16 July 2021: Lab/In Vitro Research
lncRNA MEG3 Downregulation Relieves Intracerebral Hemorrhage by Inhibiting Oxidative Stress and Inflammation in an miR-181b-Dependent Manner
Bo Xie1ABCDEFG, Mingliang Qiao1BCD, Jialong Xuan2BCEF*DOI: 10.12659/MSM.929435
Med Sci Monit 2021; 27:e929435
Abstract
BACKGROUND: This study was designed to illustrate the effects and latent mechanism of lncRNA maternally expressed gene 3 (MEG3) on intracerebral hemorrhage (ICH)-induced brain injury.
MATERIAL AND METHODS: An ICH rat model was generated to determine the role of lncRNA MEG3 in ICH. The interaction between lncRNA MEG3 and microRNA (miR)-181b were confirmed by Starbase and dual-luciferase reporter assay. One hour (h) or 3 days after ICH stimulation, rat neurological injury was evaluated by modified Neurological Severity Score (mNSS). Brain water content and cell apoptosis were assessed using brain edema assessment and flow cytometry (FCM), respectively. Caspase3 activity was also determined. Enzyme-linked immunosorbent assay (ELISA) was applied to evaluate the levels of pro-inflammatory cytokines. Moreover, the representative biomarkers of oxidative stress were evidenced using detection kits.
RESULTS: The lncRNA MEG3 level in ICH rat brain tissues was higher than that in the sham group. miR-181b was a direct target of lncRNA MEG3 and it was downregulated in brain tissues of ICH rats. Notably, we found that neurobehavioral scores, brain water content, and neuronal apoptosis were decreased and caspase3 activity was reduced in MEG3-shRNA-treated ICH rats, while we observed the opposite result in ICH+MEG3-shRNA+miR-181b inhibitor rats. Further analyses revealed that MEG3-shRNA inhibited inflammatory cytokines release and reduced oxidative stress. All these results were reversed by miR-181b inhibitor. In addition, MEG3-shRNA activated the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) pathway, which was reversed by miR-181b inhibitor.
CONCLUSIONS: MEG3-shRNA restrained oxidative stress and inflammation following ICH in an miR-181b-dependent manner.
Keywords: Intracranial Hemorrhage, Hypertensive, Cerebral Hemorrhage, Cytokines, Down-Regulation, Phosphatidylinositol 3-Kinases, Proto-Oncogene Proteins c-akt
Background
Intracerebral hemorrhage (ICH), a common subtype of stroke, represents 10–15% of all strokes, and is associated with high mortality and disability [1]. Despite remarkable advances in the understanding of the development of ICH during the past decade, therapeutic options for ICH are largely ineffective [2]. Increasing evidence suggests that multiple pathways are involved in the development of ICH, such as inflammation, oxidative stress, excitotoxicity, and apoptosis, which combine to cause brain damage [3,4]. Moreover, activated inflammatory factors, including TNF-α, IL-1β, and IL-6, induce brain edema and neuronal apoptosis [5]. In addition, oxidative stress is a prominent factor in the pathogenesis of neuronal injury, which results in neurological dysfunction by regulating cellular signaling and suppressing neuronal activity [6]. For example, Xu et al revealed that miR-146a protects against ICH by suppressing inflammatory response and oxidative stress [7]. Therefore, anti-inflammatory approaches and exploring the mechanisms responsible for ICH damage may be a novel strategy for treating ICH.
lncRNAs, which are non-decoding RNAs over 200 nucleotides in length, have been reported to be pivotal regulators in the development of human diseases, including diabetes [8], psoriasis [9], and ICH [10]. Previous studies have shown that lncRNAs participate in multiple biological processes, such as cell viability, apoptosis, and differentiation [11]. lncRNA MEG3 has been reported to be related to progression of various cancers, including gastric cancer [12], prostate cancer [13], and cervical cancer [14]. lncRNA MEG3 was found to affect secondary brain injury in CNS injury models [15]. A recent study has reported that lncRNA MEG3 expression was significantly upregulated in an ICH mouse model and in oxygen-and-glucose-deprivation (OGD)/hemin (OGD/H)-induced brain microvascular endothelial cells [16]. However, the role and mechanism of lncRNA MEG3 in ICH remain largely unclear. miRNAs, a group of small non-coding RNA molecules consisting of 20–22 nucleotides, can regulate target genes by binding with the 3′-UTRs of mRNAs [17]. Various reports have confirmed that miRNAs act as a vital regulator in the pathology of ICH [18–21]. The expression of plasma circulating miR-181b was reported to be decreased in patients with ICH compared with that in the control group [22]. In addition, a study has revealed that miR-181b inhibition promotes endoplasmic reticulum (ER) stress-induced brain damage following ICH by targeting Heat Shock Protein A5 (HSPA5) [23]. Interestingly, previous studies have suggested an interaction between lncRNA MEG3 and miR-181b [24–26]. However, whether lncRNA MEG3 affects ICH-induced brain damage through miR-181b remains unclear.
We thus aimed to explore the function of and relationship between lncRNA MEG3 and miR-181b in ICH models and to explore the underlying mechanism.
Material and Methods
ANIMALS:
A total of 60 male Sprague-Dawley rats (age 6–8 weeks, weight 220±5 g) were provided by the Experimental Animal Center of Shanghai and were housed in standard condition (22±1°C, 55±5% humidity, 12-h light/dark cycle) with free access to food and water. All animal care and procedures were approved by the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Moreover, this study was approved by Animal Ethics Committee of Weihai Central Hospital.
ESTABLISHMENT OF THE INTRACEREBRAL HEMORRHAGE MODEL:
To establish the intracerebral hemorrhage model, rats were injected intraperitoneally with bacterial collagenase type VII (Sigma Aldrich; Merck KGaA) according to the methods described in a previous study [27]. Briefly, rats were anesthetized with intraperitoneal injection of 400 mg/kg 5% chloral hydrate. Then, a burr hole was drilled at the injection site (0.2 mm posterior to the bregma, 3.0 mm left lateral to the midline, 6 mm in depth below the skull). Subsequently, the bacterial collagenase type VII (0.23 U dissolved in 1 μl saline; 0.5 μl/min) was slowly injected into the central striatum of the rat. To prevent backflow, the syringe needle was held in place for a further 10 min. Subsequently, the holes were sealed with bone wax and the rats were placed in a cage with a heating pad at a temperature of 37°C for a period of time. Sham rats were injected with saline instead of collagenase. For intracerebral hemorrhage therapy, after ICH induction for 1 h, control-shRNA, lncRNA MEG3-shRNA, lncRNA MEG3-shRNA+inhibitor control, or lncRNA MALAT1-shRNA+miR-181b inhibitor were delivered to rats by intracerebral injection as previously described [28]. For intracerebral injection, control-shRNA (2 μg/2 μl), lncRNA MEG3-shRNA (2 μg/2 μl), lncRNA MEG3-shRNA (2 μg/2 μl) +inhibitor control (2 μg/2 μl), or lncRNA MALAT1-shRNA (2 μg/2 μl) +miR-181b inhibitor (2 μg/2 μl) were added to 1.25 μl Entranster™ in vivo transfection reagent, and then the solution was mixed gently and left for 15 min. Treatment was performed once a day for 3 consecutive days. Rats were grouped into 6 groups: Sham, ICH, ICH+control-shRNA, ICH+lncRNA MEG3-shRNA, ICH+lncRNA MEG3-shRNA+inhibitor control, and ICH+lncRNA MEG3-shRNA+miR-181b inhibitor group.
CELL CULTURE:
Neurons in ICH rats brain tissues were separated and digested for 1 h. Then, the neurons were grown in neurobasal medium (Gibco; USA) with B27 supplement, 10% FBS (Gibco; USA), 100 U/ml penicillin/streptomycin, and 0.5 mmol/l glutamine in a humidified atmosphere with 5% CO2 at 37°C.
The 293T cell line was obtained from the ATCC. The cells were cultured in DMEM with 10% FBS, 1% GlutaMax, and 1% penicillin/streptomycin (Abcam, MA) in a humidified atmosphere with 5% CO2 at 37°C.
CELL APOPTOSIS ASSAY:
An annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis detection kit (Beyotime, Shanghai, China) was used to evaluate neuronal apoptosis following the manufacturer’s protocol. After that, cell apoptosis was assessed using a BD FACSCalibur flow cytometer (BD Biosciences) and these data were calculated using Kaluza analysis software.
DUAL-LUCIFERASE REPORTER ASSAY:
Bioinformatics software was used to predict the binding sites between miR-181b and lncRNA MEG3. Then, the lncRNA MEG3 3′UTR, which contains the miR-181b binding site or mutated target site, were compounded by genomic PCR and cloned into pGL-3-Luc (Promega, USA) to generate the reporter vector MEG3-wild-type (MEG3-WT) or MEG3-mutated-type (MEG3-MUT). Then, 293T cells were transfected with MEG3 wild-type or mutant portion combined with miR-181b mimic or mimic control by applying Lipofectamine 2000 (Invitrogen) in accordance with the manufacturer’s manual. Then, the luciferase activity was determined using the Dual-Luciferase Reporter Assay System (Promega, USA).
