19 November 2021: Clinical Research
A Novel Method for the Production of an Autologous Anti-Inflammatory and Anti-Catabolic Product (Cytorich) from Human Blood: A Prospective Treatment for the COVID-19-Induced Cytokine Storm
Irina Brokhman1ABCDEF*, Alyssia M.T. Watkin1BE, Jeffrey C. Bacher1B, Stephen A. Glazer2DE, Anthony M. Galea1ACDEFGDOI: 10.12659/MSM.934365
Med Sci Monit 2021; 27:e934365
Abstract
BACKGROUND: Autologous blood-derived products can target specific inflammatory molecular pathways and have potentially beneficial therapeutic effects on inflammatory pathologies. The purpose of this study was to assess in vitro the anti-inflammatory and anti-catabolic potential of an autologous blood product as a possible treatment for COVID-19-induced cytokine storm.
MATERIAL AND METHODS: Blood samples from healthy donors and donors who had recovered from COVID-19 were incubated using different techniques and analyzed for the presence of anti-inflammatory, anti-catabolic, regenerative, pro-inflammatory, and procatabolic molecules.
RESULTS: The highest concentrations of therapeutic molecules for targeting inflammatory pathways were found in the blood that had been incubated for 24 h at 37°C, whereas a significant increase was observed after 6 h of incubation in blood from COVID-19-recovered donors. Beneficially, the 6-h incubation process did not downregulate anti-COVID-19 immunoglobulin G concentrations. Unfortunately, increases in matrix metalloproteinase 9, tumor necrosis factor α, and interleukin-1 were detected in the product after incubation; however, these increases could be blocked by adding citric acid, with no effect on the concentration of the target therapeutic molecules. Our data allow for safer and more effective future treatments.
CONCLUSIONS: An autologous blood-derived product containing anti-inflammatory and anti-catabolic molecules, which we term Cytorich, has a promising therapeutic role in the treatment of a virus-induced cytokine storm, including that associated with COVID-19.
Keywords: Anti-Inflammatory Agents, COVID-19, TIMP2 Protein, Human, TIMP1 Protein, Human, MMP9 Protein, Human, IL1RN Protein, Human, Anabolic Agents, COVID-19, cytokine release syndrome, Female, Humans, Interleukin-1beta, Male, Matrix Metalloproteinase 9, Metabolism, young adult
Background
Recently, the novel coronavirus SARS-CoV-2, which causes COVID-19, has spread rapidly, leading to a global pandemic. Serious complications and in some cases death caused by COVID-19 have been associated with the excessive and/or uncontrolled release of pro-inflammatory cytokines, resulting in a “cytokine storm.” Cytokine storms have previously been found to be directly correlated with tissue injury and an unfavorable prognosis in severe viral influenza [1].
Interleukin (IL)-1 is a pro-inflammatory cytokine that has been shown to be markedly increased during cytokine storms. IL-1 has also been identified as a therapeutic target for patients with attributes of macrophage activation syndrome (MAS), which is characterized by excessive activation and expansion of cytotoxic CD8+ T cells and macrophages, generating a cytokine storm [2]. Research has demonstrated that the expression and activation of matrix metalloproteinases (MMPs), such as MMP-9, can be upregulated via IL-1 in various cell types [3,4]. MMP-9 is known to regulate acute lung injury by disrupting the function of the airway epithelial barrier and degrading a broad spectrum of extracellular matrix proteins [5–8]. A recent study in mice showed that MMP-9 deficiency in lung structural cells protected the animals from virus-induced mortality [9]. The enzymatic activities of MMPs are strictly controlled by the 4 members of the tissue inhibitor of metalloproteinase (TIMP) family of endogenous inhibitors: TIMP-1, TIMP-2, TIMP-3, and TIMP-4 [10]. TIMPs are secreted proteins that generally have a positive effect on cell growth and survival [11]. Each TIMP protein has a distinct role in regulating MMP enzymes; for instance, MMP-3 and MMP-9 are inhibited more effectively by TIMP-1 than TIMP-2, whereas MMP-2 is more effectively inhibited by TIMP-2 than TIMP-1 [12,13].
The pro-inflammatory cytokine IL-1 has been shown to drive upregulation of MMP enzyme expression and activation in different cell types [3,4]. We therefore reasoned that a therapeutic intervention directed at MMP9 and IL-1 suppression using natural endogenous TIMPs and the IL-1 inhibitor IL-1ra could have a positive synergistic effect on the prevention of COVID-19-induced cytokine storm by blocking inflammatory (IL-1) and catabolic (MMPs) pathways. This intervention is also extremely likely to generate a positive synergistic effect on the regulation of the inflammatory process in severe COVID-19 cases. At the moment, effective treatments for SARS-CoV-2 are very limited; therefore, additional therapeutic strategies are urgently needed.
Blood product convalescent plasma (CP) containing a high neutralizing antibody titer was used to treat severe acute respiratory syndrome, Middle East respiratory syndrome, and the 2009 influenza A virus subtype H1N1 pandemic with satisfactory efficacy and safety. Clinical trials have been approved for the CP treatment of COVID-19, including by Health Canada; however, recent research has demonstrated some uncertainty regarding the therapeutic efficacy of CP as a COVID-19 treatment [14,15]. Consequently, there is increased urgency for additional COVID-19 treatments, especially in countries where access to the vaccines and supportive care for people infected with the virus are limited. This paper reports on a novel method for the creation of an autologous acellular blood product that contains anti-inflammatory and anti-catabolic components enriched by TIMP-1, TIMP-2, and IL-1ra. This method generates a novel immunomodulatory product capable of targeting cytokine storm, including that caused by COVID-19, through IL-1 and MMP-9 downregulation. The product can likely minimize the progression of COVID-19 infections and improve patient outcomes.
Material and Methods
ETHICS STATEMENT:
All experimental procedures and clinical trials performed in this study were approved by the Chesapeake International Review Board (approval numbers: CR00082114 and CR00277448). All participants consented to the study by signing an informed consent form.
BLOOD SAMPLE COLLECTION:
For the in vitro studies, peripheral blood was collected by venipuncture under sterile conditions from 25 healthy male and female volunteer donors who were nonvaccinated and had not been previously exposed to COVID-19 and 22 nonvaccinated recovered COVID-19 patients with mild cases confirmed by real-time reverse-transcription polymerase chain reaction assay (aged 21 to 60 years). The presence of anti-SARS-CoV-2 antibodies in the samples from recovered COVID-19 patients was confirmed using Lyher Novel Coronavirus (2019-nCoV) IgM/IgG Antibody Combo Test Kit (Colloidal Gold) qualitative analysis.
PREPARATION OF THE AUTOLOGOUS BLOOD PRODUCT:
The surfaces of the glass blood sample tubes (10 mL, Covidien) and vacutainer tubes were cleaned using 10% betadine solution and 70% alcohol. Peripheral blood was collected from study participants by venipuncture under aseptic conditions and transferred to the prepared tubes. To test the effect of the internal surface of containers on cytokine production, 2 types of tubes were used: 10-mL Monoject glass blood collection tube (Covidien) and polystyrene BD vacutainer 10-ml no gel. Incubation was carried out aseptically at 37°C for either 24 h or various time intervals (see results section). After the incubation time (3.5, 6, and 24 h), samples were centrifuged for 10 min at 4000 rpm, passed through a Millipore filter (0.22 μm, SLGP033RS), aliquoted, and stored at −80°C. The final cytokine and anti-SARS-CoV-2 antibody concentrations were measured using a multiplex assay (Immunology Multiplex Assay for TIMP-1 and TIMP-2, and MMP-9, EMD Millipore; Bio-Plex Pro™ Human Cytokine 27-plex Assay, Bio-Rad) and enzyme-linked immunosorbent assay (ELISA) method (High-sensitivity SARS-CoV-2 S1 IgG Quantitative ELISA, BioVendor) according to the suppliers’ protocols. Cytokine and antibody concentrations of incubated blood samples were compared with those of unprocessed control samples (0 h). For the sodium citrate (SC) experiments the sample tubes were filled with either 0.5 mL of 4% SC anticoagulant (Baxter, JB7747) or saline (0.9% sodium chloride, Baxter, JB 533) using sterile syringes and needles after cleaning and before incubation.
