26 October 2020: Articles 
COVID-19-Induced Diabetic Ketoacidosis and Acute Respiratory Distress Syndrome in an Obese 24-Year-Old Type I Diabetic
Educational Purpose (only if useful for a systematic review or synthesis), Rare coexistence of disease or pathology
Sukhdev Singh1E*, Allison Foster1E, Zohaib Khan1E, Aisha Siddiqui1E, Muhammed Atere2E, Jay M. Nfonoyim3EDOI: 10.12659/AJCR.925586
Am J Case Rep 2020; 21:e925586
Abstract
BACKGROUND: In early 2020, severe acute respiratory syndrome-corona virus 2 caused an outbreak of a viral pneumonia that rapidly progressed to a global pandemic. Most cases presented with mild respiratory symptoms and required only supportive care with instructions to self-quarantine at home. Others had more severe symptoms that became complicated by acute respiratory distress syndrome (ARDS) and required hospitalization.
CASE REPORT: In this report, we present the case of a young patient in New York City who presented to our hospital with coronavirus disease 2019-induced diabetic ketoacidosis (DKA) that progressed to ARDS and subsequent death. The patient was managed for DKA on presentation with insulin protocol and acidosis management. However, it became evident that he had underlying respiratory complications, which later presented as ARDS requiring mechanical ventilation and antibiotics.
CONCLUSIONS: We recommend that clinicians be aware of this potentially fatal complication in all patients with pre-existing diabetes. Simultaneously, a low threshold for intubation should be advocated for patients with concurrent COVID-19 and type I diabetes mellitus since the potential for poor clinical outcomes from respiratory demise may be lessened by early respiratory intervention.
Keywords: COVID-19, Diabetes Mellitus, Type 1, Diabetic Ketoacidosis, Respiratory Distress Syndrome, Adult, SARS Virus, Betacoronavirus, COVID-19, Coronavirus Infections, Obesity, Pandemics, Pneumonia, Viral, SARS-CoV-2, young adult
Background
Coronavirus disease 2019 (COVID-19), caused by a coronavirus strain severe acute respiratory syndrome-coronavirus-2 (SARSCoV-2), rapidly caused a pandemic in early 2020. After being discovered in Wuhan, China, SARS-CoV-2 was found to present with a variety of symptoms, including fever, chills, and dyspnea. As the number of cases rose, critically ill patients were developing acute respiratory distress syndrome (ARDS) requiring mechanical ventilation at an alarming rate.
Diabetic ketoacidosis (DKA) is a life-threatening complication of diabetes mellitus and occurs because of the absence of insulin preventing glucose uptake in certain tissues, resulting in a hyperglycemic state. The demand for energy by tissue drives fatty acid metabolism, producing ketones and ultimately an anion gap metabolic acidosis. Additionally, DKA can predispose patients to infections because of a weakened immune system. Inflammatory cytokines, increased cortisol, and metabolic dysfunction create a devastating picture for DKA patients who develop infections as their immune systems are not adequately equipped to fight off such infections. Furthermore, diabetic patients experiencing infections, trauma, or other stressors have an increased susceptibility to DKA.
In this case report, we present a patient with DKA precipitated by SARS-CoV-2-induced COVID-19 further complicated by ARDS. This combination was fatal in our patient. We emphasize the importance of having a high clinical suspicion in such cases and intervening early to prevent rapid deterioration.
Case Report
A 24-year-old obese man with a known medical history of diabetes mellitus type 1 and hyperlipidemia presented to the Emergency Department (ED) for nausea, nonbilious vomiting, and 1 episode of hematemesis. He had subjective fevers, fatigue, dry cough, and chills; however, he denied dyspnea, chest pain, diarrhea, constipation, recent ill contacts, or any recent travel. The patient was noncompliant with his medications. Vital signs on presentation were temperature 36.4°C, pulse rate 122 beats/ min, blood pressure 141/84 mmHg, respiratory rate 16 breaths/ min, and oxygen saturation of 95% on room air as measured by a pulse oximeter. On re-examination, the patient was hypothermic, with a temperature of 32.9°C measured rectally. A subsequent Foley probe temperature was obtained and found to be 36.8°C.
