angiotensin receptor blocker (ARB), and revascularization at baseline. Furthermore, we also performed multivariate Cox analysis of MACEs in subgroups. All statistical testing was 2-sided. When
Results
BASELINE CHARACTERISTICS:
A total of 636 patients were included in our study. Table 1 demonstrates the baseline characteristics. Follow-up mean duration was 4.2 years (interquartile range, 4.1–4.4 years). Men were 66.8% of the subjects, who were 25 to 80 years old (mean age: 60.42±9.83 years). No therapy and statin monotherapy groups had a higher heart rate (no therapy, 70.51±9.33 bpm; beta-blocker monotherapy, 69.72±9.98 bpm; statin monotherapy, 70.28±10.00 bpm; cotherapy, 67.63±10.83 bpm; P=.008) and a higher percentage of hypertension (no therapy, 51.1%; beta-blocker monotherapy, 69.0%; statin monotherapy, 61.1%; cotherapy, 70.0%; P=.001). No therapy and beta-blocker monotherapy groups had higher total cholesterol levels (no therapy, 4.37±1.06 mmol/L; beta-blocker monotherapy, 4.35±1.20 mmol/L; statin monotherapy, 4.09±1.09 mmol/L; cotherapy, 4.08±0.95 mmol/L; P=.008) and triglyceride levels (no therapy, 1.99±1.45 mmol/L; beta-blocker monotherapy, 1.76±1.04 mmol/L; statin monotherapy, 1.51±0.80 mmol/L; cotherapy, 1.62±0.98 mmol/L; P=.010). Statin monotherapy and cotherapy groups had higher percentage in the use of aspirin (no therapy, 94.2%; beta-blocker monotherapy, 80.3%; statin monotherapy, 100.0%; cotherapy, 99.3%; P<.001); clopidogrel (no therapy, 57.6%; beta-blocker monotherapy, 47.9%; statin monotherapy, 77.2%; cotherapy, 80.9%; P<.001); and higher revascularization at baseline (no therapy, 63.3%; beta-blocker monotherapy, 67.6%; statin monotherapy, 76.5%; cotherapy, 76.9%; P=.014). The baseline characteristics of subjects presenting MACEs are shown in Supplementary Table 1.
CLINICAL OUTCOMES:
During the follow-up of 4.2±0.3 years, there were 98 (15.4%) MACEs, including zero cardiovascular deaths (0.0%), 8 MIs (1.3%), 73 ischemia-driven revascularizations (11.5%), 17 cases of cardiac function NYHA III or IV (2.7%), and 14 strokes (2.2%). The follow-up data showed the rates of MACEs in the no therapy group, beta-blocker monotherapy group, statin monotherapy group, and cotherapy group were 28.8% (40/139), 19.7% (14/71), 14.8% (22/149), and 7.9% (22/277) (P<.001), respectively (Supplementary Table 2). Relative to the no therapy group, the cumulative incidence of MACEs was gradually decreasing in the beta-blocker monotherapy group, statin monotherapy group, and cotherapy group (P<.001) (Figure 1). Cumulative event curves of ischemia-driven revascularization were similar to those of MACEs (P<.001) (Supplementary Figure 2). Cumulative event curves of MI, progress to NYHA III or IV, and stroke are shown in Supplementary Figure 2.
Univariate and multivariate Cox regression models were used to reveal the impact of consistent beta-blocker and statin treatment on MACEs in ACS patients. In univariable Cox regression model (Model 1), compared with no therapy group, the hazard ratios (HRs) for MACEs in the statin monotherapy group and the cotherapy group were 0.50 (95% confidence interval 0.30–0.84, P=.009) and 0.25 (95% CI 0.15–0.43, P<.001). Multivariate analysis indicated that the statin monotherapy group and the cotherapy group had a lower risk of MACEs than the no therapy group (Model 2, statin monotherapy group, HR 0.50, 95% CI 0.30–0.85, P=.010; cotherapy group, HR 0.26, 95% CI 0.15–0.43, P<.001; Model 3, statin monotherapy group, HR 0.48, 95% CI 0.28–0.80, P=.006; cotherapy group, HR 0.24, 95% CI 0.14–0.41, P<.001; Model 4, statin monotherapy group, HR 0.35, 95% CI 0.20–0.60, P<.001; cotherapy group, HR 0.16, 95% CI 0.09–0.28, P<.001; compared with the no therapy group). There was no significant difference the relative risk of MACEs between the no therapy group and the beta-blocker monotherapy group (Table 2).
Furthermore, relative to the no therapy group, the statin monotherapy group and the cotherapy group showed a lower risk of ischemia-driven revascularization and cardiac function NYHA III or IV progression. There were no cardiovascular deaths, 8 MIs, and 14 strokes during the follow-up. The incidence of cardiovascular death, MI, and stroke was low and did not allow for further analysis (Supplementary Tables 3, 4).