STATISTICAL ANALYSIS:
All statistical tests were performed using GraphPad Prism version 5.01. Statistical comparisons were performed using analyses of variance (ANOVAs): 1-way ANOVA test to analyze differences between the means of 2 or more independent groups and 2-way ANOVA test to analyze the difference between the means of more than 2 groups. Data are represented by means±standard error of the means (SEMs), and significance is defined by
Results
OPTIMAL CONDITIONS FOR PRODUCING ANTI-INFLAMMATORY AND ANTI-CATABOLIC MOLECULES VIA INCUBATED HUMAN BLOOD:
Producing IL-1ra in adherent human monocytes has been previously described [16,17]. The induction of IL-1ra protein synthesis by human peripheral mononuclear cells cultured in plastic tubes is achieved by serum activation, followed by exposure to granulocyte-macrophage colony-stimulating factor, endotoxin, and surface-bound immunoglobulin (IgG) [18]. To develop the optimal conditions to maximize therapeutic IL-1ra production in human blood cells in the absence of nonautologous biologically active protein, we examined different physical conditions for human blood incubation, concentrating on 2 major factors: the effect of internal surface material on IL-1ra production and the impact of incubation time and agitation on IL1-ra production.
EFFECT OF INTERNAL SURFACE MATERIAL ON IL-1RA PRODUCTION:
Glass surfaces have previously been shown to stimulate the activation of primary human monocytes and may result in increased IL-1ra concentration [19]. We compared IL-1ra levels in healthy donor blood incubated for 24 h at 37°C in either glass or polystyrene vacutainer tubes; a statistically significant increase in IL-1ra concentration was observed in the blood exposed to a glass surface (Figure 1A). This finding confirmed that the use of glass tubes increases IL-1ra production under the incubation conditions employed.
EFFECT OF INCUBATION TIME AND AGITATION ON IL1-RA PRODUCTION:
To maximize cell activation and IL-1ra secretion, the contact between the incubated blood cells and the internal glass surface of the collection tubes needs to be increased. Rocking platforms are widely used in small-scale cell cultures to distribute nutrients evenly [20]. We theorized that this platform could also be used to increase the exposure of incubated blood cells with the internal glass surface. We incubated human blood in glass vacutainer tubes at 37°C for 3.5 h or 24 h, with and without agitation on a rocker platform. We identified the optimal incubation time and evaluated the effect of agitation on cell activation and IL-1ra production. A 2-way ANOVA test revealed a significant increase of IL-1ra production in the blood after 24 h of incubation only; no significant increase in IL-1ra concentration was observed at 3.5 h (Figure 1B). Surprisingly, agitated cultures produced significantly lower levels of IL-1ra (Figure 1B), possibly due to the prevention of cell-cell contacts or insufficient adhesion to the glass surface, presumably adversely affecting monocyte activation and downregulating IL-1ra synthesis. An alternative explanation for this decrease could be that agitation may stimulate the production of IL-1ra inhibitors.
Monocytes and mononuclear phagocytes have been previously shown to produce TIMP-1 and TIMP-2 [21]. Since TIMPs are key inhibitors of MMPs, an increased concentration of TIMPs in autologous blood products (ABPs) could have an anti-catabolic effect. Hence, we examined the concentration levels of TIMP-1, TIMP-2, TIMP-3, and TIMP-4 in whole blood cells incubated using the optimal conditions for IL-1ra production (24 h at 37°C nonagitated). TIMP-1 and TIMP-2 levels were significantly increased after 24 h of incubation, whereas TIMP-3 and TIMP-4 concentrations did not drastically change (Figure 1C). TIMP-1 and TIMP-2 are significant MMP-2 and MMP-9 inhibitors (gelatinase A and B) [22], and their increased concentration in a prospective ABP has the potential to prevent a future lung injury [5–9].
BLOOD INCUBATION LED TO INCREASED MMP CONCENTRATIONS THAT COULD BE REDUCED WITH SC:
The desired anti-inflammatory IL-1ra and anti-catabolic TIMPs are produced by activated blood monocytes [16–18,21]; however, monocyte activation processes have also been associated with MMP upregulation [23], which could have a negative impact on the therapeutic effect of the final ABP [7–9]. To determine whether our optimized blood incubation conditions lead to catabolic molecular enrichment of MMPs, we assessed the concentrations of MMP-9, MMP-2, MMP-3, and MMP-7. After incubation at 37°C for 24 h, MMP-9 levels did indeed significantly increase compared with the base level (unincubated blood) (Figure 2A). In contrast, MMP-2, MMP-3, and MMP-7 did not display any significant change in concentration (Figure 2A). Since MMP-9 has been suggested to play a proactive role in lung epithelium inflammation, it is necessary to block its production in the final ABP. The secretion of MMPs from white blood cells is stimulated by the process of fibrinolysis [24], and MMP-9 levels are strongly affected by the presence of anticoagulants [25]. A well-known and safe anticoagulant is SC, which binds to calcium in the blood in a soluble complex and depresses prothrombin activity in a dose-dependent manner [26]. We reasoned that adding SC to incubated blood may affect the extracellular and intracellular calcium-dependent cell regulatory pathways, preventing MMP-9 production. This would allow us to avoid the potential MMP-9-induced negative catabolic effect in our prospective ABP treatment. To test this hypothesis, we examined the MMP-9 concentration in untreated blood and blood incubated in the presence of SC at a 1: 19 ratio (4%). We observed an increase in MMP-9 concentration after 24 h of incubation (Figure 2B), but the production of MMP-9 was significantly downregulated with the addition of SC (Figure 2B). The final ABP containing incubated blood and SC was found to contain similar MMP-9 levels to the nonincubated control.
Previous studies have shown that monocyte incubation in the presence of human serum stimulates the production of IL-1β pro-inflammatory cytokine [16]. Additionally, the cultivation of human monocytes on glass in vitro has been shown to increase their cytotoxic activities [27]. Furthermore, upregulated levels of the pro-inflammatory cytokines TNF-α and IL-1 have also been reported in incubated human serum [28,29]. To investigate whether the incubation protocol results in increased levels of pro-inflammatory cytokines in the final product, we compared TNF-α and IL-1β concentrations in nonincubated blood, incubated blood, and the final product (ABP+SC). As was expected, the incubated blood with no SC displayed a significant increase in TNF-α and IL-1β concentrations (Figure 3A, 3B), potentially generating adverse events if used as a treatment method. This increase in pro-inflammatory factors was successfully blocked by SC addition prior to incubation (Figure 3A, 3B), and this blockage by SC was found to be dose-dependent (Figure 3C, 3D). The effect of SC on the ABP allows us to negate the concerns regarding pro-inflammatory factors in the ABP, facilitating the use of ABP as a potentially valuable treatment option.
SC DOES NOT AFFECT ANTI-INFLAMMATORY, ANTI-CATABOLIC, OR REGENERATIVE FACTOR ENRICHMENT:
Calcium is known to be a physiological regulator of protein synthesis [30]; therefore, its extracellular depletion by SC might also result in the decreased enrichment of the desired anti-inflammatory (IL-1ra) and anti-catabolic (TIMPs) factors in incubated blood. We compared the concentrations of target therapeutic molecules (IL-1ra, platelet-derived growth factor [PDGF], TIMP-1, and TIMP-2) in the blood samples incubated in the presence or absence of SC (Figure 4A–4D). There was no substantial reduction in the concentration of IL-1ra, PDGF, TIMP-1, or TIMP-2 caused by the presence of SC. Thus, although we found SC to be a negative regulator of TNF-α, IL-1, and MMP-9 (Figure 3) as desired, it did not affect the enrichment of anti-inflammatory or anti-catabolic proteins (Figure 4).
A PROSPECTIVE AUTOLOGOUS TREATMENT FOR COVID-1-INDUCED CYTOKINE STORM:
The data presented above have revealed an efficient technique, which employs the incubation of venous blood at 37°C for 24 h in glass vacutainer tubes in the presence of SC, for producing excessive concentrations of IL-1ra, TIMP-1, and TIMP-2 by human blood cells. To optimize our existing protocol and analyze how this protocol would be efficient for blood cells previously exposed to SARS-CoV-2, we measured IL-1ra, TIMP-1, and TIMP-2 concentrations in samples from recovered COVID-19 patients after incubations of 6 or 24 h (at 37°C with 4% SC). One-way ANOVA tests revealed significant IL-1ra enrichment in the samples of immunized patients starting at 6 h of incubation (Figure 5A). Increased concentrations of TIMP-1 and TIMP-2 in unincubated control samples from recovered COVID-19 patients were observed compared with the nonexposed healthy donors (Figure 4B, 4C vs Figure 5B, 5C; TIMP-1 in healthy blood was 6000 pg/mL vs 20 962.3 pg/mL in COVID recovered blood, and TIMP-2 in healthy blood was 3900 pg/mL vs 24 204.1 pg/mL in COVID-19 recovered blood). Significant TIMP-1 enrichment was detected after 6 h of incubation in recovered COVID-19 patients’ blood samples (Figure 5B); however, the concentration of TIMP-2 did not change compared to the baseline (Figure 5C). We were also able to confirm that the protocol to produce an ABP enriched in IL-1ra and TIMP-1 from donor patients recovered from COVID-19 does not upregulate the pro-inflammatory cytokines IL-1 or TNF-α or the production of catabolic MMP-9 (Figure 6A–6C). Since it is important to consider the potential of using this protocol to create an ABP capable of preventing a cytokine storm in COVID-19 patients, it is essential to confirm that this method does not affect the concentration of already formed anti-SARS-CoV-2 antibodies. To explore this, we compared SARS-CoV-2 IgG concentration levels before and after the 6-h incubation, and we found no downregulation of the antibody concentration (Figure 6D).
