19 October 2021: Clinical Research
Proper Partial Pressure of Arterial Oxygen for Patients with Traumatic Brain Injury
Hong Wu1ACE, Liang Gong2BD, Jia-Cheng Gu1DF, Hong-Wei Xing3BC, Zhong-Xin Qian2DF, Qing Mao1ABG*DOI: 10.12659/MSM.932318
Med Sci Monit 2021; 27:e932318
Abstract
BACKGROUND: The partial pressure of arterial oxygen (PaO₂) is critical to the outcome of patients with traumatic brain injury (TBI). However, it is not clear what range of PaO2 should be maintained to improve patient outcome. The aim of this study was to explore the PaO2 value needed in the acute phase of TBI and provide new evidence for clinical practice.
MATERIAL AND METHODS: A total of 153 patients with TBI were enrolled retrospectively. Univariate and multivariate logistic regression analyses were conducted on sex, Glasgow Coma Scale (GCS) score on admission, PaO₂ within 6 h of admission, oxygenation index, and other factors. The Glasgow Outcome Score (GOS) of the patient at discharge was used as an indicator of outcome. The good outcome group had GOS ≥4, and the poor outcome group had GOS <4.
RESULTS: The 153 patients were divided into a good outcome group (n=62) and poor outcome group (n=91). There was a significant difference in sex, admission GCS, surgery, airway status, PaO₂, and oxygen index within 6 h of admission between the 2 groups. Logistic regression analysis showed that PaO₂ <60 mmHg, male sex, and admission GCS score of 3 to 12 were independent risk factors for a poor outcome.
CONCLUSIONS: Patients with TBI having PaO₂ <60 mmHg within 6 h after admission were more likely to have poor outcomes. The upper limit value of PaO₂ that affects the outcome of TBI in patients has not been found.
Keywords: Brain Concussion, Hyperbaric Oxygenation, Partial Pressure, Brain Injuries, Traumatic, Female, Humans, Male, Oxygen
Background
In 2012, traumatic brain injury (TBI) accounted for 37% of all deaths due to traumatic diseases in Europe [1]. In China, patients with TBI are generally severely injured, and the proportion of patients with TBI with severe head trauma is 22%. Although there are reports in the literature stating that the management of TBI in China is better than that in Europe, the mortality rate of patients with TBI after Intensive Care Unit (ICU) admission is still as high as 11.4% [2]. Poor outcomes in patients with TBI can cause social and family burdens [3]. After the occurrence of TBI, patients can develop lung infections, atelectasis, and acute respiratory distress syndrome. They can also develop other lung diseases due to disorders of consciousness, vomiting and aspiration, mechanical ventilation [4], and prolonged bed rest. This will reduce the alveolar ventilation function and decrease the partial pressure of oxygen (PaO2), causing hypoxemia. Nerve cells experience edema and even necrosis after insufficient oxygen supply, hindering the recovery of nerve structure and function after TBI, prolonging the patient’s hospital stay, and even causing death. The American Craniocerebral Trauma Foundation stated in the “Guidelines for the Management of Severe Head Injury” that patients should maintain PaO2 >60 mmHg after TBI [5]. The British “Guidelines for the Safe Transfer of Brain Injury” require patients with TBI to maintain PaO2 >97.5 mmHg [6]. However, they were controversial about the lower limit of low PaO2 and did not set an upper limit.
In addition, a PaO2 that is too high is not good for the outcome of patients with TBI; however, its limit has not yet been determined [7–9]. Studies have reported that the relationship between early arterial oxygenation and long-term functional and cognitive outcomes after TBI is U-shaped. Controlling PaO2 within 24 h of admission in patients with TBI within the range of mild hyperoxia (150–200 mmHg) could improve outcomes [10]. At the same time, high PaO2 (>200 mmHg) within the first 24 h of admission in patients with TBI is associated with higher postoperative mortality and short-term functional deterioration [7]. Hence, 200 mmHg is worth discussing as the threshold of high PaO2.
For patients with TBI, the thresholds for low and high PaO2 are uncertain. Therefore, monitoring and maintaining a patient’s PaO2 after TBI within a reasonable range can be beneficial for rehabilitation after TBI. This study aimed to explore the relationship between PaO2 and outcome in patients with acute TBI in the neurosurgical intensive care unit (NICU), find the appropriate range of PaO2 in patients with acute TBI, and provide new evidence for clinical practice.
