27 February 2021: Database Analysis
Sites and Causes of Infection in Patients with Sepsis-Associated Liver Dysfunction: A Population Study from the Medical Information Mart for Intensive Care III
Jinfeng Lin1BCEF, Chunfeng Gu2CD, Suyan Zhang1B, Lijun Tian1B, Ke Ren1B, Zhilong Cao1B, Xudong Han1AG*DOI: 10.12659/MSM.928928
Med Sci Monit 2021; 27:e928928
Abstract
BACKGROUND: Little is known about the relationship between the site of infection, type of pathogen, and the occurrence of sepsis-associated liver dysfunction (SALD). This population study aimed to identify the sites and types of infection in SALD patients.
MATERIAL AND METHODS: We conducted a retrospective observational study using the Medical Information Mart for Intensive Care III. Patients with sepsis were divided into a SALD group and a control group. We evaluated the effect of the location of culture-positive specimens and the distribution of pathogens on the occurrence of SALD and then compared the clinical outcomes.
RESULTS: A total of 14 596 admissions were included, and the incidence of SALD was 11.96%. Positive bile culture (odds ratio [OR] 7.450, P<0.001), peritoneal fluid culture (OR 3.616, P<0.001), and blood culture (OR 1.957, P<0.001) were correlated with the occurrence of SALD. Infection with Enterococcus faecium (OR 3.065, P<0.001), Bacteroides fragilis (OR 2.061, P<0.001), Klebsiella oxytoca (OR 2.066, P<0.001), Enterobacter aerogenes (OR 1.92, P=0.001), and Aspergillus fumigatus (OR 2.144, P=0.001) were correlated with the occurrence of SALD. The Intensive Care Unit mortality and hospital mortality were higher in the SALD group than in the control group (24.7% vs 9.0%, P<0.001; 34.2% vs 13.8%, P<0.001, respectively).
CONCLUSIONS: SALD should be considered for patients with sepsis whose infection site is the biliary system, abdominal cavity, or blood and the pathogen is Enterococcus faecium, B. fragilis, K. oxytoca, Enterobacter aerogenes, or A. fumigatus. When SALD occurs in patients with sepsis, the above infection sites and pathogens should be considered first.
Keywords: database, Sepsis, Bacterial Infections, Critical Care, Databases, Factual, Hospital Mortality, Incidence, Intensive Care Units, Liver Diseases, Risk Factors
Background
Sepsis is common in patients in the Intensive Care Unit (ICU). Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection [1]. The Surviving Sepsis Campaign guidelines indicate that improving the perfusion of tissues, including the liver, in patients with sepsis is critical to improve the prognosis [2]. Measures include timely and effective fluid resuscitation, appropriate use of vasoactive drugs, and effective treatment of primary infections, among others [2]. The liver plays an important role in maintaining metabolic and immunological homeostasis. During sepsis, the liver can regulate immune defense through mechanisms such as bacterial clearance, production of acute-phase proteins or cytokines, and metabolic adaptation to inflammation [3]. However, the liver is also affected in sepsis. During sepsis, the infection itself, hyperactivity of the inflammatory response, failure of the microcirculation, and adverse effects of treatment can cause liver injury [4]. According to the clinical presentation, sepsis-associated liver dysfunction (SALD) can be divided into 2 major patterns: hypoxic hepatitis (due to ischemia and shock) and cholestasis (due to altered bile metabolism) [3,5]. Because of the lack of uniform diagnostic criteria, the precise incidence of SALD remains difficult to determine. The mean incidence of liver dysfunction in sepsis patients is 39.9% [6]. SALD leads to increased mortality and poor prognosis in patients with sepsis. Early identification and treatment will improve the prognosis of patients with sepsis and liver injury [7]. Infection is the root cause of the occurrence and development of sepsis and SALD [8]. Different infection sites and pathogen types lead to different types of organ failure resulting from sepsis [9]. Clarifying the relationship of infection sites and pathogen types with the occurrence of SALD in patients with sepsis would aid in the early identification and treatment of SALD, and there are few such studies in the literature. Medical Information Mart for Intensive Care III (MIMIC-III) was developed at the Massachusetts Institute of Technology. MIMIC-III includes clinical data of patients admitted to the Beth Israel Deaconess Medical Center in Boston, Massachusetts, between June 2001 and October 2012, and includes 58 000 hospital admissions for 38 645 adults [10]. The current population study aimed to use data from the MIMIC-III database version 1.4 (v1.4) to identify the sites of infection and infections in patients with SALD.
Material and Methods
PATIENTS:
We conducted a retrospective study using the MIMIC-III database v1.4. The patient information in the database was deidentified for privacy protection, and the requirement for individual informed consent was waived. One of us (Jinfeng Lin) obtained access to the database (certificate number 32304761).
Patients admitted to the hospital with sepsis were included in this study. Patients younger than 18 years and hospitalized for less than 48 h were excluded. The study subjects were divided into a control group and a SALD group. The SALD group was further divided into a hypoxic hepatitis group, a cholestasis group, and a combined hypoxic hepatitis and cholestasis group.
According to the definition of sepsis [1], we identified patients with confirmed infection (positive specimen culture) and sequential organ failure assessment (SOFA) score ≥ 2 as sepsis patients in this study.
SEPSIS-ASSOCIATED LIVER DYSFUNCTION:
Hypoxic hepatitis was defined as serum aminotransaminase (alanine aminotransferase [ALT] or aspartate aminotransferase [AST]) levels >1000 IU/L [11]. Cholestasis was defined as a total serum bilirubin level >3 mg/dL [12]. The exclusion criteria were previous liver disease, such as autoimmune hepatitis, alcoholic cirrhosis, biliary cirrhosis, and otherwise unspecified cirrhosis, and obstruction of the bile duct.
STUDY PROCESS:
According to the inclusion and exclusion criteria, the subjects of this study were selected and grouped. The age, sex, SOFA score, lactate level, and white blood cell count of the 2 groups measured at admission were recorded. Moreover, the levels of ALT, AST, total bilirubin, and albumin and the international normalized ratio (INR) of each group during hospitalization were compared. We studied the location of culture-positive specimens, the distribution of pathogens, and the effect of each on the occurrence of SALD. Finally, we compared the clinical outcomes, including length of stay in the ICU and hospital, ICU and hospital mortality, and the 1-year cumulative survival rate between the 2 groups.