Discussion
The ongoing COVID-19 pandemic has a high morbidity and mortality rate worldwide. Despite extraordinary efforts from governments and scientific communities, including employing safety measures and accelerated vaccine development, COVID-19 deaths will continue to rise. Furthermore, SARS-CoV-2 changes rapidly by acquiring genetic mutations and will plausibly generate more contagious variants. Accumulating evidence suggests that the high mortality and severity of the SARS-CoV-2 infection is directly associated with generating a cytokine storm [31]. IL-1, TNF-α, and IL-6 have been consistently reported as being central to cytokine storm regulation. A treatment algorithm based on effective inhibition of pro-inflammatory cytokines could be crucial for improving patient outcomes [32]; for example, Anakinra, a recombinant humanized IL-1 receptor antagonist, has been effectively used to treat MAS [33]. Recent data have indicated that IL-6 holds a key role in cytokine storm pathophysiology and lung tissue damage, probably by inducing MMP-9 expression in macrophages via Cox-2-dependent and -independent mechanisms [34]. An ABP containing therapeutically active proteins that target specific inflammatory molecular pathways, such as IL-1 or MMP-9, could provide a potential strategy to treat or manage inflammatory pathologies, including the COVID-19-induced cytokine storm.
An autologous blood product containing therapeutically active proteins that target specific inflammatory molecular pathways is a potential strategy to treat or manage inflammatory pathologies [35,36]. Thus, an autologous blood treatment product Orthokine [37] exploits the ability of incubated blood cells to produce an increasing concentration of IL-1ra in the absence of pro-inflammatory cytokine enrichment. Orthokine was recently introduced to the osteoarthritis market and was found to be effective in clinical trials [37]. However, others have reported a large variability in the concentration of growth factors and anti-inflammatory cytokines measured in this product, with significantly high levels of IL-1b, TNF-α, and IL-6 detected in the final product [28,38] that would negatively affect the safety of using it therapeutically. Here we report a novel method for creating an ABP containing an anti-inflammatory and anti-catabolic component (conditioned blood serum enriched with IL-1ra, TIMP-1, and TIMP-2) based on detailed cytokine analysis. Our experimental data also revealed that an approximately 10-fold enrichment of IL-1ra is achieved by incubating whole human blood in a closed vacutainer system at 37°C for 24 h (Figures 1, 2). Although previous reports argue that the therapeutic concentration of IL-1ra is sufficient to saturate IL-1 receptors and block the IL-1 pathway [39,40], the results of our preliminary clinical trial (data not shown) indicate that the 10-fold enrichment we achieve is effective enough to reduce inflammation symptoms in osteoarthritic knees, presumably due to the synergistic effect of TIMP-1 and TIMP-2 proteins. In an attempt to minimize blood tissue manipulation and exposure to a potentially hazardous environment, we utilized a closed vacutainer glass system for the incubation process. A glass surface was previously found to be an efficient inducer of IL-1ra production [19], and we confirmed this experimentally for the system presented here (Figure 1).
The present study demonstrated for the first time that conditioned blood serum contains increased levels of MMP-9 (Figure 2). Fortunately, we determined that modifying the process of preparing the ABP by adding 4% SC to the patients’ blood before incubation blocks MMP-9 (Figure 2). The mechanism behind this may involve limiting the mobilization of gelatinase-rich granules from human neutrophils and/or altering plasmin activation that upregulates MMP secretion from white blood cells [25,41,42]. Since MMPs are calcium-dependent proteinases [43] SC-induced extracellular calcium concentration changes may negatively affect MMP-9 enrichment in the incubated blood. Our data demonstrated that the incubation process leads to excessive IL-1 and TNF-α production (Figure 3), which is consistent with previously published results [28,29]. Activated monocytes produce therapeutically active IL-1ra during incubation [16–18]; however, the same activation causes the release of pro-inflammatory cytokines with systemic toxicity and pyrogenic potential when administrated as part of the treatment [16,23,27]. This study is the first to demonstrate that supplementation with 4% SC can block IL-1β and TNF-α enrichment in a dose-dependent manner (Figure 3). It has been previously shown that IL-1β and MMP-9 transcription is affected by thrombin [44], so it would be interesting to study the molecular and chemical mechanisms of SC-mediated TNF-α and IL-1β downregulation in monocyte cultures.
In an attempt to create an autologous treatment for the COVID-19-mediated cytokine storm, we validated this protocol for COVID-19-recovered patients. Approximately 10-fold IL-1ra enrichment was shown after 6 h of incubation, presenting a great advantage in reducing incubation time (Figure 5). Surprisingly, elevated TIMP-1 and TIMP-2 concentrations were detected in control samples from recovered COVID-19 patients versus healthy volunteer donors. Evidently, viral infections can stimulate long-term TIMP-1 and TIMP-2 production by human blood cells. Additionally, the incubation process upregulated the concentration of TIMP-1 but not that of TIMP-2. We provide evidence that the optimal blood incubation conditions for IL-1ra, TIMP-1, and TIMP-2 production facilitate the synthesis of pro-inflammatory IL-1β, TNF-α, and catabolic MMP-9; however, this can be simply rectified by addition of SC. In comparison, our process did not demonstrate any effect on the concentration of SARS-CoV-2 IgG. Our data have demonstrated a simple protocol for creating ABP for the potential treatment of cytokine storms, including those caused by COVID-19, which is now extremely important globally.
Conclusions
Our present in vitro study demonstrates that ABP (Cytorich) containing IL-1β and MMP-9 antagonists has a potentially promising therapeutic effect for treating virus-induced cytokine storms. Similar inflammatory pathways are also involved in the pathogenesis of other immune pathological conditions, such as hemophagocytic lymphohistiocytosis/MAS-like syndrome, as well as septic shock. Hence, it is likely that this method and ABP will be beneficial in the treatment of various pathologies. Imminent future research is critical to facilitate the use of this ABP in treatment; large, double-blind controlled clinical studies will be required to confirm the clinical benefits for a new ABP treatment.
Figures
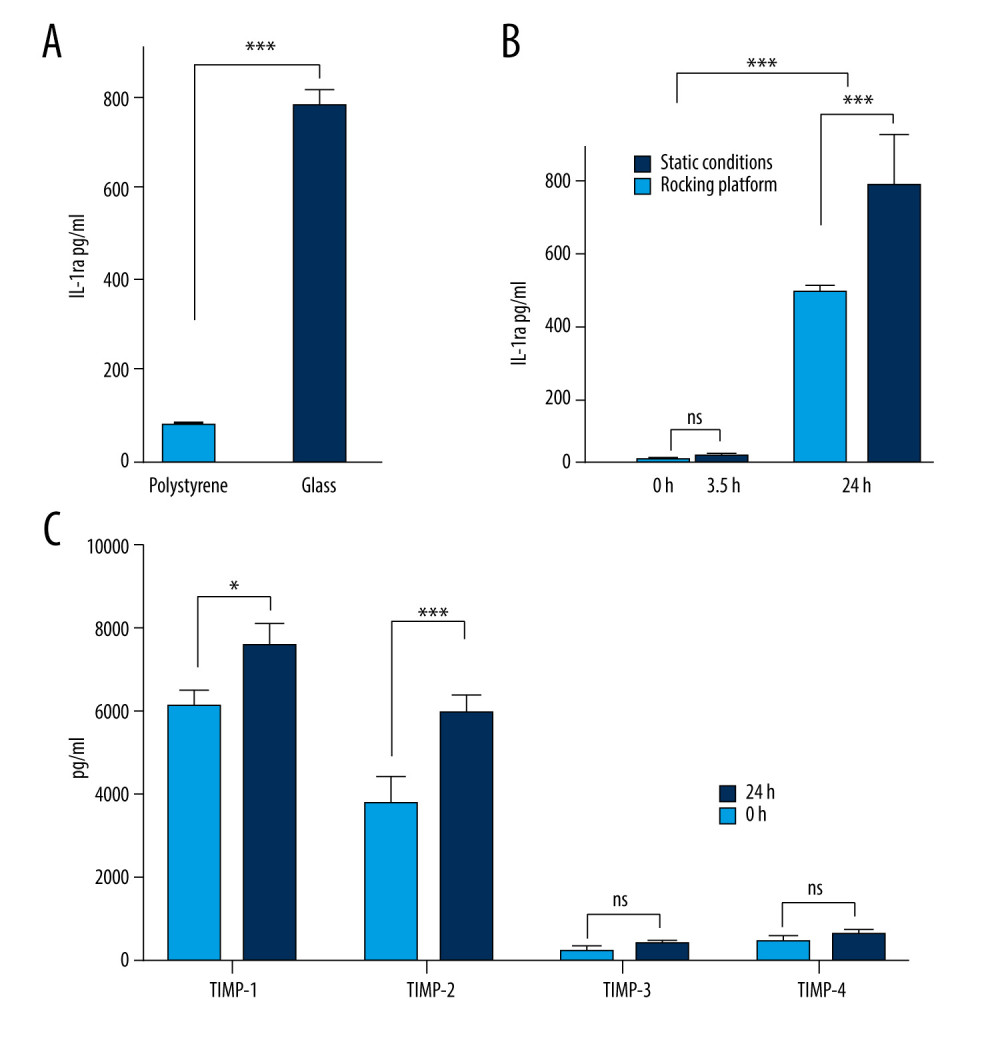 Figure 1. Production of anti-inflammatory or anti-catabolic molecules in incubated healthy human blood samples. (A) Interleukin (IL)-1ra production is significantly increased by incubation in glass surfaces (*** P<0.0001). (B) Comparison of the IL-1ra levels in human blood samples incubated for 3.5 h or 24 h, in the presence or absence of agitation (static vs rocking; * P<0.001). (C) Analysis of tissue inhibitor of metalloproteinase (TIMP)-1, TIMP-2, TIMP-3, and TIMP-4 concentrations in human blood samples before (baseline level) and after incubation at 37°C for 24 h. Two-way revealed a statistically significant (* P<0.001, *** P<0.0001) increase in the levels of TIMP-1 and TIMP-2 at 24 h after incubation. The figure was created using ImageJ software version 1.53e; Java 1.8.0_172.