SUBGROUP ANALYSIS:
We also conducted a subgroup analysis between groups. In the univariate Cox regression model (Model 1), the cotherapy group showed a lower MACE occurrence than the beta-blocker monotherapy group (HR 0.39, 95% CI 0.20–0.76, P=.005). Further variables were adjusted for in Model 2, Model 3, and Model 4, and there were no significant changes of HR for MACEs in the cotherapy group (Model 2, HR 0.39, 95% CI 0.20–0.77, P=.006; Model 3, HR 0.37, 95% CI 0.19–0.73, P=.004; Model 4, HR 0.28, 95% CI 0.13–0.59, P=.001) (Table 3, Figure 2A).
In addition, relative to the statin monotherapy group, the cotherapy group showed a significant 49% reduction in MACE occurrence (HR 0.51, 95% CI 0.28–0.92, P=.025). This reduction of MACEs was not attenuated when adjusting for addition variables in Model 2, Model 3, and Model 4 (Model 2, HR 0.50, 95% CI 0.28–0.91, P=.023; Model 3, HR 0.52, 95% CI 0.29–0.95, P=.034; Model 4, HR 0.54, 95% CI 0.29–0.98, P=.044) (Table 3, Figure 2B).
Discussion
LIMITATIONS:
Our study had some limitations. Subjects enrolled were from a single center, which was limited to the native Chinese population. In addition, 6.3% of subjects were lost to follow-up, which could result in biases. These facts may limit generalizing our findings.
Conclusions
Results from our current study indicated that using beta-blocker and statin combination therapy lowered the risk of MACEs in ACS patients.
Figures
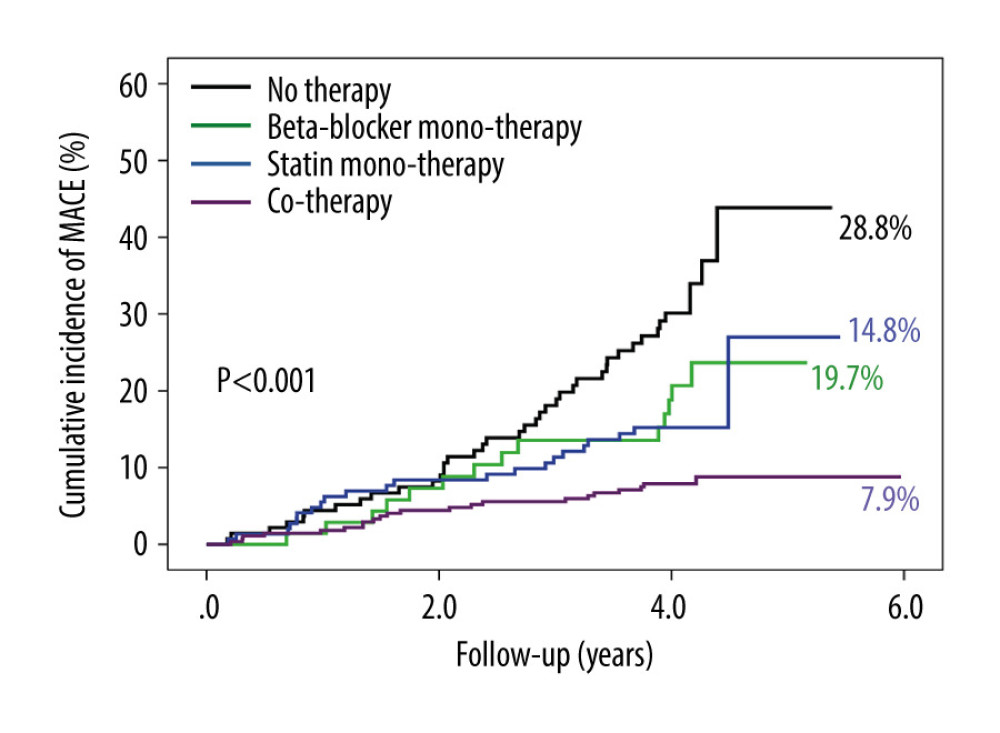 Figure 1. The cumulative incidence of major adverse cardiovascular events (MACEs). Compared with no therapy group, the cumulative incidence of MACEs gradually decreased in the beta-blocker monotherapy group, statin monotherapy group, and cotherapy group (P<.001).