STATISTICAL ANALYSIS:
Continuous variables are presented as the median and 25–75% interquartile range. Categorical data are expressed as numbers and percentages. The Mann-Whitney U test (or Kruskal-Wallis H) or chi-squared test (or Fisher’s exact test) was used for univariable analysis as appropriate. An unconditional multiple logistic regression model was used to determine the relationship of infection sites and pathogen types with the occurrence of SALD. An unconditional multiple logistic regression model was used to determine the relationship between pathogens and SALD. The results of the logistic regression analysis were reported as adjusted odds ratios (ORs) with 95% confidence intervals (CIs). Kaplan-Meier curves and log-rank tests were used to compare cumulative 1-year survival rates of patients in each group. We used MS Excel and SPSS 25 (SPSS, Chicago, IL, USA) for data management and calculations. A 2-sided
Result
SCREENING OF PATIENTS:
According to the sepsis 3.0 diagnostic criteria, the number of cases with confirmed infection and a SOFA score ≥2 points in the MIMIC-III clinical database v1.4 was 17 549. The incidence of sepsis was 28.63%. The number of patients younger than 18 years old and/or hospitalized for less than 48 h was 1996. The number of patients with previous liver disease was 957. As a result, a total of 14 596 patients were included in this retrospective study, of which 12 850 were in the control group and 1746 were in the SALD group (284 in the hypoxic hepatitis group, 1162 in the cholestasis group, and 300 in the hypoxic hepatitis and cholestasis group). The incidence of SALD was 11.96%. The specific process is shown in Figure 1.
PATIENT CHARACTERISTICS ON ADMISSION:
The SOFA score (7 vs 4, P<0.001), lactic acid level (2.3 vs 1.7, P<0.001), and white blood cell count (12.2 vs 10.6, P<0.001) of patients in the SALD group were significantly higher than those in the control group. The details are shown in Table 1.
LIVER BLOOD LABORATORY DATA:
Figure 2 shows the levels of ALT, AST, total bilirubin, and albumin and the INR in each group. The INR of the SALD group was higher than that of the control group (P<0.001), while albumin was lower than that of the control group (P<0.001).
LOCATION OF CULTURE-POSITIVE SPECIMENS:
A total of 42 412 positive culture results were detected in the 2 groups. There were 35 006 cases in the control group and 7406 cases in the SALD group. The types of positive specimens in the control group were mainly sputum and urine, which accounted for 25.18% and 25.15%, respectively. The types of positive specimens in the SALD group were mainly sputum, urine, and blood, which accounted for 23.41%, 16.81%, and 16.39%, respectively. The positive rates of blood culture (16.39% vs 12.91%, P<0.001), catheter tip culture (3.48% vs 2.46%, P<0.001), peritoneal fluid culture (3.07% vs 0.88%, P<0.001), and bile culture (2.44% vs 0.42%, P<0.001) were significantly higher in the SALD group than in the control group. The details are shown in Table 2.
THE EFFECT OF THE LOCATION OF CULTURE-POSITIVE SPECIMENS ON THE OCCURRENCE OF SALD:
The results of logistic regression analysis suggested that positive bile culture (OR 7.450, 95% CI 5.263–10.548, P<0.001), followed by positive peritoneal fluid culture (OR 3.616, 95% CI 2.736–4.779, P<0.001) and positive blood culture (OR 1.957, 95% CI 1.755–2.183, P<0.001), was most closely correlated to the occurrence of SALD. The details are shown in Table 3.
DISTRIBUTION OF PATHOGENS:
The pathogens of the 2 groups were mainly bacteria and fungi. In the control group, gram-positive bacteria, gram-negative bacteria, and fungi accounted for 43.06%, 29.23%, and 17.59% of the pathogens, respectively. In the SALD group, gram-positive bacteria, gram-negative bacteria, and fungi accounted for 38.06%, 29.57%, and 21.42% of the pathogens, respectively.
Gram-positive bacteria were significantly more abundant in the control group than in the SALD group (43.06% vs 38.06%, P<0.001), while fungi were significantly more abundant in the SALD group than in the control group (21.42% vs 17.59%, P<0.001). The details are shown in Table 4.
For gram-positive bacteria, there were more Enterococcus and Enterococcus faecium in the SALD group than in the control group (7.76% vs 6.44%, P<0.001 and 1.59% vs 0.53%, P<0.001, respectively). For gram-negative bacteria, Enterobacter cloacae was more abundant in the SALD group than in the control group (1.92% vs 1.17%, P<0.001). For fungi, yeast and Candida albicans were more abundant in the SALD group than in the control group (16.18% vs 14.81%, P=0.003 and 3.59% vs 1.90%, P<0.001, respectively). The details are shown in Table 5.
THE EFFECT OF PATHOGEN TYPE ON THE OCCURRENCE OF SALD:
Fungi were most closely correlated to the occurrence of SALD (OR 2.045, 95% CI 1.847–2.264, P<0.001). The details are shown in Table 6.
Subgroup analysis showed that Enterococcus faecium infection was most closely correlated to the occurrence of SALD among gram-positive bacteria (OR 3.065, 95% CI 2.344–4.008, P<0.001). Among gram-negative bacteria, infection with Bacteroides fragilis (OR 2.061, 95% CI 1.522–2.791, P<0.001), Klebsiella oxytoca (OR 2.066, 95% CI 1.511–2.824, P<0.001), and Enterobacter aerogenes (OR 1.92, 95% CI 1.297–2.841, P=0.001) was closely correlated to the occurrence of SALD. Among fungi, infection with Aspergillus fumigatus (OR 2.144, 95% CI 1.374–3.347, P=0.001) was most closely correlated to the occurrence of SALD. The details are shown in Table 7.
PROGNOSIS OF THE 2 GROUPS:
Patients in both groups had multiple ICU stays during one hospitalization period, so the total number of all ICU stays was greater than the number of hospital stays. The number of patients in the ICU in the 2 groups was 14 587 (control group) and 2158 (SALD group). The ICU stay (OR 6.2 [CI 2.8–14.7] vs OR 4.0 [CI 2.0–9.0], P<0.001) and hospital stay (OR 18.5 [CI 9.98–30.99] vs OR 12.8 [CI 7.34–21.68], P<0.001) of the SALD group were longer than those of the control group. Moreover, the ICU mortality and hospital mortality were significantly higher in the SALD group than in the control group (24.7% vs 9.0%, P<0.001; 34.2% vs 13.8%, P<0.001, respectively). The details are shown in Table 8.
Kaplan-Meier curves and log-rank tests showed that the 1-year cumulative probability of survival for the control group was significantly higher than for the SALD group (log-rank=264.005; P<0.001). In the SALD group, the 1-year cumulative probability of survival for the cholestasis subgroup was the highest. The details are shown in Figure 3.
Discussion
We conducted retrospective research using the MIMIC-III database to study the correlation of infection sites and pathogen types with the occurrence of SALD. The results showed that the incidence of SALD was 11.96%. Infection in the biliary tract, abdominal cavity, or blood and infection with
According to the clinical presentation, SALD can be divided into 2 major patterns: cholestatic dysfunction and hypoxic hepatitis [5]. Some studies define hypoxic hepatitis as an acute elevation of serum aminotransferase levels (20-fold the upper limit of normal) [11,13]. In our research, we defined SALD as elevation of ALT and/or AST levels >1000 IU/L and/or total serum bilirubin levels >3 mg/dL. The incidence of SALD remains imprecisely known, most likely because of the lack of unified diagnostic criteria. The incidence of sepsis-associated liver dysfunction or liver failure ranges from 1.3% to 46% in all patients with sepsis [6]. In this study, according to the above diagnostic criteria, the incidence of SALD was 11.96%.