On physical examination, the patient was in mild distress, lethargic, and had delayed responses to questioning; however, he was alert and oriented to person, place, and time. His mucous membranes were dry. The remainder of his physical examination was unremarkable.
His blood glucose on arrival was 507 mg/dL, his last measured hemoglobin A1c was 15.8% according to his chart, and his electrolyte panel showed a bicarbonate level of 2 mEq/L. Laboratory studies were significant for ketonuria (160 mg/dL) and elevated creatinine (1.4 mg/dL). His calculated anion gap was 30.6 mEq/L. Testing for bacteremia with blood cultures, urinary tract infection with urine culture, and nasopharyngeal swab with reverse transcription polymerase chain reaction (RTPCR) for SARS-CoV-2 was done in the ED using the New York State Wadsworth Center test. Initial chest X-ray (Figure 1) revealed no cardiopulmonary disease. Electrocardiogram results showed sinus tachycardia with normal axis intervals and negative acute ST changes. Relevant daily laboratory results are shown in Tables 1–3.
During the ED course he was assessed for DKA with metabolic encephalopathy and sepsis. On admission to the Medical Intensive Care Unit, he received 3 liters of fluids in addition to vancomycin, cefepime, 150 mEq of sodium bicarbonate infusion, potassium repletion with 100 mL, and insulin intravenously on 6 units/h (insulin infusion protocol). On hospital day 1, he remained lethargic and fell asleep multiple times while being examined. Given his clinical picture, he was diagnosed with DKA with an anion gap metabolic acidosis secondary to medication noncompliance paired with an upper-respiratory infection, and acute kidney injury secondary to DKA. His oxygen saturation at the time was 99% oxygen on room air. Laboratory results were notable for increased leukocyte count to 12.4 from 10.8 the previous day. Cardiac troponins were negative. On hospital day 2, he began showing signs of respiratory distress and became febrile (temp 38.3°C) and tachypneic. SARS-CoV-2 nasopharyngeal swab result was positive. Given his deteriorating respiratory status, the decision was made to intubate and mechanically ventilate with a respiratory rate of 14 breaths/min, tidal volume of 500 mL, fraction of inspired oxygen (FiO2) of 80%, and positive end-expiratory pressure (PEEP) of 15 cm of H2O. Postintubation arterial blood gas testing showed respiratory improvement. A chest X-ray (Figure 2) at this time revealed mild perihilar patchy areas of opacity on the right with increased vascular markings on the left, indicating probable COVID-19 pneumonia. On hospital day 3, he remained febrile with an increased temperature of 40°C, and azithromycin and hydroxychloroquine were added to his regimen for COVID-19. On day 4, his blood glucose levels stabilized; however, he remained febrile and became tachycardic at a rate of 118 beats/min. An attempt to titrate FiO2 to 60% was unsuccessful as his oxygen saturation again dropped to 86%, prompting an increase back to 80%. Arterial blood gas testing showed improvements with a blood pH of 7.40. On day 7, he continued to desaturate, with levels as low as 75% oxygen saturation and remained febrile. His leukocyte count increased further, raising concern for a superimposed fungal or bacterial infection. A sputum sample was collected and sent to the laboratory for culture. At this time, vancomycin and cefepime were discontinued and replaced with meropenem for a broader antibiotic coverage along with voriconazole for fungal coverage. During day 8, sputum results returned with yeast isolates, although no specific pathogen was isolated at the time. A chest X-ray (Figure 3) showed interval worsening of his infiltrates bilaterally. On day 9, he remained intermittently febrile. Attempts to titrate FiO2 on the ventilator were unsuccessful again, and he had multiple desaturations. Arterial blood gasses revealed worsening hyper-capnia with pCO2 level of 61 mmHg. A chest X-ray (Figure 4) at the time revealed diffuse fluffy infiltrates throughout both lungs, consistent with ARDS. Later that day he desaturated to 50% despite maximum PEEP and FiO2. A computed tomography scan of the chest was not performed because of the patient’s body habitus. The patient subsequently became severely hypotensive, with a blood pressure reading of 40/20 mmHg and bradycardia. He eventually became pulseless and an advanced cardiovascular life support protocol was initiated. Unfortunately, he could not be resuscitated, and the cause of death was hypoxic respiratory failure secondary to SARSCoV-2-induced COVID-19 complicated by ARDS.