Figure 1. The cumulative incidence of major adverse cardiovascular events (MACEs). Compared with no therapy group, the cumulative incidence of MACEs gradually decreased in the beta-blocker monotherapy group, statin monotherapy group, and cotherapy group (P<.001). 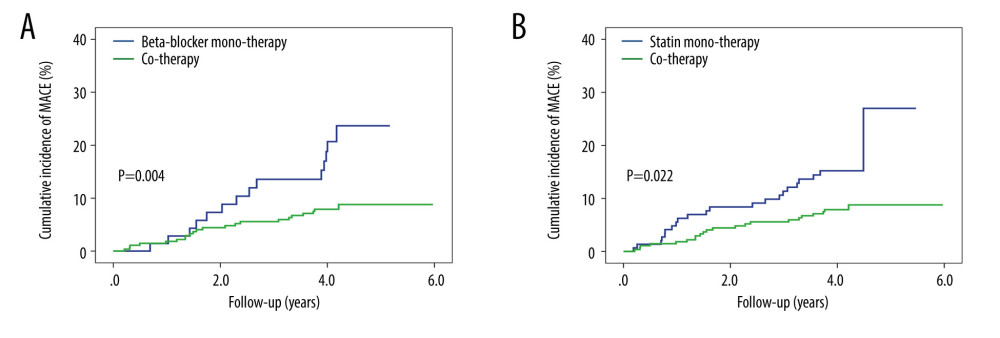 Figure 2. The cumulative incidence of major adverse cardiovascular events (MACEs) in the subgroup. (A) Patients in the cotherapy group showed a lower MACE occurrence than the beta-blocker monotherapy group (P=.004). (B) Patients in the cotherapy group showed a lower MACE occurrence than the statin monotherapy group (P=.022).
Figure 2. The cumulative incidence of major adverse cardiovascular events (MACEs) in the subgroup. (A) Patients in the cotherapy group showed a lower MACE occurrence than the beta-blocker monotherapy group (P=.004). (B) Patients in the cotherapy group showed a lower MACE occurrence than the statin monotherapy group (P=.022). Tables
Table 1. Baseline clinical characteristics of the study patients according to risk category of always beta-blocker and statin treatment.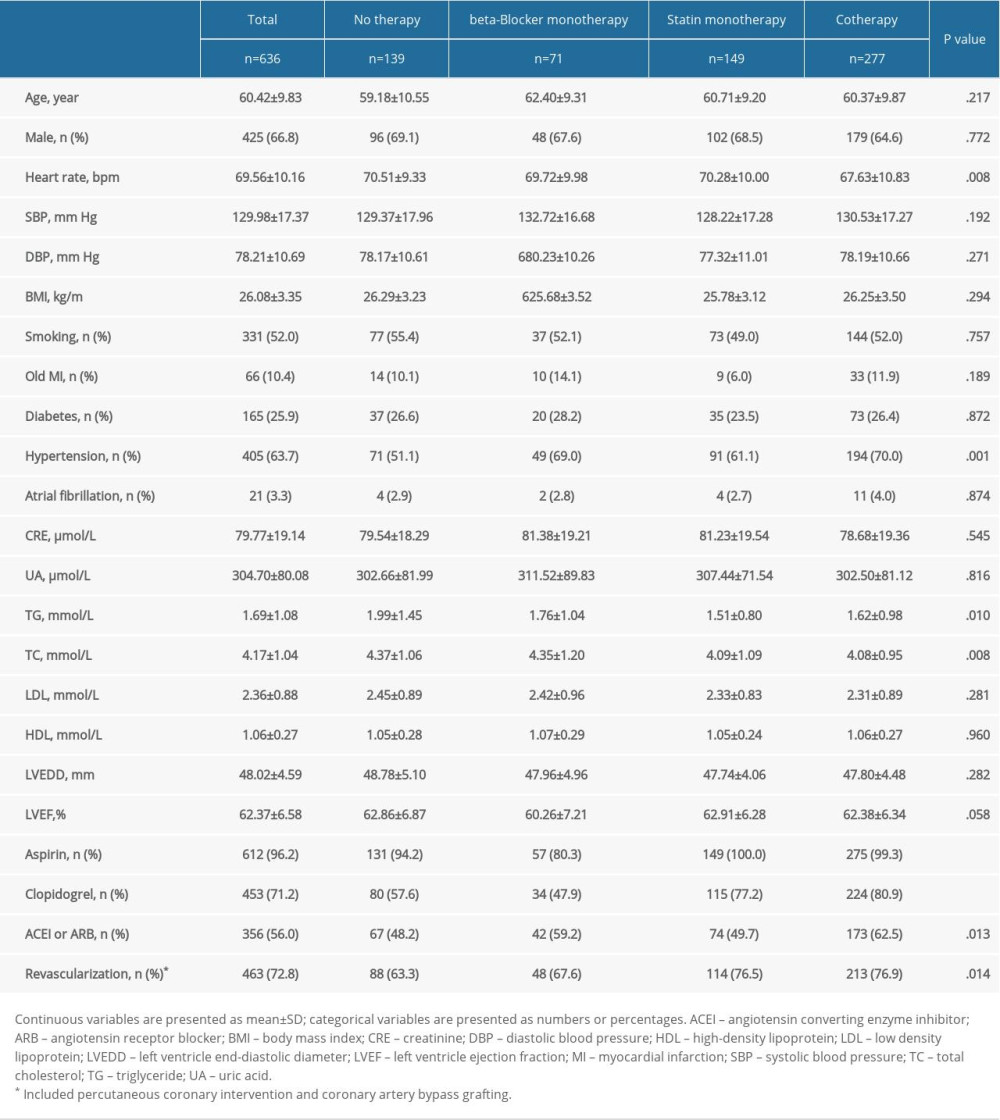 Table 2. Univariate and multivariate Cox analysis according to risk category of always beta-blocker and statin.