EVALUATION OF NEUROLOGICAL SCORES:
To evaluate neurological function after treatment, the mNSS method was conducted at 1 h or 3 days after ICH. The mNSS test included motor, sensory, reflex, and balance tests. Neurological function was graded on a 0–18 scale.
BRAIN EDEMA MEASUREMENT:
The brain water content of rat brain tissue was measured to assess brain edema according to a published protocol [29]. Three days after ICH or sham induction, rats were randomly selected and anesthetized with chloral hydrate. Then, the brain tissue was separated into contralateral and ipsilateral hemispheres and cerebellum. We determined the wet weight of samples, and then placed them in an oven at 100°C for 24 h to get the dry weight. Water content was calculated following this formula: Brain water content (%)=(wet weight-dry weight)/wet weight×100%.
DETECTION OF CASPASE-3 ACTIVITY:
The Caspase-3 Assay Kit (Abcam, China) was utilized to analyze caspase-3 activity in nerve cells following the manufacturer’s protocol. Briefly, nerve cells were lysed, centrifuged, and collected. Then, supernatant was co-incubated with caspase-3 reagent. After that, the optical density (OD) of caspase-3 activity was detected using a microplate reader (Thermo LabSystem, Finland) at 405 nm.
ELISA ASSAY:
Three days after ICH induction, blood and cerebrospinal fluid (CSF) were harvested from all rats through piercing the heart and foramen magnum prior to sacrifice. Blood samples were centrifuged at 1000×g for 5 min at 4°C, and CSF samples were immediately centrifuged at 12 000 g for 30 min at 4°C. Then, the levels of inflammatory factors tumor necrosis factor (TNF)-α (cat no. PT516) and interleukin (IL)-1β (cat no. PI303) in serum and CSF were measured using ELISA kits (Beyotime, Shanghai, China) referring to the product instructions.
OXIDATIVE STRESS MEASUREMENT:
Tissue homogenate was treated with HEPES buffer, mixed, and centrifuged at 1500 g at 4°C. Then, the supernatant was collected and the thiobarbituric acid reaction method was applied to measure malondialdehyde (MDA) levels in brain tissue. Moreover, a SOD assay kit (Abcam, China) was applied to determine the superoxide dismutase (SOD) activity in brain tissue referring to the manufacturer’s instructions.
QUANTITATIVE REVERSE TRANSCRIPTION PCR (QRT-PCR):
Total RNA from brain tissues cells was extracted by TRIzol reagent (Invitrogen, UK) following the manufacturer’s instructions. Then, total RNA was reverse transcribed into cDNA with a Reverse Transcription Kit (Fermentas, USA). The expression of lncRNA MEG3 was detected by a SYBR Green PCR Master Mix Kit (TaKaRa, Japan). Primers were obtained from Sangon Biotech (China). The reaction was run in triplicate using the ABI PRISM 7900 sequence detection system (Applied Biosystems, USA). Primer sequences used for qPCR were:
The relative gene expression was evaluated using 2−ΔΔCq method [30].
WESTERN BLOT ANALYSIS:
Brain tissue sample protein was collected and separated using RIPA buffer (Beyotime). The concentration of samples was determined using the BCA Protein Assay Kit (Solarbio, USA). After that, the samples were mixed, boiled, centrifuged, resolved by 10% SDS-PAGE, and subsequently transferred onto PVDF membranes. After blocking, the membranes were cultivated in 4°C overnight with primary antibodies (1: 1000 dilution) against p-AKT (cat no. ab38449; Abcam), AKT (cat no. ab18785; Abcam), and GAPDH (cat no. ab9485; Abcam), respectively. After washing with PBST, membranes were cultivated with the secondary antibody (1: 2000; cat no. ab7097; Abcam) for 2 h at room temperature. Finally, proteins were visualized using ECL western blotting detection kits (Pierce Biotechnology) referring to the manufacturer’s protocol.
STATISTICAL ANALYSES:
Statistical analyses were conducted using SPSS 20.0. All results are expressed by mean±SD. Mean differences among groups were estimated with the unpaired
Results
LNCRNA MEG3 WAS OVEREXPRESSED IN BRAIN TISSUES OF ICH RATS:
Firstly, we assessed the lncRNA MEG3 level from brain tissues of ICH rats using qRT-PCR after ICH models establishment. Figure 1 shows that that compared to sham group, lncRNA MEG3 was upregulated in ICH rats.
MIR-181B DIRECTLY INTERACTED WITH LNCRNA MEG3:
To explore the potential mechanisms of lncRNA MEG3, the TargetScan target prediction program was applied to make bioinformatics predictions. Our data disclosed a potential lncRNA MEG3 binding site on miR-181b (Figure 2A). Then, we applied the dual-luciferase reporter system to confirm the relationship between lncRNA MEG3 and miR-181b. These results indicated that upregulation of miR-181b dramatically suppressed luciferase activity of MEG3-WT reporter but had no effects in the MEG3-MUT group (Figure 2B). These observations indicated that miR-181b directly binds to lncRNA MEG3.
MIR-181B WAS DOWNREGULATED IN ICH RATS BRAIN TISSUES:
To further elucidate whether miR-181b is mediated via lncRNA MEG3, we used qRT-PCR analysis to evaluate the miR-181b level in brain tissues of rats in the sham group and ICH group. Our findings suggested that miR-181b was notably downregulated in the brain tissues of ICH rats compared to the sham group (Figure 3). Our results revealed that miR-181b was downregulated in ICH rats brain tissues, suggesting that lncRNA MEG3 is involved in ICH progression by negatively regulating miR-181b levels.
MIR-181B INHIBITOR REVERSED THE EFFECTS OF LNCRNA MEG3-SHRNA ON MIR-181B EXPRESSION IN ICH RATS BRAIN TISSUES:
We further assessed the relationship between miR-181b and lncRNA MEG3 in ICH. Control-shRNA, MEG3-shRNA, inhibitor control, or miR-181b inhibitor were injected into ICH rats, and miR-181b and lncRNA MEG3 levels were measured using qRT-PCR analysis. As presented in Figure 4A, compared to control-shRNA, MEG3-shRNA remarkably suppressed lncRNA MEG3 expression in ICH rat brain tissues. Moreover, we found that miR-181b expression in the ICH+miR-181b inhibitor group was markedly decreased compared with the ICH+inhibitor control group (Figure 4B). In addition, compared with the control-shRNA group, MEG3-shRNA significantly upregulated miR-181b levels in ICH rats. The effects of MEG3-shRNA on miR-181b were reversed by miR-181b inhibitor in ICH rats brain tissues (Figure 4C). In conclusion, these observations suggest that miR-181b interferes with lncRNA MEG3 functions in ICH.
MIR-181B INHIBITED BRAIN EDEMA AND REDUCED NEUROLOGICAL DAMAGE IN ICH RATS:
To investigate the functions of miR-181b in ICH rats, we also determined brain edema and neurological severity scores of ICH mice. At 1 h after ICH stimulation, the neurobehavioral scores in the ICH groups were obviously improved compared to the sham group, but there was no obvious neurological damage in the other groups (Figure 5A). Meanwhile, as displayed in Figure 5B, after ICH induction for 3 days, intraventricular injection of lncRNA MEG3-shRNA resulted in the reduction of neurobehavioral scores. However, compared to the inhibitor control group, the neurobehavioral scores of the MEG3-shRNA+miR-181b inhibitor group were significantly increased, demonstrating that miR-181b relieved neurological injury in ICH. As brain edema serves a vital role in ICH-induced brain damage, we next detected the brain water content in different groups. Our data in Figure 5C show that brain edema in the ICH group was clearly increased, which was subsequently decreased following MEG3-shRNA treatment. However, downregulation of miR-181b led to a marked increase in brain water content (Figure 5C). These findings reveal that miR-181b can alleviate brain damage in ICH rats.
MIR-181B INHIBITOR REVERSED THE EFFECTS OF LNCRNA MEG3-SHRNA ON NEURONS APOPTOSIS IN ICH RATS:
To further analyze the function of miR-181b in ICH, we analyzed the apoptosis of neurons and related apoptotic pathways. Results suggested that neuronal apoptosis and caspase-3 activity in ICH groups were higher than that in the sham group. Moreover, compared with the ICH+control-shRNA group, the neuronal apoptosis and caspase-3 activity in the ICH+MEG3-shRNA group were notably attenuated. However, miR-181b inhibitor significantly promoted neuronal apoptosis and caspase-3 activity (Figure 6A–6C). Therefore, our findings indicate that miR-181b inhibits neuronal apoptosis.
