06 May 2021: Clinical Research
Establishment and Verification of a Prediction Model for Symptomatic Radiation Pneumonitis in Patients with Esophageal Cancer Receiving Radiotherapy
Liu-Ting Yang1ABCDEFG, Lei Zhou1BC, Long Chen1ACDE, Shi-Xiong Liang1ADE, Jiang-Qiong Huang1DEF, Xiao-Dong Zhu12ACDEF*DOI: 10.12659/MSM.930515
Med Sci Monit 2021; 27:e930515
Abstract
BACKGROUND: This study aimed to determine the value of the significant index in predicting symptomatic radiation pneumonitis (RP) in esophageal cancer patients, establish a nomogram prediction model, and verify the model.
MATERIAL AND METHODS: The patients enrolled were divided into 2 groups: a model group and a validation group. According to the logistic regression analysis, the independent predictors for symptomatic RP were obtained, and the nomogram prediction model was established according to these independent predictors. The consistency index (C-index) and calibration curve were used to evaluate the accuracy of the model, and the prediction ability of the model was verified in the validation group. Recursive partitioning analysis (RPA) was used for the risk stratification analysis.
RESULTS: The ratio of change regarding the pre-albumin at the end of treatment (P=0.001), platelet-to-lymphocyte ratio during treatment (P=0.027), and neutrophil-to-lymphocyte ratio at the end of treatment (P=0.001) were the independent predictors for symptomatic RP. The C-index of the nomogram model was 0.811. According to the risk stratification of RPA, the whole group was divided into 3 groups: a low-risk group, a medium-risk group, and a high-risk group. The incidence of symptomatic RP was 0%, 16.9%, and 57.6%, respectively. The receiver operating characteristic curve also revealed that the nomogram model has good accuracy in the validation group.
CONCLUSIONS: The developed nomogram and corresponding risk classification system have superior prediction ability for symptomatic RP and can predict the occurrence of RP in the early stage.
Keywords: Anti-Inflammatory Agents, Esophageal Neoplasms, nomograms, Nutrition Assessment, Radiation Pneumonitis, Blood Platelets, Lymphocytes, Neutrophils, Radiotherapy, Reproducibility of Results, Risk
Background
Esophageal cancer (EC) is one of the most common digestive system cancers, and the number of cases has been increasing year by year. In 2018, EC was ranked as the seventh most frequently diagnosed cancer and the sixth leading cause of cancer-related death worldwide [1]. Since most patients have EC too far advanced for radical resection when diagnosed, radiotherapy (RT) remains one of the main treatment methods, especially for local advanced EC. Several studies have suggested that the effect of concurrent chemo-RT is equivalent to surgical treatment [2–4].
However, the sensitivity of normal lung tissue to radiation limits the use of RT in thoracic tumors. Radiation-induced lung injury is the most common complication of EC patients after RT, which manifests as radiation pneumonia in the acute stage and radiation-induced pulmonary fibrosis in the chronic stage. This causes dry cough, dyspnea, and shortness of breath after activity, which affects the quality of life of patients, and even threatens life. Symptomatic radiation pneumonitis (RP) is of grade ≥2, with obvious clinical symptoms, which affects the quality of life and requires clinical intervention. Most of these patients can recover after active treatment. However, this still has a certain impact on the life of the patients. It is of great significance for patients to determine the occurrence of symptomatic RP as early as possible, allowing the corresponding positive measures to be performed as soon as possible, thereby improving the quality of life.
In the past, the prediction of RP focused on dosimetry. From the results of previous studies, we know that there is a very close relationship between RP and radiation dosage in the lungs. The recognized dose constraints were defined for total lungs as follows: V5 <60%, V20 <30%, V30 <20%, mean lung dose (MLD) <20 Gy. Once the radiation dose to the lungs exceeds the threshold, the incidence of RP will increase significantly. The radiation dose to the lungs in our study was strictly controlled. However, more studies reported that even though the lung dose was strictly controlled, these patients still presented with radiation-induced lung injury, indicating that non-dosimetry factors play a considerable role in the development of radiation-induced pneumonia. Furthermore, the present data on RP are mostly from lung cancer, and the results of different studies vary. The present study aimed to evaluate the significant index before and during RT in predicting symptomatic RP in EC patients, establish a nomogram prediction model according to independent prediction factors, and verify the established model, hoping to predict the occurrence of symptomatic RP effectively at the earliest time.
Material and Methods
PATIENTS:
Inclusion criteria were: (1) newly diagnosed and pathologically confirmed EC; (2) receipt of ≥56 Gy of RT for a curative aim; (3) availability of clinicopathologic, dosimetric, and laboratory data; (4) complete pulmonary imaging within 6 months after RT; (5) no obvious signs of infection before treatment. Exclusion criteria were: (1) previous history of thoracic RT; (2) pregnant and lactating women; (3) patients with acute infectious disease.
METHODS:
The patients enrolled were divided into 2 groups: a model group and a validation group. Four types of data were collected as follows: (1) clinicopathologic characteristics of patients, such as age, smoking, drinking, height, weight, basic lung diseases, pathologic type, and tumor location; (2) dosimetry factors, such as radiation dose and lung exposure; (3) treatment technology and scheme, such as RT technology, chemotherapy scheme, and cycle; (4) hematologic index, such as neutrophil count, lymphocyte count, platelet count, monocyte count, albumin, and pre-albumin. According to the logistic regression analysis, the independent predictors for symptomatic RP were obtained, and the nomogram prediction model was established according to these independent predictors. The consistency index (C-index) and calibration curve were used to evaluate the accuracy of the model, and the prediction ability of the model was verified in the validation group. Recursive partitioning analysis (RPA) was used for the risk stratification analysis.
PRETREATMENT ASSESSMENT: The clinicopathologic information, imaging data, and laboratory test results were all acquired from medical records. The clinicopathologic parameters included age, sex, smoking, drinking, smoking index, pulmonary bulla, pathologic diagnosis, tumor location and length, tumor-lymph node-metastasis stage [5], radiation dose, and chemotherapy.
RADIOTHERAPY:
All of the patients were treated with intensity-modulated RT (IMRT). The delineation of target volumes and organs at risk (OARs) referred to the Radiotherapy and Oncology Group (RTOG) guidelines. All RT plans were delivered with 6 MV of photon beams. The prescribed doses of RT were 56.00–69.96 Gy at 1.8–2.2 Gy per fraction, once daily, and 5 fractions per week. The plans were normalized to 95% of the planning tumor volume (PTV) received at 100% of the prescribed dose. The dose constraints were defined for OARs as follows: total lungs: V5 <60%, V20 <30%, V30 <20%, MLD <20 Gy; maximum point dose of the spinal cord <45 Gy; heart: V30 <40%, V40 <30%.
CHEMOTHERAPY:
A total of 113 patients received chemotherapy among the 131 cases in the model group, and 38 patients received chemotherapy among the 43 cases in the validation group. The chemotherapy plan was based on platinum. The chemotherapy plans were as follows: platinum+5-fluorouracil; taxus+platinum; etoposide+cisplatin. During the treatment, the corresponding symptomatic treatment was taken when the patient presented with adverse reactions. When the patient could not tolerate the follow-up chemotherapy, the chemotherapy was suspended.
COLLECTION OF HEMATOLOGIC INDEX:
The hematologic index was obtained at 3 different time points: pretreatment, at 3 to 4 weeks during RT, and at the end of RT. The platelet-to-lymphocyte ratio (PLR), neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR) and systemic immune inflammation index (SII) were calculated as follows: PLR=P/L; NLR=N/L; LMR=L/M; SII=P×N/L (neutrophil count [N], lymphocyte count [L], platelet count [P], and monocyte count [M]).
MEASUREMENT OF BODY MASS INDEX (BMI):
The height and weight of each patient were measured before and at the end of the RT. The BMI was obtained using the formula: BMI=weight (kg)/square of height (m2).
FOLLOW-UP:
Patients were followed up at 1 month and 3 months after completion of the RT, and subsequently every 3 months for the first year for the evaluation of RP. If the patient had obvious cough, expectoration, shortness of breath, dyspnea, and other discomforts after or during the RT, chest computed tomography was performed for further diagnosis. The endpoint of the present study was symptomatic RP defined as grade ≥2 RP occurring within 6 months after RT.
STATISTICAL ANALYSIS:
All data were analyzed using SPSS 24.0 and R 3.4.2. The characteristics of patients were calculated as the proportion for categorical variables, or mean±standard deviation for continuous variables. The differences between variables were evaluated by chi-square test,
Results
PATIENT CHARACTERISTICS:
A total of 174 cases of EC, which was confirmed by pathology, were enrolled in the present study. Among these, 131 cases in April 2013 to December 2018 were assigned to the modeling group and 43 cases in January 2019 to August 2020 were assigned to the validation group. The detailed characteristics of the enrolled population are listed in Table 1.
INCIDENCE OF RP:
RP was graded in accordance with the classification criteria of the American RTOG and the European tumor treatment research cooperative group. The incidence of symptomatic RP was 23.7% and 18.6% in the primary and validation cohort, respectively (Figure 1, Table 2).
PREDICTIVE FACTORS OF SYMPTOMATIC RP IN THE MODELING GROUP:
The factors significantly correlated with symptomatic RP were MLD (P=0.021), V5 (P=0.011), V10 (P=0.007), V15 (P=0.028), and V20 (P=0.028) before RT, whereas during the RT, albumin (P<0.001), and pre-albumin (P<0.001) at the end of the RT; the ratio of change regarding the pre-albumin at the end of treatment (the pre/end ratio of albumin, P<0.001), the pre-albumin, PLR, LMR, and SII during the RT, and the NLR, PLR, LMR, SII, and D-dimer at the end of RT were also closely correlated with symptomatic RP. The further multivariate analysis indicated that the pre/end ratio of pre-albumin, PLR during the RT, and NLR at the end of RT were independent predictors of symptomatic RP (Tables 3–5).