The persistence or development of liver failure after sepsis is strongly associated with outcome [14], and the mortality rate of sepsis patients with liver dysfunction or failure ranges from 54% to 68% [6]. Raurich et al [15] retrospectively studied 181 patients with septic shock. They found that the mortality of patients with ischemic hepatitis (defined as having a value of serum aminotransferases ≥1000 IU/L) was 84.0%. In our study, the hospital mortality of SALD patients was 34.2%. The mortality was lower than that found by Raurich et al [15], which may be related to the different research subjects. In that study [15], the research subjects were patients with septic shock, and their conditions were more serious than those of the patients in our study.
In MIMIC-III, the median length of ICU stay is 2.1 days (Q1–Q3: 1.2–4.6), and the median length of hospital stay is 6.9 days (Q1–Q3: 4.1–11.9) [10]. In the current study, the median length of ICU stay was 6.2 days (Q1–Q3: 2.8–14.7) in the SALD group and 4.0 days (Q1–Q3: 2.0–9.0) in the control group, and the median length of hospital stay was 18.5 days (Q1–Q3: 9.98–30.99) and 12.8 days (Q1–Q3: 7.34–21.68), respectively. Compared with other critically ill patients in the MIMIC-III database, patients in the SALD group had the longest ICU and hospital stays, followed by the patients in the control group and MIMIC-III.
Infection is the root cause of sepsis. We suspected that the infection site and the type of pathogen were related to the occurrence of SALD. In our study, we found that the positive rate of peritoneal fluid, bile, and blood culture in the SALD group was significantly higher than in the control group. The results of the distribution of infection sites were similar to the results of Raurich et al [15] (mainly abdominal infection).
There are a few studies on the correlation between infection sites and the occurrence of SALD. Most previous studies have focused on the role of bacterial infections in the occurrence of acute-on-chronic liver failure (ACLF). The results show that spontaneous peritonitis is the most common cause of ACLF [16–18], which is different from the results of our study. In the current study, biliary infection was most closely corelated with the occurrence of SALD (OR 7.450), followed by abdominal infection (OR 3.616) and bloodstream infection (OR 1.957). The specific mechanism needs to be clarified by relevant basic research.
Similarly, few studies exist on the relationship between different pathogens and the occurrence of SALD. The main organisms triggering ACLF are gram-positive bacteria, followed by gram-negative bacteria [19]. In a study by Mücke et al [17], the ACLF-inducing bacteria were gram-positive bacteria (52.1%,
The above 5 pathogens were 4 bacteria and 1 fungus with distinct pathogenic characteristics. These 4 bacteria possess certain common characteristics. Most of them are opportunistic pathogenic bacteria and can cause abdominal and bloodstream infections. These common features may be part of the mechanism leading to SALD. In immunocompromised mice,
The current study is a retrospective study, and there may be data bias. The conclusion needs to be confirmed through more prospective multi-center randomized controlled trials. Second, this study is an observational study, and the specific mechanism needs further investigation.
Conclusions
An awareness of SALD should be maintained for sepsis patients whose infection site is the bile, abdominal cavity, or blood and the pathogen is
Figures
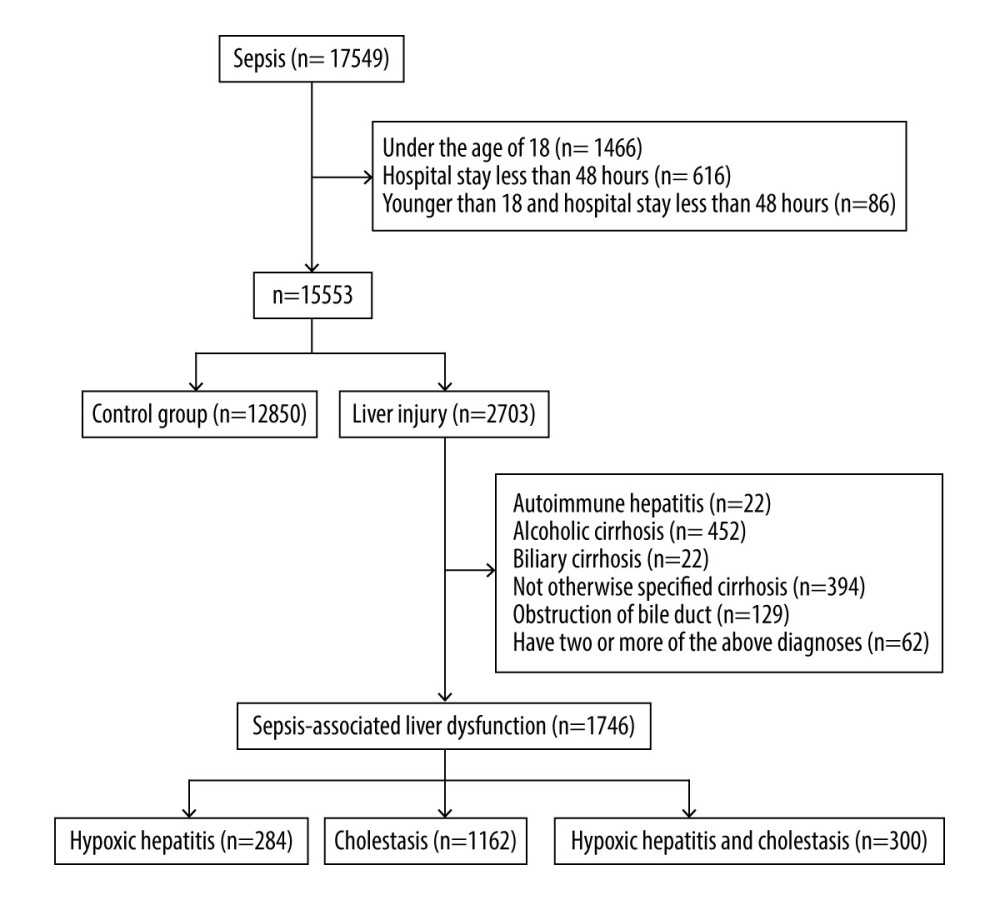 Figure 1. Flow chart for the study selection process. A total of 14 596 patients were included in this retrospective study, of which 12 850 were in the control group and 1746 were in the SALD group. The incidence of SALD was 11.96%. SALD, sepsis-associated liver dysfunction.