Table 2. Univariate and multivariate Cox analysis according to risk category of always beta-blocker and statin.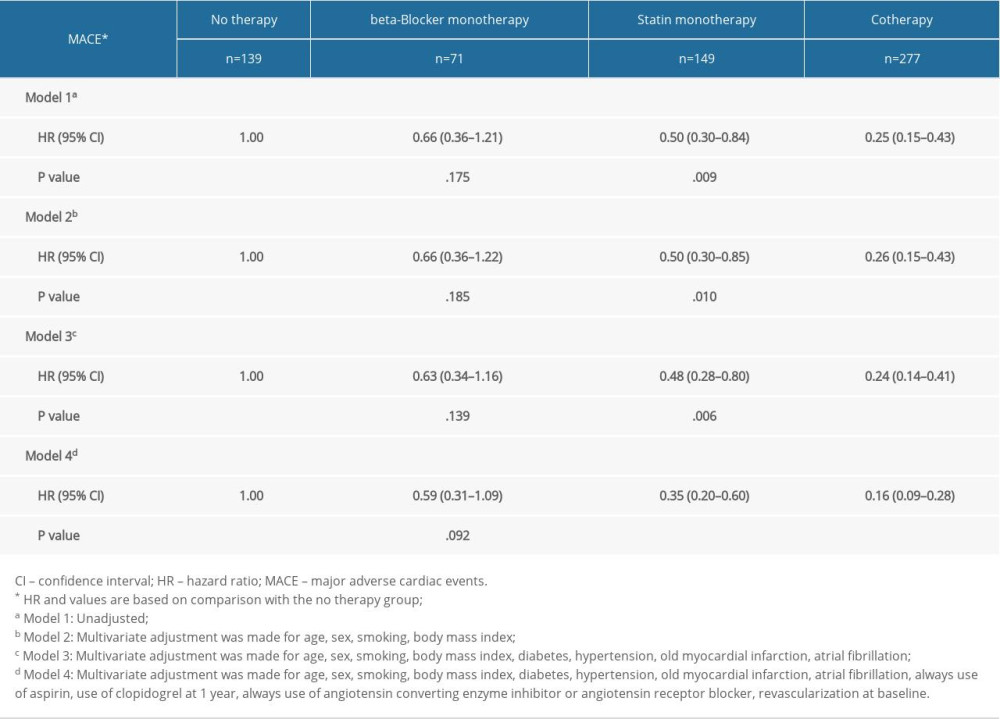 Table 3. Multivariate Cox analysis of MACE in subgroups.
Table 3. Multivariate Cox analysis of MACE in subgroups.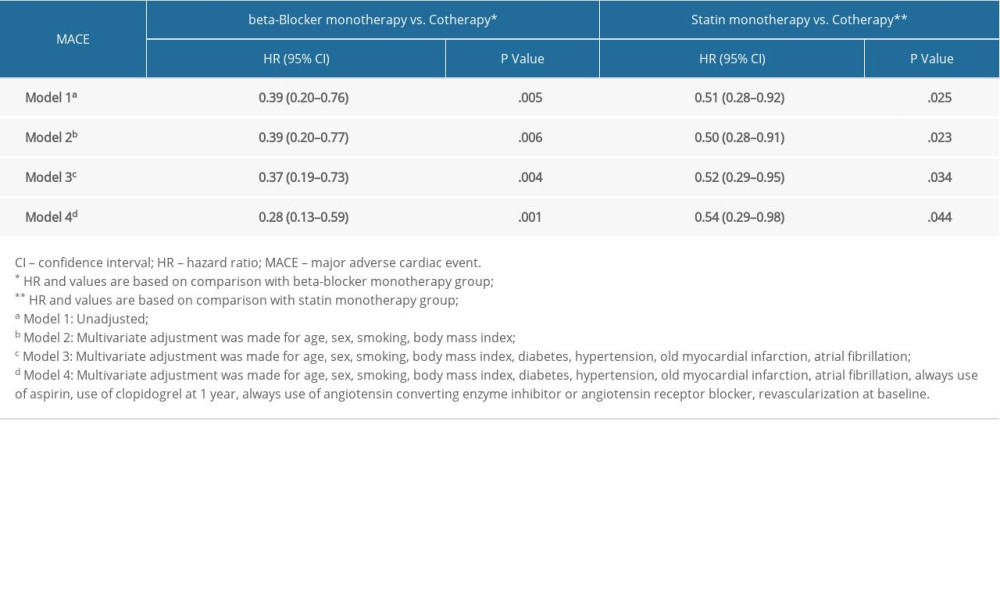 Supplementary Table 1. Baseline clinical characteristics of patients with MACE.