Discussion
Coronavirus is an enveloped positive-sense ribonucleic acid virus named for the spiked projections on its envelope [1]. SARS-CoV-2 is the virus responsible for the COVID-19 pandemic. Patients infected with SARS-CoV-2 may be asymptomatic; however, a variety of presentations, including fever, dry cough, dyspnea, nasal congestion, anorexia, diarrhea, headache, fatigue, nausea, vomiting, myalgias, chills, and chest tightness, have been documented [2]. Although coronaviruses usually present as mild respiratory infections or the common cold, SARS-CoV-2 has proven to be a serious health threat causing severe COVID-19 pneumonia, sepsis, ARDS, multiorgan failure, and ultimately death [3]. Despite the clear link between worsened outcomes with comorbid conditions such as hyper-tension, diabetes, glucocorticoid use, pulmonary disease, and other immunocompromised conditions, a case of COVID-19 with concurrent DKA had not yet been reported in the United States at the time our patient presented.
At the time of this publication there have been nearly 5 million reported cases of SARS-CoV-2 and over 160 000 deaths in the United States [4]. Currently, advanced age is considered a crucial risk factor for determining progression to ARDS [5]. Our patient was 24 years old, mildly obese with a body mass index of 32.1, and had a history of poorly controlled diabetes mellitus type I. He initially presented with hyperglycemia along with altered mental status and was later diagnosed with DKA. As per hospital protocol, he was tested for SARS-CoV-2 via RT-PCR, which confirmed infection. Considering his ultimate death, it is important to recognize the special considerations necessary for patients presenting concurrently with DKA and COVID-19 to improve outcome.
DKA is a complication of diabetes mellitus that can result in death if not diagnosed and treated promptly [6]. It is an acute metabolic dysfunction due to an absolute insulin deficiency with peripheral insulin resistance and increased secretion of glucagon, cortisol, catecholamines, and growth hormone [6]. DKA can be precipitated by infections, stress, trauma, or inadequate insulin supplementation [6]. Even in diabetic patients with adequate glycemic control, the risk for developing severe infections leading to DKA is not eliminated [7]. Our patient presented with a glucose level of 507 mg/dL; poor glycemic control, as in this patient, in addition to infections, are frequently the precipitating factors for the development of DKA.
When patients with type I diabetes mellitus have an acute insult, such as an episode of DKA, the immune system is suppressed by elevations in cortisol, which can lead to complications such as sepsis [8–10]. Numerous studies have demonstrated that the development of ketoacidosis and hyper-glycemia precipitate an inflammatory state due to elevations in proinflammatory cytokines as well as oxidative stress [8–10]. Hyperglycemia induces macrophages to produce interleukin (IL)-1, IL-6, C-reactive peptide, and tumor necrosis factor-alpha, which reduce the body’s responsiveness to insulin [8–11].