ESTABLISHMENT OF THE NOMOGRAM MODEL:
According to the multivariate analysis results, the RMS software package of r3.4.2 was used to establish the nomogram prognosis model (Figure 2). According to internal validation, the C-index of the nomogram model was 0.811 (95% confidence interval [CI]: 0.723–0.900, Akaike information criterion=115.5), which was higher than any other predictors. The area under the curve (AUC) values of the pre/end ratio of pre-albumin, PLR during the RT, and NLR at the end of RT alone were 0.689 (95% CI: 0.602–0.768), 0.645 (95% CI: 0.556–0.727), and 0.678 (95% CI: 0.591–0.757), respectively. The AUC of the nomogram model was 0.811 (95% CI: 0.733–0.874), evidently improving the prediction ability (Figure 3, Table 6). The calibration curve revealed that the nomogram model has good accuracy in predicting the occurrence of symptomatic pneumonia (Figure 4). The nomogram model could be used to differentiate the patients that have RP grade ≥2 with 74.19% sensitivity,76.77% specificity, and 76.15% accuracy. According to the nomogram model, the scores of each variable were as follows: when the pre/end ratio of pre-albumin was 0–7, the scores were 0–100, respectively; when the PLR was 0 and 1, the scores were 0 and 18, respectively; when the NLR was 0 and 1, the scores were 0 and 23, respectively. The sum of the scores of all variables of each patient was the total points. According to the risk probability corresponding to the nomogram, the probability of symptomatic RP can be predicted.
NOMOGRAM MODEL AND RISK STRATIFICATION:
In the present study, the nomogram model had a better prediction ability for symptomatic RP. According to the nomogram model, RPA was carried out for the total score of the prediction of symptomatic RP in the modeling group, and the risk of patients was stratified. All patients were divided into 3 risk groups: low (score <23), medium (23≤ score <55), and high (score ≥55). It can be observed from the table that there was a significant difference in the incidence of symptomatic RP in the different risk stratification groups (P<0.001). The incidence was 0% in the low-risk group, 17.14% in the medium-risk group, and 57.58% in the high-risk group (Table 7). It was found that the incidence of symptomatic RP in the high-risk group was higher than that in the low-risk group and medium-risk group (P<0.001).
VERIFICATION OF THE NOMOGRAM MODEL:
The nomogram model was validated in the validation group. According to the nomogram model, the total score of each case in the validation group was calculated. According to external validation, the AUC of the nomogram model in the validation group was 0.854 (95% CI: 0.696–1.000), which was higher than any other predictors. The AUC values of the pre/end ratio of pre-albumin, PLR during the RT, and NLR at the end of RT alone was 0.718 (95% CI: 0.562–0.873), 0.698 (95% CI: 0.484–0.913), and 0.738 (95% CI: 0.562–0.913), respectively (Figure 5, Table 8). The ROC curve also revealed that the nomogram model had good accuracy in the validation group. According to the total score in the validation group, the incidence was 6.7% in the low-risk group, 11.1% in the medium-risk group, and 50% in the high-risk group (Table 9). It was found that the incidence of symptomatic RP in the high-risk group was higher than that in low-risk group and medium-risk group (P<0.05).
Discussion
EC is one of the most common malignant tumors in the digestive system. Most of these patients cannot undergo an operation and therefore receive comprehensive RT treatment. However, the related adverse reactions caused by RT can seriously affect the quality of life of these patients, and can even lead to the interruption of RT, thereby affecting the efficacy. Radiation-induced lung injury is the most common complication in EC patients after RT. Once this occurs, it is difficult to reverse. The present study aimed to determine the correlation between the significant indexes and symptomatic RP in patients with EC before and during RT. Under the premise that the lung dose is effectively controlled, the present study revealed that the pre/end ratio of pre-albumin, PLR during RT, and NLR at the end of RT were independent predictors of symptomatic RP. On the basis of this, a prediction tool that is convenient for clinical use was developed to facilitate clinicians in RT, the early prediction of the risk of symptomatic RP, and timely intervention, which could maximize the benefits of the treatment.
In recent decades, many researchers have devoted themselves to investigating factors that can predict the occurrence and severity of RP. The research results mainly included the following 4 aspects: (1) clinical characteristics of patients, such as age [6–10], smoking [11–15], lung function [16–18], and basic lung diseases [19, 20]; (2) dosimetry factors, such as radiation dose [21,22] and lung exposure [7,23]; (3) treatment technology and scheme, such as RT technology [24–26] and chemotherapy scheme [27]; (4) individual genetic sensitivity, such as tumor transforming growth factor-β1(TGF-β1) gene polymorphism [28]. However, different studies still show great differences in these results. With the development of RT technology and the popularization of IMRT, the dose for lungs in EC has been well controlled, which was basically controlled within the limited range. However, the incidence of radiation-induced lung injury remains high. In clinical practice, patients with the same stage received almost the same dose in both lungs, but the occurrence and severity of radiation-induced lung injury may be completely different, revealing that to a certain extent, when predicting the radiation-induced lung injury, in addition to considering conventional factors, some new independent prediction factors also need to be given increasing attention.
In a strict sense, RP is a kind of aseptic inflammation and an inflammatory response to radiation injury. Therefore, it can be assumed that the inflammatory index is correlated with RP to some extent. Some inflammatory markers, such as C-reactive protein and lactate dehydrogenase, have been reported to be associated with radiation-induced pneumonia [29], whereas some cytokines, such as TGF-β1, interleukin-1α (IL-1α), IL-6, and IL-10, have also been shown to be associated with radiation-induced pneumonia [30–32]. However, some of these indicators have not been widely used in clinics, or the cost was a little high. Therefore, these could not be routinely used in clinics. Whole blood cell count is a routine, cheap, and simple detection method that can provide information on blood components. Among these, the NLR has been proven to be an important marker of inflammation. Some studies have reported that NLR is a marker of the severity and deterioration of lung diseases (such as pneumonia [33] and chronic obstructive pulmonary disease [COPD] [34]). A study on COPD patients revealed that the NLR of patients with acute exacerbation is significantly higher than that of patients with a stable stage, and is the most sensitive of all inflammatory serum markers [34]. There have been few reports on the relationship between RP and inflammatory indexes. In the study conducted by Lee et al [35], NLR was used to predict RP. It was found that the NLR value of patients with symptomatic pneumonia was significantly higher than that of patients without symptoms, when imaging changes occurred (4.99 vs 2.90, respectively). These were the independent predictors of symptoms of RT. However, when RP occurred, it was no longer significant to measure NLR. In the study conducted by Shaverdian et al [36], it was considered that patients with high NLR before RT had a lower incidence of symptomatic radiation-induced pneumonia. Platelets release proinflammatory mediators such as chemokines and cytokines [37] to mediate the inflammatory response. Therefore, PLR was also considered as an inflammatory marker that can be used to predict inflammation and mortality in many diseases [38]. In addition to NLR, the investigators attempted to describe the dynamic changes of PLR, LMR, and SII in the course of RT to determine whether these have more predictive significance for RP. In the present study, it was found that NLR, PLR, and SII gradually increased in the course of the RT, but decreased near the end of the RT, and that these were ultimately higher than the level before RT. The increase in inflammatory index in the symptomatic radiation pneumonitis group was higher than that in the nonsymptomatic group, whereas the change trend of LMR was just the opposite. The further multivariate analysis revealed that PLR during RT and NLR at the end of RT were independent predictors of symptomatic RP.
EC patients are often accompanied by light or heavy malnutrition before, during, and after treatment. For patients undergoing RT, malnutrition can also reduce the sensitivity of tumor cells to RT, increase the positioning error of RT, reduce the tolerance to RT, and thereby increase the adverse reactions after RT. In the study conducted by Wen et al [39], it was found that human hemoglobin was significantly correlated with acute radiation esophagitis, and that the total lymphocyte count was significantly correlated with the acute adverse reactions in the blood system. The study conducted by Hill et al [40] revealed that 75.5% of patients have different degrees of weight loss through the RT treatment for gastrointestinal cancer. Weight loss was more serious in patients with unplanned RT interruption and failure to complete the planned chemotherapy cycle. Radiotoxicity was closely correlated with the patient-generated subjective global assessment score (
Therefore, it was speculated that RP and malnutrition are correlated with the same cytokines in the process of occurrence and development. Patients with malnutrition are more likely to develop RP, and similar conclusions have been reached in the present study. However, can nutritional support therapy be used to improve the nutritional status of patients, and reduce the incidence of radiation-induced pneumonia in patients with chest RT? Feijó et al [43] conducted a nutritional intervention on gastric cancer patients, and found that the level of IL-6 in the nutritional intervention group was significantly lower. That is, patients without nutritional intervention were significantly more prone to inflammation than patients with nutritional intervention. In the study conducted by Fabian et al [50], TNF-α and IL-6 in the serum of breast cancer patients decreased after 5 months of eicosapentaenoic acid/docosahexaenoic acid nutrition supplementation. However, this still requires a large number of prospective, randomized studies to confirm.
