08 August 2021: Clinical Research
Magnetic Resonance Imaging (MRI)-Targeted Biopsy in Patients with Prostate-Specific Antigen (PSA) Levels <20 ng/mL: A Single-Center Study in Northeastern China
Zhihong DaiDOI: 10.12659/MSM.930234
Med Sci Monit 2021; 27:e930234
Abstract
BACKGROUND: We investigated the feasibility of applying magnetic resonance imaging (MRI)-targeted biopsy (TB) in patients with prostate-specific antigen (PSA) levels <20 ng/mL.
MATERIAL AND METHODS: We retrospectively analyzed 218 patients with PSA levels <20 ng/mL and suspicious lesions according to the Prostate Imaging Recording and Data System version 2.0 (PI-RADS v2). All 218 men underwent transperineal MRI-TB, followed by template-guided 12-core systematic biopsy (SB). Of the 218 patients undergoing TB, 100 received MRI-ultrasound-assisted software fusion biopsy (FB) and 118 received cognitive biopsy (CB). Clinically significant prostate cancer (csPCa) was defined as a Gleason score ≥3+4.
RESULTS: The overall TB positive rate was similar to that of SB (P=0.156), but with a higher diagnostic rate for csPCa (P=0.034). SB misdiagnosed csPCa in 11.47% of cases; TB misdiagnosed csPCa in 5.50% of cases. SB+TB detected more tumors with a Gleason score of 7 than did SB alone (43 vs 22). Detection rates of csPCa were similar for CB and FB (P=0.217). In total, 47 men had 2 MRI-determined suspicious areas. Of 265 suspicious areas, 143 (53.96%) had a PI-RADS v2 score of 3; 92 (34.71%) had a score of 4; and 30 (11.32%) had a score of 5. The positive detection rates for csPCa in patients with PI-RADS v2 scores of 3, 4, and 5, were 11.19%, 48.91%, and 80.00%, respectively.
CONCLUSIONS: TB increased the positive biopsy detection rate but missed some cases of csPCa. TB combined with SB may be the most suitable biopsy for patients with PSA <20 ng/mL.
Keywords: Biopsy, Magnetic Resonance Imaging, Cine, Prostatic Neoplasms, Biopsy, Large-Core Needle, Feasibility Studies, Image-Guided Biopsy, Kallikreins, Magnetic Resonance Imaging, Interventional, Multimodal Imaging, Neoplasm Grading, Prostate, Prostate-Specific Antigen
Background
Transrectal ultrasound-guided (TRUS) systematic biopsy is the current criterion standard for diagnosing prostate cancer (PCa) [1]. Previous studies have shown that the positive detection rate of biopsies can be related to an abnormal increase in the serum levels of prostate-specific antigen (PSA) [2]. However, this method has several disadvantages, including a low detection rate for clinically significant prostate cancer (csPCa) [3], upgrading of the postoperative Gleason score [4], a high false-negative rate [5], and the overdetection of non-clinically significant PCa [6].
In 1983, Hricak et al [7] were the first to report the diagnostic value of multiparametric magnetic resonance imaging (mpMRI) for the diagnosis of PCa. With the development of multiparametric imaging and technology, which allows for the combination of anatomical and functional data, an increasing number of physicians are now using mpMRI to guide biopsies [8]. A previous study by Lo et al [9] found that the overall negative- and positive-predictive values of mpMRI were 80% to 91% and 52% to 57%, respectively. Since then, a large number of studies have reported that, compared with SB, mpMRI-guided targeted biopsy (TB) appears to have a higher detection rate for csPCa and a reduced level of detection for non-clinically significant PCa [10,11]. The mpMRI is currently regarded as an important method for improving the detection rate of csPCa.
The most widely accepted indication for biopsy is a PSA value >4 ng/mL. The detection rate increases with increasing PSA level. However, in patients with a PSA level <20 ng/mL, the detection rate of SB is only 20% to 32% and can cause some cases of csPCa to miss the optimal treatment time [12,13]. In contrast, the prognosis for patients with a PSA level <20 ng/mL is significantly better than that of those with PSA levels above 20 ng/mL [14]. Therefore, it is critical to identify a novel diagnostic method, such as mpMRI-TB, for patients with PSA levels <20 ng/mL. Unfortunately, little is known about the efficacy of mpMRI-TB for patients with PSA values <20 ng/mL, particularly in northeastern China.
In this study, we describe the use of mpMRI-TB in patients with PSA values <20 ng/mL in northeastern China. We evaluated the feasibility of mpMRI-TB for patients with PSA levels <20 ng/mL.