Figure 1. Flow chart for the study selection process. A total of 14 596 patients were included in this retrospective study, of which 12 850 were in the control group and 1746 were in the SALD group. The incidence of SALD was 11.96%. SALD, sepsis-associated liver dysfunction. 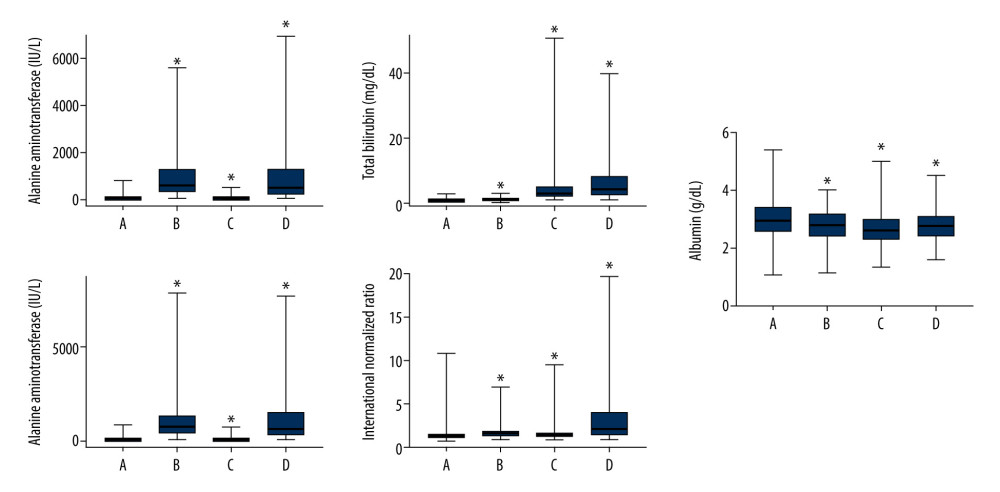 Figure 2. Liver blood laboratory data. The INR of the SALD group was higher than that of the control group (P<0.001). Albumin was lower than that of the control group (P<0.001). (A) control group; (B) hypoxic hepatitis; (C) cholestasis; (D) hypoxic hepatitis and cholestasis. * P<0.001. ALT – alanine aminotransferase; AST – aspartate aminotransferase; INR – international normalized ratio; SALD – sepsis-associated liver dysfunction.
Figure 2. Liver blood laboratory data. The INR of the SALD group was higher than that of the control group (P<0.001). Albumin was lower than that of the control group (P<0.001). (A) control group; (B) hypoxic hepatitis; (C) cholestasis; (D) hypoxic hepatitis and cholestasis. * P<0.001. ALT – alanine aminotransferase; AST – aspartate aminotransferase; INR – international normalized ratio; SALD – sepsis-associated liver dysfunction. 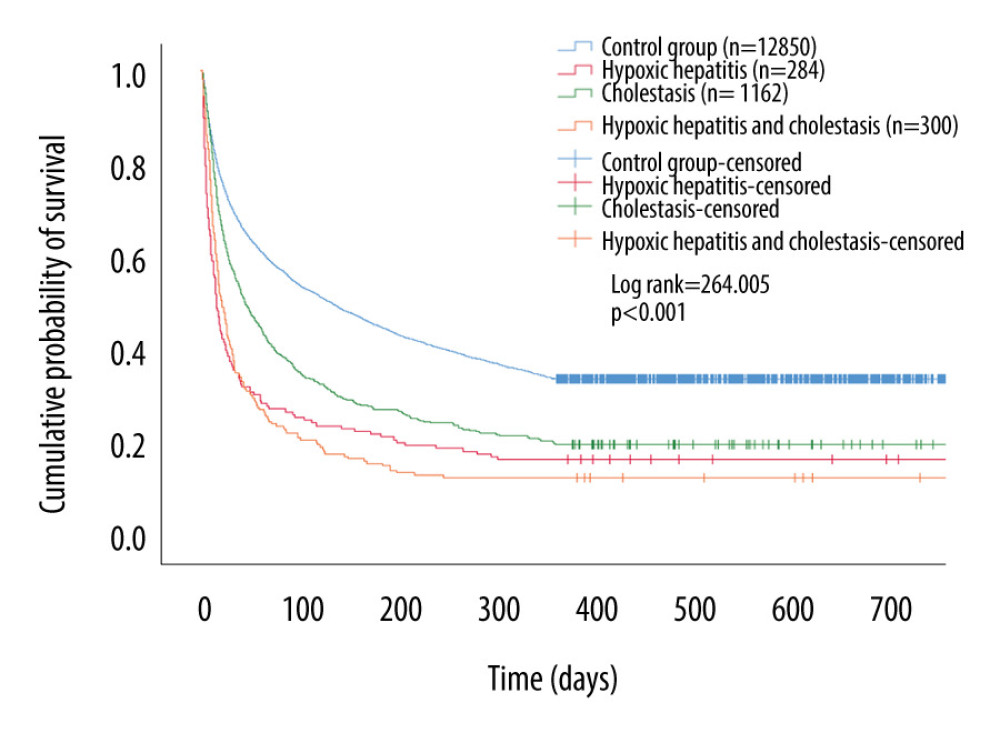 Figure 3. Kaplan-Meier survival curves of each groups. The 1-year cumulative probability of survival for the control group was significantly higher than that for the SALD group (log-rank=264.005; P<0.001). SALD – sepsis-associated liver dysfunction.
Figure 3. Kaplan-Meier survival curves of each groups. The 1-year cumulative probability of survival for the control group was significantly higher than that for the SALD group (log-rank=264.005; P<0.001). SALD – sepsis-associated liver dysfunction. Tables
Table 1. Patient characteristics on admission in the two groups.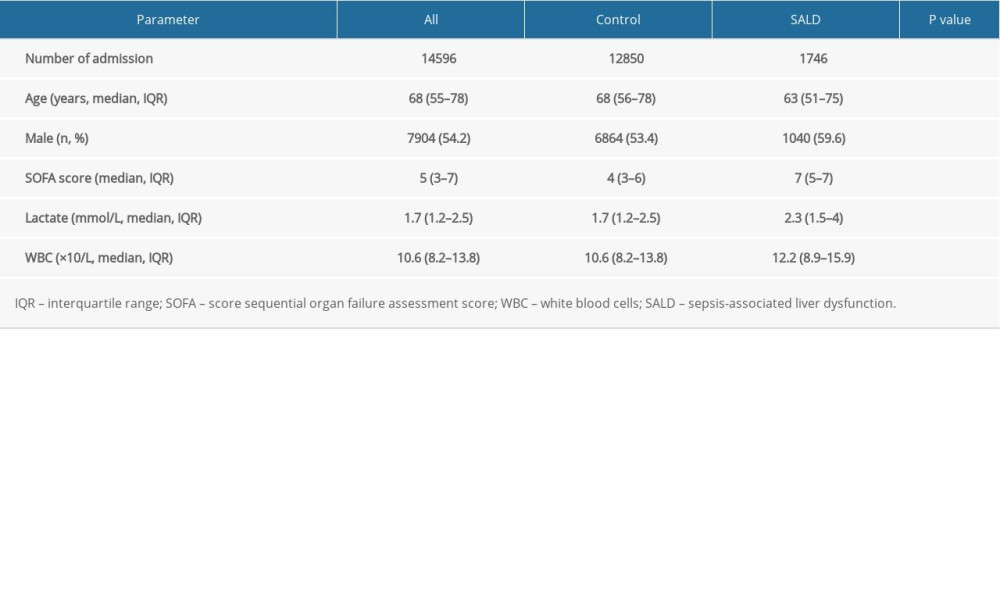 Table 2. Location of culture-positive specimens in the 2 groups.