Additionally, there are limited data regarding the effects of SARS-CoV-2 on the development of DKA. SARS-CoV-2 is known to interact with the renin-angiotensin-aldosterone system (RAAS), which may provide a unique mechanism to the development of DKA [12]. SARS-CoV-2 uses angiotensin-converting enzyme 2 (ACE-2) as an entry receptor for the virus to enter cells, and ACE-2 has high expressivity in the lungs and pancreas [12]. The virus enters cells, leading to an inflammatory response and the downregulation of ACE-2, resulting in inflammation of the targeted organs and elevations in angiotensin II [12]. On the basis of these mechanisms, injury of pancreatic beta cells may precipitate DKA in a similar fashion to the way lung injury precipitates ARDS [12]. The management of these patients is crucial considering that the disruption of RAAS complicates fluid resuscitation, and elevations in angiotensin II can result in ARDS [12]. Angiotensin II potentiates the production of aldosterone, which heightens the potential for hypokalemia in DKA patients who already have electrolyte imbalances [12].
Management of DKA consists of correcting the hypovolemia by fluid resuscitation, administering insulin to decrease hyperglycemia, correcting electrolyte imbalances, stabilizing the myocardium, and identifying the precipitating event [9]. In this case the precipitating event was probably an early infection with COVID-19. The regimen for our patient included intravenous fluids, intravenous insulin, sodium bicarbonate administration for myocardial protection from hyperkalemia, and potassium repletion and correction; however, evidence-based medicine guidance on recommended treatment for his COVID-19 infection was unavailable at the time our patient presented [9,10,13]. Although various treatment options are being investigated for the treatment of COVID-19, conflicting evidence currently prevents recommendation of one treatment over another. Further studies are necessary to determine which medications for COVID-19 should be recommended in patients with DKA.
Despite DKA being associated with numerous complications, ARDS is a rare complication from treatment and is characterized as refractory hypoxic respiratory failure due to pulmonary edema and decreased lung compliance [14]. The injury to the lungs may be produced by direct insults to the alveolar epithelium, indirectly through damage to the vascular endothelium, or simultaneously, as in this case. Pneumonia is the most common cause of ARDS from direct injury, whereas sepsis accounts for many cases of indirect injury [15]. Once damage has been inflicted on the endothelium or alveolar epithelium, an extensive release of cytokines ensues, causing widespread inflammation [15]. One proposed mechanism for the occurrence of ARDS in patients with DKA is an alteration in the permeability of the alveolar-capillary membrane resulting in entrance of fluid into the alveolar airspace, loss of surfactant, and subsequently reduced lung compliance leading to collapse [14,15]. The alteration in permeability is worsened in patients with SARS-CoV-2 since entry of the virus via ACE-2 receptors in the lung increases inflammation and subsequently permeability [12]. Low albumin in the setting of post-volume resuscitation in DKA has also been theorized to predispose the development of ARDS. This is most likely due to low oncotic pressure causing pulmonary blood vessels to allow fluid to leak into the lung parenchyma and alveoli [16]. Our patient initially had normal albumin levels on presentation; however, on day 4 of hospitalization, the patient’s albumin level was 2.0 g/dL. In this patient the fluid resuscitation necessary to correct DKA most probably attributed to the development of ARDS and was worsened by a superimposed COVID-19 infection.
ARDS is diagnosed by assessing the timing of symptoms, patient’s oxygenation status, determining the origin of the pulmonary edema, and findings on imaging. As per the Berlin definition, ARDS must occur within 7 days after a known clinical insult or sudden worsening of respiratory function with imaging suggestive of pulmonary edema, with other etiologies such as fluid overload and cardiogenic causes ruled out [17]. Although the prognosis of ARDS alone is poor, new data have shown that DKA in COVID-19 patients has increased mortality compared with historical DKA mortality rates [18,19]. Considering the difficulties of diagnosing ARDS, combined with the elevated mortality of DKA in COVID-19 patients, it is crucial to recognize this clinical picture early and manage accordingly [18,19].