Material and Methods
PATIENTS:
This study included 218 patients who underwent initial biopsy with PSA levels ranging from 4 ng/mL to <20 ng/mL and with suspicious lesions in the prostate upon MRI. In addition, we considered patient age, a range of influential factors, and whether there were abnormal results from a digital rectal examination (DRE). All patients were treated in the Second Affiliated Hospital of Dalian Medical University (Dalian, China) between January 2018 and March 2021. Written informed consent was obtained from all patients. This study was approved by the Ethics Committee of the Second Affiliated Hospital of Dalian Medical University (2020037). All patients first underwent transperineal mpMRI-TB and then underwent template-guided 12-core systematic biopsy (SB). According to the different methods of TB, we divided the patients into an MRI software fusion-TB (FB) group (n=100) and a cognitive fusion biopsy (CB) group (n=118).
MPMRI TECHNIQUE:
The mpMRI procedure was performed with a 3-Tesla MRI system and included T1-weighted imaging, T2-weighted imaging, diffusion-weighted imaging, and an apparent diffusion coefficient. Prostate volume was measured using the following formula: (maximum anterior-posterior diameter)×(maximum transverse diameter)×(maximum cranio-caudal diameter)×0.52 [15]. All mpMRI examinations were reviewed by an expert genitourinary radiologist with 5 years of experience in prostate mpMRI, who had interpreted >400 prostate mpMRI examinations using the Prostate Imaging Reporting and Data System version 2 (PI-RADS v2) [15], where scores of 1=unlikely, 2=equivocal, 3=likely, and 4=highly likely to harbor csPCa. All lesions with PI-RADS scores ≥3 were considered as suspicious for cancer.
METHODS AND PROCEDURES:
All patients were administered levofloxacin tablets (500 mg) as a perioperative antibiotic treatment. Patients were also given an enema with 500 mL saline 2 h before the procedure. All patients with a PI-RADS score ≥3 underwent TB. All procedures were performed by the same urologists, who had independently completed more than 100 transperineal prostate biopsies. Patients were first given local anesthesia in the skin and prostate tip and around the prostate capsule with 0.1% lidocaine (20 mL), while in the lithotomy position. When CB was performed, the operator first reviewed the MRI scans to confirm the suspicious areas. Then, using real-time TRUS images, the surgeon performed transperineal biopsy of the suspicious region. For FB, the surgeon used the Fusion Software and transrectal TRUS platform (MIM software Co., Ltd., Cleveland, OH, USA). The radiologist and surgeon were involved in conducting these biopsies. A radiologist, who was experienced in prostate mpMRI, marked the suspicious lesions on MRI and correlated this to the real-time TRUS images for SB. To confirm the suspicious areas, the patients underwent an average of 2 needles. Biopsy cores were individually labeled.
After the completion of the mpMRI-TB, patients underwent SB, according to the standard 5-mm template brachytherapy grid, resulting in the SB+TB group.
DEFINITION OF CSPCA:
There is still no consensus on the definition of csPCa, although one of the most widely accepted indicators for csPCa is a Gleason score ≥3+4 [16–18]. Therefore, we regarded a Gleason score ≥3+4 as indicating csPCa.
DATA ANALYSIS:
All analyses were conducted with SPSS version 23 (IBM SPSS Statistics for Windows, version 23.0, Inc., Chicago, IL, USA). Patient characteristics, MRI findings, and pathological evaluations were compared between the 2 groups using the
Results
COMPARISON BETWEEN THE SB AND SB+TB GROUPS:
There was a significant difference in PCa detection rates between the SB (43.12%) and SB+TB (52.75%) groups (P=0.001). There was also a significantly significant difference in the rate of csPCa detection between the 2 groups, with 75 patients (34.40%) having csPCa detected in the SB+TB group compared with only 50 patients (22.94%) in the SB group (P=0.001). The combination of SB and TB detected more tumors with a Gleason score of 7 than did SB alone (43 vs 22), but the detection rate for tumors with a Gleason score >8 was similar between in the 2 groups (Table 2). When using TB, the detection rates for PCa and csPCa were 45.87% (100/218) and 28.90% (63/218), respectively. When considering the diagnostic performance of only SB or TB, the overall positive detection rate of TB was higher than that of SB (45.87% vs 43.12%), although this difference was not statistically significant (P=0.156). A significantly higher number of cases of csPCa were diagnosed in the TB group (28.90% vs 22.94%; P=0.034). However, compared with a lower misdiagnosis rate with SB+TB, TB misdiagnosed csPCa in 5.50% of cases and SB misdiagnosed csPCa in 11.47% of cases (Figure 1).We also recorded the number of adverse reactions within the first 3 days after biopsy. Of the 13 (5.96%) patients that had adverse reactions after biopsy, 8 had hematuria, 4 had urinary retention, and 1 patient had fever after surgery, with a body temperature of up to 39°C, which resolved after anti-infective treatment.