Table 2. Location of culture-positive specimens in the 2 groups.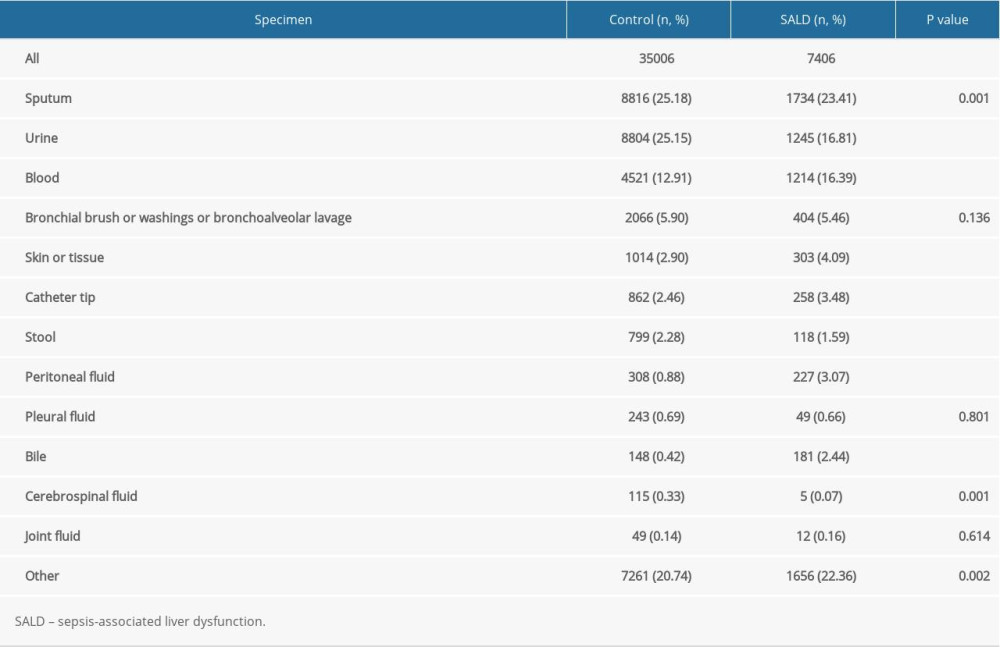 Table 3. The effect of location of culture-positive specimens on the occurrence of SALD.
Table 3. The effect of location of culture-positive specimens on the occurrence of SALD.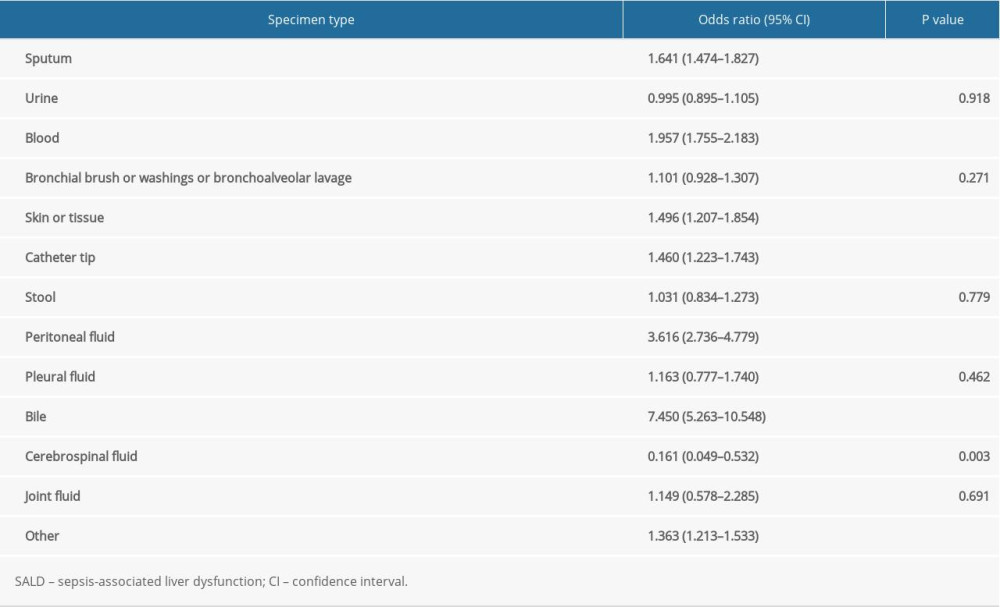 Table 4. Distribution of pathogens in the 2 groups.
Table 4. Distribution of pathogens in the 2 groups.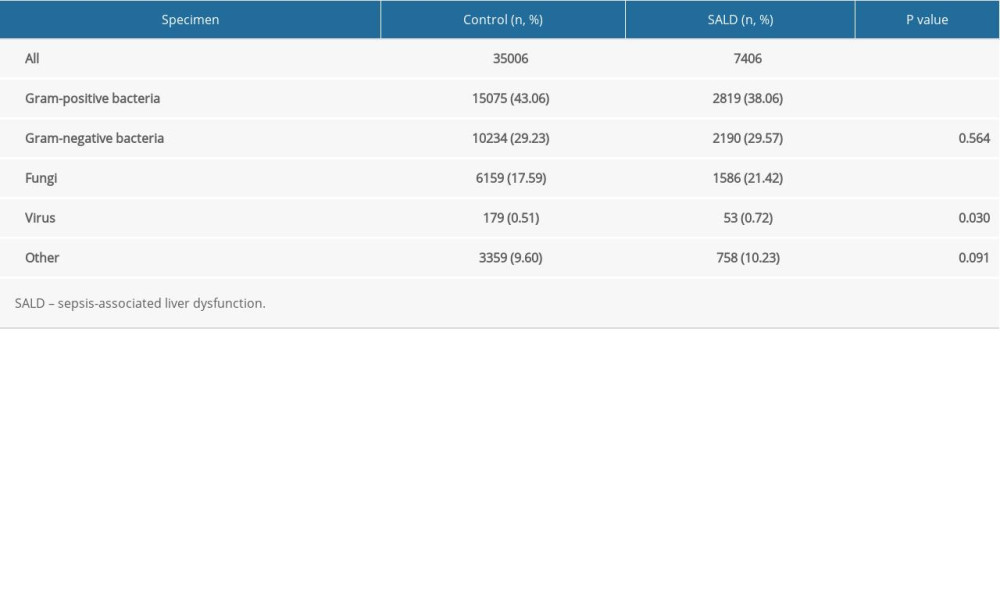 Table 5. Subgroup analysis of distribution of pathogens in the 2 groups.