Material and Methods
STUDY POPULATION:
This retrospective observational study included patients with TBI admitted to the Department of Neurosurgery, Renji Hospital, Shanghai Jiao Tong University School of Medicine and the Department of Neurosurgery, Shanghai Punan Hospital between March 2020 and December 2020. The patients included in this study were admitted to the NICU within 6 h after TBI (patients with TBI with GCS ≥13 at the time of admission were included in the NICU due to disease progression) and were aged ≥18 years. Their computed tomography (CT) scan of the head showed traumatic lesions of the brain (brain contusion, skull fracture, diffuse axonal injury, epidural/subdural hematoma), and they had complete arterial blood gas data during hospitalization. The exclusion criteria were a time of stay in the NICU less than 3 days, head CT showing no obvious abnormalities in the brain, and incomplete or lost chest CT on admission and arterial blood gas data during hospitalization.
METHODS AND TBI MANAGEMENT:
According to the guidelines, the patients with TBI were evaluated and treated with decompressive hemicraniectomy (DHC) [11]. The patients were diagnosed and treated according to the “Guidelines for the Management of Severe Head Trauma, Fourth Edition”, published by the Head Trauma Foundation in 2017 [5]. A Codman intracranial pressure probe was used to monitor intracranial pressure, which was maintained at <22 mmHg. Cerebral perfusion pressure was maintained at 60 to 70 mmHg, PaCO2 at 35 to 40 mmHg [12], and SaO2 at >92% [13]. Using physical cooling methods and antipyretic drugs, an ice blanket was used to maintain the core body temperature at 35.0 to 37.5°C.
The following data were collected: sex, age (elderly: >65 years), Glasgow Coma Scale (GCS) score at admission, head CT examination report at admission, chest CT examination report at admission, and treatment methods (DHC, drugs). All arterial blood samples were analyzed with a GEM Premier 3000 blood gas analyzer. At the same time, PaO2 within 6 h of admission, oxygenation index (OI) within 6 h of admission, and airway status within 6 h of admission (noninvasive oxygen inhalation, tracheal intubation) were collected. OI was calculated as PaO2/fraction of inspiration O2 (FiO2). For patients with mechanical ventilation, we collected FiO2 through ventilator parameters. For patients with oxygen inhalation, we collected inspired oxygen flow (L/min), and at this time, the following calculation was used: FiO2=21+inspired oxygen flow×4. The American Craniocerebral Trauma Foundation stated in the “Guidelines for the Management of Severe Head Injury” that patients should maintain PaO2 >60 mmHg after TBI [5]. The British “Guidelines for the Safe Transfer of Brain Injury” require patients with TBI to maintain PaO2 >97.5 mmHg [6]. According to these guidelines, the lower limit of PaO2 is assumed to be 60 mmHg and 97.5 mmHg, respectively. Since the guidelines do not require an upper limit, and in previous studies, 200 mmHg has been controversial, this study assumed a PaO2 of 200 mmHg as the upper limit. Patients were divided into 4 groups, according to PaO2 levels: <60 mmHg; 60 to 97.5 mmHg; 97.5 to 200 mmHg; and ≥200 mmHg.
OUTCOME VARIABLES:
The primary outcomes were the Glasgow Outcome Scale (GOS) [14]. The GOS is evaluated by analyzing the functional status of patients at discharge. We set the good outcome group as GOS ≥4 points, and the poor outcome group as GOS <4 points.
STATISTICAL ANALYSIS:
The variables considered in the statistical analysis included sex, age, GCS at admission, chest and lung diseases at admission, treatment methods (DHC, drugs), airway status within 6 h of admission, hospitalization time, PaO2 within 6 h of admission, and OI within 6 h of admission. Statistical analysis was performed using SPSS version 25.0 (IBM Corp, Armonk, NY, USA).