Supplementary Table 1. Baseline clinical characteristics of patients with MACE.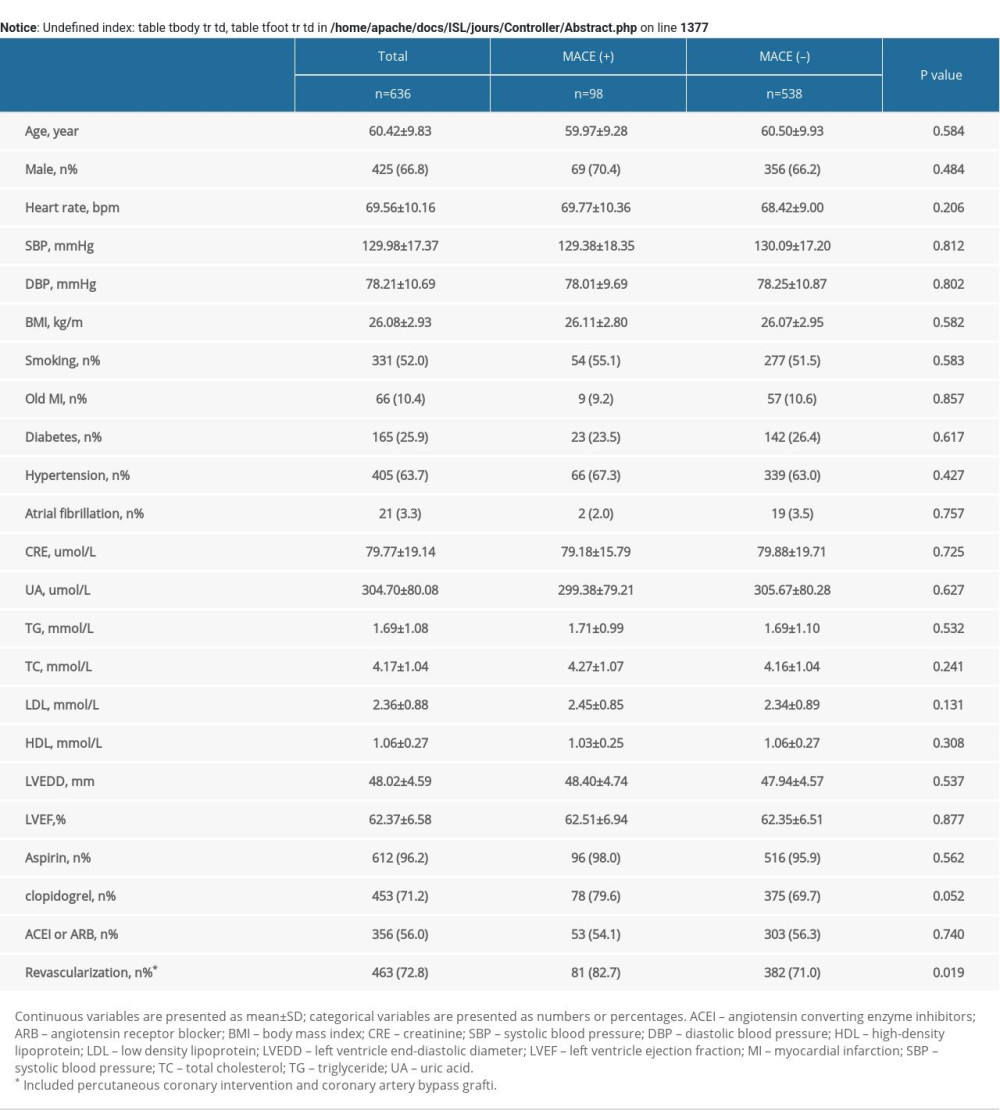 Supplementary Table 2. MACE of the study patients according to risk category of always beta-blocker and statin treatment.
Supplementary Table 2. MACE of the study patients according to risk category of always beta-blocker and statin treatment.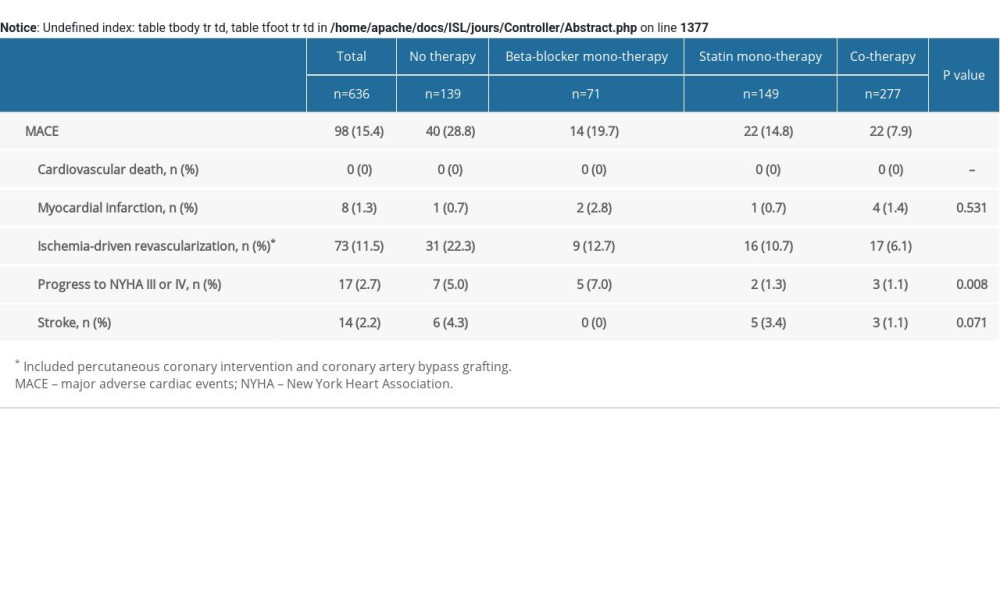 Supplementary Table 3. Multivariate COX analysis of ischemia-driven revascularization according to risk category of always beta-blocker and statin treatment.