We were able to find a few recent studies on the prediction model of RP [51–53]. Yu et al [51] combined IL8 and chemokine (C-C motif) ligand 2 at baseline level and 2 clinical variables to construct a nomogram. The model achieved good predictability (AUC=0.863, accuracy=80.0%, sensitivity=100%, specificity=76.5%). Du et al [53] combined genetic and nongenetic factors to establish a multiple linear regression model for the assessment of grade ≥2 RP risk. This model can successfully distinguish the RP ≥2 population with 92.0% sensitivity and 100% specificity. However, the above prediction models were based on lung cancer. Moreover, inflammatory cytokines and single-nucleotide polymorphism sites have not been routinely detected in clinical studies. In our study, the nomogram model also achieved good predictability (AUC=0.811, accuracy=76.15%, sensitivity=74.19%, specificity=76.77%). In addition, the 3 factors included were obtained by conventional, cheap, and simple detection methods. Therefore, our model is more convenient for clinical use.
The predictive model of symptomatic RP for patients with EC receiving RT established in the present study has good predictability. However, there are still limitations: (1) the present study was a single-center retrospective study with a small number of cases; (2) the relationship between continuous variables and symptomatic RP was linearly processed, which may have led to bias; (3) the validation of the nomogram was limited to cases in our research center, and the validation of external data was not strictly performed. However, under the premise that the lung dose is effectively controlled, this study shifted the focus of RP from dosimetry to inflammation and nutritional status, which also suggests to us whether reducing the inflammatory reaction or improving the nutritional status of patients during RT can reduce the incidence risk of pneumonia. Multicenter prospective studies are needed to confirm whether this model can be widely used for the prediction of symptomatic RP after RT for EC.
Conclusions
Under the premise that the lung dose is effectively controlled, the pre/end ratio of pre-albumin, PLR during RT, and NLR at the end of RT were the independent predictors of symptomatic RP. The nomogram chart based on these predictors has a better predictability for symptomatic RP, but it still needs more multicenter prospective studies to verify it.
Figures
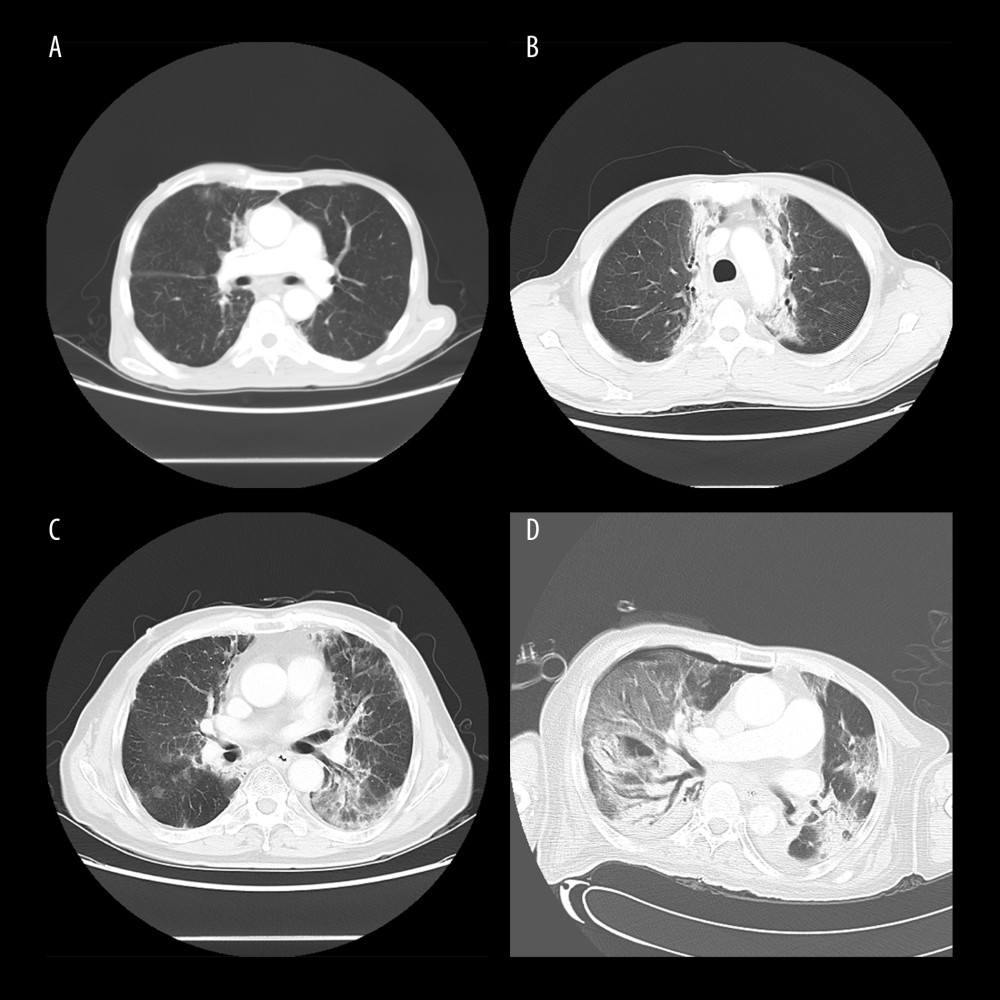 Figure 1. Computed tomography (CT) manifestations of radiation pneumonitis (RP). (A) CT manifestations of grade 1 RP. (B) CT manifestations of grade 2 RP. (C) CT manifestations of grade 3 RP. (D) CT manifestations of grade 4 RP.
Figure 1. Computed tomography (CT) manifestations of radiation pneumonitis (RP). (A) CT manifestations of grade 1 RP. (B) CT manifestations of grade 2 RP. (C) CT manifestations of grade 3 RP. (D) CT manifestations of grade 4 RP. 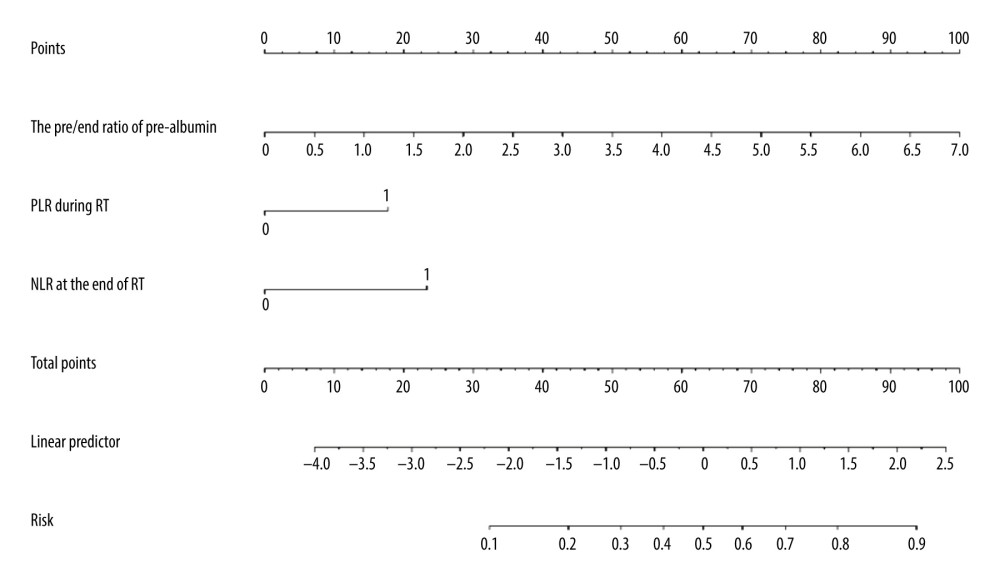 Figure 2. Nomogram predicting the development of symptomatic radiation pneumonitis for esophageal cancer.
Figure 2. Nomogram predicting the development of symptomatic radiation pneumonitis for esophageal cancer. 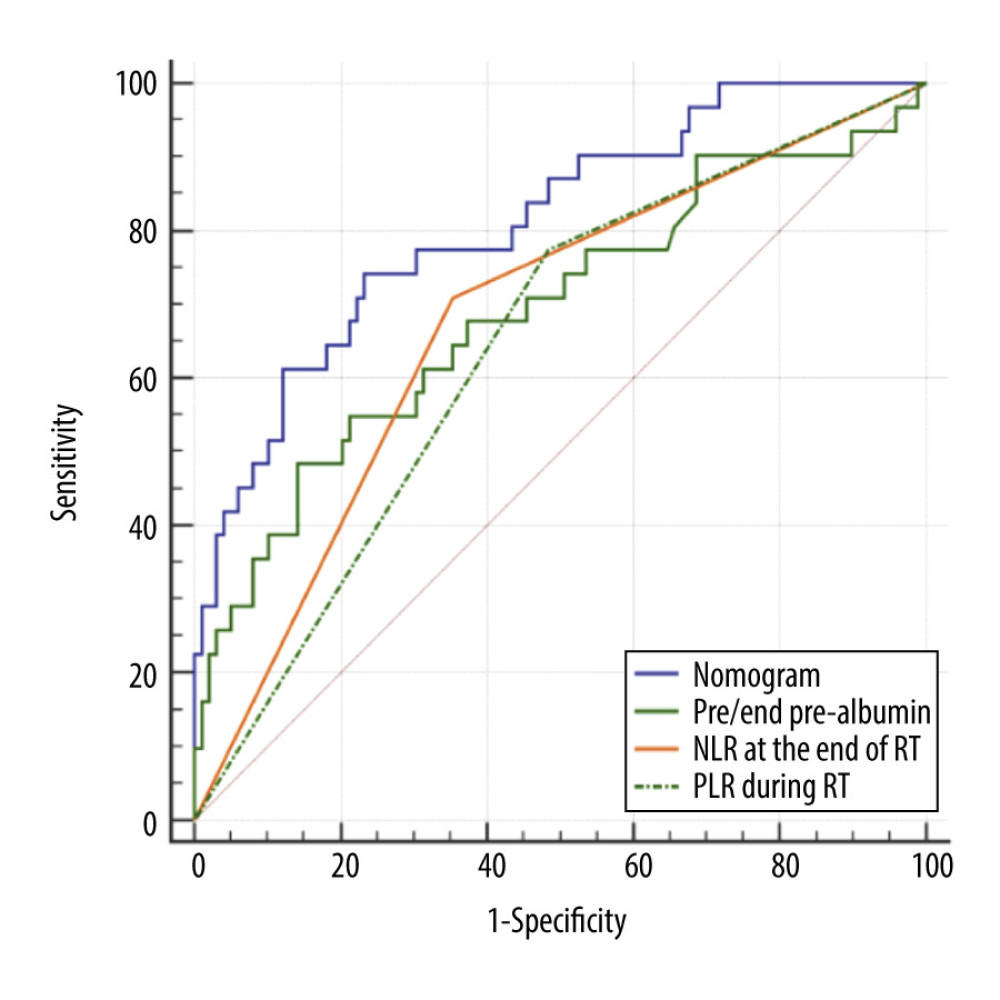 Figure 3. Receiver operating characteristic curve based on the sensitivity and specificity of the pre/end ratio of pre-albumin, platelet-to-lymphocyte ratio during radiation therapy (RT), neutrophil-to-lymphocyte ratio at the end of RT, and nomogram model in the model group.