Table 5. Subgroup analysis of distribution of pathogens in the 2 groups.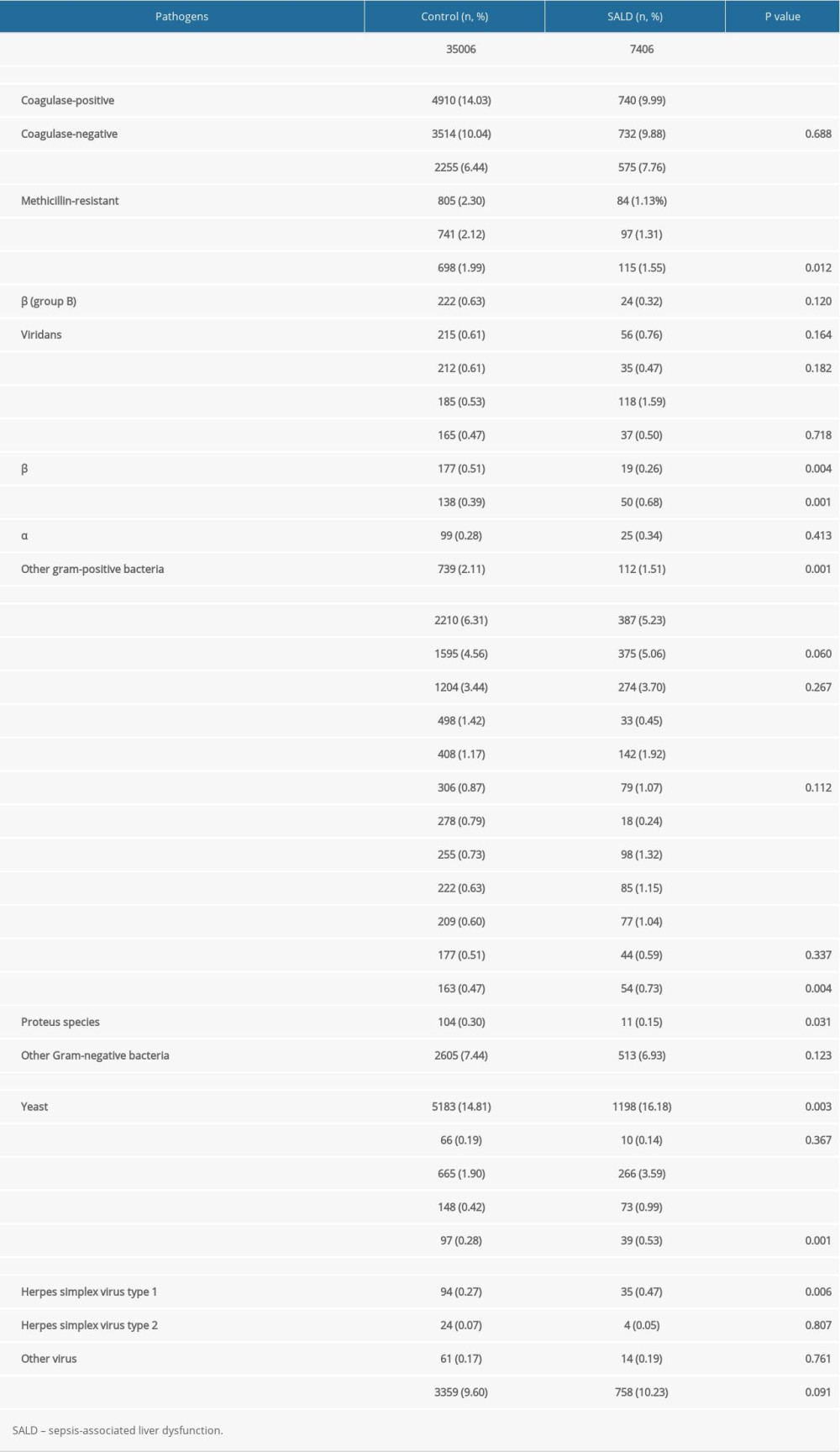 Table 6. The effect of pathogen type on the occurrence of SALD.
Table 6. The effect of pathogen type on the occurrence of SALD.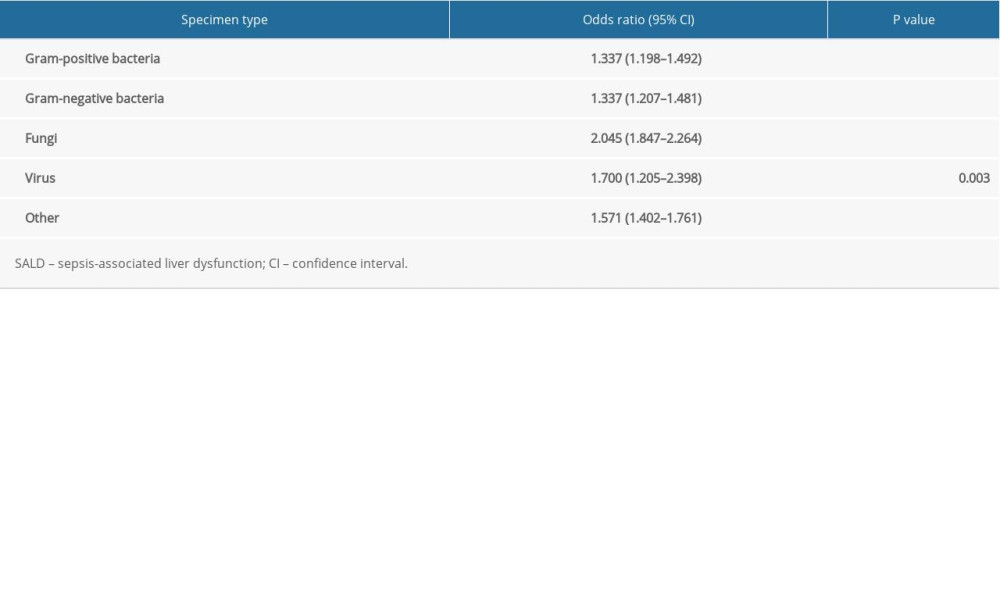 Table 7. Subgroup analysis of the effect of pathogen type on the occurrence of SALD.