MIR-181B INHIBITOR ABOLISHED THE FUNCTIONS OF LNCRNA MEG3-SHRNA IN INFLAMMATORY RESPONSE AND OXIDATIVE STRESS IN ICH:
Given that immunoreaction and oxidative stress are critical indicators in the pathogenesis of ICH, we next determined the levels of inflammatory cytokine (TNF-α and IL-1β) in ICH rat serum and CSF using ELISA. We observed that the expression of TNF-α (Figure 7A, 7B) and IL-1β (Figure 7C, 7D) in the CSF and serum were obviously increased in ICH rats. Figure 7 also demonstrates that MEG3-shRNA remarkably attenuated TNF-α and IL-1β levels in ICH rats CSF and serum in comparison with the control-shRNA group. These decreases were reversed following miR-181b inhibitor treatment.
Abnormal oxidative stress resulted in ICH-stimulated brain damage. Thus, we then investigated whether miR-181b plays a role in oxidative stress in ICH by determining MDA and SOD levels in the serum of rats. Compared to the sham group, we observed higher MDA levels (Figure 7E) and lower SOD activity (Figure 7F) in the serum of ICH rats. The MDA expression was markedly suppressed and the activity of SOD was strongly increased in the ICH+MEG3-shRNA group compared to the ICH+control-shRNA group. However, after miR-181b inhibition, these results were abolished. Taken together, our data demonstrate that lncRNA MEG3-shRNA prevents ICH-stimulated oxidative stress and inflammation through regulating miR-181b levels.
LNCRNA MEG3 REGULATED THE PI3K/AKT PATHWAY IN ICH RATS BY TARGETING MIR-181B:
Previous reports have confirmed that miR-181b exerts functions by regulating the PI3K/AKT pathway [31,32]. Therefore, in this study, we explored the effects and molecular mechanism of miR-181b in the PI3K/AKT pathway in ICH rats. As exhibited in Figure 8, the expressions of p-AKT protein were remarkably suppressed and the ratio of p-AKT/AKT was significantly decreased in ICH rats tissues compared with the sham group. Compared with the ICH+control-shRNA group, the p-AKT protein level and p-AKT/AKT ratio in the ICH+MEG3-shRNA group were markedly increased. However, these effects were abolished by miR-181b inhibitor. Our findings indicated that lncRNA MEG3 is crucial for CH development by targeting miR-181b.
Discussion
ICH is a serious public health problem causing high mortality and disability around the world [33]. Moreover, previous reports have confirmed that many self-destructive processes, including inflammation, apoptosis, and oxidative stress, are vital regulators leading to ICH-induced brain injury [34]. Despite progress in research and preclinical identification, the pathophysiological mechanism and effective treatments for ICH have not been found. Therefore, discovering the pathogenesis of ICH and finding effective therapeutic strategies are greatly needed.
lncRNAs are reported to regulate many diseases. A study by Chen et al demonstrated that lncRNA Mtss1 promotes inflammatory responses and secondary brain injury after ICH by targeting miR-709 in mice [35]. However, the roles of lncRNA in ICH have not been fully investigated. Moreover, the role of lncRNA MEG3 in ICH remains largely unclear. Thus, the present study was designed to determine the effects of lncRNA MEG3 and particular mechanisms in brain tissues in ICH. Firstly, we conducted an ICH rat model and then determined the lncRNA MEG3 levels in ICH and sham rats by qRT-PCR. We observed lncRNA MEG3 levels in ICH rats were higher than that in the sham group, suggesting that lncRNA MEG3 participates in the pathogenic process of ICH. lncRNA MEG3, a maternally expressed gene related to many human cancers, was found to act as a linker to activate some miRNAs [36]. Recently, Shen et al discovered lncRNA MEG3 acts as an endogenous RNA to mediate HOXA11 expression through targeting miR-181a in multiple myeloma [37]. miR-181b was found to be an important regulatory miRNA relating to many human diseases. miR-181b acts as either a carcinogen or a tumor-inhibiting factor in many tumors [38]. In addition, based on TargetScan and dual-luciferase reporter assay, we found that lncRNA MEG3 directly regulated the miR-181b level by binding to the 3′UTR of miR-181b. Meanwhile, it was reported that downregulation of lncRNA MEG3 inhibited osteosarcoma cell growth, migration, and invasion and promoted apoptosis through targeting miR-127 [39]. We speculated that inhibited lncRNA MEG3 expression or altered miR-181b expression could influence the development of ICH. To confirm our hypothesis, we injected control-shRNA, lncRNA MEG3-shRNA, inhibitor control, or miR-181b inhibitor into brain tissues 1 h after ICH induction. Our data confirmed that lncRNA MEG3-shRNA remarkably inhibited lncRNA MEG3 expression in ICH brain tissues and enhanced miR-181b expression, while miR-181b inhibitor reversed these effects. Furthermore, the level of miR-181b was depressed in the ICH+miR-181b inhibitor group. In conclusion, our data show that miR-181b inhibitor interfered with lncRNA MEG3 functions in brain tissues of ICH rats.
Our research further evaluated whether lncRNA MEG3-shRNA has a protective effect on ICH rats. After 1 h or 3 days after ICH induction, we evaluated neurobehavioral scores using mNSS to assess the neurological damage in ICH rats. Our data revealed that lncRNA MEG3-shRNA decreased neurological injury, as evidenced by lower neurological scores, which were inducted by ICH stimulation for 1 h or 3 days. However, the neurological scores in the ICH+lncRNA MEG3-shRNA+miR-181b inhibitor group were obviously increased compared to the inhibitor control group. Brain edema is an important marker of inflammatory response and tissue injury during ICH [40]. Thus, reducing brain edema could be a novel method for ICH treatment. Results from brain edema assessment indicated that lncRNA MEG3-shRNA significantly suppressed the cerebral water content, which was abolished by miR-181b inhibitor.
Neuronal apoptosis, inflammatory response and oxidative stress are 3 vital elements of brain damage [41]. The present study shows that neuronal apoptosis and oxidative stress were remarkably alleviated following lncRNA MEG3-shRNA treatment, and we observed the opposite effects in the lncRNA MEG3-shRNA+miR-181b inhibitor group. The results of the caspase3 activity assay suggested that the activity of caspase3 was conspicuously inhibited, and this inhibition was reversed by miR-181b inhibitor. To further illustrate the mechanism of lncRNA MEG3 in ICH, we assessed the roles of lncRNA MEG3 in pro-inflammatory cytokine secretion and oxidative stress. Our results show that lncRNA MEG3-shRNA decreased the levels of TNF-α and IL-1β in ICH rat serum and CSF. Previous reports found that oxidative stress is highly related to ICH-induced brain damage [42,43]. In accordance with previous reports, we found that lncRNA MEG3-shRNA injection alleviated oxidative damage in the brain, as evidenced by decreased MDA level, and promoted SOD activity. However, these effects were reversed by miR-181b inhibitor, suggesting that the protective effects of lncRNA MEG3-shRNA in ICH rats occur by targeting miR-181b.
Chen et al found that the PI3K/AKT pathway participates in the progression of ICH [44]. Thus, we further assessed whether the PI3K/AKT pathway is involved in the action of miR-181b in ICH rats. Results from western blotting suggested that lncRNA MEG3-shRNA remarkably increased the p-AKT expression and increased p-AKT/AKT ratio in the ICH rats brain tissues, while inhibition of miR-181b obviously reduced the protein expression of p-AKT and decreased the ratio of p-AKT/AKT.
Conclusions
Our observations show that lncRNA MEG3-shRNA exerts neuroprotective effects during ICH-induced brain injury via suppressing oxidative stress and inflammation in an miR-181b-dependent manner, indicating that lncRNA MEG3 may be a novel agent for clinical treatment of ICH.
Figures
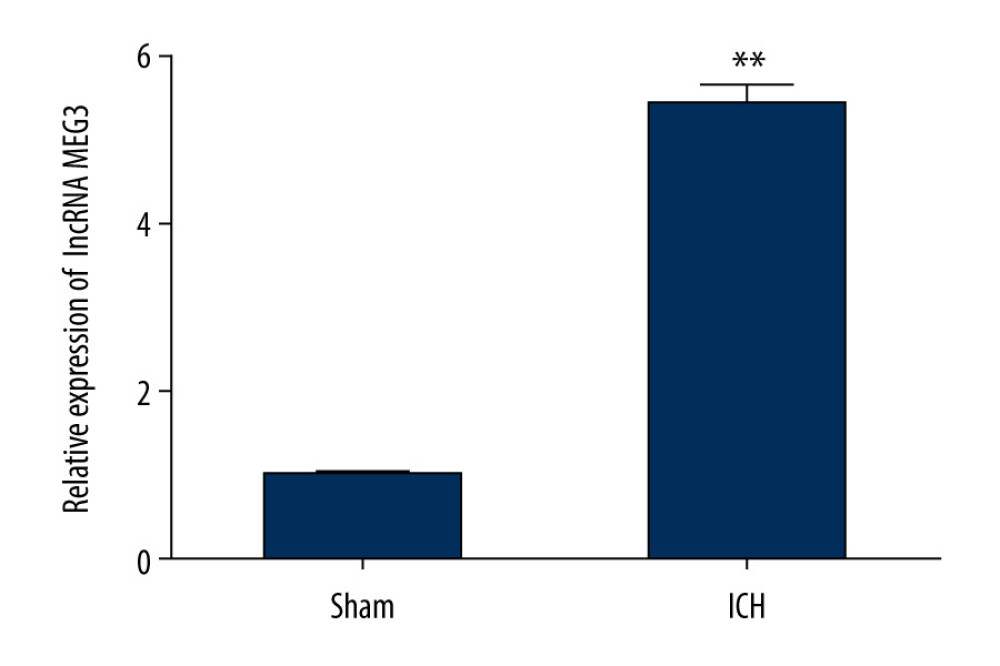 Figure 1. The expression level of LncRNA MEG3 in brain tissues of ICH rats. Three days after ICH induction, lncRNA MEG3 levels in brain tissues from sham and ICH rats were determined using qRT-PCR. ** P<0.01 vs sham.