Figure 3. Receiver operating characteristic curve based on the sensitivity and specificity of the pre/end ratio of pre-albumin, platelet-to-lymphocyte ratio during radiation therapy (RT), neutrophil-to-lymphocyte ratio at the end of RT, and nomogram model in the model group. 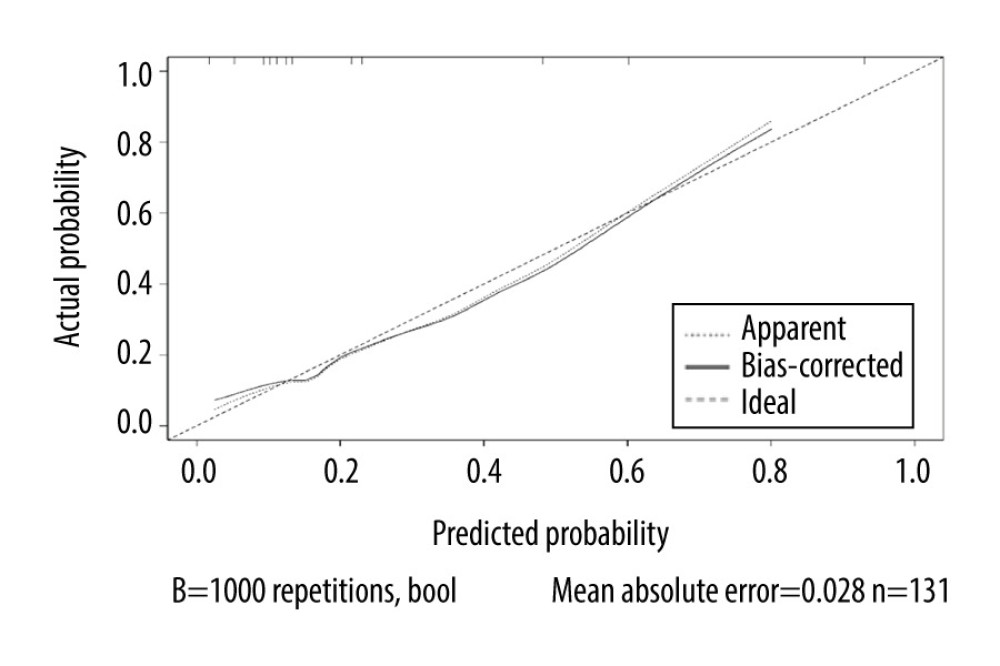 Figure 4. Calibration curves of the nomogram predicting symptomatic radiation pneumonitis in the primary cohort.
Figure 4. Calibration curves of the nomogram predicting symptomatic radiation pneumonitis in the primary cohort. 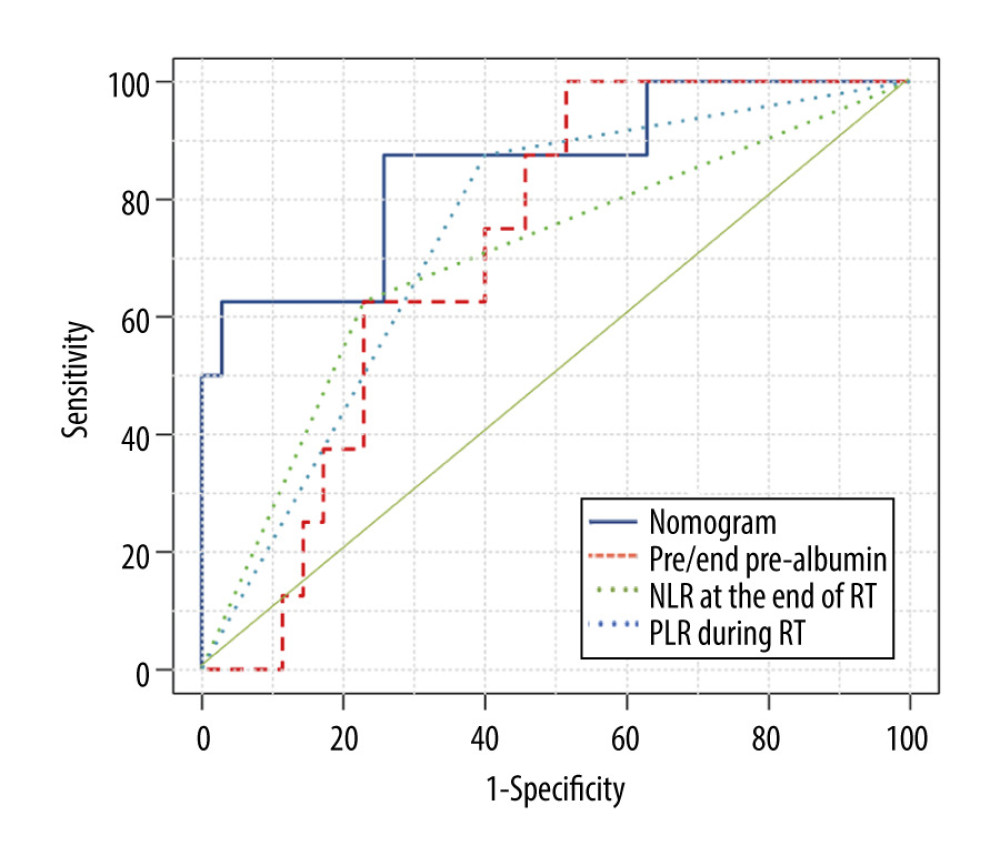 Figure 5. Receiver operating characteristic curve based on the sensitivity and specificity of the pre/end ratio of pre-albumin, platelet-to-lymphocyte ratio during radiation therapy (RT), neutrophil-to-lymphocyte ratio at the end of RT, and nomogram model in the validation group.
Figure 5. Receiver operating characteristic curve based on the sensitivity and specificity of the pre/end ratio of pre-albumin, platelet-to-lymphocyte ratio during radiation therapy (RT), neutrophil-to-lymphocyte ratio at the end of RT, and nomogram model in the validation group. Tables
Table 1. Baseline characteristics of all patients.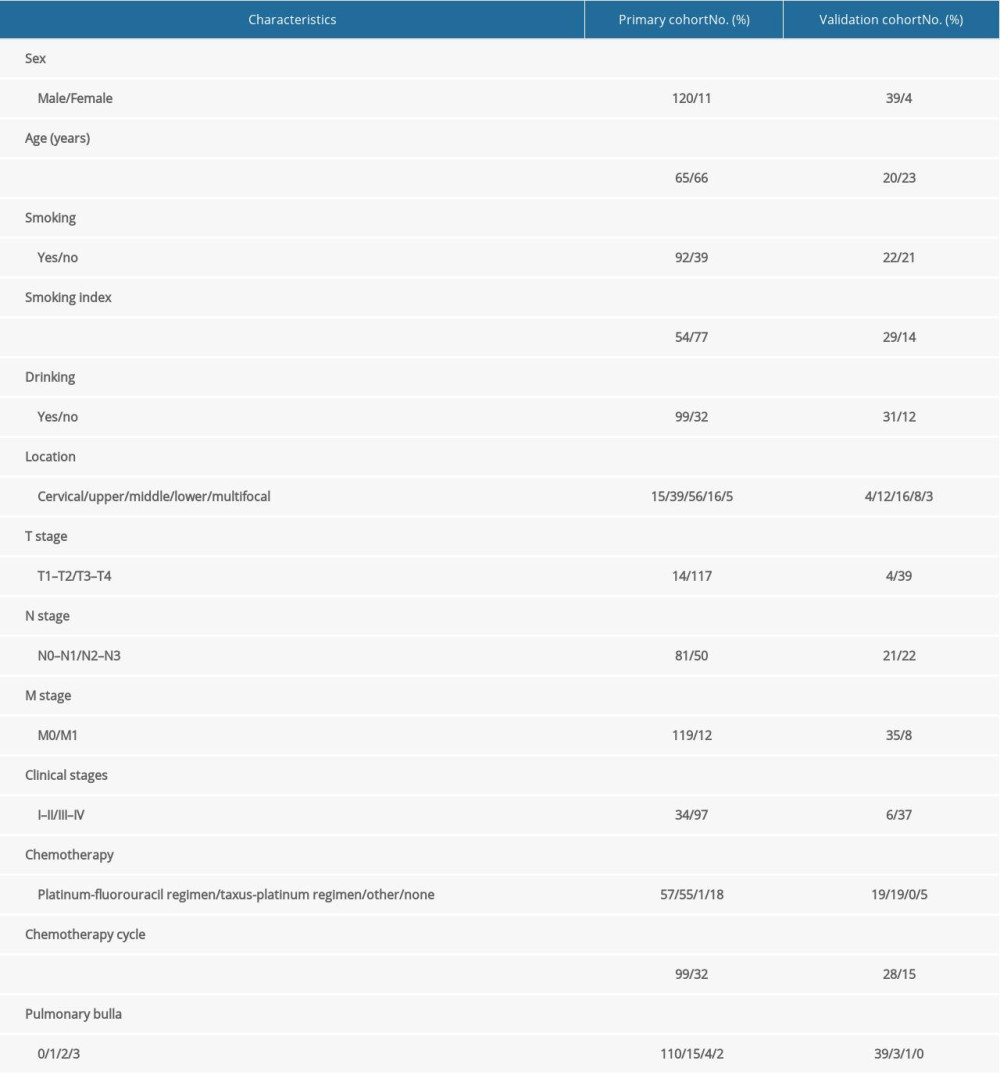 Table 2. Occurrence of radiation pneumonia in esophageal cancer.