Table 7. Subgroup analysis of the effect of pathogen type on the occurrence of SALD.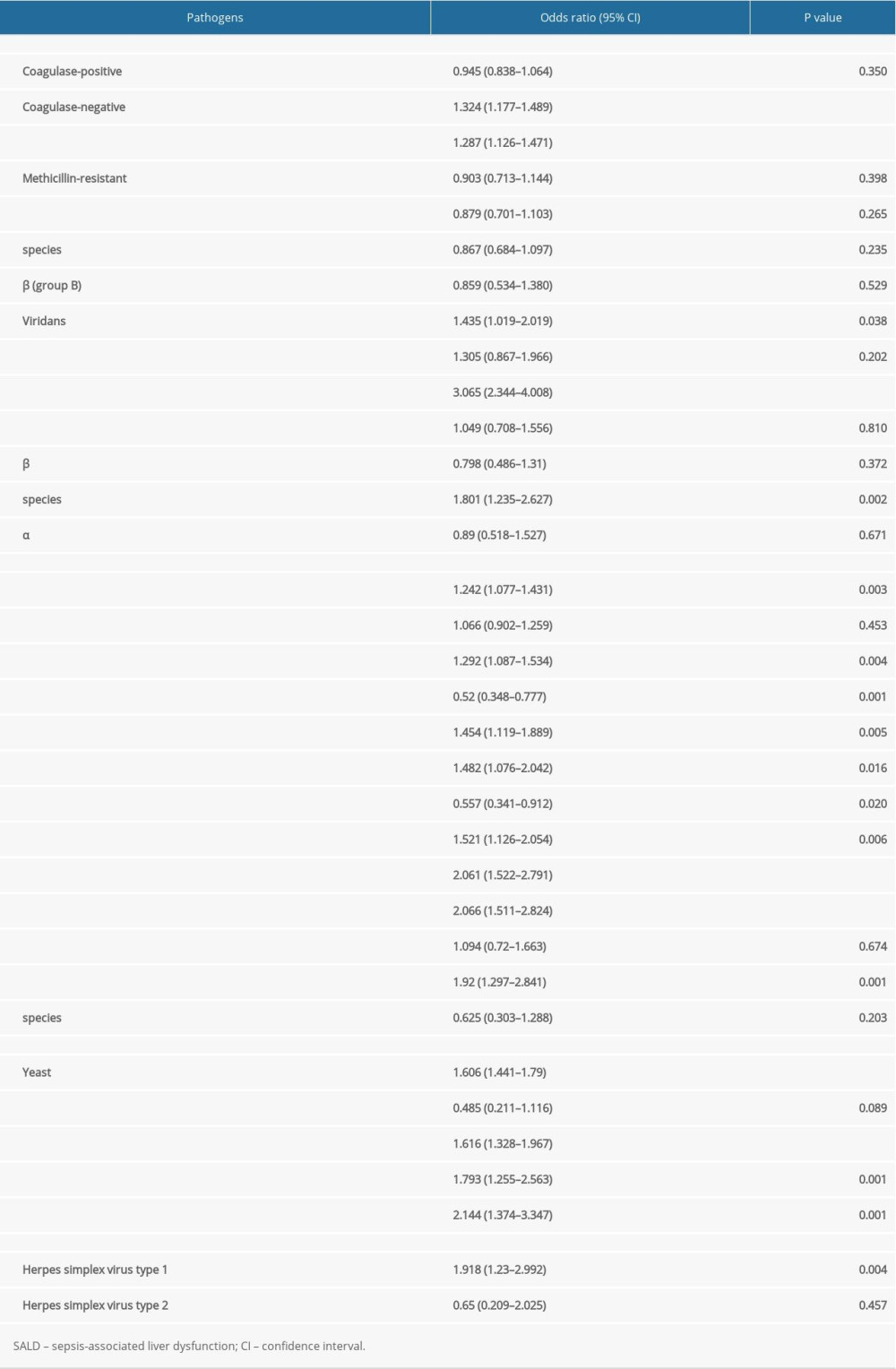 Table 8. Prognosis of the 2 groups.
Table 8. Prognosis of the 2 groups.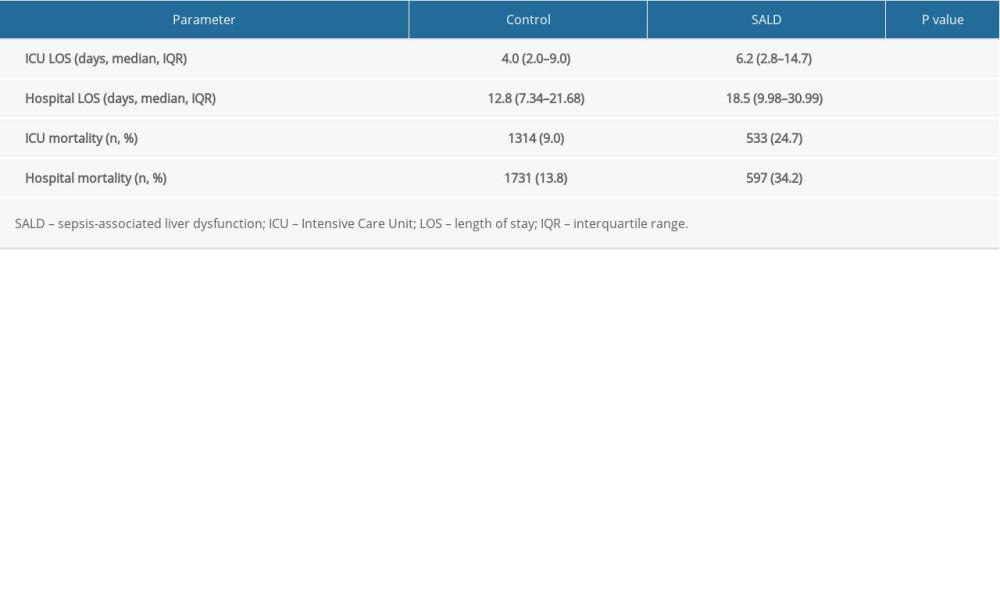
References
1. Singer M, Deutschman CS, Seymour CW, The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3): JAMA, 2016; 315(8); 775-87
2. Rhodes A, Evans LE, Alhazzani W, Surviving sepsis campaign: International Guidelines for Management of Sepsis and Septic Shock. 2016: Intensive Care Med, 2017; 43(3); 304-77
3. Strnad P, Tacke F, Koch A, Trautwein C, Liver – guardian, modifier and target of sepsis: Nat Rev Gastroenterol Hepatol, 2017; 14(1); 55-66
4. Woźnica EA, Inglot M, Woźnica RK, Łysenko L, Liver dysfunction in sepsis: Adv Clin Exp Med, 2018; 27(4); 547-51
5. Horvatits T, Trauner M, Fuhrmann V, Hypoxic liver injury and cholestasis in critically ill patients: Curr Opinion Crit Care, 2013; 19(2); 128-32
6. Yan J, Li S, Li S, The role of the liver in sepsis: Int Rev Immunol, 2014; 33(6); 498-510
7. Canabal JM, Kramer DJ, Management of sepsis in patients with liver failure: Curr Opinion Crit Care, 2008; 14(2); 189-97
8. Nesseler N, Launey Y, Aninat C, Clinical review: the liver in sepsis: Crit Care, 2012; 16(5); 235
9. Lelubre C, Vincent JL, Mechanisms and treatment of organ failure in sepsis: Nat Rev Nephrol, 2018; 14(7); 417-27
10. Johnson AEW, Pollard TJ, Shen L, MIMIC-III, a freely accessible critical care database: Sci Data, 2016; 3; 160035
11. Fuhrmann V, Kneidinger N, Herkner H, Hypoxic hepatitis: Underlying conditions and risk factors for mortality in critically ill patients: Intensive Care Med, 2009; 35(8); 1397-405
12. Jenniskens M, Langouche L, Vanwijngaerden YM, Cholestatic liver (dys)function during sepsis and other critical illnesses: Intensive Care Med, 2016; 42(1); 16-27
13. Fuhrmann V, Kneidinger N, Herkner H, Impact of hypoxic hepatitis on mortality in the Intensive Care Unit: Intensive Care Med, 2011; 37(8); 1302-10
14. Brun-Buisson C, Meshaka P, Pinton P, Vallet BEPISEPSIS Study Group, EPISEPSIS: A reappraisal of the epidemiology and outcome of severe sepsis in French Intensive Care Units: Intensive Care Med, 2004; 30(4); 580-88
15. Raurich JM, Pérez O, Llompart-Pou JA, Incidence and outcome of ischemic hepatitis complicating septic shock: Hepatol Res, 2009; 39(7); 700-5
16. Bajaj JS, O’Leary JG, Reddy KR, Survival in infection-related acute-on-chronic liver failure is defined by extrahepatic organ failures: Hepatology, 2014; 60(1); 250-56
17. Mücke MM, Rumyantseva T, Mücke VT, Bacterial infection-triggered acute-on-chronic liver failure is associated with increased mortality: Liver Int, 2018; 38(4); 645-53
18. Fernández J, Acevedo J, Wiest R, Bacterial and fungal infections in acute-on-chronic liver failure: Prevalence, characteristics and impact on prognosis: Gut, 2018; 67(10); 1870-80
19. Yang L, Wu T, Li J, Bacterial infections in acute-on-chronic liver failure: Semin Liver Dis, 2018; 38(2); 121-33
20. Jouvion G, Brock M, Droin-Bergère S, Ibrahim-Granet O: Virulence, 2012; 3(1); 43-50
Figures
 Figure 1. Flow chart for the study selection process. A total of 14 596 patients were included in this retrospective study, of which 12 850 were in the control group and 1746 were in the SALD group. The incidence of SALD was 11.96%. SALD, sepsis-associated liver dysfunction.
Figure 1. Flow chart for the study selection process. A total of 14 596 patients were included in this retrospective study, of which 12 850 were in the control group and 1746 were in the SALD group. The incidence of SALD was 11.96%. SALD, sepsis-associated liver dysfunction. Figure 2. Liver blood laboratory data. The INR of the SALD group was higher than that of the control group (P<0.001). Albumin was lower than that of the control group (P<0.001). (A) control group; (B) hypoxic hepatitis; (C) cholestasis; (D) hypoxic hepatitis and cholestasis. * P<0.001. ALT – alanine aminotransferase; AST – aspartate aminotransferase; INR – international normalized ratio; SALD – sepsis-associated liver dysfunction.