Figure 1. Production of anti-inflammatory or anti-catabolic molecules in incubated healthy human blood samples. (A) Interleukin (IL)-1ra production is significantly increased by incubation in glass surfaces (*** P<0.0001). (B) Comparison of the IL-1ra levels in human blood samples incubated for 3.5 h or 24 h, in the presence or absence of agitation (static vs rocking; * P<0.001). (C) Analysis of tissue inhibitor of metalloproteinase (TIMP)-1, TIMP-2, TIMP-3, and TIMP-4 concentrations in human blood samples before (baseline level) and after incubation at 37°C for 24 h. Two-way revealed a statistically significant (* P<0.001, *** P<0.0001) increase in the levels of TIMP-1 and TIMP-2 at 24 h after incubation. The figure was created using ImageJ software version 1.53e; Java 1.8.0_172. ![Optimal blood incubation conditions for interleukin (IL)1-ra production facilitate the synthesis of matrix metalloproteinase (MMP)-9, which is reversed by sodium citrate (SC) addition. (A) Comparison of the MMP-9, MMP-2, MMP-3, and MMP-7 protein concentrations in human blood samples before (baseline level) and after incubation at 37°C for 24 h. Two-way analysis of variance (ANOVA) revealed a statistically significant increase in the levels of MMP-9 at 24 h after incubation (*** P<0.001; nonsignificant [ns], P>0.01). (B) Two-way ANOVA revealed that the presence of SC has a negative impact on MMP-9 secretion by blood cells compared with saline controls (* P<0.01). The figure was created using ImageJ software version 1.53e; Java 1.8.0_172.](https://jours.isi-science.com/imageXml.php?i=medscimonit-27-e934365-g002.jpg&idArt=934365&w=1000) Figure 2. Optimal blood incubation conditions for interleukin (IL)1-ra production facilitate the synthesis of matrix metalloproteinase (MMP)-9, which is reversed by sodium citrate (SC) addition. (A) Comparison of the MMP-9, MMP-2, MMP-3, and MMP-7 protein concentrations in human blood samples before (baseline level) and after incubation at 37°C for 24 h. Two-way analysis of variance (ANOVA) revealed a statistically significant increase in the levels of MMP-9 at 24 h after incubation (*** P<0.001; nonsignificant [ns], P>0.01). (B) Two-way ANOVA revealed that the presence of SC has a negative impact on MMP-9 secretion by blood cells compared with saline controls (* P<0.01). The figure was created using ImageJ software version 1.53e; Java 1.8.0_172.
Figure 2. Optimal blood incubation conditions for interleukin (IL)1-ra production facilitate the synthesis of matrix metalloproteinase (MMP)-9, which is reversed by sodium citrate (SC) addition. (A) Comparison of the MMP-9, MMP-2, MMP-3, and MMP-7 protein concentrations in human blood samples before (baseline level) and after incubation at 37°C for 24 h. Two-way analysis of variance (ANOVA) revealed a statistically significant increase in the levels of MMP-9 at 24 h after incubation (*** P<0.001; nonsignificant [ns], P>0.01). (B) Two-way ANOVA revealed that the presence of SC has a negative impact on MMP-9 secretion by blood cells compared with saline controls (* P<0.01). The figure was created using ImageJ software version 1.53e; Java 1.8.0_172. ![Sodium citrate (SC) prevents pro-inflammatory cytokine enrichment in a dose-dependent manner. Analysis of interleukin (IL)-1β (A) and tumor necrosis factor (TNF)-α (B) concentrations in human blood samples before incubation (1) or after incubation at 37°C for 24 h in the presence of either saline (2) or 4% SC (3). Dose-dependent effects of SC concentration on IL-1β (C) and TNF-α (D) production. The concentration in human blood samples is shown before incubation (1), after incubation at 37°C for 24 h (2), after incubation with the SC 5-fold less than the working concentration (3), and after incubation with the SC at 4% working concentration (4). A 2-way analysis of variance (ANOVA) reveals a statistically significant decrease in IL-1β and TNF-α concentrations in 24-h incubated blood in the presence of SC in a dose-dependent manner, compared with the saline control (*** P<0.001; nonsignificant [ns], P>0.01). The figure was created using ImageJ software version 1.53e; Java 1.8.0_172.](https://jours.isi-science.com/imageXml.php?i=medscimonit-27-e934365-g003.jpg&idArt=934365&w=1000) Figure 3. Sodium citrate (SC) prevents pro-inflammatory cytokine enrichment in a dose-dependent manner. Analysis of interleukin (IL)-1β (A) and tumor necrosis factor (TNF)-α (B) concentrations in human blood samples before incubation (1) or after incubation at 37°C for 24 h in the presence of either saline (2) or 4% SC (3). Dose-dependent effects of SC concentration on IL-1β (C) and TNF-α (D) production. The concentration in human blood samples is shown before incubation (1), after incubation at 37°C for 24 h (2), after incubation with the SC 5-fold less than the working concentration (3), and after incubation with the SC at 4% working concentration (4). A 2-way analysis of variance (ANOVA) reveals a statistically significant decrease in IL-1β and TNF-α concentrations in 24-h incubated blood in the presence of SC in a dose-dependent manner, compared with the saline control (*** P<0.001; nonsignificant [ns], P>0.01). The figure was created using ImageJ software version 1.53e; Java 1.8.0_172.
Figure 3. Sodium citrate (SC) prevents pro-inflammatory cytokine enrichment in a dose-dependent manner. Analysis of interleukin (IL)-1β (A) and tumor necrosis factor (TNF)-α (B) concentrations in human blood samples before incubation (1) or after incubation at 37°C for 24 h in the presence of either saline (2) or 4% SC (3). Dose-dependent effects of SC concentration on IL-1β (C) and TNF-α (D) production. The concentration in human blood samples is shown before incubation (1), after incubation at 37°C for 24 h (2), after incubation with the SC 5-fold less than the working concentration (3), and after incubation with the SC at 4% working concentration (4). A 2-way analysis of variance (ANOVA) reveals a statistically significant decrease in IL-1β and TNF-α concentrations in 24-h incubated blood in the presence of SC in a dose-dependent manner, compared with the saline control (*** P<0.001; nonsignificant [ns], P>0.01). The figure was created using ImageJ software version 1.53e; Java 1.8.0_172. ![Sodium citrate (SC) does not affect anti-inflammatory, anti-catabolic, and regenerative protein enrichment. Protein concentrations of interleukin (IL)-1ra (A), platelet-derived growth factor (PDGF) (B), tissue inhibitor of metalloproteinase (TIMP)-1 (C), and TIMP-2 (D) in human blood samples are shown before incubation (1), after incubation at 37°C for 24 h in the presence of saline as a control (2), and after incubation at 37°C for 24 h in the presence of 4% SC (3). One-way analysis of variance (ANOVA) tests reveal that SC did not decrease the protein concentrations in 24-h incubated blood or in the final product (* P<0.01; *** P<0.0001; nonsignificant [ns], P>0.01). The figure was created using ImageJ software version 1.53e; Java 1.8.0_172.](https://jours.isi-science.com/imageXml.php?i=medscimonit-27-e934365-g004.jpg&idArt=934365&w=1000) Figure 4. Sodium citrate (SC) does not affect anti-inflammatory, anti-catabolic, and regenerative protein enrichment. Protein concentrations of interleukin (IL)-1ra (A), platelet-derived growth factor (PDGF) (B), tissue inhibitor of metalloproteinase (TIMP)-1 (C), and TIMP-2 (D) in human blood samples are shown before incubation (1), after incubation at 37°C for 24 h in the presence of saline as a control (2), and after incubation at 37°C for 24 h in the presence of 4% SC (3). One-way analysis of variance (ANOVA) tests reveal that SC did not decrease the protein concentrations in 24-h incubated blood or in the final product (* P<0.01; *** P<0.0001; nonsignificant [ns], P>0.01). The figure was created using ImageJ software version 1.53e; Java 1.8.0_172.