Figure 1. The expression level of LncRNA MEG3 in brain tissues of ICH rats. Three days after ICH induction, lncRNA MEG3 levels in brain tissues from sham and ICH rats were determined using qRT-PCR. ** P<0.01 vs sham. 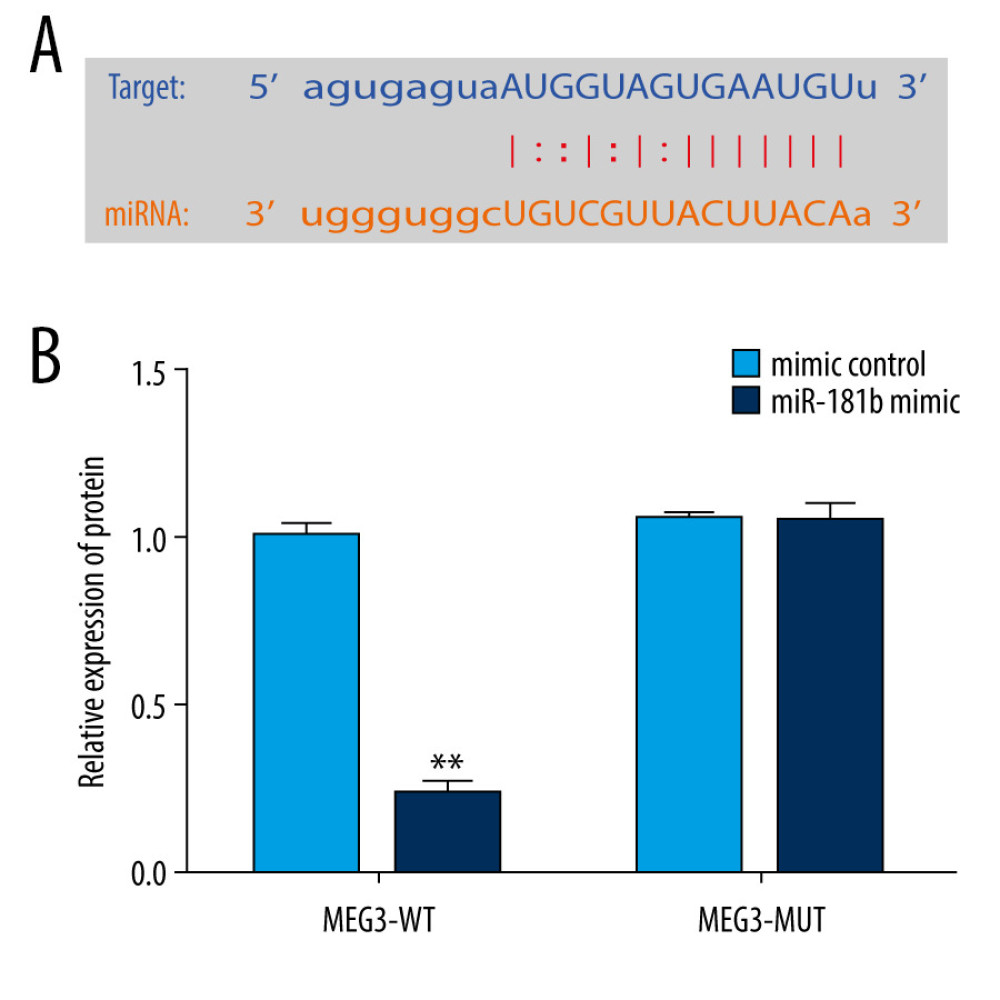 Figure 2. miR-181b directly interacted with lncRNA MEG3. (A) The relationship between lncRNA MEG3 and 3′UTR of miR-181b was predicted. (B) Dual-luciferase reporter activities of reporters containing wild-type or mutant MEG3 were analyzed. ** P<0.01 vs mimic control.
Figure 2. miR-181b directly interacted with lncRNA MEG3. (A) The relationship between lncRNA MEG3 and 3′UTR of miR-181b was predicted. (B) Dual-luciferase reporter activities of reporters containing wild-type or mutant MEG3 were analyzed. ** P<0.01 vs mimic control. 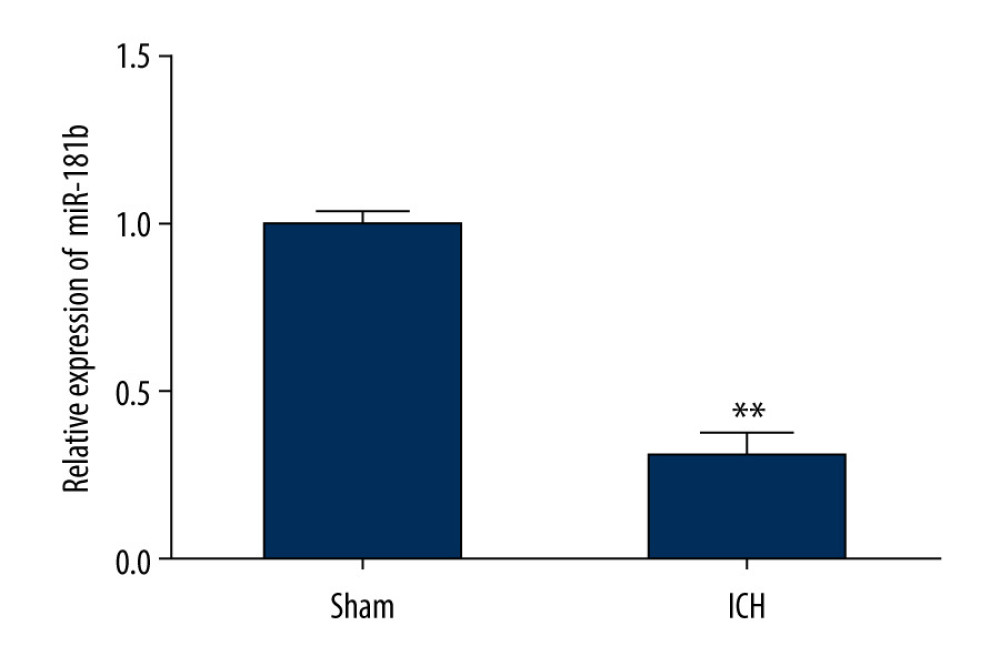 Figure 3. The level of miR-181b in brain tissues from sham and ICH rats. Three days after ICH induction, the expression of miR-181b in brain tissues of sham and ICH rats was measured by qRT-qPCR. ** P<0.01 vs sham.
Figure 3. The level of miR-181b in brain tissues from sham and ICH rats. Three days after ICH induction, the expression of miR-181b in brain tissues of sham and ICH rats was measured by qRT-qPCR. ** P<0.01 vs sham. 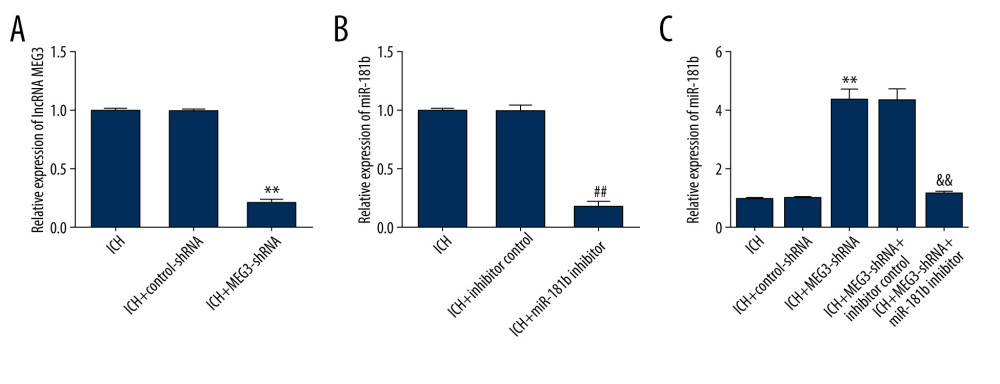 Figure 4. Effect of lncRNA MEG3-shRNA or miR-181b inhibitor on miR-181b expression in ICH rats brain tissues. After ICH stimulation for 1 h, control-shRNA, MEG3-shRNA, inhibitor control, or miR-181b inhibitor were injected into ICH rats. Three days after ICH induction, (A) qRT-PCR analysis of MEG3-shRNA on lncRNA MEG3 expression; (B) The inhibitory effect of miR-181b inhibitor on miR-181b levels was detected using qRT-PCR; (C) qRT-PCR assay was performed to measure miR-181b level in brain tissues of ICH rats after MEG3-shRNA + miR-181b inhibitor or MEG3-shRNA +inhibitor control treatment. ** P<0.01 vs ICH+control-shRNA; ## P<0.01 vs ICH+inhibitor control; && P<0.01 vs ICH+MEG3-shRNA+inhibitor control.