Table 2. Occurrence of radiation pneumonia in esophageal cancer.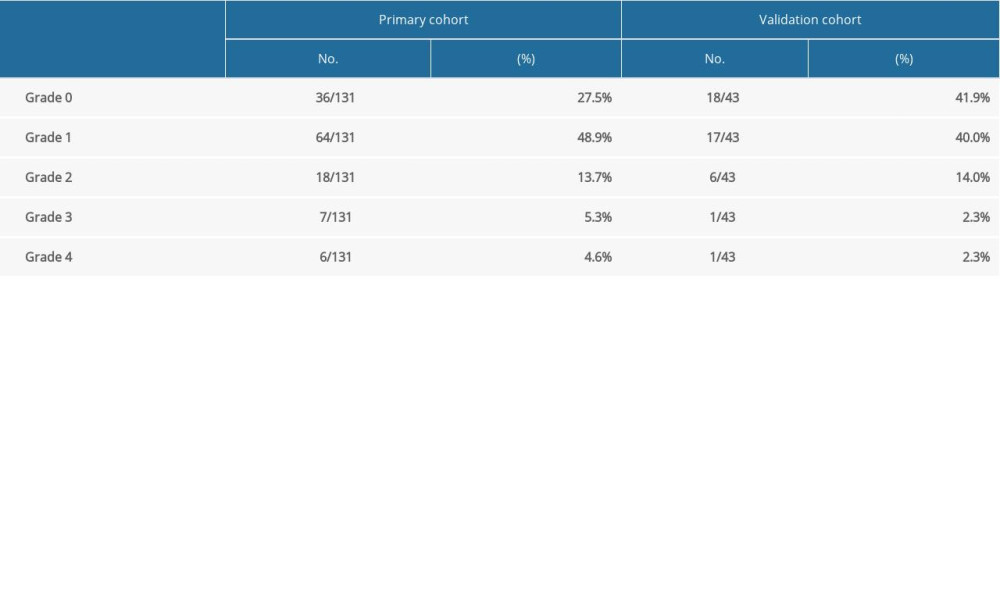 Table 3. Univariate analysis of clinicopathologic, dosimetric, and inflammatory parameters in predicting symptomatic radiation pneumonitis.
Table 3. Univariate analysis of clinicopathologic, dosimetric, and inflammatory parameters in predicting symptomatic radiation pneumonitis.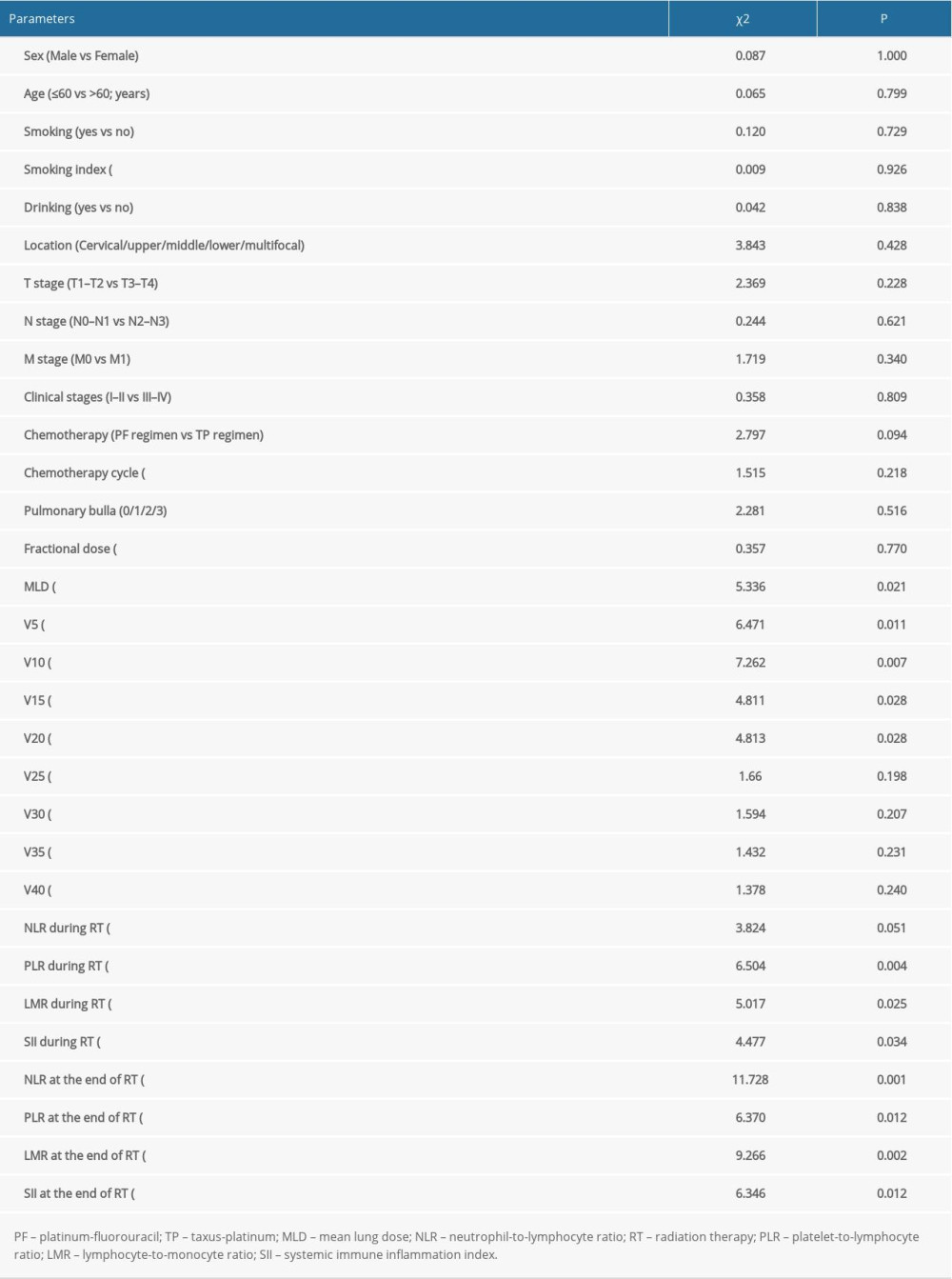 Table 4. Univariate analysis of nutritional parameters and coagulation function in predicting symptomatic radiation pneumonitis.
Table 4. Univariate analysis of nutritional parameters and coagulation function in predicting symptomatic radiation pneumonitis.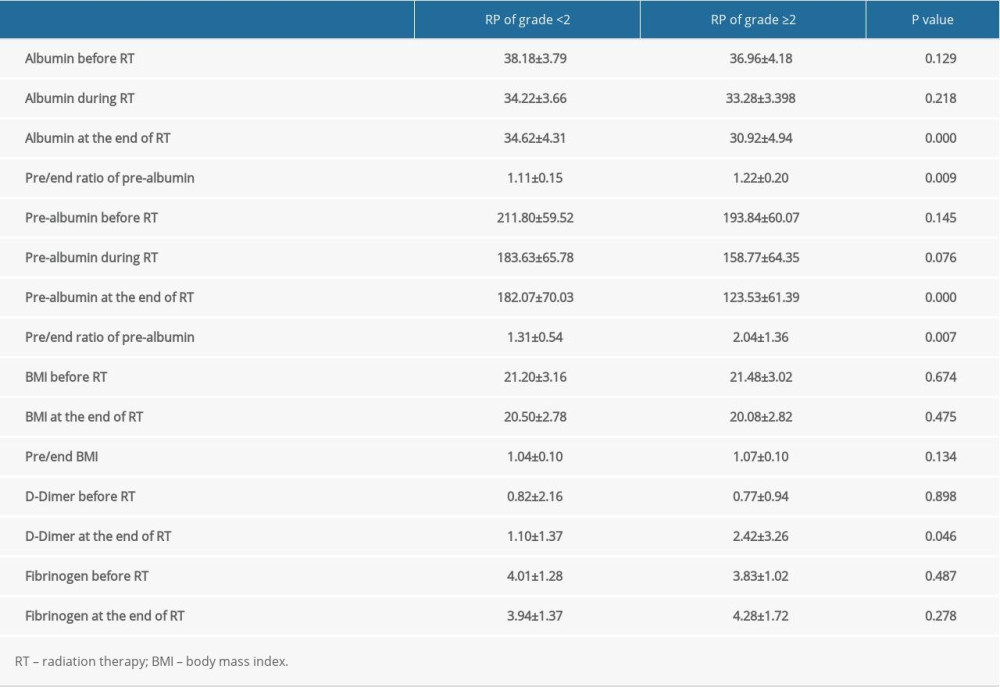 Table 5. Multivariate analysis of potential parameters in predicting symptomatic radiation pneumonitis.