The Kruskal-Wallis test was used to compare GOS among the 4 groups, according to PaO2. The Mann-Whitney test was used to analyze the difference of PaO2 in patients with different outcomes. The Pearson chi-squared test was used to evaluate the relationship between each categorical variable and the GOS upon discharge. Finally, we adopted the forward stepwise multivariate logistic regression analysis to analyze the differences among significant variables in the chi-squared test (sex, GCS on admission, and airway status, PaO2, and OI within 6 h of admission) and variables found in previous studies (age, chest, and lung diseases, DHC). Values of
Results
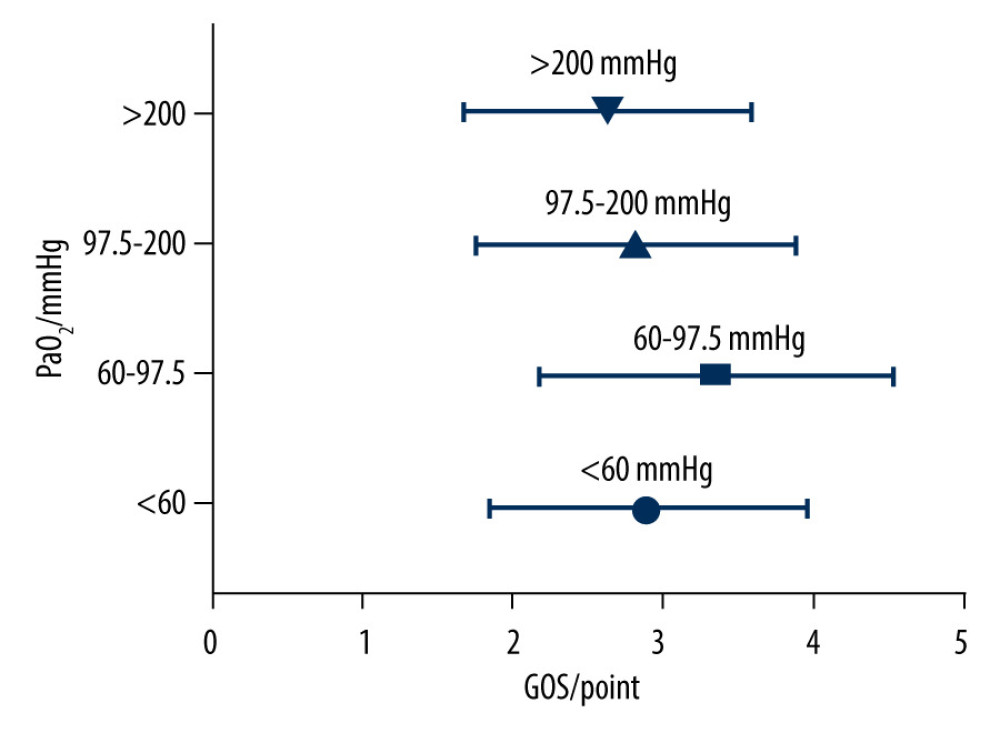
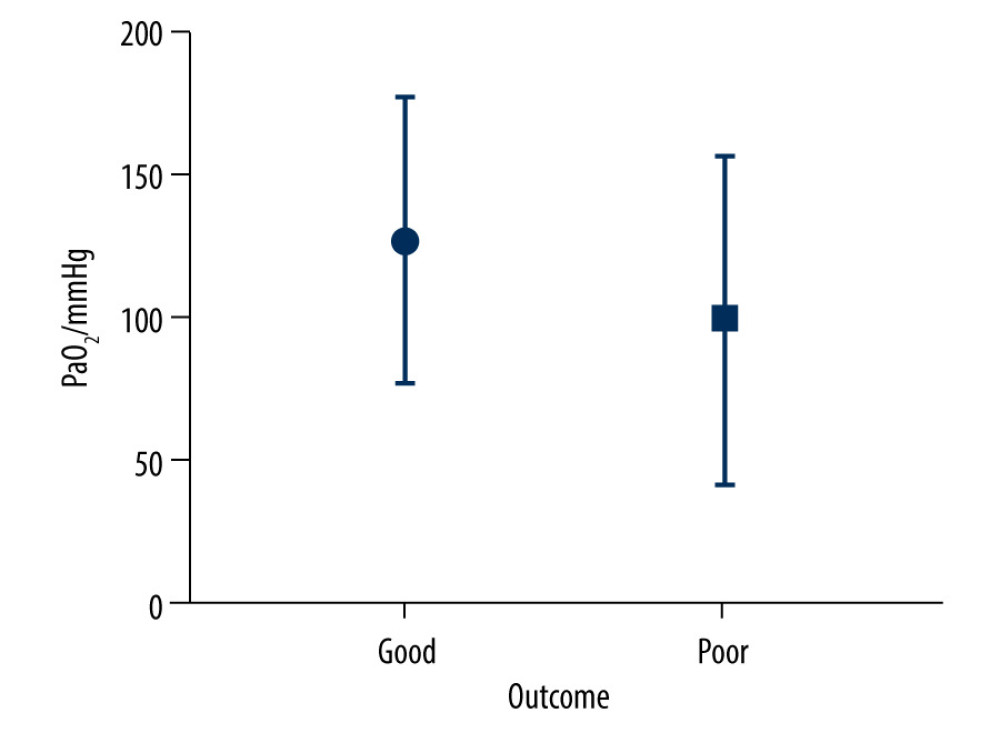
As shown in Figure 1, there were significant differences (
Overall, 153 patients (109 men and 44 women) were included in this study, of which 55 (35.9%) patients were elderly, 25 patients (16.3%) had an initial GCS of 13 to 15, 45 patients (29.4%) had an initial GCS of 9 to 12, and 83 patients (54.2%) had an initial GCS of 3 to 8. Chest CT examination on admission revealed that 52 patients (34.0%) had various degrees of chest and lung diseases, such as chronic obstructive pulmonary disease, emphysema, rib fracture, lung exudation, pneumothorax, and pleural effusion. A total of 129 patients (84.3%) underwent DHC after admission, based on the CT scan and the surgeon’s decision. There was no significant difference in outcome between patients with DHC and without DHC (
In this study, we also conducted multivariate logistic regression analysis. In Table 2, the independent risk factors associated with the outcome of the 153 patients with TBI are summarized. The following were significantly associated with poor TBI outcome: PaO2 within 6 h of admission of <60 mmHg (odds ratio [OR], 5.314; 95% confidence interval [CI], 1.134–24.905;
Discussion
PaO2 is one of the factors affecting the outcome of TBI. An increase or decrease in PaO2 will lead to an increase in the mortality of patients with TBI [10,15,16]. However, the recommended range of PaO2 in patients with TBI remains controversial. This study reviewed the PaO2 levels of 153 TBI patients within 6 h after admission to the NICU. The results showed that 17% of patients developed hypoxemia within 6 h after admission, and 77% of them had poor outcomes. More than half of the patients had PaO2 levels lower than 97.5 mmHg within 6 h after admission, and 76% of them had a poor outcome. Only 31.4% of patients had PaO2 levels of 97.5 to 200 mmHg within 6 h after admission, and 71% of them had a good outcome.