Figure 4. Effect of lncRNA MEG3-shRNA or miR-181b inhibitor on miR-181b expression in ICH rats brain tissues. After ICH stimulation for 1 h, control-shRNA, MEG3-shRNA, inhibitor control, or miR-181b inhibitor were injected into ICH rats. Three days after ICH induction, (A) qRT-PCR analysis of MEG3-shRNA on lncRNA MEG3 expression; (B) The inhibitory effect of miR-181b inhibitor on miR-181b levels was detected using qRT-PCR; (C) qRT-PCR assay was performed to measure miR-181b level in brain tissues of ICH rats after MEG3-shRNA + miR-181b inhibitor or MEG3-shRNA +inhibitor control treatment. ** P<0.01 vs ICH+control-shRNA; ## P<0.01 vs ICH+inhibitor control; && P<0.01 vs ICH+MEG3-shRNA+inhibitor control. 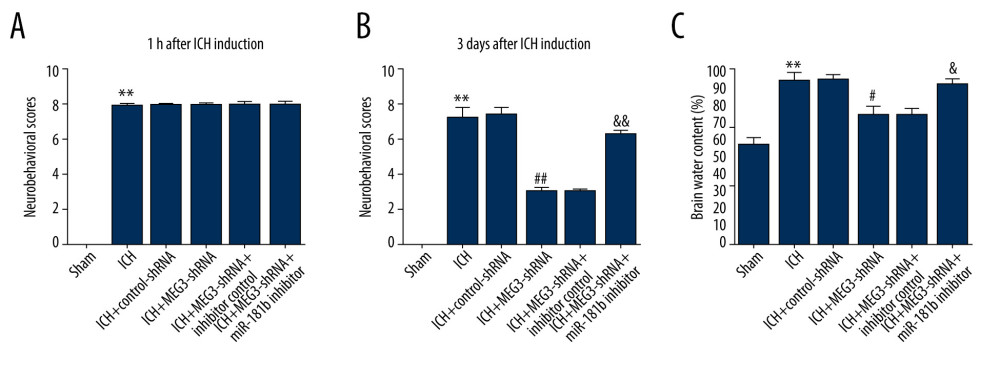 Figure 5. Effects of lncRNA MEG3-shRNA on neurological damage in ICH rats. After ICH induction, ICH rats were treated with control-shRNA, lncRNA MEG3-shRNA, inhibitor control, or miR-181b inhibitor. (A) At 1 hour following ICH or sham operation, the neurobehavioral scores of rats were measured by neurological severity score method. (B) The mNSS test was applied to assess neurobehavioral scores at 3 days after ICH or sham operation in different groups. (C) Three days after ICH induction, brain water contents were assessed using the wet/dry method in different groups. ** P<0.01 vs Sham; #, ## P<0.05, 0.01 vs ICH+control-shRNA; &, && P<0.05, 0.01 vs ICH+MEG3-shRNA+inhibitor control.
Figure 5. Effects of lncRNA MEG3-shRNA on neurological damage in ICH rats. After ICH induction, ICH rats were treated with control-shRNA, lncRNA MEG3-shRNA, inhibitor control, or miR-181b inhibitor. (A) At 1 hour following ICH or sham operation, the neurobehavioral scores of rats were measured by neurological severity score method. (B) The mNSS test was applied to assess neurobehavioral scores at 3 days after ICH or sham operation in different groups. (C) Three days after ICH induction, brain water contents were assessed using the wet/dry method in different groups. ** P<0.01 vs Sham; #, ## P<0.05, 0.01 vs ICH+control-shRNA; &, && P<0.05, 0.01 vs ICH+MEG3-shRNA+inhibitor control. 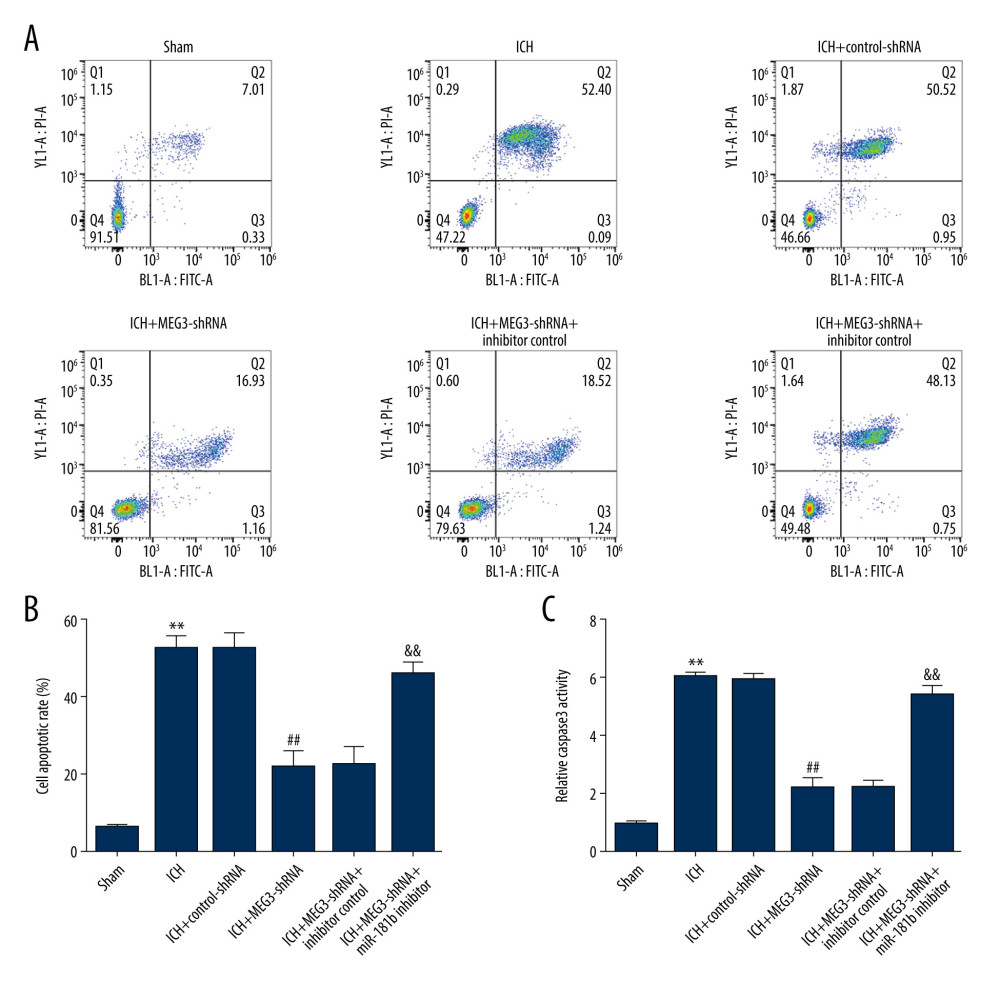 Figure 6. miR-181b inhibitor abolished the effects of lncRNA MEG3-shRNA on neuronal apoptosis in ICH rats. Rats were grouped into 6 groups: Sham, ICH, ICH+control-shRNA, ICH+lncRNA MEG3-shRNA, ICH+lncRNA MEG3-shRNA+inhibitor control, and ICH+lncRNA MEG3-shRNA+miR-181b inhibitor. Three days after ICH induction, neuronal apoptosis was calculated using flow cytometry in different groups (A) and quantitative analysis of apoptotic cells was performed (B). (C) The activity of caspase-3 in different groups was determined. ** P<0.01 vs Sham; ## P<0.01 vs ICH+control-shRNA; && P<0.01 vs ICH+MEG3-shRNA+inhibitor control.