Figure 4. Sodium citrate (SC) does not affect anti-inflammatory, anti-catabolic, and regenerative protein enrichment. Protein concentrations of interleukin (IL)-1ra (A), platelet-derived growth factor (PDGF) (B), tissue inhibitor of metalloproteinase (TIMP)-1 (C), and TIMP-2 (D) in human blood samples are shown before incubation (1), after incubation at 37°C for 24 h in the presence of saline as a control (2), and after incubation at 37°C for 24 h in the presence of 4% SC (3). One-way analysis of variance (ANOVA) tests reveal that SC did not decrease the protein concentrations in 24-h incubated blood or in the final product (* P<0.01; *** P<0.0001; nonsignificant [ns], P>0.01). The figure was created using ImageJ software version 1.53e; Java 1.8.0_172. ![Production of anti-inflammatory and anti-catabolic molecules in incubated blood samples from recovered COVID-19 patients. Blood samples from immunized (COVID-19 recovered) patients were incubated for 0, 6, or 24 h at 37°C with 4% sodium citrate (SC) and then analyzed for concentrations of interleukin (IL)-1ra (A), tissue inhibitor of metalloproteinase (TIMP)-1 (B), and TIMP-2 (C). IL-1ra (A) was significantly enriched starting after 6 h of incubation in 37°C (1-way ANOVA test). TIMP-1 was significantly enriched after 6 h of incubation (B), whereas TIMP-2 concentration remained unchanged (C) (1-way ANOVA test; *** P<0.0001; nonsignificant [ns], P>0.01). The figure was created using ImageJ software version 1.53e; Java 1.8.0_172.](https://jours.isi-science.com/imageXml.php?i=medscimonit-27-e934365-g005.jpg&idArt=934365&w=1000) Figure 5. Production of anti-inflammatory and anti-catabolic molecules in incubated blood samples from recovered COVID-19 patients. Blood samples from immunized (COVID-19 recovered) patients were incubated for 0, 6, or 24 h at 37°C with 4% sodium citrate (SC) and then analyzed for concentrations of interleukin (IL)-1ra (A), tissue inhibitor of metalloproteinase (TIMP)-1 (B), and TIMP-2 (C). IL-1ra (A) was significantly enriched starting after 6 h of incubation in 37°C (1-way ANOVA test). TIMP-1 was significantly enriched after 6 h of incubation (B), whereas TIMP-2 concentration remained unchanged (C) (1-way ANOVA test; *** P<0.0001; nonsignificant [ns], P>0.01). The figure was created using ImageJ software version 1.53e; Java 1.8.0_172.
Figure 5. Production of anti-inflammatory and anti-catabolic molecules in incubated blood samples from recovered COVID-19 patients. Blood samples from immunized (COVID-19 recovered) patients were incubated for 0, 6, or 24 h at 37°C with 4% sodium citrate (SC) and then analyzed for concentrations of interleukin (IL)-1ra (A), tissue inhibitor of metalloproteinase (TIMP)-1 (B), and TIMP-2 (C). IL-1ra (A) was significantly enriched starting after 6 h of incubation in 37°C (1-way ANOVA test). TIMP-1 was significantly enriched after 6 h of incubation (B), whereas TIMP-2 concentration remained unchanged (C) (1-way ANOVA test; *** P<0.0001; nonsignificant [ns], P>0.01). The figure was created using ImageJ software version 1.53e; Java 1.8.0_172. ![Optimal blood incubation condition protocol for interleukin (IL)-1ra and tissue inhibitor of metalloproteinase (TIMP) enrichment did not affect SARS-CoV-2 immunoglobulin (Ig)G concentrations. Samples from immunized patients were analyzed for matrix metalloproteinase (MMP)-9 (A), IL-1β (B), tumor necrosis factor (TNF)-α (C), and anti- SARS-CoV-2 IgG (D) concentrations in control or after 6- or 24-h incubation at 37°C with 4% sodium citrate (SC) (nonsignificant [ns], P>0.01). The figure was created using ImageJ software version 1.53e; Java 1.8.0_172.](https://jours.isi-science.com/imageXml.php?i=medscimonit-27-e934365-g006.jpg&idArt=934365&w=1000) Figure 6. Optimal blood incubation condition protocol for interleukin (IL)-1ra and tissue inhibitor of metalloproteinase (TIMP) enrichment did not affect SARS-CoV-2 immunoglobulin (Ig)G concentrations. Samples from immunized patients were analyzed for matrix metalloproteinase (MMP)-9 (A), IL-1β (B), tumor necrosis factor (TNF)-α (C), and anti- SARS-CoV-2 IgG (D) concentrations in control or after 6- or 24-h incubation at 37°C with 4% sodium citrate (SC) (nonsignificant [ns], P>0.01). The figure was created using ImageJ software version 1.53e; Java 1.8.0_172.
Figure 6. Optimal blood incubation condition protocol for interleukin (IL)-1ra and tissue inhibitor of metalloproteinase (TIMP) enrichment did not affect SARS-CoV-2 immunoglobulin (Ig)G concentrations. Samples from immunized patients were analyzed for matrix metalloproteinase (MMP)-9 (A), IL-1β (B), tumor necrosis factor (TNF)-α (C), and anti- SARS-CoV-2 IgG (D) concentrations in control or after 6- or 24-h incubation at 37°C with 4% sodium citrate (SC) (nonsignificant [ns], P>0.01). The figure was created using ImageJ software version 1.53e; Java 1.8.0_172. References
1. Tisoncik J, Korth M, Simmons C, Into the eye of the cytokine storm: Microbiol Mol Biol Rev, 2012; 76(1); 14-32
2. Shakoory B, Carcillo JA, Chatham WW, Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial: Crit Care Med, 2016; 44(2); 275-81
3. Nee LE, McMorrow T, Campbell E, TNF-alpha and IL-1β-mediated regulation of MMP-9 and TIMP-1 in renal proximal tubular cells: Kidney Int, 2004; 66(4); 1376-86
4. Origuchi T, Migita K, Nakashima T, IL-1-mediated expression of membrane type matrix-metalloproteinase in rheumatoid osteoblasts: Clin Exp Rheumatol, 2000; 18; 333-39
5. Elkington PT, Friedland JS, Matrix metalloproteinases in destructive pulmonary pathology: Thorax, 2006; 61(3); 259-66
6. Je H, Suk M, Yoon D, Inhibition of matrix metalloproteinase-9 prevents neutrophilic inflammation in ventilator-induced lung injury: Am J Physiol Lung Cell Mol Physiol, 2006; 291(4); 580-87
7. Albaiceta G, Gutiérrez-Fernández A, Parra D, Lack of matrix metalloproteinase-9 worsens ventilator-induced lung injury: Am J Physiol Lung Cell Mol Physiol, 2008; 294(3); 535-43
8. Vermeer P, Denker J, Estin M, MMP9 modulates tight junction integrity and cell viability in human airway epithelia: Am J Physiol Lung Cell Mol Physiol, 2009; 296(5); 751-62
9. Rojas-Quintero J, Wang X, Tipper J, Matrix metalloproteinase-9 deficiency protects mice from severe influenza A viral infection: J Clin Invest, 2018; 3(24); e99022
10. Woolley E, Roberts R, Evanson M, Inhibition of human collagenase activity by a small molecular weight plasma protein: Biochem Biophys Res Commun, 1975; 66; 747-54
11. Page-McCaw A, Ewald AJ, Werb Z, Matrix metalloproteinases and the regulation of tissue remodelling: Nat Rev Mol Cell Biol, 2007; 8; 221-33
12. Hernandez-Barrantes S, Toth M, Bernardo MM, Binding of active (57 kDa) membrane type 1-matrix metalloproteinase (MT1-MMP) to tissue inhibitor of metalloproteinase (TIMP)-2 regulates MT1-MMP processing and pro-MMP-2 activation: J Biol Chem, 2000; 275(16); 12080-89