PaO2 <60 mmHg was a risk factor for poor prognosis in patients with TBI, whereas PaO2 60 to 97.5 mmHg was not. Studies have reported that the occurrence and duration of hypoxemia in patients with TBI before admission and during treatment after admission significantly increases the mortality rate [17,18]. Some researchers used 60 mmHg as the threshold for hypoxemia in patients with TBI [19]. However, the PaO2 benefit interval is not equal to the definition interval of hypoxemia in patients with TBI. Davis et al reported that PaO2 <110 mmHg is associated with an increase in mortality [20] and reported that even if the patient survives TBI, delayed hypoxemia will exacerbate structural damage and cause long-term behavioral defects [21]. The “Guidelines for the Safe Transfer of Brain Injury” require patients with TBI to maintain PaO2 >97.5 mmHg [6]. Although the guideline specifies the required PaO2 in the transfer process, it reflects that patients with TBI need a higher level of PaO2 in the acute stage. In the present study, we showed that in the 60 to 97.5 mmHg group, the proportion of patients with poor outcomes was enormous. Due to the similar results reported in many studies, clinicians still need to pay enough attention to PaO2 levels in the acute phase of TBI and maintain a high level.
Maintaining PaO2 at 250 to 486 mmHg within 72 h after admission in patients with a severely high blood oxygen concentration is detrimental to the outcome of patients with TBI. However, the threshold of high blood oxygen concentration is still controversial. TBI can improve the in-hospital all-cause survival rate [12]. There are also reports in the literature stating that patients with TBI should maintain a PaO2 level between 110 and 487 mmHg [20]. The present study showed that the outcome of patients in the PaO2 ≥200 mmHg group was not significantly different from that of patients in the 97.5 to 200 mmHg group. It may be because 200 mmHg is not the cut-off value of high blood oxygen concentration, and there was still a good outcome in PaO2 ≥200 mmHg, which agrees with previous literature [10,20,22–24]. Although the upper limit of PaO2 cannot be determined, it widens the upper limit of PaO2 to improve the outcome of patients with TBI.
Sex has a wide range of influencing factors in TBI. It has been reported that female sex hormones, including androgen and progesterone, have neuroprotective effects, and they act on the steroidal central nervous system to reduce nerve injury after TBI [25,26]. Bazarian et al believe that, in mild TBI, the prognosis of women is worse than that of men [27]. In the present study, male sex was a risk factor affecting the outcome of patients with TBI. We agree with the analysis of Mollayeva et al [28]: many factors influence the outcome of TBI by sex, which should be analyzed from multiple perspectives in the future.