COMPARISON BETWEEN CB AND FB:
There were 218 patients (with a total of 265 suspicious lesions) with PI-RADS v2 score ≥3 and who underwent either CB or FB. Basic patient characteristics, mpMRI imaging results, and pathological outcomes were compared between the CB and FB groups. The basic characteristics of patients, including age, PSA, PSAD, and prostate volume, were not significantly different. From our analysis, we found no difference between CB and FB in the overall PCa detection rate (52.54% vs 53.00%; P=0.842) or in the detection rate of csPCa (36.44% vs 32.00%; P=0.217). Furthermore, the difference in the time spent for biopsy was statistically significant between the CB (23.42 min) and FB (35.16 min) groups (P=0.023) (Table 3).
OUTCOMES OF PI-RADS V2:
When considering patients with a positive result on mpMRI, we found that 47 patients had 2 suspicious lesions. In these cases, we recorded the imaging results from each lesion. Of the 265 suspicious lesions, 143 (53.96%) had a PI-RADS v2 score of 3; 92 (34.71%) had a score of 4; and 30 (11.32%) had a score of 5. In the group of patients with 3 as a PI-RADS v2 score, 49 (34.27%) lesions proved to be cancerous, but only 16 (11.19%) of the suspicious lesions were indicated as clinically significant cancer. In the group of patients with 4 as a PI-RADS v2 score, 58 (63.04%) lesions proved to be cancerous, and 45 (48.91%) suspicious lesions were indicated as clinically significant cancer. In the group of patients with 5 as a PI-RADS v2 score, 27 (90.00%) lesions proved to be cancerous, and 24 (80.00%) suspicious lesions were indicated as clinically significant cancer. These data showed that as the PI-RADS v2 score increased, there was a higher detection rate for all cancers, especially clinically significant cancers (Figure 2).
Discussion
A change in the serum levels of PSA and an abnormal DRE are regarded as the most commonly used indicators for the clinical diagnosis of PCa. For patients with a PSA level >20 ng/mL, the rate of PCa diagnosis can be as high as 89.8% [19]. However, for patients with a PSA <20 ng/mL, especially in the diagnostic gray zone (4–10 ng/mL), the positive rate for PCa detection using TRUS-guided biopsy has much lower sensitivity. Over the last few years, the use of mpMRI to diagnose PCa has gradually revolutionized traditional diagnostic methods, not only for its high sensitivity in recognizing csPCa, but also because of its ability to rule out indolent tumors [20]. Olivier et al [21] evaluated 2562 patients from 43 studies and showed that MRI-guided biopsies had a similar overall PCa detection rate as TRUS-guided biopsy, increased detection rates of csPCa, and reduced rates of insignificant PCa. However, little research has been conducted in China, particularly for patients with a PSA <20 ng/mL.
Here, we report the positive detection rate of PCa using SB and TB in patients with a PSA <20 ng/mL in northeastern China. We showed that the detection rate for any PCa in the SB+TB group was 52.75%, while the detection rate for csPCA in the SB+TB group was 34.40%; these detection rates were significantly higher than those of only SB. Compared with SB+TB, TB misdiagnosed csPCa in 5.50% of cases while SB misdiagnosed csPCa in 11.47% of cases. Similar to the findings of previous reports [22–24], combined SB and TB showed a higher positive detection rate for any cancer and csPCa, when compared with SB and TB. The present data showed that csPCa would have been missed in 5.2% to 7.2% of patients who underwent only TB and in 7.6% to 10.9% of patients who underwent only SB. Diamand et al also showed that a combination of SB and TB significantly increased concordance with final histopathology results [25].TB increases the positive detection rate of biopsy; however, it is possible that some cases of csPCa could have been be missed from this study or previously published studies. Our present study also found that SB and TB detected more tumors with a Gleason score of 7 (43 vs 22) than did SB, with a similar detection rate for tumors with a Gleason score >8. Therefore, we propose that the combination of TB and SB may be the most suitable form of biopsy for patients with a PSA <20 ng/mL.
Currently, mpMRI can be achieved by various methods, including in-bore biopsy performed in the MRI suite using real-time MRI-guidance [26]; FB, in which specialized software is used to fuse MRI and TRUS images, thereby achieving direct biopsy by MRI-TRUS fusion guidance [26]; and CB, in which MRI is used to display the suspicious lesion and to cognitively target the lesion by TRUS guidance [27]. Although some studies have reported that an in-bore biopsy can improve the detection rate [28,29], this particular technique needs more expensive materials and takes longer to complete; therefore, clinicians prefer to use TB and SB techniques because they are relatively economical and convenient [30]. In our study, we compared the detection rates for any cancer and clinically significant cancer between CB and SB. There was no significant difference between CB and FB; however, the rates of cancer and clinically significant cancer were higher in the CB group than in the SB group. These findings differed from those published in 2016 [21], in which the authors compared 3 different techniques for PCa biopsy, including TRUS, CB, and FB; the sensitivity for FB was 0.89, compared with 0.86 for CB. This may be associated with our more limited experience of the early stages of FB, whereby the ultrasound screenshot layer did not exactly correspond to the MRI layer outlined previously, causing the suspicious area to be biased in fusion images, especially in some smaller lesions.