Table 5. Multivariate analysis of potential parameters in predicting symptomatic radiation pneumonitis.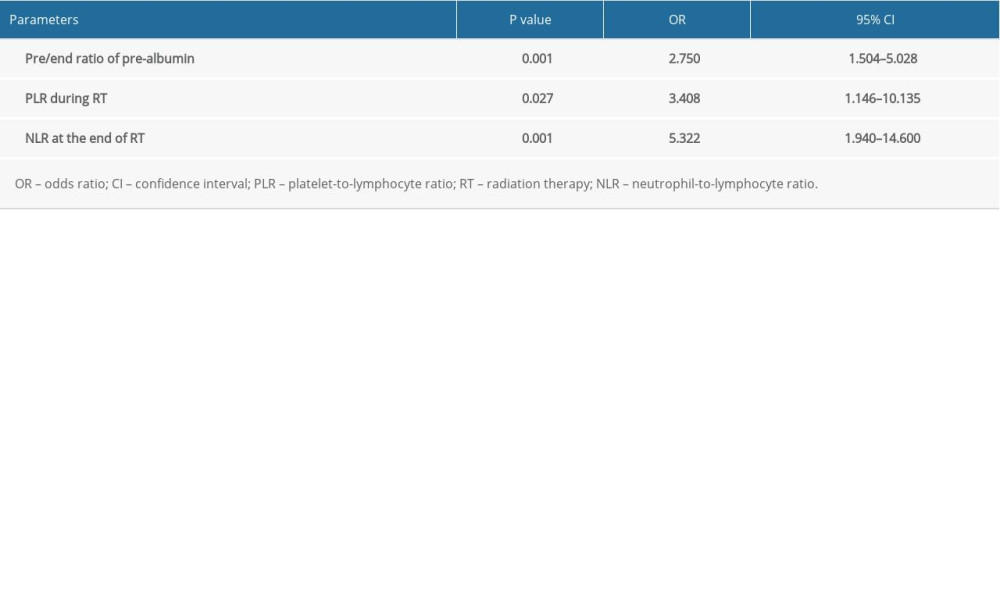 Table 6. Areas under the receiver operating characteristic (ROC) curve for the independent predictors and nomogram model in the modeling group.
Table 6. Areas under the receiver operating characteristic (ROC) curve for the independent predictors and nomogram model in the modeling group.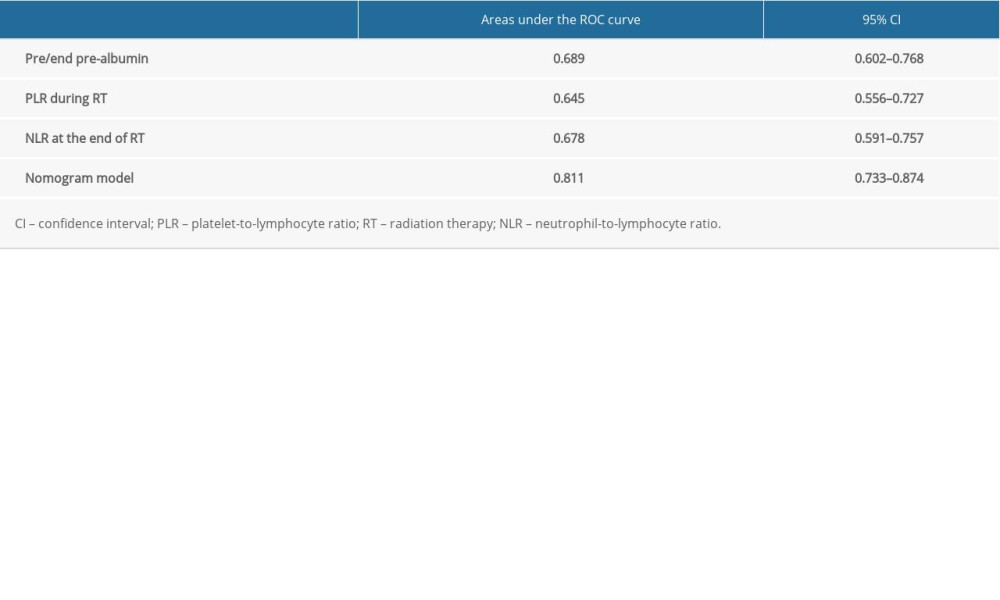 Table 7. Incidence of symptomatic radiation pneumonitis (RP) in the modeling group.
Table 7. Incidence of symptomatic radiation pneumonitis (RP) in the modeling group.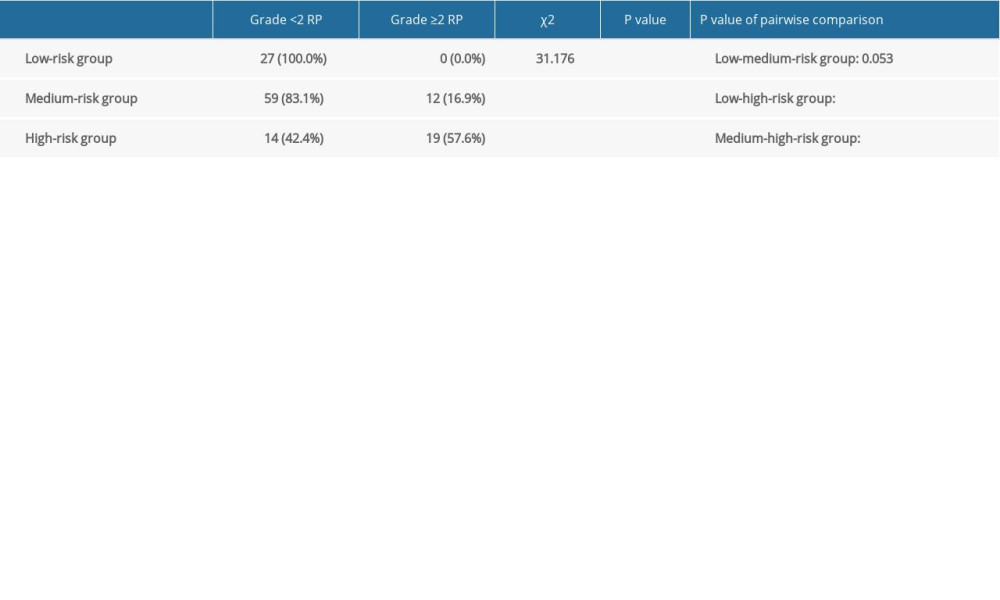 Table 8. Areas under the receiver operating characteristic (ROC) curve for the independent predictors and nomogram model in the validation group.
Table 8. Areas under the receiver operating characteristic (ROC) curve for the independent predictors and nomogram model in the validation group.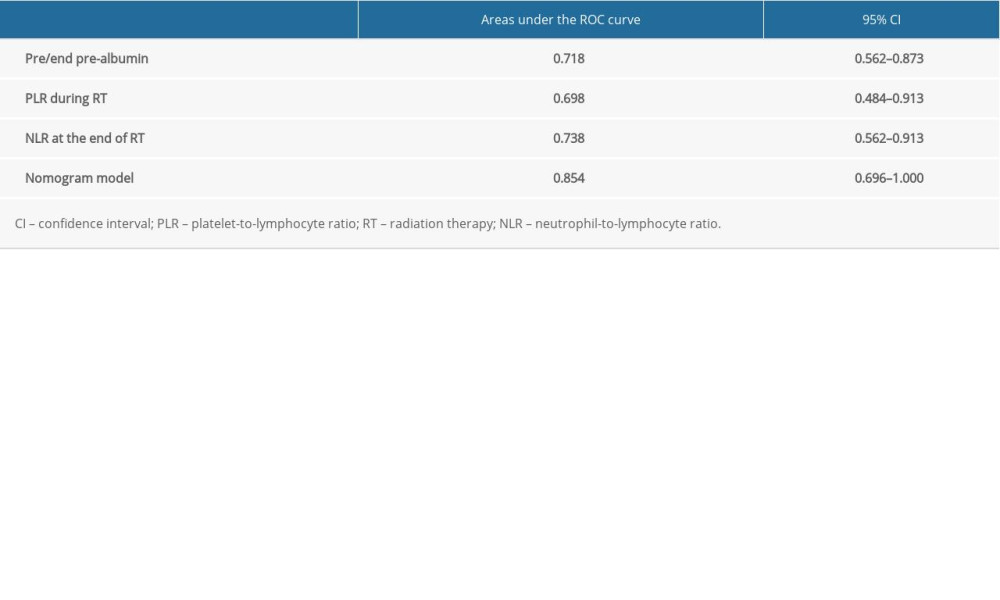 Table 9. Incidence of symptomatic radiation pneumonitis (RP) in the validation group.