GCS, as a common measure of the degree of coma and injury after TBI, has also been proven to be a predictor of TBI outcome [14,29,30]. It is worth noting that the present study did not analyze outcome according to the type of TBI. For instance, the outcome of acute epidural hemorrhage may be better than that of acute subdural hemorrhage or cerebral contusion and laceration, but when acute epidural hemorrhage causes cerebral hernia, the outcome is still poor. Therefore, we regard the admission GCS as the basis for judging the condition of the injury rather than the type of TBI. When the GCS was low, whether it was because of a severe brain injury or respiratory failure, it led to a poor outcome in patients. Our results showed that GCS on admission was an independent risk factor for the outcome of TBI.
TBI can cause a series of inflammatory reactions and cause acute lung injury [31,32], which can cause pulmonary ventilation disorders and even increase the fatality rate of TBI [33,34]. If the patient has a history of chronic obstructive pulmonary disease, chronic bronchitis, lung surgery, or other chest and lung diseases before or after TBI, acute lung injury is worsened. In this study, the results showed that 34.0% of the patients with TBI had traumatic and non-traumatic chest diseases on admission. We believe that this may be due to some chest and lung diseases included in this study, such as rib fracture and a small amount of pulmonary exudation, which are not related to the outcome of patients with TBI. However, severe underlying lung diseases can cause hypoxemia, leading to poor recovery of neurological function [35]. Therefore, the oxygenation of patients with TBI combined with chest and lung diseases should gain the attention of clinicians.
Patients with TBI can have atelectasis a short time after DHC, leading to the decrease of PaO2. Although DHC can reduce mortality, intracranial pressure, and length of stay in patients with TBI, compared with drug therapy, patients with TBI who underwent DHC have a higher proportion of survival and poor outcomes [36]. The present study found no significant difference between DHC and drug treatment between the 2 groups, which may be because GOS at discharge was not enough to evaluate the impact of DHC on prognosis. In addition, the severity of patients who only needed to receive drug treatment was generally less than that of patients who needed DHC, and their airway self-protection ability and gas exchange function was better than those of patients after DHC to maintain good PaO2 in the acute phase. Although DHC can increase the proportion of patients with poor prognosis, it can reduce the mortality of patients in the acute stage and is still one of the necessary means for the treatment of severe TBI.
Patients with a GCS ≤10 points who receive endotracheal intubation in the hospital can benefit from functional outcomes [37]. The present study showed that 90.1% of the patients with TBI in the poor outcome group were intubated at the time of admission to the NICU, and the airway status was significantly different among TBI patients with different prognoses. However, airway status in the acute phase was not associated with independent risk factors for outcome. This may have been because most patients with TBI were given tracheal intubation for surgery rather than for respiratory failure in the emergency room. On the other hand, most patients with TBI were admitted to the NICU after emergency surgery. Their consciousness disorder was challenging to recover in a short period, and they could not have the tracheal intubation removed quickly. The evaluation of tracheal intubation should be closely related to the evaluation time of GCS; therefore, the influence of airway status on the outcome of patients with TBI needs to be further explored.
This study has certain limitations. First, it is a single-center study. Second, the upper limit of high blood oxygen concentration is still uncertain. Third, this study should have collected preoperative GCS, which may be more representative than the GCS evaluated in the emergency room. Finally, whether patients with TBI receive tracheal intubation in the emergency room may be more significant than the postoperative state of tracheal intubation. Therefore, more large-scale multi-center studies are warranted to further investigate this issue.
Conclusions
In summary, PaO2 <60 mmHg within 6 h after admission was an independent risk factor for the outcome of TBI. PaO2 within 6 h after admission had a U-shaped effect on the outcome of patients with TBI. The upper limit value of PaO2 that affects the outcome of patients with TBI has not been determined. It is worthy of clinicians’ attention to consider factors such as sex and admission GCS, which cause the decrease of PaO2 in patients with TBI.