Supplementary Table 3. Multivariate COX analysis of ischemia-driven revascularization according to risk category of always beta-blocker and statin treatment.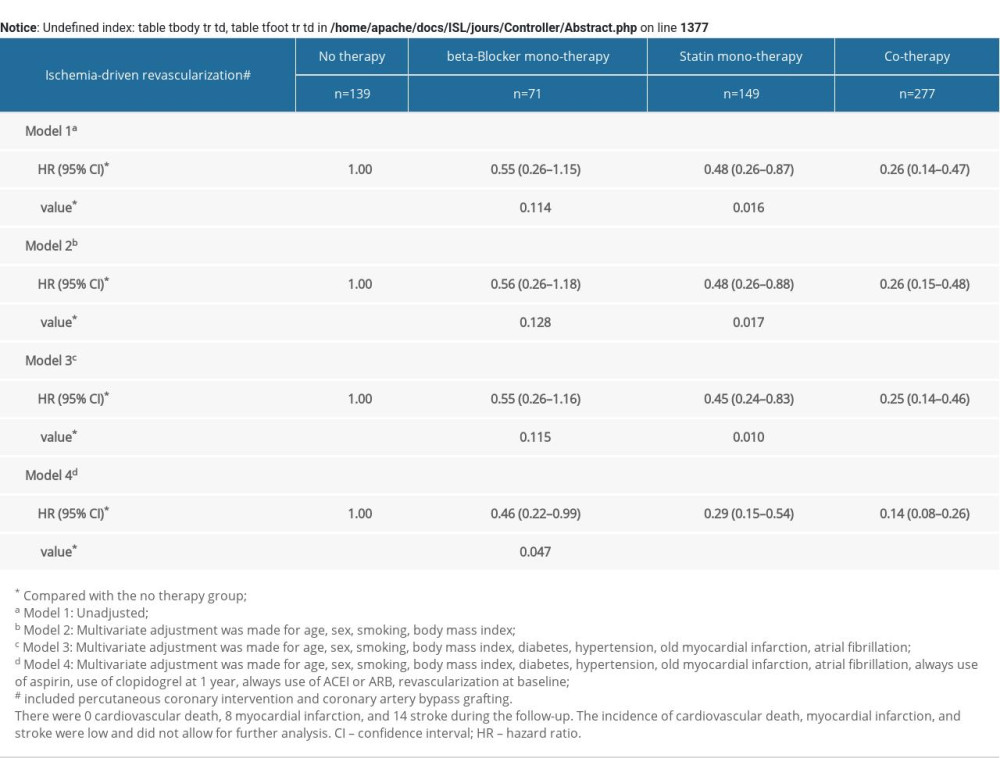 Supplementary Table 4. Multivariate COX analysis of progress to NYHA III or IV according to risk category of always b-blocker and statin treatment.