Figure 6. miR-181b inhibitor abolished the effects of lncRNA MEG3-shRNA on neuronal apoptosis in ICH rats. Rats were grouped into 6 groups: Sham, ICH, ICH+control-shRNA, ICH+lncRNA MEG3-shRNA, ICH+lncRNA MEG3-shRNA+inhibitor control, and ICH+lncRNA MEG3-shRNA+miR-181b inhibitor. Three days after ICH induction, neuronal apoptosis was calculated using flow cytometry in different groups (A) and quantitative analysis of apoptotic cells was performed (B). (C) The activity of caspase-3 in different groups was determined. ** P<0.01 vs Sham; ## P<0.01 vs ICH+control-shRNA; && P<0.01 vs ICH+MEG3-shRNA+inhibitor control. 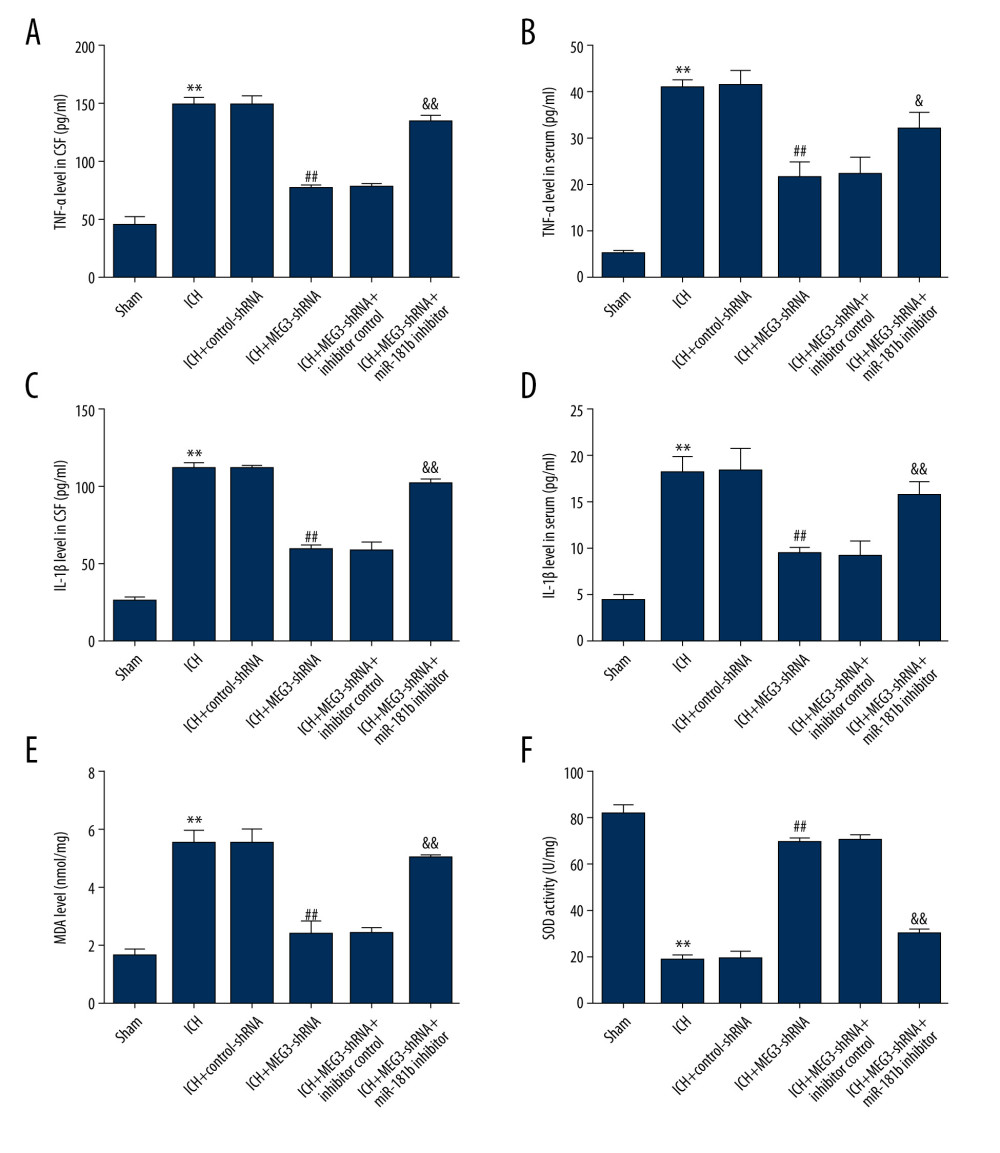 Figure 7. miR-181b inhibitor reversed the effects of lncRNA MEG3-shRNA on inflammatory response and oxidative stress in ICH rats. Rats were divided into 6 groups: Sham, ICH, ICH+control-shRNA, ICH+lncRNA MEG3-shRNA, ICH+lncRNA MEG3-shRNA+inhibitor control, and ICH+lncRNA MEG3-shRNA+miR-181b inhibitor. Three days after ICH induction, levels of TNF-α (A, B) and IL-1β (C, D) in the rat CSF and serum of all rats were measured using ELISA. MDA (E) and SOD activity (F) in the serum of rats were determined. ** P<0.01 vs Sham; ## P<0.01 vs ICH+control-shRNA; &, && P<0.05, 0.01 vs ICH+MEG3-shRNA+inhibitor control.
Figure 7. miR-181b inhibitor reversed the effects of lncRNA MEG3-shRNA on inflammatory response and oxidative stress in ICH rats. Rats were divided into 6 groups: Sham, ICH, ICH+control-shRNA, ICH+lncRNA MEG3-shRNA, ICH+lncRNA MEG3-shRNA+inhibitor control, and ICH+lncRNA MEG3-shRNA+miR-181b inhibitor. Three days after ICH induction, levels of TNF-α (A, B) and IL-1β (C, D) in the rat CSF and serum of all rats were measured using ELISA. MDA (E) and SOD activity (F) in the serum of rats were determined. ** P<0.01 vs Sham; ## P<0.01 vs ICH+control-shRNA; &, && P<0.05, 0.01 vs ICH+MEG3-shRNA+inhibitor control. 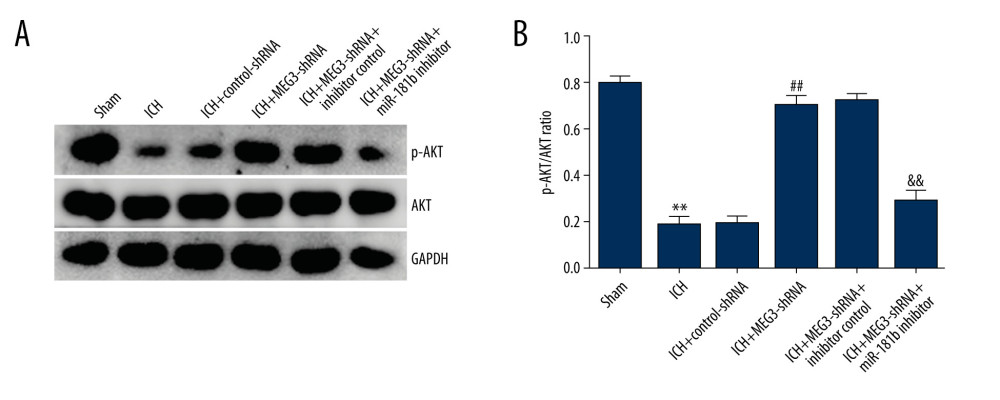 Figure 8. (A, B) Effect of lncRNA MEG3 on PI3K/AKT pathway in ICH rats by targeting miR-181b. One hour after ICH induction, control-shRNA, lncRNA MEG3-shRNA, inhibitor control, or miR-181b inhibitor were injected into rats. Three days after ICH induction, the protein levels of p-AKT and AKT were assessed using western blotting in different groups. The ratio of p-AKT/AKT was calculated and presented. ** P<0.01 vs sham; ## P<0.01 vs ICH+control-shRNA; && P<0.01 vs ICH+MEG3-shRNA+inhibitor control.