References
1. Majdan M, Plancikova D, Brazinova A, Epidemiology of traumatic brain injuries in Europe: A cross-sectional analysis: Lancet Public Health, 2016; 1(2); e76-e83
2. Gao G, Wu X, Feng J, Clinical characteristics and outcomes in patients with traumatic brain injury in China: A prospective, multicentre, longitudinal, observational study: Lancet Neurol, 2020; 19(8); 670-77
3. Jiang JY, Gao GY, Feng JF, Traumatic brain injury in China: Lancet Neurol, 2019; 18(3); 286-95
4. Hu PJ, Pittet JF, Kerby JD, Acute brain trauma, lung injury, and pneumonia: More than just altered mental status and decreased airway protection: Am J Physiol Lung Cell Mol Physiol, 2017; 313(1); L1-115
5. Carney N, Totten AM, O’Reilly C, Guidelines for the management of severe traumatic brain injury, fourth edition: Neurosurgery, 2017; 80(1); 6-15
6. Nathanson MH, Andrzejowski J, Dinsmore J, Guidelines for safe transfer of the brain-injured patient: trauma and stroke, 2019: Guidelines from the Association of Anaesthetists and the Neuro Anaesthesia and Critical Care Society: Anaesthesia, 2020; 75(2); 234-46
7. Brenner M, Stein D, Hu P, Association between early hyperoxia and worse outcomes after traumatic brain injury: Arch Surg, 2012; 147(11); 1042-46
8. Helmerhorst HJ, Roos-Blom MJ, van Westerloo DJ, de Jonge E, Association between arterial hyperoxia and outcome in subsets of critical illness: A systematic review, meta-analysis, and meta-regression of cohort studies: Crit Care Med, 2015; 43(7); 1508-19
9. Harpsø M, Granfeldt A, Løfgren B, Deakin CD, No effect of hyperoxia on outcome following major trauma: Open Access Emerg Med, 2019; 11; 57-63
10. Alali AS, Temkin N, Vavilala MS, Matching early arterial oxygenation to long-term outcome in severe traumatic brain injury: Target values: J Neurosurg, 2019; 132(2); 537-44
11. Hawryluk GWJ, Rubiano AM, Totten AM, Guidelines for the management of severe traumatic brain injury: 2020 Update of the decompressive craniectomy recommendations: Neurosurgery, 2020; 87(3); 427-34
12. Asher SR, Curry P, Sharma D: J Neurosurg Anesthesiol, 2013; 25(2); 168-73
13. Manley G, Knudson MM, Morabito D, Hypotension, hypoxia, and head injury: Frequency, duration, and consequences: Arch Surg, 2001; 136(10); 1118-23
14. Teasdale G, Jennett B, Assessment of coma and impaired consciousness. A practical scale: Lancet, 1974; 2(7872); 81-84