Supplementary Table 4. Multivariate COX analysis of progress to NYHA III or IV according to risk category of always b-blocker and statin treatment.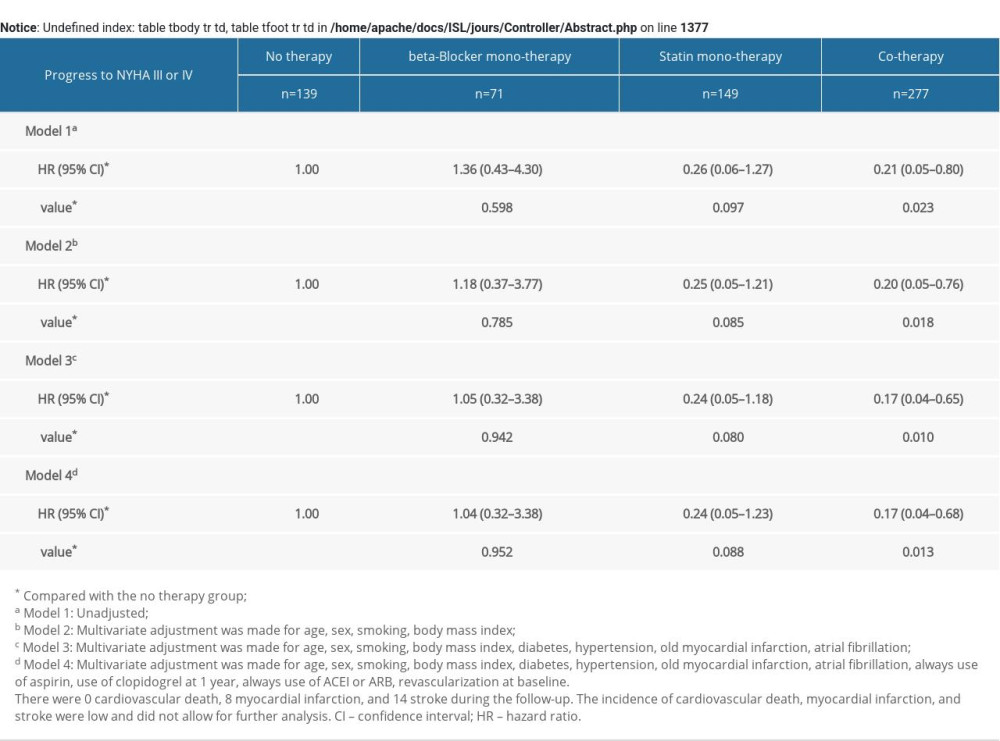
References
1. Task Force Members; ESC Committee for Practice Guidelines (CPG), ESC National Cardiac Societies: 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk: Atherosclerosis, 2019; 290; 140-205
2. Mark L, Janosi A, Ferenci T, Toth PP, Recommendations of statin treatment after acute coronary syndrome: Hungarian experiences: Atherosclerosis, 2020; 303; 53-54
3. Mortensen MB, Nordestgaard BG, Statin use in primary prevention of atherosclerotic cardiovascular disease according to 5 major guidelines for sensitivity, specificity, and number needed to treat: JAMA Cardiol, 2019; 4(11); 1131-38
4. Fleisher LA, Beckman JA, Brown KA, 2009 ACCF/AHA focused update on perioperative beta blockade incorporated into the ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: Circulation, 2009; 120(21); e169-276
5. Mortensen MB, Falk E, Li D, Statin trials, cardiovascular events, and coronary artery calcification: Implications for a trial-based approach to statin therapy in MESA: JACC Cardiovasc Imaging, 2018; 11(2 Pt 1); 221-30
6. Joo SJ, Kim SY, Choi JH, Effect of beta-blocker therapy in patients with or without left ventricular systolic dysfunction after acute myocardial infarction: Eur Heart J Cardiovasc Pharmacother, 2020 [Online ahead of print]
7. Wu VC, Chen SW, Ting PC, Selection of β-blocker in patients with cirrhosis and acute myocardial infarction: A 13-year nationwide population-based study in Asia: J Am Heart Assoc, 2018; 7(19); e008982
8. Peyracchia M, Errigo D, Raposeiras R, Beta-blocker therapy reduces mortality in patients with coronary artery disease treated with percutaneous revascularization: A meta-analysis of adjusted results: J Cardiovasc Med (Hagerstown), 2018; 19(7); 337-43
9. Cediel G, Carrillo X, Garcia C, β-Blocker treatment and prognosis in acute coronary syndrome associated with cocaine consumption: The RUTI-Cocaine Study: Int J Cardiol, 2018; 260; 7-10
10. Hognestad A, Dickstein K, Myhre E, Effect of combined statin and beta-blocker treatment on one-year morbidity and mortality after acute myocardial infarction associated with heart failure: Am J Cardiol, 2004; 93(5); 603-6
11. Zeymer U, Jünger C, Zahn R, Effects of a secondary prevention combination therapy with an aspirin, an ACE inhibitor and a statin on 1-year mortality of patients with acute myocardial infarction treated with a beta-blocker. Support for a polypill approach: Curr Med Res Opin, 2011; 27(8); 1563-70
12. Lau WC, Froehlich JB, Jewell ES, Impact of adding aspirin to beta-blocker and statin in high-risk patients undergoing major vascular surgery: Ann Vasc Surg, 2013; 27(4); 537-45
13. Bouchard D, Carrier M, Demers P, Statin in combination with β-blocker therapy reduces postoperative stroke after coronary artery bypass graft surgery: Ann Thorac Surg, 2011; 91(3); 654-59
14. Kip KE, Hollabaugh K, Marroquin OC, Williams DO, The problem with composite end points in cardiovascular studies: The story of major adverse cardiac events and percutaneous coronary intervention: J Am Coll Cardiol, 2008; 51(7); 701-7
15. Cannon CP, Brindis RG, Chaitman BRAmerican College of Cardiology Foundation/American Heart Association Task Force on Clinical Data Standards, American College of Emergency Physicians, Emergency Nurses Association, National Association of Emergency Medical Technicians, National Association of EMS Physicians, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Patient Care, Society of Thoracic Surgeons, 2013 ACCF/AHA key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes and coronary artery disease: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Acute Coronary Syndromes and Coronary Artery Disease Clinical Data Standards): Circulation, 2013; 127(9); 1052-89
16. Bouchard D, Carrier M, Demers P, Statin in combination with beta-blocker therapy reduces postoperative stroke after coronary artery bypass graft surgery: Ann Thorac Surg, 2011; 91(3); 654-59
17. Krum H, Bailey M, Meyer W, Impact of statin therapy on clinical outcomes in chronic heart failure patients according to beta-blocker use: Results of CIBIS II: Cardiology, 2007; 108(1); 28-34
18. van Haelst PL, van Doormaal JJ, May JF, Secondary prevention with fluvastatin decreases levels of adhesion molecules, neopterin and C-reactive protein: Eur J Intern Med, 2001; 12(6); 503-9