The PI-RADS v2 score, created by the European Society of Urogenital Radiology and the American College of Radiology, plays a vital role in predicting PCa [31]. A previous study evaluated the use of PI-RADS v2 for the detection of PCa and showed that it improved diagnostic performance when assessing suspicious lesions [32]. Another study from 2018 included 171 patients with suspicious PCa lesions, and of the patients showing a positive result on mpMRI, the positive rates of csPCa in patients with PI-RADS scores of 3, 4, and 5 were 12%, 60%, and 83%, respectively [4]. We assessed 265 suspicious lesions in this study, and the positive rates of csPCa in patients with PI-RADS scores of 3, 4, and 5 were 11.19%, 48.91%, and 80.00%, respectively. These findings were confirmed by pathology results and showed similar levels of detection as previous studies. When the PI-RADS score was upgraded, there was an increase in the positive detection rate for clinically significant cancer.
This study had several limitations. First, this was a retrospective study, carried out in a single center and with a limited number of patients. Second, we retrospectively analyzed CB and FB in the TB group without randomization. Third, the positive detection rate for clinically significant cancer may be associated with the surgeon’s experience and learning curve. Although our research has some limitations, our results show that the combination of TB with SB could improve the positive detection rate of PCa or csPCa and may be the most suitable type of biopsy for patients with a PSA <20 ng/mL. We also found that the combination of SB and TB detected more tumors with a Gleason score of 7 than did SB alone.
Conclusions
For patients with a PSA <20 ng/mL in northeastern China, TB increased the positive detection rate of biopsy, but also missed some cases of csPCa. TB combined with SB can improve the positive detection rates of PCa and csPCa and may be the most suitable biopsy for patients with a PSA <20 ng/mL. The mpMRI is meaningful for guiding prostate biopsy. There is an increase in the positive detection rate for clinically significant cancer with an upgraded PI-RADS v2 score.
Figures
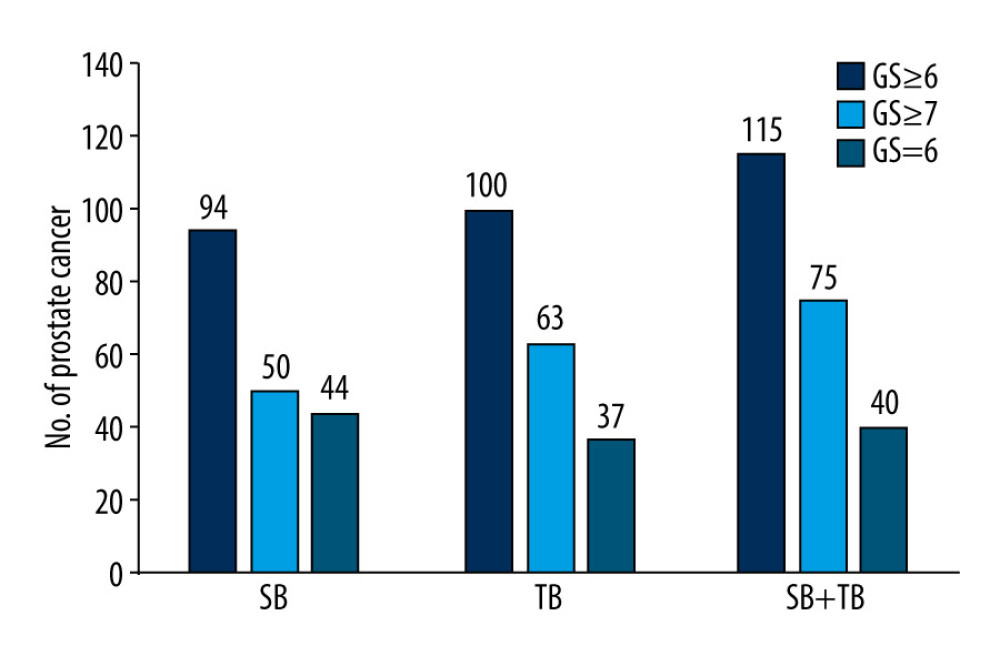 Figure 1. Diagnostic performance of systematic biopsy (SB), targeted biopsy (TB), and a combined approach in our patient cohort. The overall positive rate for TB was higher than that of SB (45.87% vs 43.12%, P=0.156), leading to a higher diagnostic rate for clinically significant prostate cancer (csPCa) (28.90% vs 22.94%; P=0.034). Compared with SB+TB, SB misdiagnosed csPCa in 11.47% of cases while TB misdiagnosed csPCa in 5.50% of cases. GS – Gleason score.