15. Qaseem A, Snow V, Fitterman N, Risk assessment for and strategies to reduce perioperative pulmonary complications for patients undergoing noncardiothoracic surgery: A guideline from the American College of Physicians: Ann Intern Med, 2006; 144(8); 575-80
16. Kavanagh BP, Perioperative atelectasis: Minerva Anestesiol, 2008; 74(6); 285-87
17. Jones PA, Andrews PJ, Midgley S, Measuring the burden of secondary insults in head-injured patients during intensive care: J Neurosurg Anesthesiol, 1994; 6(1); 4-14
18. Chi JH, Knudson MM, Vassar MJ, Prehospital hypoxia affects outcome in patients with traumatic brain injury: A prospective multicenter study: J Trauma, 2006; 61(5); 1134-41
19. McHugh GS, Engel DC, Butcher I, Prognostic value of secondary insults in traumatic brain injury: Results from the IMPACT study: J Neurotrauma, 2007; 24(2); 287-93
20. Davis DP, Meade W, Sise MJ, Both hypoxemia and extreme hyperoxemia may be detrimental in patients with severe traumatic brain injury: J Neurotrauma, 2009; 26(12); 2217-23
21. Davies M, Jacobs A, Brody DL, Friess SH, Delayed hypoxemia after traumatic brain injury exacerbates long-term behavioral deficits: J Neurotrauma, 2018; 35(5); 790-801
22. Raj R, Bendel S, Reinikainen M, Hyperoxemia and long-term outcome after traumatic brain injury: Crit Care, 2013; 17(4); R177
23. Fujita M, Oda Y, Yamashita S, Early-stage hyperoxia is associated with favorable neurological outcomes and survival after severe traumatic brain injury: A post-hoc analysis of the Brain Hypothermia Study: J Neurotrauma, 2017; 34(8); 1565-70
24. Ó Briain D, Nickson C, Pilcher DV, Udy AA, Early hyperoxia in patients with traumatic brain injury admitted to intensive care in Australia and New Zealand: A retrospective multicenter cohort study: Neurocrit Care, 2018; 29(3); 443-51
25. Arevalo MA, Azcoitia I, Garcia-Segura LM, The neuroprotective actions of oestradiol and oestrogen receptors: Nat Rev Neurosci, 2015; 16(1); 17-29
26. Raghava N, Das BC, Ray SK, Neuroprotective effects of estrogen in CNS injuries: Insights from animal models: Neurosci Neuroecon, 2017; 6; 15-29
27. Bazarian JJ, Blyth B, Mookerjee S, He H, McDermott MP, Sex differences in outcome after mild traumatic brain injury: J Neurotrauma, 2010; 27(3); 527-39
28. Mollayeva T, Mollayeva S, Colantonio A, Traumatic brain injury: Sex, gender and intersecting vulnerabilities: Nat Rev Neurol, 2018; 14(12); 711-22