Conclusions
Patients with type I diabetes that present with COVID-19 and DKA should immediately be assessed for potential respiratory compromise, as the risk for mortality from ARDS is elevated in this population subset. Because of the uncertainty of treatment regimens for COVID-19, it is apparent that early ventilatory support is the best approach in the management of patients with COVID-19-induced DKA and respiratory compromise. Despite recent publicization of young people being able to recover from COVID-19 at a higher rate than their older counterparts, we present a case of a 24-year-old young man that unfortunately did not survive a COVID-19 infection superimposed on a pre-existing condition. As such, we suggest that clinicians remain vigilant for COVID-19 in patients with DKA, as the risk of developing ARDS is higher. We also suggest that young people remain mindful of social distancing protocols, as the virus is fatal to their age group, albeit at a lesser rate.
Figures
Tables
Table 1.. Patient’s laboratory values: temperature, white blood count (WBC), blood glucose, blood pH, anion gap, and partial pressure of oxygen/fraction of inspired oxygen (PaO2/FiO2), with date of collection.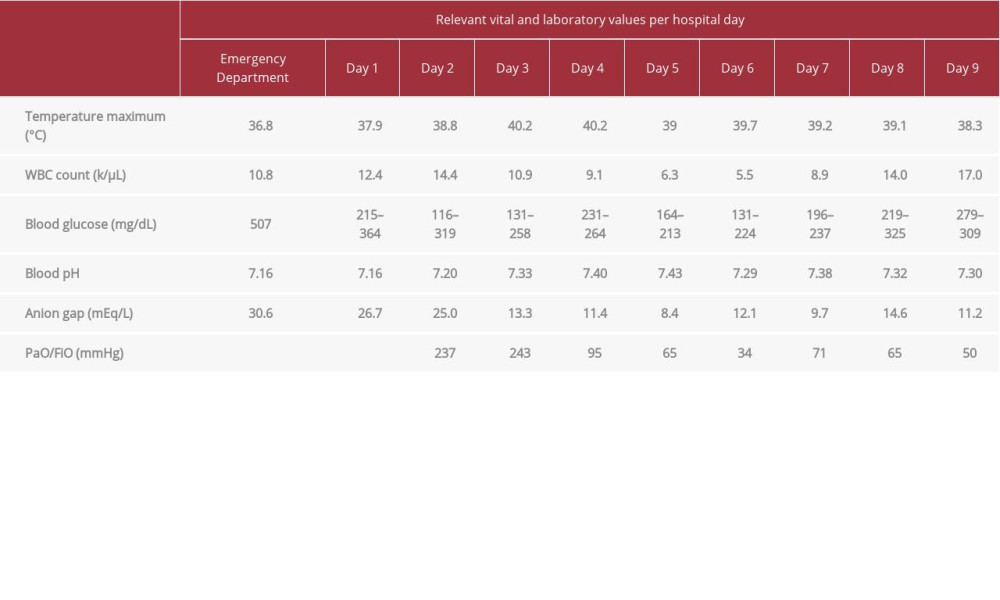 Table 2.. Patient’s laboratory values: Na and K.
Table 2.. Patient’s laboratory values: Na and K.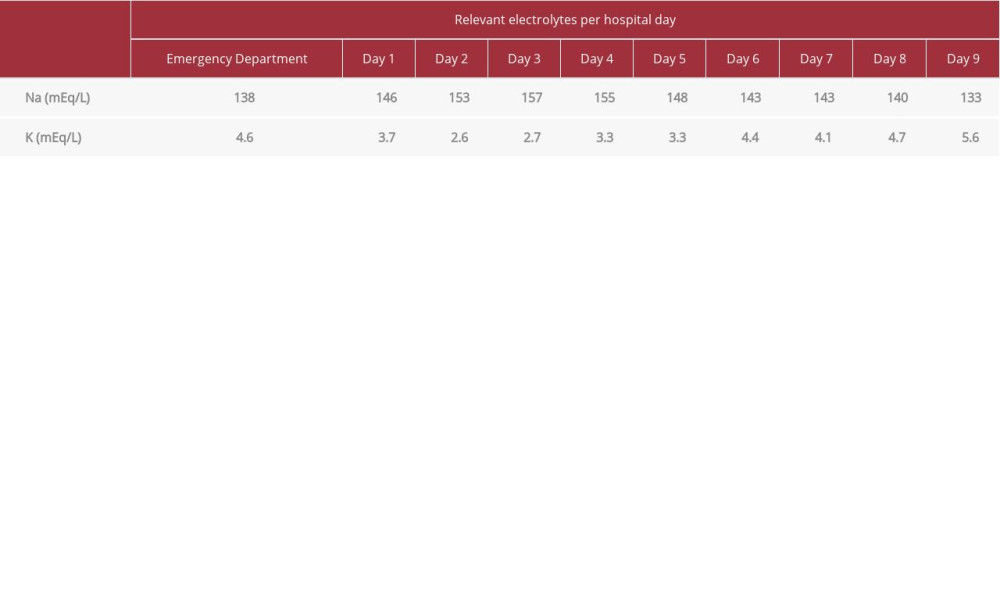 Table 3.. Patient’s fluid balance for the duration of his stay.