Figure 1. Diagnostic performance of systematic biopsy (SB), targeted biopsy (TB), and a combined approach in our patient cohort. The overall positive rate for TB was higher than that of SB (45.87% vs 43.12%, P=0.156), leading to a higher diagnostic rate for clinically significant prostate cancer (csPCa) (28.90% vs 22.94%; P=0.034). Compared with SB+TB, SB misdiagnosed csPCa in 11.47% of cases while TB misdiagnosed csPCa in 5.50% of cases. GS – Gleason score. 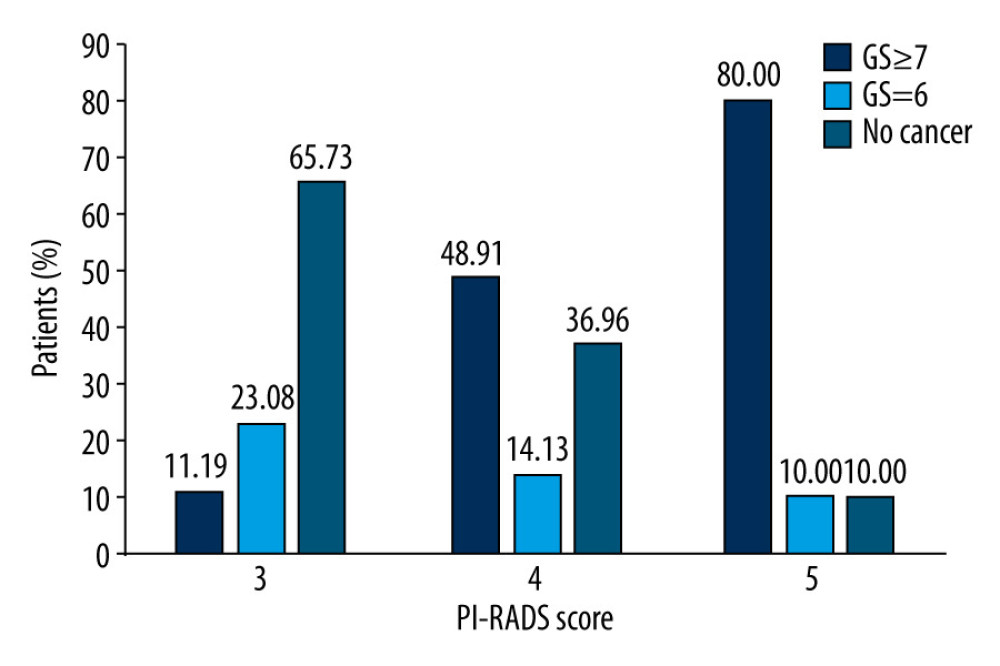 Figure 2. Diagnostic performance of Prostate Imaging Recording and Data System (PI-RADS) version 2.0 involving 218 men with 265 suspicious lesions. Of the 265 suspicious lesions, 143 (53.96%) had a PI-RADS v2 score of 3, 92 (34.71%) had a score of 4, and 30 (11.32%) had a score of 5. The positive detection rates for csPCa in patients with PI-RADS scores of 3, 4, and 5, were 11.19%, 48.91%, and 80.00%, respectively. GS – Gleason score.
Figure 2. Diagnostic performance of Prostate Imaging Recording and Data System (PI-RADS) version 2.0 involving 218 men with 265 suspicious lesions. Of the 265 suspicious lesions, 143 (53.96%) had a PI-RADS v2 score of 3, 92 (34.71%) had a score of 4, and 30 (11.32%) had a score of 5. The positive detection rates for csPCa in patients with PI-RADS scores of 3, 4, and 5, were 11.19%, 48.91%, and 80.00%, respectively. GS – Gleason score. References
1. Wilt TJ, Jones KM, Barry MJ, Follow-up of prostatectomy versus observation for early prostate cancer: N Engl J Med, 2017; 377; 132-42
2. Zhang M, Milot L, Khalvati F, Value of increasing biopsy cores per target with cognitive MRI-targeted transrectal US prostate biopsy: Radiology, 2019; 291; 83-89
3. Naji L, Randhawa H, Sohani Z, Digital rectal examination for prostate cancer screening in primary care: A systematic review and meta-analysis: Ann Fam Med, 2018; 16; 149-54
4. Kasivisvanathan V, Rannikko AS, Borghi M, MRI-targeted or standard biopsy for prostate-cancer diagnosis: N Engl J Med, 2018; 378; 1767-77
5. Descotes JL, Diagnosis of prostate cancer: Asian J Urol, 2019; 6; 129-36
6. Sherrer RL, Glaser ZA, Gordetsky JB, Comparison of biparametric MRI to full multiparametric MRI for detection of clinically significant prostate cancer: Prostate Cancer Prostatic Dis, 2019; 22; 331-36
7. Monni F, Fontanella P, Grasso A, Magnetic resonance imaging in prostate cancer detection and management: A systematic review: Minerva Urol Nefrol, 2017; 69; 567-78
8. Kam J, Yuminaga Y, Kim R, Does magnetic resonance imaging-guided biopsy improve prostate cancer detection? A comparison of systematic, cognitive fusion and ultrasound fusion prostate biopsy: Prostate Int, 2018; 6; 88-93
9. Lo G, Burton KR, Haider MA, Negative predictive value of prostate multiparametric magnetic resonance imaging among men with negative prostate biopsy and elevated prostate specific antigen: A clinical outcome retrospective cohort study: J Urol, 2019; 202(6); 1159-65
10. Stabile A, Giganti F, Emberton M, MRI in prostate cancer diagnosis: Do we need to add standard sampling? A review of the last 5 years: Prostate Cancer Prostatic Dis, 2018; 21; 473-87
11. Ahmed HU, El-Shater Bosaily A, Brown LC, Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): A paired validating confirmatory study: Lancet, 2017; 389; 815-22