19. Takemoto M, Liao JK, Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors: Arterioscler Thromb Vasc Biol, 2001; 21(11); 1712-19
20. Schouten O, Bax JJ, Dunkelgrun M, Pro: Beta-blockers are indicated for patients at risk for cardiac complications undergoing noncardiac surgery: Anesth Analg, 2007; 104(1); 8-10
21. Hedblad B, Wikstrand J, Janzon L, Low-dose metoprolol CR/XL and fluvastatin slow progression of carotid intima-media thickness: Main results from the Beta-Blocker Cholesterol-Lowering Asymptomatic Plaque Study (BCAPS): Circulation, 2001; 103(13); 1721-26
22. Wiklund O, Hulthe J, Wikstrand J, Effect of controlled release/extended release metoprolol on carotid intima-media thickness in patients with hypercholesterolemia: A 3-year randomized study: Stroke, 2002; 33(2); 572-77
Figures
 Figure 1. The cumulative incidence of major adverse cardiovascular events (MACEs). Compared with no therapy group, the cumulative incidence of MACEs gradually decreased in the beta-blocker monotherapy group, statin monotherapy group, and cotherapy group (P<.001).
Figure 1. The cumulative incidence of major adverse cardiovascular events (MACEs). Compared with no therapy group, the cumulative incidence of MACEs gradually decreased in the beta-blocker monotherapy group, statin monotherapy group, and cotherapy group (P<.001). Figure 2. The cumulative incidence of major adverse cardiovascular events (MACEs) in the subgroup. (A) Patients in the cotherapy group showed a lower MACE occurrence than the beta-blocker monotherapy group (P=.004). (B) Patients in the cotherapy group showed a lower MACE occurrence than the statin monotherapy group (P=.022).
Figure 2. The cumulative incidence of major adverse cardiovascular events (MACEs) in the subgroup. (A) Patients in the cotherapy group showed a lower MACE occurrence than the beta-blocker monotherapy group (P=.004). (B) Patients in the cotherapy group showed a lower MACE occurrence than the statin monotherapy group (P=.022). Tables
 Table 1. Baseline clinical characteristics of the study patients according to risk category of always beta-blocker and statin treatment.
Table 1. Baseline clinical characteristics of the study patients according to risk category of always beta-blocker and statin treatment. Table 2. Univariate and multivariate Cox analysis according to risk category of always beta-blocker and statin.
Table 2. Univariate and multivariate Cox analysis according to risk category of always beta-blocker and statin. Table 3. Multivariate Cox analysis of MACE in subgroups.
Table 3. Multivariate Cox analysis of MACE in subgroups. Table 1. Baseline clinical characteristics of the study patients according to risk category of always beta-blocker and statin treatment.
Table 1. Baseline clinical characteristics of the study patients according to risk category of always beta-blocker and statin treatment. Table 2. Univariate and multivariate Cox analysis according to risk category of always beta-blocker and statin.
Table 2. Univariate and multivariate Cox analysis according to risk category of always beta-blocker and statin. Table 3. Multivariate Cox analysis of MACE in subgroups.
Table 3. Multivariate Cox analysis of MACE in subgroups. Supplementary Table 1. Baseline clinical characteristics of patients with MACE.
Supplementary Table 1. Baseline clinical characteristics of patients with MACE. Supplementary Table 2. MACE of the study patients according to risk category of always beta-blocker and statin treatment.
Supplementary Table 2. MACE of the study patients according to risk category of always beta-blocker and statin treatment. Supplementary Table 3. Multivariate COX analysis of ischemia-driven revascularization according to risk category of always beta-blocker and statin treatment.
Supplementary Table 3. Multivariate COX analysis of ischemia-driven revascularization according to risk category of always beta-blocker and statin treatment. Supplementary Table 4. Multivariate COX analysis of progress to NYHA III or IV according to risk category of always b-blocker and statin treatment.
Supplementary Table 4. Multivariate COX analysis of progress to NYHA III or IV according to risk category of always b-blocker and statin treatment. In Press
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