Table 3.. Patient’s fluid balance for the duration of his stay.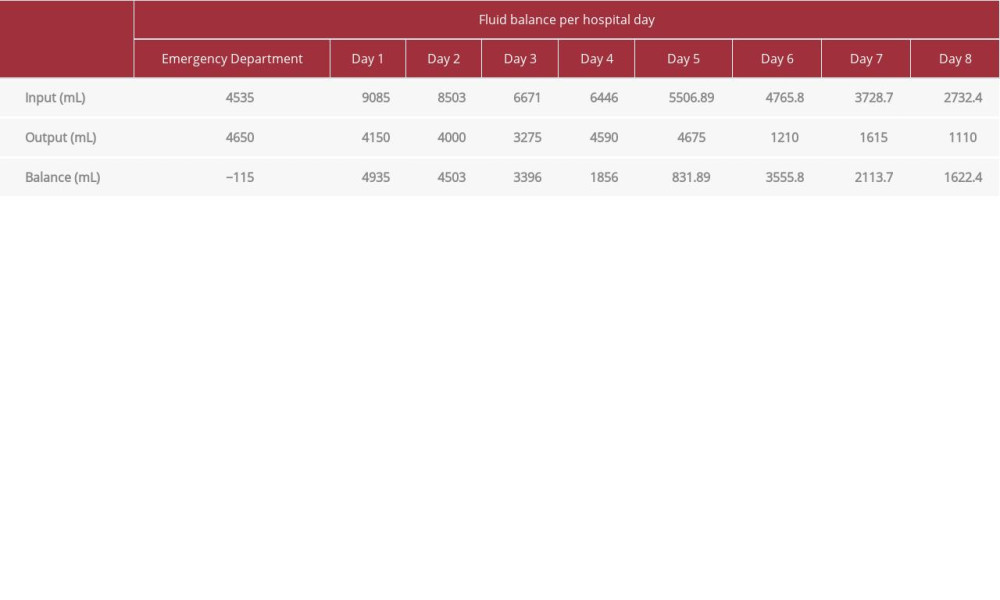
References:
1.. Lake MA, What we know so far: COVID-19 current clinical knowledge and research: Clin Med, 2020; 20(2); 124-27
2.. Guan W, Liang W, Zhao Y, Comorbidity and its impact on 1590 patients with Covid-19 in China: A nationwide analysis: Eur Resp J, 2020; 55(5); 2000547
3.. Sohrabi C, Alsafi Z, O’Neill N, World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19): Int J Surg, 2020; 76; 71-76
4.. , Coronavirus disease 2019 (COVID-19) in the U.S., 2020
5.. , Interim Clinical guidance for management of patients with confirmed 2019 novel coronavirus (2019-nCoV) infection, 2020
6.. Davis TME, Davis W, Incidence and associates of diabetic ketoacidosis in a community-based cohort: The Fremantle Diabetes Study Phase II: BMJ Open Diabetes Res Care, 2020; 8(1); e000983
7.. Gupta R, Ghosh A, Singh AK, Misra A, Clinical considerations for patients with diabetes in times of COVID-19 epidemic: Diabetes Metab Syndr Clin Res Rev, 2020; 14(3); 211-12
8.. Carey IM, Critchley JA, DeWilde S, Risk of infection in type 1 and type 2 diabetes compared with the general population: A matched cohort study: Diabetes Care, 2018; 41(3); 513-21
9.. Fayfman M, Pasquel FJ, Umpierrez GE, Management of hyperglycemic crises: Med Clin N Am, 2017; 101(3); 587-606
10.. Hussain A, Bhowmik B, Cristina do Vale Moreira N, COVID-19 and diabetes: Knowledge in progress: Diabetes Res Clin Pract, 2020; 162; 108142
11.. Lu J, Liu J, Li L, Lan Y, Liang Y, Cytokines in type 1 diabetes: Mechanisms of action and immunotherapeutic targets: Clin Transl Immunol, 2020; 9(3); e1122
12.. Chee YJ, Ng SJH, Yeoh E, Diabetic ketoacidosis precipitated by Covid-19 in a patient with newly diagnosed diabetes mellitus: Diabetes Res Clin Pract, 2020; 164; 108166
13.. Emami J, Pasutto FM, Mercer JR, Jamali F, Inhibition of insulin metabolism by hydroxychloroquine and its enantiomers in cytosolic fraction of liver homogenates from healthy and diabetic rats: Life Sci, 1998; 64(5); 325-35
14.. Mitsuishi S, Matoba K, Yamazaki H, Acute respiratory distress syndrome in diabetic ketoacidosis: Int Med, 2014; 53(14); 1581