12. Rouviere O, Puech P, Renard-Penna R, Use of prostate systematic and targeted biopsy on the basis of multiparametric MRI in biopsy-naive patients (MRI-FIRST): A prospective, multicentre, paired diagnostic study: Lancet Oncol, 2019; 20; 100-9
13. Nafie S, Mellon JK, Dormer JP, The role of transperineal template prostate biopsies in prostate cancer diagnosis in biopsy naive men with PSA less than 20 ng ml(-1.): Prostate Cancer Prostatic Dis, 2014; 17; 170-73
14. Dani H, Loeb S, The role of prostate cancer biomarkers in undiagnosed men: Curr Opin Urol, 2017; 27; 210-16
15. Osses DF, van Asten JJ, Tijsterman JD, Cognitive-targeted versus magnetic resonance imaging-guided prostate biopsy in prostate cancer detection: Curr Urol, 2018; 11; 182-88
16. Futterer JJ, Briganti A, De Visschere P, Can clinically significant prostate cancer be detected with multiparametric magnetic resonance imaging? A systematic review of the literature: Eur Urol, 2015; 68; 1045-53
17. Filson CP, Natarajan S, Margolis DJ, Prostate cancer detection with magnetic resonance-ultrasound fusion biopsy: The role of systematic and targeted biopsies: Cancer, 2016; 122; 884-92
18. Elkhoury FF, Felker ER, Kwan L, Comparison of targeted vs systematic prostate biopsy in men who are biopsy naive: The prospective assessment of image registration in the diagnosis of prostate cancer (PAIREDCAP) study: JAMA Surg, 2019; 154(9); 811-18
19. Waqas M, Shohab D, Khawaja MA, Outcome of trans rectal ultrasound guided twelve core biopsy of prostate for the detection of prostate cancer: A single centre experience: J Ayub Med Coll Abbottabad, 2018; 30; 49-53
20. Bass EJ, Freeman A, Jameson C, Prostate cancer diagnostic pathway: Is a one-stop cognitive MRI targeted biopsy service a realistic goal in everyday practice? A pilot cohort in a tertiary referral centre in the UK: Br Med J Open, 2018; 8; e024941
21. Wegelin O, van Melick HHE, Hooft L, Comparing three different techniques for magnetic resonance imaging-targeted prostate biopsies: A systematic review of in-bore versus magnetic resonance imaging-transrectal ultrasound fusion versus cognitive registration. Is there a preferred technique?: Eur Urol, 2017; 71; 517-31
22. von Below C, Wassberg C, Norberg M, Additional value of magnetic resonance-targeted biopsies to standard transrectal ultrasound-guided biopsies for detection of clinically significant prostate cancer: Scand J Urol, 2017; 51; 107-13
23. Rouvière O, Puech P, Renard-Penna R, Use of prostate systematic and targeted biopsy on the basis of multiparametric MRI in biopsy-naive patients (MRI-FIRST): A prospective, multicentre, paired diagnostic study: Lancet Oncol, 2019; 20; 100-9
24. van der Leest M, Cornel E, Israël B, Head-to-head comparison of transrectal ultrasound-guided prostate biopsy versus multiparametric prostate resonance imaging with subsequent magnetic resonance-guided biopsy in biopsy-naïve men with elevated prostate-specific antigen: A large prospective multicenter clinical study: Eur Urol, 2019; 75; 570-78
25. Diamand R, Oderda M, Al Hajj Obeid W, A multicentric study on accurate grading of prostate cancer with systematic and MRI/US fusion targeted biopsies: Comparison with final histopathology after radical prostatectomy: World J Urol, 2019; 37; 2109-17