Figure 8. (A, B) Effect of lncRNA MEG3 on PI3K/AKT pathway in ICH rats by targeting miR-181b. One hour after ICH induction, control-shRNA, lncRNA MEG3-shRNA, inhibitor control, or miR-181b inhibitor were injected into rats. Three days after ICH induction, the protein levels of p-AKT and AKT were assessed using western blotting in different groups. The ratio of p-AKT/AKT was calculated and presented. ** P<0.01 vs sham; ## P<0.01 vs ICH+control-shRNA; && P<0.01 vs ICH+MEG3-shRNA+inhibitor control. References
1. Hostettler IC, Seiffge DJ, Werring DJ, Intracerebral hemorrhage: An update on diagnosis and treatment: Expert Rev Neurother, 2019; 19; 679-94
2. Hostettler IC, Seiffge DJ, Werring DJ, Intracerebral hemorrhage: An update on diagnosis and treatment: Expert Rev Neurother, 2019; 19; 679-94
3. Leclerc JL, Li C, Jean S, Temporal and age-dependent effects of haptoglobin deletion on intracerebral hemorrhage-induced brain damage and neurobehavioral outcomes: Exp Neurol, 2019; 317; 22-33
4. Qu X, Wang N, Chen W, RNF34 overexpression exacerbates neurological deficits and brain injury in a mouse model of intracerebral hemorrhage by potentiating mitochondrial dysfunction-mediated oxidative stress: Sci Rep, 2019; 9; 16296
5. Li P, Jiwu C, Butin attenuates brain edema in a rat model of intracerebral hemorrhage by anti inflammatory pathway: Transl Neurosci, 2018; 9; 7-12
6. Duan X, Wen Z, Shen H, Intracerebral hemorrhage, oxidative stress, and antioxidant therapy: Oxid Med Cell Longev, 2016; 2016; 1203285
7. Qu X, Wang N, Cheng W, MicroRNA-146a protects against intracerebral hemorrhage by inhibiting inflammation and oxidative stress: Exp Ther Med, 2019; 18; 3920-28
8. Jiao H, Xie D, Qiao Y, LncRNA PRINS is involved in the development of nephropathy in patients with diabetes via interaction with Smad7: Exp Ther Med, 2019; 17; 3203-8
9. Jia HY, Zhang K, Lu WJ, LncRNA MEG3 influences the proliferation and apoptosis of psoriasis epidermal cells by targeting miR-21/caspase-8: BMC Mol Cell Biol, 2019; 20; 46
10. Zhang J, Dong B, Hao J, LncRNA Snhg3 contributes to dysfunction of cerebral microvascular cells in intracerebral hemorrhage rats by activating the TWEAK/Fn14/STAT3 pathway: Life Sci, 2019; 237; 116929
11. Zhao W, Geng D, Li S, LncRNA HOTAIR influences cell growth, migration, invasion, and apoptosis via the miR-20a-5p/HMGA2 axis in breast cancer: Cancer Med, 2018; 7; 842-55
12. Wei GH, Wang X, lncRNA MEG3 inhibit proliferation and metastasis of gastric cancer via p53 signaling pathway: Eur Rev Med Pharmacol Sci, 2017; 21; 3850-56
13. Wu M, Huang Y, Chen T, LncRNA MEG3 inhibits the progression of prostate cancer by modulating miR-9–5p/QKI-5 axis: J Cell Mol Med, 2019; 23; 29-38
14. Wang X, Wang Z, Wang J, LncRNA MEG3 has anti-activity effects of cervical cancer: Biomed Pharmacother, 2017; 94; 636-43
15. Zhang L, Wang H, Long non-coding RNA in CNS injuries: A new target for therapeutic intervention: Mol Ther Nucleic Acids, 2019; 17; 754-66
16. Li Z, Han L, Liang Q, Huang Z, Long noncoding RNA MEG3 contributes to dysfunction of brain microvascular endothelial cells after intracerebral hemorrhage by regulating the miR-1930–5p/Mllt1 axis: Brain Res Bull, 2021; 166; 1-11
17. Abdi J, Rastgoo N, Li L, Role of tumor suppressor p53 and micro-RNA interplay in multiple myeloma pathogenesis: J Hematol Oncol, 2017; 10; 169
18. Shao G, Zhou C, Ma K, MiRNA-494 enhances M1 macrophage polarization via Nrdp1 in ICH mice model: J Inflamm (Lond), 2020; 17; 17
19. Yu A, Zhang T, Zhong W, miRNA-144 induces microglial autophagy and inflammation following intracerebral hemorrhage: Immunol Lett, 2017; 182; 18-23
20. Wang J, Zhu Y, Jin F, Differential expression of circulating microRNAs in blood and haematoma samples from patients with intracerebral haemorrhage: J Int Med Res, 2016; 44; 419-32
21. Gareev I, Yang G, Sun J, Circulating MicroRNAs as potential noninvasive biomarkers of spontaneous intracerebral hemorrhage: World Neurosurg, 2020; 133; e369-75
22. Gareev I, Yang G, Sun J, Circulating microRNAs as potential noninvasive biomarkers of spontaneous intracerebral hemorrhage: World Neurosurg, 2020; 133; e369-75
23. Wang Z, Fang L, Shi H, Yang Z, miR-181b regulates ER stress induced neuron death through targeting Heat Shock Protein A5 following intracerebral haemorrhage: Immunol Lett, 2019; 206; 1-10
24. Pang X, Feng G, Shang W, Inhibition of lncRNA MEG3 protects renal tubular from hypoxia-induced kidney injury in acute renal allografts by regulating miR-181b/TNF-alpha signaling pathway: J Cell Biochem, 2019; 120(8); 12822-31
25. Han X, Zheng Z, Wang C, Wang L, Association between MEG3/miR-181b polymorphisms and risk of ischemic stroke: Lipids Health Dis, 2018; 17(1); 292
26. Liu X, Hou L, Huang W, The mechanism of long non-coding RNA MEG3 for neurons apoptosis caused by hypoxia: Mediated by miR-181b-12/15-LOX signaling pathway: Front Cell Neurosci, 2016; 10; 201
27. Wei N, Wei Y, Li B, Baicalein promotes neuronal and behavioral recovery after intracerebral hemorrhage via suppressing apoptosis, oxidative stress and neuroinflammation: Neurochem Res, 2017; 42; 1345-53
28. Yuan B, Shen H, Lin L, MicroRNA367 negatively regulates the inflammatory response of microglia by targeting IRAK4 in intracerebral hemorrhage: J Neuroinflammation, 2015; 12; 206
29. Qu X, Wang N, Cheng W, MicroRNA-146a protects against intracerebral hemorrhage by inhibiting inflammation and oxidative stress: Exp Ther Med, 2019; 18; 3920-28
30. Livak KJ, Schmittgen TD, Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method: Methods, 2001; 25; 402-8
31. Zheng J, Wu C, Xu Z, Hepatic stellate cell is activated by microRNA-181b via PTEN/Akt pathway: Mol Cell Biochem, 2015; 398; 1-9
32. Zheng H, Liu J, Tycksen E, MicroRNA-181a/b-1 over-expression enhances osteogenesis by modulating PTEN/PI3K/AKT signaling and mitochondrial metabolism: Bone, 2019; 123; 92-102
33. Chen HS, Hsieh CF, Chau TT, Risk factors of in-hospital mortality of intracerebral hemorrhage and comparison of ICH scores in a Taiwanese population: Eur Neurol, 2011; 66; 59-63
34. Liu XC, Jing LY, Yang MF, Enhanced neuroprotection of minimally invasive surgery joint local cooling lavage against ICH-induced inflammation injury and apoptosis in rats: Cell Mol Neurobiol, 2016; 36; 647-55
35. Chen JX, Wang YP, Zhang X, lncRNA Mtss1 promotes inflammatory responses and secondary brain injury after intracerebral hemorrhage by targeting miR-709 in mice: Brain Res Bull, 2020; 162; 20-29
36. Long J, Pi X, lncRNA-MEG3 suppresses the proliferation and invasion of melanoma by regulating CYLD expression mediated by sponging miR-499–5p: Biomed Res Int, 2018; 2018; 2086564
37. Shen X, Bai H, Zhu H, Long non-coding RNA MEG3 functions as a competing endogenous RNA to regulate HOXA11 expression by sponging miR-181a in multiple myeloma: Cell Physiol Biochem, 2018; 49; 87-100
38. Hori D, Dunkerly-Eyring B, Nomura Y, miR-181b regulates vascular stiffness age dependently in part by regulating TGF-beta signaling: PLoS One, 2017; 12; e174108
39. Wang Y, Kong D, Knockdown of lncRNA MEG3 inhibits viability, migration, and invasion and promotes apoptosis by sponging miR-127 in osteosarcoma cell: J Cell Biochem, 2018; 119; 669-79
40. Li P, Jiwu C, Butin attenuates brain edema in a rat model of intracerebral hemorrhage by anti inflammatory pathway: Transl Neurosci, 2018; 9; 7-12
41. Tang G, Yang H, Chen J, Metformin ameliorates sepsis-induced brain injury by inhibiting apoptosis, oxidative stress and neuroinflammation via the PI3K/Akt signaling pathway: Oncotarget, 2017; 8; 97977-89
42. Duan X, Wen Z, Shen H, Intracerebral hemorrhage, oxidative stress, and antioxidant therapy: Oxid Med Cell Longev, 2016; 2016; 1203285
43. Hu X, Tao C, Gan Q, Oxidative stress in intracerebral hemorrhage: Sources, mechanisms, and therapeutic targets: Oxid Med Cell Longev, 2016; 2016; 3215391
44. Chen S, Peng J, Sherchan P, TREM2 activation attenuates neuroinflammation and neuronal apoptosis via PI3K/Akt pathway after intracerebral hemorrhage in mice: J Neuroinflammation, 2020; 17; 168
Figures
 Figure 1. The expression level of LncRNA MEG3 in brain tissues of ICH rats. Three days after ICH induction, lncRNA MEG3 levels in brain tissues from sham and ICH rats were determined using qRT-PCR. ** P<0.01 vs sham.
Figure 1. The expression level of LncRNA MEG3 in brain tissues of ICH rats. Three days after ICH induction, lncRNA MEG3 levels in brain tissues from sham and ICH rats were determined using qRT-PCR. ** P<0.01 vs sham. Figure 2. miR-181b directly interacted with lncRNA MEG3. (A) The relationship between lncRNA MEG3 and 3′UTR of miR-181b was predicted. (B) Dual-luciferase reporter activities of reporters containing wild-type or mutant MEG3 were analyzed. ** P<0.01 vs mimic control.