13. Strongin Y, Collier I, Bannikov G, Mechanism of cell surface activation of 72-kDa type IV collagenase. Isolation of the activated form of the membrane metalloprotease: J Biol Chem, 1995; 270; 5331-38
14. Agarwal A, Mukher A, Kumar G, Convalescent plasma in the management of moderate covid-19 in adults in India: Open label phase II multicentre randomised controlled trial (PLACID Trial) [erratum BMJ. 2020;371:4232]: BMJ, 2020; 371; 3939
15. Wesley H, Thomas G, Stewart , Passive Immunity Trial for Our Nation (PassITON): study protocol for a randomized placebo-control clinical trial evaluating COVID-19 convalescent plasma in hospitalized adults: BMC Trials, 2021; 22; 221
16. Danis A, Kulesz J, Nelson S, Brooks M, Cytokine regulation of human monocyte interleukin-1 (IL-1) production in vitro. Enhancement of IL-1 production by interferon (IFN) gamma, tumour necrosis factor-alpha, IL-2 and IL-1, and inhibition by IFN-alpha: Clin Exp Immunol, 1990; 80; 435-43
17. Hannum H, Wilcox J, Arend P, Interleukin-1 receptor antagonist activity of a human interleukin-1 inhibitor: Nature, 1990; 25(343); 336-40
18. Poutsiaka D, Clark D, Vannier E, Dinarello C, Production of interleukin-1 receptor antagonist and interleukin-1 β by peripheral blood mononuclear cells is differentially regulated: Blood, 1991; 1(78); 1275-81
19. Linder S, Pinkowski W, Aepfelbacher M, Adhesion. Cytoskeletal architecture and activation status of primary human macrophages on a diamond-like carbon coated surface: Biomaterials, 2002; 23; 767-73
20. Singh V, Disposable bioreactor for cell culture using wave-induced agitation: Cytotechnology, 1999; 30; 149-58
21. Shapiro D, Kobayashi K, Welgus G, Identification of TIMP-2 in human alveolar macrophages. Regulation of biosynthesis is opposite to that of metalloproteinases and TIMP-1: J Biol Chem, 1992; 15(267); 13890-94
22. Reynolds J, Collagenases and tissue inhibitors of metalloproteinases: A functional balance in tissue degradation: Oral Dis, 1996; 2; 70-76
23. Elkington T, Green A, Friedland S, Analysis of matrix metalloproteinase secretion by macrophages: Methods Mol Biol, 2009; 531; 253-65
24. Menshikov Y, Elizarova P, Kudryashova E, Plasmin-independent gelatinase B (matrix metalloproteinase-9) release by monocytes under the influence of urokinase: Biochemistry (Mosc), 2001; 66; 954-59
25. Makowski S, Ramsby L, Use of citrate to minimize neutrophil matrix metalloproteinase-9 in human plasma: Anal Biochem, 2003; 15(322); 283-86
26. Quick J, Stefanini M, The chemical state of the calcium reacting in the coagulation of blood: J Gen Physiol, 1948; 32; 191-202
27. Kaplan G, In vitro differentiation of human monocytes. Monocytes cultured on glass are cytotoxic to tumor cells but monocytes cultured on collagen are not: J Exp Med, 1983; 15; 2061-72
28. Rutgers M, Saris B, Dhert J, Creemers B, Cytokine profile of autologous conditioned serum for treatment of osteoarthritis, in vitro effects on cartilage metabolism and intra-articular levels after injection: Arthritis Res Ther, 2010; 12; 114
29. Sawyere M, Lanz I, Dahlgren A, Cytokine and growth factor concentrations in canine autologous conditioned serum: Vet Surg, 2016; 45; 582-86
30. Chin V, Cade C, Brostrom CO, Calcium-dependent regulation of protein synthesis at translational initiation in eukaryotic cells: J Biol Chem, 1987; 262; 16509-14
31. Hojyo S, Uchida M, Tanaka K, How COVID-19 induces cytokine storm with high mortality: Inflamm Regen, 2020; 40; 37
32. Peng X, Wang Y, Xi X, Promising therapy for heart failure in patients with severe COVID-19: Calming the cytokine storm: Cardiovasc Drugs Ther, 2021; 35; 231-47
33. Rajasekaran S, Kruse K, Kovey K, Therapeutic role of anakinra, an interleukin-1 receptor antagonist, in the management of secondary hemophagocytic lymphohistiocytosis/sepsis/multiple organ dysfunction/macrophage activating syndrome in critically ill children: Pediatr Crit Care Med, 2014; 15(5); 401-8
34. Kothari P, Pestana R, Mesraoua R, IL-6–Mediated induction of matrix metalloproteinase-9 is modulated by JAK-dependent IL-10 expression in macrophages: J Immunol, 2014; 192(1); 10
35. Meijer H, Reinecke J, Becker C, The production of anti-inflammatory cytokines in whole blood by physico-chemical induction: Inflamm Res, 2003; 52; 404-7
36. van Drumpt R, van der Weegen W, King W, Safety and treatment effectiveness of a single autologous protein solution injection in patients with knee osteoarthritis: Biores Open Access, 2016; 5(1); 261-68
37. Baltzer AW, Moer C, Jansen A, Krauspe R, Autologous conditioned serum (Orthokine) is an effective treatment for knee osteoarthritis: Osteoarthritis Cartilage, 2009; 17; 152-60
38. Sawyere M, Lanz I, Dahlgren A, Cytokine and growth factor concentrations in canine autologous conditioned serum: Vet Surg, 2016; 45; 582-86
39. Eming A, Krieg T, Davidson JM, Inflammation in wound repair: Molecular and cellular mechanisms: J Invest Dermatol, 2007; 127; 514
40. Granowitz V, Clark D, Mancilla J, Dinarello CA, Interleukin-1 receptor antagonist competitively inhibits the binding of interleukin-1 to the type II interleukin-1 receptor: J Biol Chem, 1991; 266; 14147-50
41. Wilhelm M, Collier E, Marmer L, SV40-transformed human lung fibroblasts secrete a 92-kDa type IV collagenase which is identical to that secreted by normal human macrophages: J Biol Chem, 1989; 264; 17213-21
42. Mollinedo F, Nakajima M, Llorens A, Major co-localization of the extracellular-matrix degradative enzymes heparanase and gelatinase in tertiary granules of human neutrophils: Biochem J, 1997; 327; 917-23
43. Fosang J, Neame J, Last K, The interglobular domain of cartilage aggrecan is cleaved by PUMP, gelatinases, and cathepsin B: J Biol Chem, 1992; 267; 19470-74
44. Chang J, Hsu A, Ko H, Thrombin regulates matrix metalloproteinase-9 expression in human monocytes: Biochem Biophys Res Commun, 2009; 385; 241-46
Figures
 Figure 1. Production of anti-inflammatory or anti-catabolic molecules in incubated healthy human blood samples. (A) Interleukin (IL)-1ra production is significantly increased by incubation in glass surfaces (*** P<0.0001). (B) Comparison of the IL-1ra levels in human blood samples incubated for 3.5 h or 24 h, in the presence or absence of agitation (static vs rocking; * P<0.001). (C) Analysis of tissue inhibitor of metalloproteinase (TIMP)-1, TIMP-2, TIMP-3, and TIMP-4 concentrations in human blood samples before (baseline level) and after incubation at 37°C for 24 h. Two-way revealed a statistically significant (* P<0.001, *** P<0.0001) increase in the levels of TIMP-1 and TIMP-2 at 24 h after incubation. The figure was created using ImageJ software version 1.53e; Java 1.8.0_172.