26. Panebianco V, Valerio MC, Giuliani A, Clinical utility of multiparametric magnetic resonance imaging as the first-line tool for men with high clinical suspicion of prostate cancer: Eur Urol Oncol, 2018; 1; 208-14
27. Hansen N, Patruno G, Wadhwa K, Magnetic resonance and ultrasound image fusion supported transperineal prostate biopsy using the ginsburg protocol: Technique, learning points, and biopsy results: Eur Urol, 2016; 70; 332-40
28. Faria R, Soares MO, Spackman E, Optimising the diagnosis of prostate cancer in the era of multiparametric magnetic resonance imaging: A cost-effectiveness analysis based on the prostate mr imaging study (PROMIS): Eur Urol, 2018; 73; 23-30
29. Quentin M, Blondin D, Arsov C, Prospective evaluation of magnetic resonance imaging guided in-bore prostate biopsy versus systematic transrectal ultrasound guided prostate biopsy in biopsy naive men with elevated prostate specific antigen: J Urol, 2014; 192; 1374-79
30. Giganti F, Moore CM, A critical comparison of techniques for MRI-targeted biopsy of the prostate: Transl Androl Urol, 2017; 6; 432-43
31. Lai WS, Gordetsky JB, Thomas JV, Factors predicting prostate cancer upgrading on magnetic resonance imaging-targeted biopsy in an active surveillance population: Cancer, 2017; 123; 1941-48
32. Kasel-Seibert M, Lehmann T, Aschenbach R, Assessment of PI-RADS v2 for the detection of prostate cancer: Eur J Radiol, 2016; 85; 726-31
Figures
 Figure 1. Diagnostic performance of systematic biopsy (SB), targeted biopsy (TB), and a combined approach in our patient cohort. The overall positive rate for TB was higher than that of SB (45.87% vs 43.12%, P=0.156), leading to a higher diagnostic rate for clinically significant prostate cancer (csPCa) (28.90% vs 22.94%; P=0.034). Compared with SB+TB, SB misdiagnosed csPCa in 11.47% of cases while TB misdiagnosed csPCa in 5.50% of cases. GS – Gleason score.
Figure 1. Diagnostic performance of systematic biopsy (SB), targeted biopsy (TB), and a combined approach in our patient cohort. The overall positive rate for TB was higher than that of SB (45.87% vs 43.12%, P=0.156), leading to a higher diagnostic rate for clinically significant prostate cancer (csPCa) (28.90% vs 22.94%; P=0.034). Compared with SB+TB, SB misdiagnosed csPCa in 11.47% of cases while TB misdiagnosed csPCa in 5.50% of cases. GS – Gleason score. Figure 2. Diagnostic performance of Prostate Imaging Recording and Data System (PI-RADS) version 2.0 involving 218 men with 265 suspicious lesions. Of the 265 suspicious lesions, 143 (53.96%) had a PI-RADS v2 score of 3, 92 (34.71%) had a score of 4, and 30 (11.32%) had a score of 5. The positive detection rates for csPCa in patients with PI-RADS scores of 3, 4, and 5, were 11.19%, 48.91%, and 80.00%, respectively. GS – Gleason score.
Figure 2. Diagnostic performance of Prostate Imaging Recording and Data System (PI-RADS) version 2.0 involving 218 men with 265 suspicious lesions. Of the 265 suspicious lesions, 143 (53.96%) had a PI-RADS v2 score of 3, 92 (34.71%) had a score of 4, and 30 (11.32%) had a score of 5. The positive detection rates for csPCa in patients with PI-RADS scores of 3, 4, and 5, were 11.19%, 48.91%, and 80.00%, respectively. GS – Gleason score. Tables
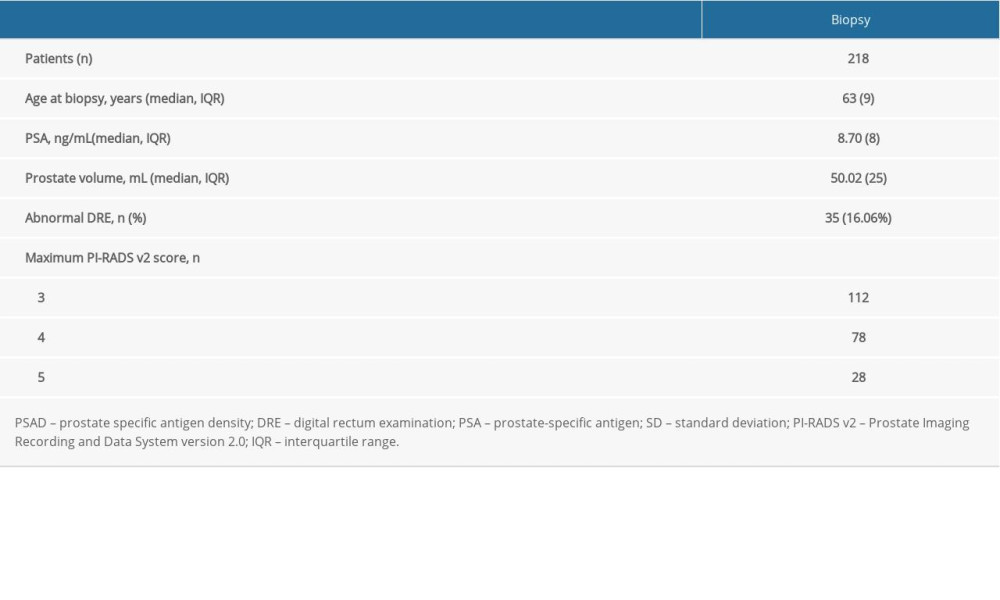 Table 1. Basic characteristics of the study population.