Figure 2. miR-181b directly interacted with lncRNA MEG3. (A) The relationship between lncRNA MEG3 and 3′UTR of miR-181b was predicted. (B) Dual-luciferase reporter activities of reporters containing wild-type or mutant MEG3 were analyzed. ** P<0.01 vs mimic control. Figure 3. The level of miR-181b in brain tissues from sham and ICH rats. Three days after ICH induction, the expression of miR-181b in brain tissues of sham and ICH rats was measured by qRT-qPCR. ** P<0.01 vs sham.
Figure 3. The level of miR-181b in brain tissues from sham and ICH rats. Three days after ICH induction, the expression of miR-181b in brain tissues of sham and ICH rats was measured by qRT-qPCR. ** P<0.01 vs sham. Figure 4. Effect of lncRNA MEG3-shRNA or miR-181b inhibitor on miR-181b expression in ICH rats brain tissues. After ICH stimulation for 1 h, control-shRNA, MEG3-shRNA, inhibitor control, or miR-181b inhibitor were injected into ICH rats. Three days after ICH induction, (A) qRT-PCR analysis of MEG3-shRNA on lncRNA MEG3 expression; (B) The inhibitory effect of miR-181b inhibitor on miR-181b levels was detected using qRT-PCR; (C) qRT-PCR assay was performed to measure miR-181b level in brain tissues of ICH rats after MEG3-shRNA + miR-181b inhibitor or MEG3-shRNA +inhibitor control treatment. ** P<0.01 vs ICH+control-shRNA; ## P<0.01 vs ICH+inhibitor control; && P<0.01 vs ICH+MEG3-shRNA+inhibitor control.
Figure 4. Effect of lncRNA MEG3-shRNA or miR-181b inhibitor on miR-181b expression in ICH rats brain tissues. After ICH stimulation for 1 h, control-shRNA, MEG3-shRNA, inhibitor control, or miR-181b inhibitor were injected into ICH rats. Three days after ICH induction, (A) qRT-PCR analysis of MEG3-shRNA on lncRNA MEG3 expression; (B) The inhibitory effect of miR-181b inhibitor on miR-181b levels was detected using qRT-PCR; (C) qRT-PCR assay was performed to measure miR-181b level in brain tissues of ICH rats after MEG3-shRNA + miR-181b inhibitor or MEG3-shRNA +inhibitor control treatment. ** P<0.01 vs ICH+control-shRNA; ## P<0.01 vs ICH+inhibitor control; && P<0.01 vs ICH+MEG3-shRNA+inhibitor control. Figure 5. Effects of lncRNA MEG3-shRNA on neurological damage in ICH rats. After ICH induction, ICH rats were treated with control-shRNA, lncRNA MEG3-shRNA, inhibitor control, or miR-181b inhibitor. (A) At 1 hour following ICH or sham operation, the neurobehavioral scores of rats were measured by neurological severity score method. (B) The mNSS test was applied to assess neurobehavioral scores at 3 days after ICH or sham operation in different groups. (C) Three days after ICH induction, brain water contents were assessed using the wet/dry method in different groups. ** P<0.01 vs Sham; #, ## P<0.05, 0.01 vs ICH+control-shRNA; &, && P<0.05, 0.01 vs ICH+MEG3-shRNA+inhibitor control.
Figure 5. Effects of lncRNA MEG3-shRNA on neurological damage in ICH rats. After ICH induction, ICH rats were treated with control-shRNA, lncRNA MEG3-shRNA, inhibitor control, or miR-181b inhibitor. (A) At 1 hour following ICH or sham operation, the neurobehavioral scores of rats were measured by neurological severity score method. (B) The mNSS test was applied to assess neurobehavioral scores at 3 days after ICH or sham operation in different groups. (C) Three days after ICH induction, brain water contents were assessed using the wet/dry method in different groups. ** P<0.01 vs Sham; #, ## P<0.05, 0.01 vs ICH+control-shRNA; &, && P<0.05, 0.01 vs ICH+MEG3-shRNA+inhibitor control. Figure 6. miR-181b inhibitor abolished the effects of lncRNA MEG3-shRNA on neuronal apoptosis in ICH rats. Rats were grouped into 6 groups: Sham, ICH, ICH+control-shRNA, ICH+lncRNA MEG3-shRNA, ICH+lncRNA MEG3-shRNA+inhibitor control, and ICH+lncRNA MEG3-shRNA+miR-181b inhibitor. Three days after ICH induction, neuronal apoptosis was calculated using flow cytometry in different groups (A) and quantitative analysis of apoptotic cells was performed (B). (C) The activity of caspase-3 in different groups was determined. ** P<0.01 vs Sham; ## P<0.01 vs ICH+control-shRNA; && P<0.01 vs ICH+MEG3-shRNA+inhibitor control.
Figure 6. miR-181b inhibitor abolished the effects of lncRNA MEG3-shRNA on neuronal apoptosis in ICH rats. Rats were grouped into 6 groups: Sham, ICH, ICH+control-shRNA, ICH+lncRNA MEG3-shRNA, ICH+lncRNA MEG3-shRNA+inhibitor control, and ICH+lncRNA MEG3-shRNA+miR-181b inhibitor. Three days after ICH induction, neuronal apoptosis was calculated using flow cytometry in different groups (A) and quantitative analysis of apoptotic cells was performed (B). (C) The activity of caspase-3 in different groups was determined. ** P<0.01 vs Sham; ## P<0.01 vs ICH+control-shRNA; && P<0.01 vs ICH+MEG3-shRNA+inhibitor control. Figure 7. miR-181b inhibitor reversed the effects of lncRNA MEG3-shRNA on inflammatory response and oxidative stress in ICH rats. Rats were divided into 6 groups: Sham, ICH, ICH+control-shRNA, ICH+lncRNA MEG3-shRNA, ICH+lncRNA MEG3-shRNA+inhibitor control, and ICH+lncRNA MEG3-shRNA+miR-181b inhibitor. Three days after ICH induction, levels of TNF-α (A, B) and IL-1β (C, D) in the rat CSF and serum of all rats were measured using ELISA. MDA (E) and SOD activity (F) in the serum of rats were determined. ** P<0.01 vs Sham; ## P<0.01 vs ICH+control-shRNA; &, && P<0.05, 0.01 vs ICH+MEG3-shRNA+inhibitor control.
Figure 7. miR-181b inhibitor reversed the effects of lncRNA MEG3-shRNA on inflammatory response and oxidative stress in ICH rats. Rats were divided into 6 groups: Sham, ICH, ICH+control-shRNA, ICH+lncRNA MEG3-shRNA, ICH+lncRNA MEG3-shRNA+inhibitor control, and ICH+lncRNA MEG3-shRNA+miR-181b inhibitor. Three days after ICH induction, levels of TNF-α (A, B) and IL-1β (C, D) in the rat CSF and serum of all rats were measured using ELISA. MDA (E) and SOD activity (F) in the serum of rats were determined. ** P<0.01 vs Sham; ## P<0.01 vs ICH+control-shRNA; &, && P<0.05, 0.01 vs ICH+MEG3-shRNA+inhibitor control. Figure 8. (A, B) Effect of lncRNA MEG3 on PI3K/AKT pathway in ICH rats by targeting miR-181b. One hour after ICH induction, control-shRNA, lncRNA MEG3-shRNA, inhibitor control, or miR-181b inhibitor were injected into rats. Three days after ICH induction, the protein levels of p-AKT and AKT were assessed using western blotting in different groups. The ratio of p-AKT/AKT was calculated and presented. ** P<0.01 vs sham; ## P<0.01 vs ICH+control-shRNA; && P<0.01 vs ICH+MEG3-shRNA+inhibitor control.
Figure 8. (A, B) Effect of lncRNA MEG3 on PI3K/AKT pathway in ICH rats by targeting miR-181b. One hour after ICH induction, control-shRNA, lncRNA MEG3-shRNA, inhibitor control, or miR-181b inhibitor were injected into rats. Three days after ICH induction, the protein levels of p-AKT and AKT were assessed using western blotting in different groups. The ratio of p-AKT/AKT was calculated and presented. ** P<0.01 vs sham; ## P<0.01 vs ICH+control-shRNA; && P<0.01 vs ICH+MEG3-shRNA+inhibitor control. In Press
06 Mar 2024 : Clinical Research
Prevalence and Variation of Medical Comorbidities in Oral Surgery Patients: A Retrospective Study at Jazan ...Med Sci Monit In Press; DOI: 10.12659/MSM.943884
08 Mar 2024 : Clinical Research
Evaluation of Foot Structure in Preschool Children Based on Body MassMed Sci Monit In Press; DOI: 10.12659/MSM.943765
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