Figure 1. Production of anti-inflammatory or anti-catabolic molecules in incubated healthy human blood samples. (A) Interleukin (IL)-1ra production is significantly increased by incubation in glass surfaces (*** P<0.0001). (B) Comparison of the IL-1ra levels in human blood samples incubated for 3.5 h or 24 h, in the presence or absence of agitation (static vs rocking; * P<0.001). (C) Analysis of tissue inhibitor of metalloproteinase (TIMP)-1, TIMP-2, TIMP-3, and TIMP-4 concentrations in human blood samples before (baseline level) and after incubation at 37°C for 24 h. Two-way revealed a statistically significant (* P<0.001, *** P<0.0001) increase in the levels of TIMP-1 and TIMP-2 at 24 h after incubation. The figure was created using ImageJ software version 1.53e; Java 1.8.0_172.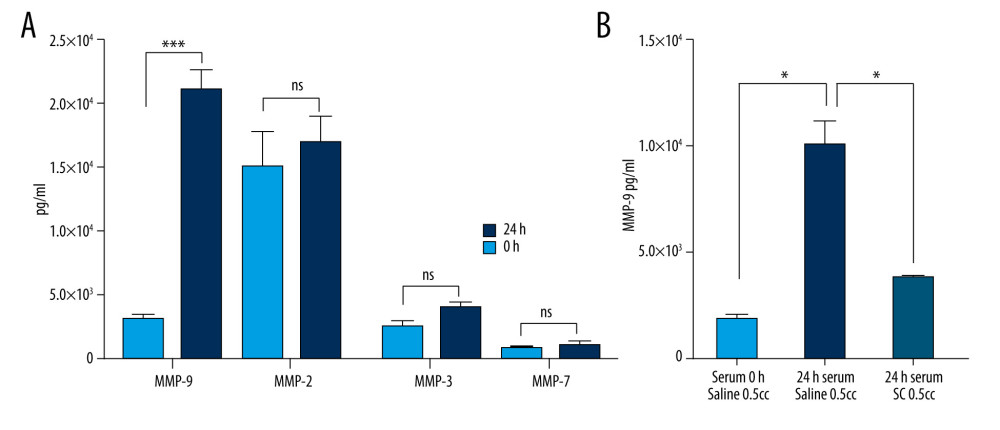 Figure 2. Optimal blood incubation conditions for interleukin (IL)1-ra production facilitate the synthesis of matrix metalloproteinase (MMP)-9, which is reversed by sodium citrate (SC) addition. (A) Comparison of the MMP-9, MMP-2, MMP-3, and MMP-7 protein concentrations in human blood samples before (baseline level) and after incubation at 37°C for 24 h. Two-way analysis of variance (ANOVA) revealed a statistically significant increase in the levels of MMP-9 at 24 h after incubation (*** P<0.001; nonsignificant [ns], P>0.01). (B) Two-way ANOVA revealed that the presence of SC has a negative impact on MMP-9 secretion by blood cells compared with saline controls (* P<0.01). The figure was created using ImageJ software version 1.53e; Java 1.8.0_172.
Figure 2. Optimal blood incubation conditions for interleukin (IL)1-ra production facilitate the synthesis of matrix metalloproteinase (MMP)-9, which is reversed by sodium citrate (SC) addition. (A) Comparison of the MMP-9, MMP-2, MMP-3, and MMP-7 protein concentrations in human blood samples before (baseline level) and after incubation at 37°C for 24 h. Two-way analysis of variance (ANOVA) revealed a statistically significant increase in the levels of MMP-9 at 24 h after incubation (*** P<0.001; nonsignificant [ns], P>0.01). (B) Two-way ANOVA revealed that the presence of SC has a negative impact on MMP-9 secretion by blood cells compared with saline controls (* P<0.01). The figure was created using ImageJ software version 1.53e; Java 1.8.0_172.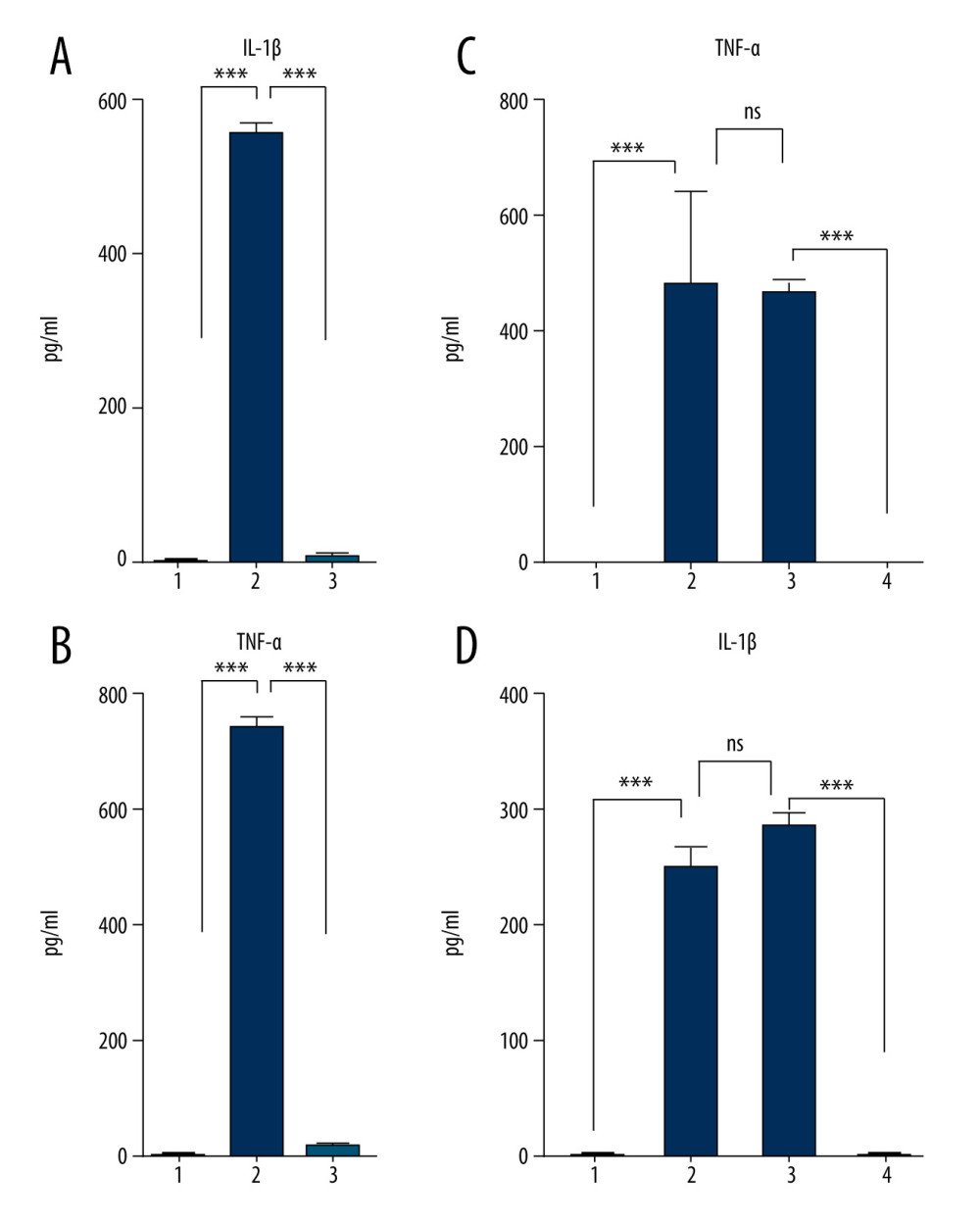 Figure 3. Sodium citrate (SC) prevents pro-inflammatory cytokine enrichment in a dose-dependent manner. Analysis of interleukin (IL)-1β (A) and tumor necrosis factor (TNF)-α (B) concentrations in human blood samples before incubation (1) or after incubation at 37°C for 24 h in the presence of either saline (2) or 4% SC (3). Dose-dependent effects of SC concentration on IL-1β (C) and TNF-α (D) production. The concentration in human blood samples is shown before incubation (1), after incubation at 37°C for 24 h (2), after incubation with the SC 5-fold less than the working concentration (3), and after incubation with the SC at 4% working concentration (4). A 2-way analysis of variance (ANOVA) reveals a statistically significant decrease in IL-1β and TNF-α concentrations in 24-h incubated blood in the presence of SC in a dose-dependent manner, compared with the saline control (*** P<0.001; nonsignificant [ns], P>0.01). The figure was created using ImageJ software version 1.53e; Java 1.8.0_172.