Table 1. Basic characteristics of the study population.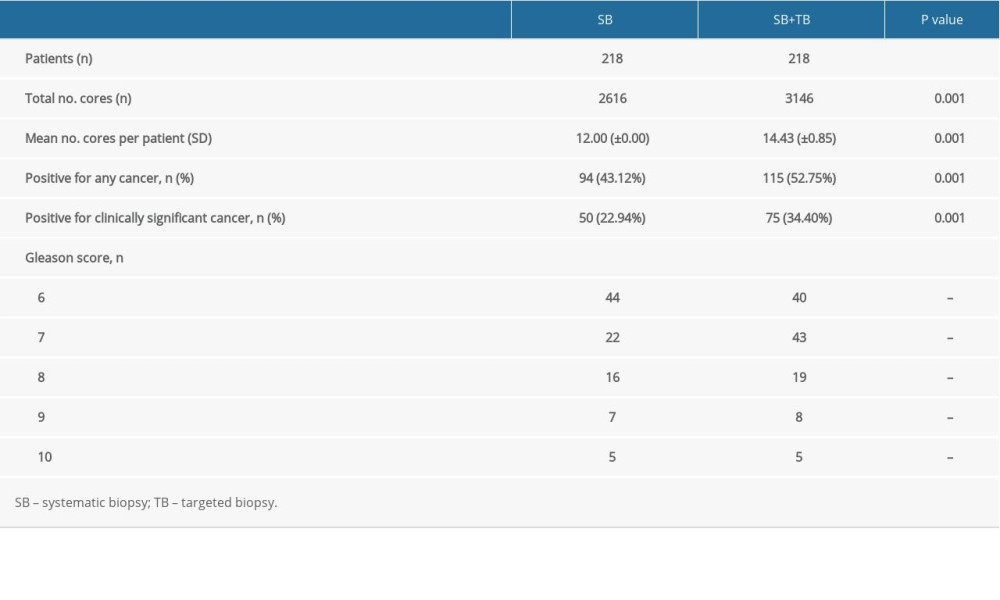 Table 2. A Comparative analysis of the SB and SB+TB groups.
Table 2. A Comparative analysis of the SB and SB+TB groups.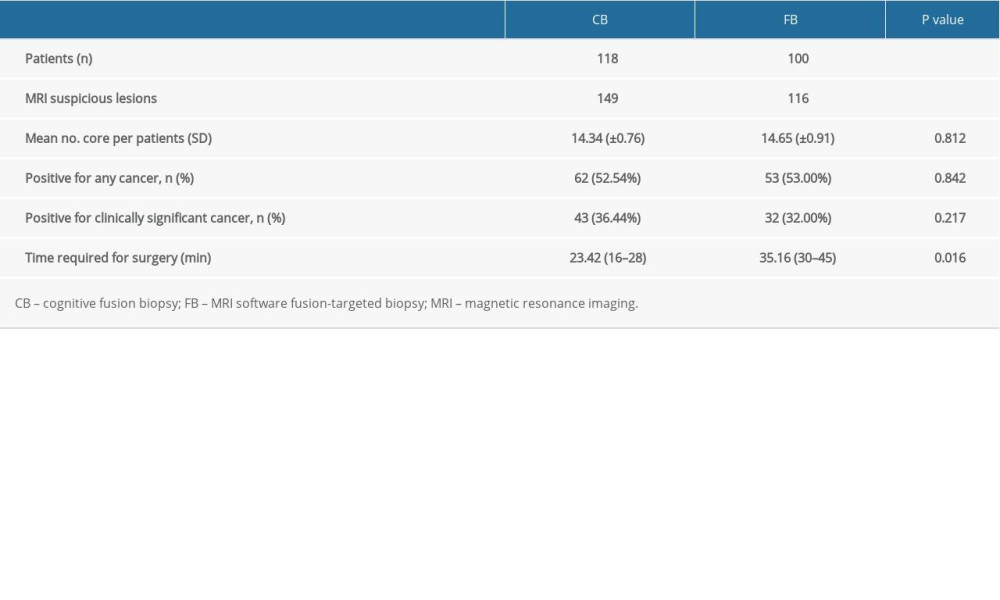 Table 3. A comparative analysis of the CB and FB groups.
Table 3. A comparative analysis of the CB and FB groups. Table 1. Basic characteristics of the study population.
Table 1. Basic characteristics of the study population. Table 2. A Comparative analysis of the SB and SB+TB groups.
Table 2. A Comparative analysis of the SB and SB+TB groups. Table 3. A comparative analysis of the CB and FB groups.
Table 3. A comparative analysis of the CB and FB groups. In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