Table 9. Incidence of symptomatic radiation pneumonitis (RP) in the validation group.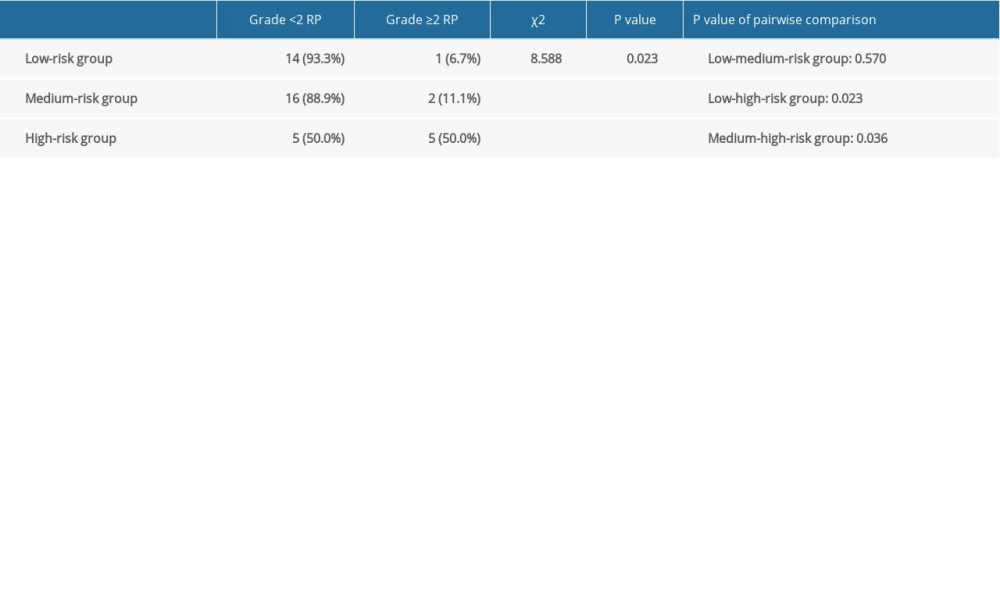
References
1. Bray F, Ferlay J, Soerjomataram I, Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries: Cancer J Clin, 2018; 68(6); 394-424
2. Hironaka S, Ohtsu A, Boku N, Nonrandomized comparison between definitive chemoradiotherapy and radical surgery in patients with T(2–3)N(any) M(0) squamous cell carcinoma of the esophagus: Int J Radiat Oncol, 2003; 57(2); 425-33
3. Chan A, Wong A, Is combined chemotherapy and radiation therapy equally effective as surgical resection in localized esophageal carcinoma?: Int J Radiat Oncol Biol Phys, 1999; 45(2); 265-70
4. Chiu PW, Chan AC, Leung SF, Multicenter prospective randomized trial comparing standard esophagectomy with chemoradiotherapy for treatment of squamous esophageal cancer: Early results from the Chinese University Research Group for Esophageal Cancer (CURE): J Gastroint Surg, 2005; 9(6); 794-802
5. Rice TW, Ishwaran H, Ferguson MK, Cancer of the esophagus and esophagogastric junction: An eighth edition staging primer: J Thorac Oncol, 2017; 12(1); 36-42
6. Parashar B, Edwards A, Mehta R, Chemotherapy significantly increases the risk of radiation pneumonitis in radiation therapy of advanced lung cancer: Am J Clin Oncol, 2011; 34(2); 160-64
7. Palma DA, Senan S, Tsujino K, Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: An international individual patient data meta-analysis: Int J Radiat Oncol Biol Phys, 2013; 85(2); 444-50
8. Dehing-Oberije C, De Ruysscher D, van Baardwijk A, The importance of patient characteristics for the prediction of radiation-induced lung toxicity: Radiother Oncol, 2009; 91(3); 421-26
9. Vogelius IR, Bentzen SM, A literature-based meta-analysis of clinical risk factors for development of radiation induced pneumonitis: Acta Oncol, 2012; 51(8); 975-83
10. Tsujino K, Hashimoto T, Shimada T, Combined analysis of V20, VS5, pulmonary fibrosis score on baseline computed tomography, and patient age improves prediction of severe radiation pneumonitis after concurrent chemoradiotherapy for locally advanced non-small-cell lung cancer: J Thorac Oncol, 2014; 9(7); 983-90
11. Jin H, Tucker SL, Liu HH, Dose-volume thresholds and smoking status for the risk of treatment-related pneumonitis in inoperable non-small cell lung cancer treated with definitive radiotherapy: Radiother Oncol, 2009; 91(3); 427-32
12. Hernando ML, Marks LB, Bentel GC, Radiation-induced pulmonary toxicity: A dose-volume histogram analysis in 201 patients with lung cancer: Int J Radiat Oncol Biol Phys, 2001; 51(3); 650-59
13. Bjermer L, Cai Y, Nilsson K, Tobacco smoke exposure suppresses radiation-induced inflammation in the lung: A study of bronchoalveolar lavage and ultrastructural morphology in the rat: Eur Respir J, 1993; 6(8); 1173-80
14. Nilsson K, Henriksson R, Cai YQ, Effects of tobacco-smoke on radiation-induced pneumonitis in rats: Int J Radiat Biol, 1992; 62(6); 719-27
15. Mörth C, Kafantaris I, Castegren M, Validation and optimization of a predictive model for radiation pneumonitis in patients with lung cancer: Oncol Lett, 2016; 12(2); 1144-48
16. Lee HJ, Zeng J, Vesselle HJ, Correlation of functional lung heterogeneity and dosimetry to radiation pneumonitis using perfusion SPECT/CT and FDG PET/CT imaging: Int J Radiat Oncol Biol Phys, 2018; 102(4); 1255-64
17. Wang J, Cao J, Yuan S, Poor baseline pulmonary function may not increase the risk of radiation-induced lung toxicity: Int J Radiat Oncol Biol Phys, 2013; 85(3); 798-804
18. Torre-Bouscoulet L, Muñoz-Montaño WR, Martínez-Briseño D, Abnormal pulmonary function tests predict the development of radiation-induced pneumonitis in advanced non-small cell lung cancer: Respir Res, 2018; 19(1); 72
19. Ito H, Nakayama H, Tsuboi M, Subpleural honeycombing on high resolution computed tomography is risk factor for fatal pneumonitis: Ann Thorac Surg, 2011; 91(3); 874-78
20. Sanuki N, Ono A, Komatsu E, Association of computed tomography-detected pulmonary interstitial changes with severe radiation pneumonitis for patients treated with thoracic radiotherapy: J Radiat Res, 2012; 53(1); 110-16
21. Bradley JD, Moughan J, Graham MV, A phase I/II radiation dose escalation study with concurrent chemotherapy for patients with inoperable stages I to III non-small-cell lung cancer: Phase I results of RTOG 0117: Int J Radiat Oncol Biol Phys, 2010; 77(2); 367-72
22. Roach M, Gandara DR, Yuo HS, Radiation pneumonitis following combined modality therapy for lung cancer: Analysis of prognostic factors: J Clin Oncol, 1995; 13(10); 2606-12
23. Graham MV, Purdy JA, Emami B, Clinical dose-volume histogram analysis for pneumonitis after 3D treatment for non-small cell lung cancer (NSCLC): Int J Radiat Oncol Biol Phys, 1999; 45(2); 323-29
24. Marks LB, Yorke ED, Jackson A, Use of normal tissue complication probability models in the clinic: Int J Radiat Oncol Biol Phys, 2010; 76; S10-19
25. Kim TH, Cho KH, Pyo HR, Dose-volumetric parameters for predicting severe radiation pneumonitis after three-dimensional conformal radiation therapy for lung cancer: Radiology, 2005; 235(1); 208-15
26. Liao Z, Lee JJ, Komaki R, Bayesian adaptive randomization trial of passive scattering proton therapy and intensity-modulated photon radiotherapy for locally advanced non-small-cell lung cancer: J Clin Oncol, 2018; 36(18); 1813-22
27. Arrieta O, Gallardo-Rincón D, Villarreal-Garza C, High frequency of radiation pneumonitis in patients with locally advanced non-small cell lung cancer treated with concurrent radiotherapy and gemcitabine after induction with gemcitabine and carboplatin: J Thorac Oncol, 2009; 4(7); 845-52
28. Yuan X, Liao Z, Liu Z, Single nucleotide polymorphism at rs1982073: T869C of the TGFbeta 1 gene is associated with the risk of radiation pneumonitis in patients with non-small-cell lung cancer treated with definitive radiotherapy: J Clin Oncol, 2009; 27(20); 3370-78
29. Inoue A, Kunitoh H, Sekine I, Radiation pneumonitis in lung cancer patients: A retrospective study of risk factors and the long-term prognosis: Int J Radiat Oncol Biol Phys, 2001; 49(3); 649-55
30. Kim JY, Kim YS, Kim YK, The TGF-beta1 dynamics during radiation therapy and its correlation to symptomatic radiation pneumonitis in lung cancer patients: Radiat Oncol, 2009; 4; 59
31. Chen Y, Hyrien O, Williams J, Interleukin (IL)-1A and IL-6: Applications to the predictive diagnostic testing of radiation pneumonitis: Int J Radiat Oncol Biol Phys, 2005; 62(1); 260-66
32. Arpin D, Perol D, Blay JY, Early variations of circulating interleukin-6 and interleukin-10 levels during thoracic radiotherapy are predictive for radiation pneumonitis: J Clin Oncol, 2005; 23(34); 8748-56
33. de Jager CP, Wever PC, Gemen EF, The neutrophil-lymphocyte count ratio in patients with community-acquired pneumonia: PLoS One, 2012; 7(10); e46561
34. Taylan M, Demir M, Kaya H, Alterations of the neutrophil-lymphocyte ratio during the period of stable and acute exacerbation of chronic obstructive pulmonary disease patients: Clin Respir J, 2017; 11(3); 311-17
35. Lee SJ, Lee HR, Lee TW, Usefulness of neutrophil to lymphocyte ratio in patients with chronic obstructive pulmonary disease: A prospective observational study: Korean J Intern Med, 2016; 31(5); 891-98
36. Shaverdian N, Veruttipong D, Wang J, Pretreatment immune parameters predict for overall survival and toxicity in early-stage non-small-cell lung cancer patients treated with stereotactic body radiation therapy: Clin Lung Cancer, 2016; 17(1); 39-46
37. Demirtas S, Karahan O, Yazici S, The relationship between complete blood count parameters and Fontaine’s stages in patients with peripheral arterial disease: Vascular, 2014; 22(6); 427-31
38. Balta S, Ozturk C, The platelet-lymphocyte ratio: A simple, inexpensive and rapid prognostic marker for cardiovascular events: Platelets, 2015; 26(7); 680-81
39. Wen CX, Sun LF, Wu CJ, Impact of radiotherapy and concurrent chemoradiotherapy on acute adverse reactions in patients with esophageal cancer: J Beihua Univ (Nat Sci), 2019; 20(01); 77-81
40. Hill A, Kiss N, Hodgson B, Associations between nutritional status, weight loss, radiotherapy treatment toxicity and treatment outcomes in gastrointestinal cancer patients: Clin Nutr, 2011; 30(1); 92-98
41. Akuzawa N, Naito H, Nutritional parameters affecting severity of pneumonia and length of hospital stay in patients with pneumococcal pneumonia: A retrospective cross-sectional study: BMC Pulm Med, 2015; 15; 149