Figure 3. Sodium citrate (SC) prevents pro-inflammatory cytokine enrichment in a dose-dependent manner. Analysis of interleukin (IL)-1β (A) and tumor necrosis factor (TNF)-α (B) concentrations in human blood samples before incubation (1) or after incubation at 37°C for 24 h in the presence of either saline (2) or 4% SC (3). Dose-dependent effects of SC concentration on IL-1β (C) and TNF-α (D) production. The concentration in human blood samples is shown before incubation (1), after incubation at 37°C for 24 h (2), after incubation with the SC 5-fold less than the working concentration (3), and after incubation with the SC at 4% working concentration (4). A 2-way analysis of variance (ANOVA) reveals a statistically significant decrease in IL-1β and TNF-α concentrations in 24-h incubated blood in the presence of SC in a dose-dependent manner, compared with the saline control (*** P<0.001; nonsignificant [ns], P>0.01). The figure was created using ImageJ software version 1.53e; Java 1.8.0_172.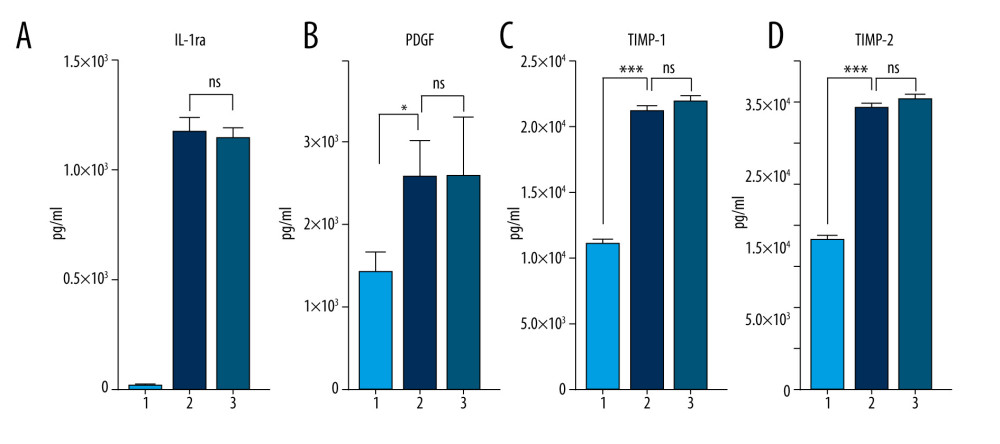 Figure 4. Sodium citrate (SC) does not affect anti-inflammatory, anti-catabolic, and regenerative protein enrichment. Protein concentrations of interleukin (IL)-1ra (A), platelet-derived growth factor (PDGF) (B), tissue inhibitor of metalloproteinase (TIMP)-1 (C), and TIMP-2 (D) in human blood samples are shown before incubation (1), after incubation at 37°C for 24 h in the presence of saline as a control (2), and after incubation at 37°C for 24 h in the presence of 4% SC (3). One-way analysis of variance (ANOVA) tests reveal that SC did not decrease the protein concentrations in 24-h incubated blood or in the final product (* P<0.01; *** P<0.0001; nonsignificant [ns], P>0.01). The figure was created using ImageJ software version 1.53e; Java 1.8.0_172.
Figure 4. Sodium citrate (SC) does not affect anti-inflammatory, anti-catabolic, and regenerative protein enrichment. Protein concentrations of interleukin (IL)-1ra (A), platelet-derived growth factor (PDGF) (B), tissue inhibitor of metalloproteinase (TIMP)-1 (C), and TIMP-2 (D) in human blood samples are shown before incubation (1), after incubation at 37°C for 24 h in the presence of saline as a control (2), and after incubation at 37°C for 24 h in the presence of 4% SC (3). One-way analysis of variance (ANOVA) tests reveal that SC did not decrease the protein concentrations in 24-h incubated blood or in the final product (* P<0.01; *** P<0.0001; nonsignificant [ns], P>0.01). The figure was created using ImageJ software version 1.53e; Java 1.8.0_172.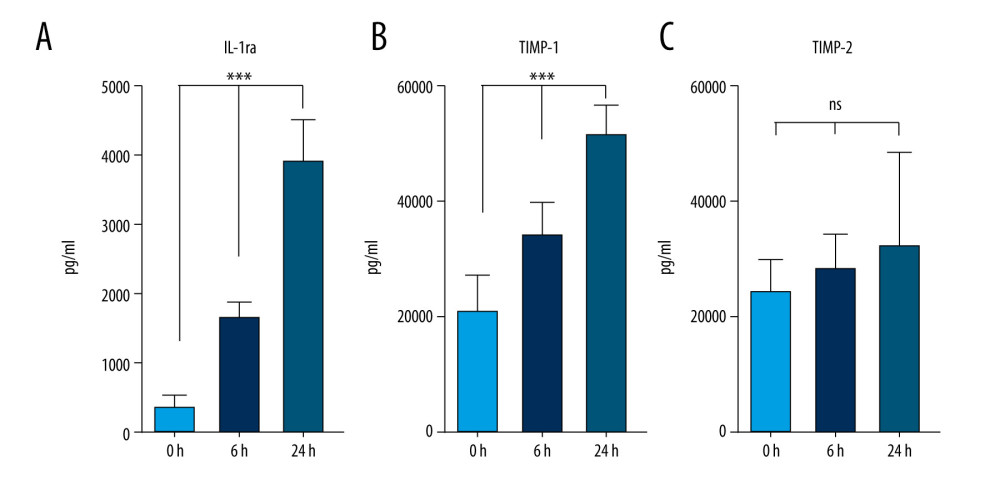 Figure 5. Production of anti-inflammatory and anti-catabolic molecules in incubated blood samples from recovered COVID-19 patients. Blood samples from immunized (COVID-19 recovered) patients were incubated for 0, 6, or 24 h at 37°C with 4% sodium citrate (SC) and then analyzed for concentrations of interleukin (IL)-1ra (A), tissue inhibitor of metalloproteinase (TIMP)-1 (B), and TIMP-2 (C). IL-1ra (A) was significantly enriched starting after 6 h of incubation in 37°C (1-way ANOVA test). TIMP-1 was significantly enriched after 6 h of incubation (B), whereas TIMP-2 concentration remained unchanged (C) (1-way ANOVA test; *** P<0.0001; nonsignificant [ns], P>0.01). The figure was created using ImageJ software version 1.53e; Java 1.8.0_172.
Figure 5. Production of anti-inflammatory and anti-catabolic molecules in incubated blood samples from recovered COVID-19 patients. Blood samples from immunized (COVID-19 recovered) patients were incubated for 0, 6, or 24 h at 37°C with 4% sodium citrate (SC) and then analyzed for concentrations of interleukin (IL)-1ra (A), tissue inhibitor of metalloproteinase (TIMP)-1 (B), and TIMP-2 (C). IL-1ra (A) was significantly enriched starting after 6 h of incubation in 37°C (1-way ANOVA test). TIMP-1 was significantly enriched after 6 h of incubation (B), whereas TIMP-2 concentration remained unchanged (C) (1-way ANOVA test; *** P<0.0001; nonsignificant [ns], P>0.01). The figure was created using ImageJ software version 1.53e; Java 1.8.0_172.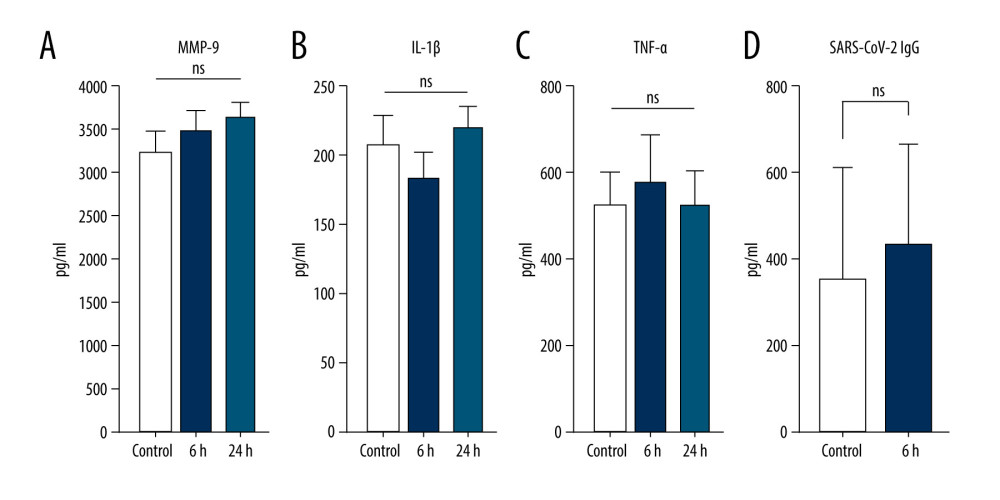 Figure 6. Optimal blood incubation condition protocol for interleukin (IL)-1ra and tissue inhibitor of metalloproteinase (TIMP) enrichment did not affect SARS-CoV-2 immunoglobulin (Ig)G concentrations. Samples from immunized patients were analyzed for matrix metalloproteinase (MMP)-9 (A), IL-1β (B), tumor necrosis factor (TNF)-α (C), and anti- SARS-CoV-2 IgG (D) concentrations in control or after 6- or 24-h incubation at 37°C with 4% sodium citrate (SC) (nonsignificant [ns], P>0.01). The figure was created using ImageJ software version 1.53e; Java 1.8.0_172.
Figure 6. Optimal blood incubation condition protocol for interleukin (IL)-1ra and tissue inhibitor of metalloproteinase (TIMP) enrichment did not affect SARS-CoV-2 immunoglobulin (Ig)G concentrations. Samples from immunized patients were analyzed for matrix metalloproteinase (MMP)-9 (A), IL-1β (B), tumor necrosis factor (TNF)-α (C), and anti- SARS-CoV-2 IgG (D) concentrations in control or after 6- or 24-h incubation at 37°C with 4% sodium citrate (SC) (nonsignificant [ns], P>0.01). The figure was created using ImageJ software version 1.53e; Java 1.8.0_172. In Press
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