29. Marmarou A, Lu J, Butcher I, IMPACT database of traumatic brain injury: Design and description: J Neurotrauma, 2007; 24(2); 239-50
30. Baum J, Entezami P, Shah K, Medhkour A, Predictors of outcomes in traumatic brain injury: World Neurosurg, 2016; 90; 525-29
31. Johnson ER, Matthay MA, Acute lung injury: Epidemiology, pathogenesis, and treatment: J Aerosol Med Pulm Drug Deliv, 2010; 23(4); 243-52
32. Perl M, Lomas-Neira J, Venet F, Pathogenesis of indirect (secondary) acute lung injury: Expert Rev Respir Med, 2011; 5(1); 115-26
33. Leone M, Albanèse J, Rousseau S, Pulmonary contusion in severe head trauma patients: Impact on gas exchange and outcome: Chest, 2003; 124(6); 2261-66
34. Rincon F, Ghosh S, Dey S, Impact of acute lung injury and acute respiratory distress syndrome after traumatic brain injury in the United States: Neurosurgery, 2012; 71(4); 795-803
35. Orliaguet G, Rakotoniaina S, Meyer PEffect of a lung contusion on the prognosis of severe head injury in the child: Ann Fr Anesth Reanim, 2000; 19(3); 164-70 [in French]
36. Lu G, Zhu L, Wang X, Decompressive craniectomy for patients with traumatic brain injury: A pooled analysis of randomized controlled trials: World Neurosurg, 2020; 133; e135-e48
37. Gravesteijn BY, Sewalt CA, Nieboer D, Tracheal intubation in traumatic brain injury: A multicentre prospective observational study: Br J Anaesth, 2020; 125(4); 505-17
Figures
Tables
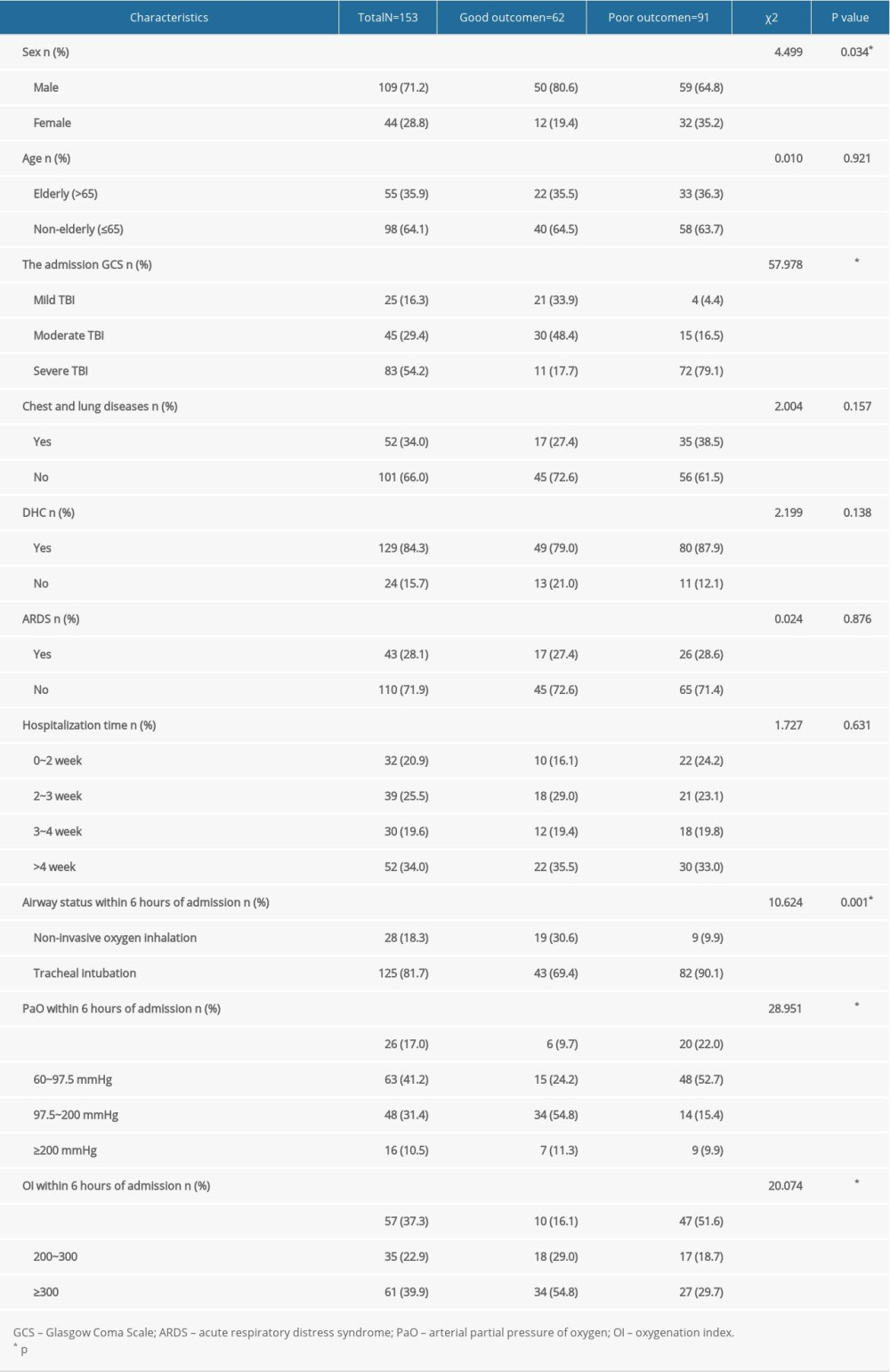 Table 1. Univariate analysis of factors for prognosis of traumatic brain injury.
Table 1. Univariate analysis of factors for prognosis of traumatic brain injury.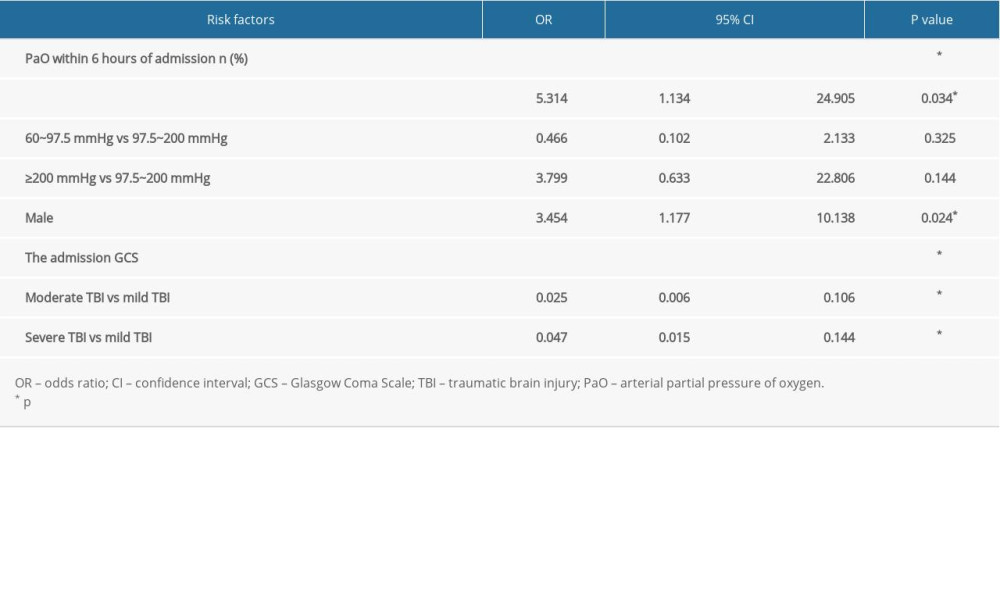 Table 2. Logistic regression analysis of factors for prognosis of traumatic brain injury.
Table 2. Logistic regression analysis of factors for prognosis of traumatic brain injury. Table 1. Univariate analysis of factors for prognosis of traumatic brain injury.
Table 1. Univariate analysis of factors for prognosis of traumatic brain injury. Table 2. Logistic regression analysis of factors for prognosis of traumatic brain injury.
Table 2. Logistic regression analysis of factors for prognosis of traumatic brain injury. In Press
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
06 Mar 2024 : Clinical Research
Prevalence and Variation of Medical Comorbidities in Oral Surgery Patients: A Retrospective Study at Jazan ...Med Sci Monit In Press; DOI: 10.12659/MSM.943884
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952