42. Lee JC, Williams GW, Kozar RA, Multitargeted feeding strategies improve nutrition outcome and are associated with reduced pneumonia in a level 1 trauma intensive care unit: J Parenter Enter Nutr, 2018; 42(3); 529-37
43. Feijó PM, Rodrigues VD, Viana MS, Effects of ω-3 supplementation on the nutritional status, immune, and inflammatory profiles of gastric cancer patients: A randomized controlled trial: Nutrition, 2019; 61; 125-31
44. Alifano M, Mansuet-Lupo A, Lococo F, Systemic inflammation, nutritional status and tumor immune microenvironment determine outcome of resected non-small cell lung cancer: PLoS One, 2014; 9(9); e106914
45. Watanobe H, Takebe K, Intravenous administration of tumor necrosis factor-alpha stimulates corticotropin releasing hormone secretion in the push-pull cannulated median eminence of freely moving rats: Neuropeptides, 1992; 22(2); 81-84
46. Plata-Salamán CR, Central nervous system mechanisms contributing to the cachexia-anorexia syndrome: Nutrition, 2000; 16(10); 1009-12
47. Strassmann G, Fong M, Freter CE, Suramin interferes with interleukin-6 receptor binding in vitro and inhibits colon-26-mediated experimental cancer cachexia in vivo: J Clin Invest, 1993; 92(5); 2152-59
48. Enomoto A, Rho MC, Fukami A, Suppression of cancer cachexia by 20S,21-epoxy-resibufogenin-3-acetate-a novel nonpeptide IL-6 receptor antagonist: Biochem Biophys Res Commun, 2004; 323(3); 1096-102
49. Matthys P, Heremans H, Opdenakker G, Anti-interferon-gamma antibody treatment, growth of Lewis lung tumours in mice and tumour-associated cachexia: Eur J Cancer, 1991; 27(2); 182-87
50. Fabian CJ, Kimler BF, Hursting SD, Omega-3 fatty acids for breast cancer prevention and survivorship: Breast Cancer Res, 2015; 17; 62
51. Yu H, Wu H, Wang W, Machine learning to build and validate a model for radiation pneumonitis prediction in patients with non-small cell lung cancer: Clin Cancer Res, 2019; 25(14); 4343-50
52. Liang B, Tian Y, Chen X, Prediction of radiation pneumonitis with dose distribution: A convolutional neural network (CNN) based model: Front Oncol, 2019; 9; 1500
53. Du L, Ma N, Dai X, Precise prediction of the radiation pneumonitis in lung cancer: An explorative preliminary mathematical model using genotype information: J Cancer, 2020; 11(8); 2329-38
Figures
 Figure 1. Computed tomography (CT) manifestations of radiation pneumonitis (RP). (A) CT manifestations of grade 1 RP. (B) CT manifestations of grade 2 RP. (C) CT manifestations of grade 3 RP. (D) CT manifestations of grade 4 RP.
Figure 1. Computed tomography (CT) manifestations of radiation pneumonitis (RP). (A) CT manifestations of grade 1 RP. (B) CT manifestations of grade 2 RP. (C) CT manifestations of grade 3 RP. (D) CT manifestations of grade 4 RP. Figure 2. Nomogram predicting the development of symptomatic radiation pneumonitis for esophageal cancer.
Figure 2. Nomogram predicting the development of symptomatic radiation pneumonitis for esophageal cancer. Figure 3. Receiver operating characteristic curve based on the sensitivity and specificity of the pre/end ratio of pre-albumin, platelet-to-lymphocyte ratio during radiation therapy (RT), neutrophil-to-lymphocyte ratio at the end of RT, and nomogram model in the model group.
Figure 3. Receiver operating characteristic curve based on the sensitivity and specificity of the pre/end ratio of pre-albumin, platelet-to-lymphocyte ratio during radiation therapy (RT), neutrophil-to-lymphocyte ratio at the end of RT, and nomogram model in the model group. Figure 4. Calibration curves of the nomogram predicting symptomatic radiation pneumonitis in the primary cohort.
Figure 4. Calibration curves of the nomogram predicting symptomatic radiation pneumonitis in the primary cohort. Figure 5. Receiver operating characteristic curve based on the sensitivity and specificity of the pre/end ratio of pre-albumin, platelet-to-lymphocyte ratio during radiation therapy (RT), neutrophil-to-lymphocyte ratio at the end of RT, and nomogram model in the validation group.
Figure 5. Receiver operating characteristic curve based on the sensitivity and specificity of the pre/end ratio of pre-albumin, platelet-to-lymphocyte ratio during radiation therapy (RT), neutrophil-to-lymphocyte ratio at the end of RT, and nomogram model in the validation group. Tables
 Table 1. Baseline characteristics of all patients.
Table 1. Baseline characteristics of all patients. Table 2. Occurrence of radiation pneumonia in esophageal cancer.
Table 2. Occurrence of radiation pneumonia in esophageal cancer. Table 3. Univariate analysis of clinicopathologic, dosimetric, and inflammatory parameters in predicting symptomatic radiation pneumonitis.
Table 3. Univariate analysis of clinicopathologic, dosimetric, and inflammatory parameters in predicting symptomatic radiation pneumonitis. Table 4. Univariate analysis of nutritional parameters and coagulation function in predicting symptomatic radiation pneumonitis.
Table 4. Univariate analysis of nutritional parameters and coagulation function in predicting symptomatic radiation pneumonitis. Table 5. Multivariate analysis of potential parameters in predicting symptomatic radiation pneumonitis.
Table 5. Multivariate analysis of potential parameters in predicting symptomatic radiation pneumonitis. Table 6. Areas under the receiver operating characteristic (ROC) curve for the independent predictors and nomogram model in the modeling group.
Table 6. Areas under the receiver operating characteristic (ROC) curve for the independent predictors and nomogram model in the modeling group. Table 7. Incidence of symptomatic radiation pneumonitis (RP) in the modeling group.
Table 7. Incidence of symptomatic radiation pneumonitis (RP) in the modeling group. Table 8. Areas under the receiver operating characteristic (ROC) curve for the independent predictors and nomogram model in the validation group.
Table 8. Areas under the receiver operating characteristic (ROC) curve for the independent predictors and nomogram model in the validation group. Table 9. Incidence of symptomatic radiation pneumonitis (RP) in the validation group.
Table 9. Incidence of symptomatic radiation pneumonitis (RP) in the validation group. Table 1. Baseline characteristics of all patients.
Table 1. Baseline characteristics of all patients. Table 2. Occurrence of radiation pneumonia in esophageal cancer.
Table 2. Occurrence of radiation pneumonia in esophageal cancer. Table 3. Univariate analysis of clinicopathologic, dosimetric, and inflammatory parameters in predicting symptomatic radiation pneumonitis.
Table 3. Univariate analysis of clinicopathologic, dosimetric, and inflammatory parameters in predicting symptomatic radiation pneumonitis. Table 4. Univariate analysis of nutritional parameters and coagulation function in predicting symptomatic radiation pneumonitis.
Table 4. Univariate analysis of nutritional parameters and coagulation function in predicting symptomatic radiation pneumonitis. Table 5. Multivariate analysis of potential parameters in predicting symptomatic radiation pneumonitis.
Table 5. Multivariate analysis of potential parameters in predicting symptomatic radiation pneumonitis. Table 6. Areas under the receiver operating characteristic (ROC) curve for the independent predictors and nomogram model in the modeling group.
Table 6. Areas under the receiver operating characteristic (ROC) curve for the independent predictors and nomogram model in the modeling group. Table 7. Incidence of symptomatic radiation pneumonitis (RP) in the modeling group.
Table 7. Incidence of symptomatic radiation pneumonitis (RP) in the modeling group. Table 8. Areas under the receiver operating characteristic (ROC) curve for the independent predictors and nomogram model in the validation group.
Table 8. Areas under the receiver operating characteristic (ROC) curve for the independent predictors and nomogram model in the validation group. Table 9. Incidence of symptomatic radiation pneumonitis (RP) in the validation group.
Table 9. Incidence of symptomatic radiation pneumonitis (RP) in the validation group. In Press
06 Mar 2024 : Clinical Research
Prevalence and Variation of Medical Comorbidities in Oral Surgery Patients: A Retrospective Study at Jazan ...Med Sci Monit In Press; DOI: 10.12659/MSM.943884
08 Mar 2024 : Clinical Research
Evaluation of Foot Structure in Preschool Children Based on Body MassMed Sci Monit In Press; DOI: 10.12659/MSM.943765
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