Figure 2. Liver blood laboratory data. The INR of the SALD group was higher than that of the control group (P<0.001). Albumin was lower than that of the control group (P<0.001). (A) control group; (B) hypoxic hepatitis; (C) cholestasis; (D) hypoxic hepatitis and cholestasis. * P<0.001. ALT – alanine aminotransferase; AST – aspartate aminotransferase; INR – international normalized ratio; SALD – sepsis-associated liver dysfunction. Figure 3. Kaplan-Meier survival curves of each groups. The 1-year cumulative probability of survival for the control group was significantly higher than that for the SALD group (log-rank=264.005; P<0.001). SALD – sepsis-associated liver dysfunction.
Figure 3. Kaplan-Meier survival curves of each groups. The 1-year cumulative probability of survival for the control group was significantly higher than that for the SALD group (log-rank=264.005; P<0.001). SALD – sepsis-associated liver dysfunction. Tables
 Table 1. Patient characteristics on admission in the two groups.
Table 1. Patient characteristics on admission in the two groups. Table 2. Location of culture-positive specimens in the 2 groups.
Table 2. Location of culture-positive specimens in the 2 groups. Table 3. The effect of location of culture-positive specimens on the occurrence of SALD.
Table 3. The effect of location of culture-positive specimens on the occurrence of SALD. Table 4. Distribution of pathogens in the 2 groups.
Table 4. Distribution of pathogens in the 2 groups. Table 5. Subgroup analysis of distribution of pathogens in the 2 groups.
Table 5. Subgroup analysis of distribution of pathogens in the 2 groups. Table 6. The effect of pathogen type on the occurrence of SALD.
Table 6. The effect of pathogen type on the occurrence of SALD. Table 7. Subgroup analysis of the effect of pathogen type on the occurrence of SALD.
Table 7. Subgroup analysis of the effect of pathogen type on the occurrence of SALD. Table 8. Prognosis of the 2 groups.
Table 8. Prognosis of the 2 groups. Table 1. Patient characteristics on admission in the two groups.
Table 1. Patient characteristics on admission in the two groups. Table 2. Location of culture-positive specimens in the 2 groups.
Table 2. Location of culture-positive specimens in the 2 groups. Table 3. The effect of location of culture-positive specimens on the occurrence of SALD.
Table 3. The effect of location of culture-positive specimens on the occurrence of SALD. Table 4. Distribution of pathogens in the 2 groups.
Table 4. Distribution of pathogens in the 2 groups. Table 5. Subgroup analysis of distribution of pathogens in the 2 groups.
Table 5. Subgroup analysis of distribution of pathogens in the 2 groups. Table 6. The effect of pathogen type on the occurrence of SALD.
Table 6. The effect of pathogen type on the occurrence of SALD. Table 7. Subgroup analysis of the effect of pathogen type on the occurrence of SALD.
Table 7. Subgroup analysis of the effect of pathogen type on the occurrence of SALD. Table 8. Prognosis of the 2 groups.
Table 8. Prognosis of the 2 groups. In Press
05 Mar 2024 : Clinical Research
Muscular Function Recovery from General Anesthesia in 132 Patients Undergoing Surgery with Acceleromyograph...Med Sci Monit In Press; DOI: 10.12659/MSM.942780
05 Mar 2024 : Clinical Research
Effects of Thermal Insulation on Recovery and Comfort of Patients Undergoing Holmium Laser LithotripsyMed Sci Monit In Press; DOI: 10.12659/MSM.942836
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