15.. Shaver CM, Bastarache JA, Clinical and biological heterogeneity in acute respiratory distress syndrome: Clin Chest Med, 2014; 35(4); 639-53
16.. Leonard RC, Asplin C, McCormick CV, Hockaday TD, Acute respiratory distress in diabetic ketoacidosis: Possible contribution of low colloid osmotic pressure: Br Med J Clin Res, 1983; 286(6367); 760-62
17.. , Acute respiratory distress syndrome: The Berlin Definition.: JAMA, 2012; 307(23); 2526-33
18.. Bellani G, Laffey JG, Pham T, Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries: JAMA, 2016; 315(8); 788-800
19.. Li J, Wang X, Chen J, COVID-19 infection may cause ketosis and ketoacidosis.: Diabetes Obes Metab, 2020 [Online ahead of print]
Figures
Tables
 Table 1.. Patient’s laboratory values: temperature, white blood count (WBC), blood glucose, blood pH, anion gap, and partial pressure of oxygen/fraction of inspired oxygen (PaO2/FiO2), with date of collection.
Table 1.. Patient’s laboratory values: temperature, white blood count (WBC), blood glucose, blood pH, anion gap, and partial pressure of oxygen/fraction of inspired oxygen (PaO2/FiO2), with date of collection. Table 2.. Patient’s laboratory values: Na and K.
Table 2.. Patient’s laboratory values: Na and K. Table 3.. Patient’s fluid balance for the duration of his stay.
Table 3.. Patient’s fluid balance for the duration of his stay. Table 1.. Patient’s laboratory values: temperature, white blood count (WBC), blood glucose, blood pH, anion gap, and partial pressure of oxygen/fraction of inspired oxygen (PaO2/FiO2), with date of collection.
Table 1.. Patient’s laboratory values: temperature, white blood count (WBC), blood glucose, blood pH, anion gap, and partial pressure of oxygen/fraction of inspired oxygen (PaO2/FiO2), with date of collection. Table 2.. Patient’s laboratory values: Na and K.
Table 2.. Patient’s laboratory values: Na and K. Table 3.. Patient’s fluid balance for the duration of his stay.
Table 3.. Patient’s fluid balance for the duration of his stay. In Press
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943275
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943411
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942864
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250








