12 November 2020: Clinical Research
Red Blood Cell Distribution Width-to-Platelet Ratio and Other Laboratory Indices Associated with Severity of Histological Hepatic Fibrosis in Patients with Autoimmune Hepatitis: A Retrospective Study at a Single Center
Xu Li1BCE, Hongqin Xu12CD, Pujun Gao1AG*DOI: 10.12659/MSM.927946
Med Sci Monit 2020; 26:e927946
Abstract
BACKGROUND: This retrospective study at a single center aimed to evaluate the role of the red blood cell distribution width (RDW)-to-platelet ratio and other laboratory indices associated with the severity of histological hepatic fibrosis on liver biopsy in patients with autoimmune hepatitis (AIH).
MATERIAL AND METHODS: We retrospectively reviewed records from 2097 adult patients who had liver biopsies. Of these patients, data from 72 with AIH and 164 with drug-induced liver injury (DILI) with complete laboratory information and medical histories were included in the analysis.
RESULTS: We found that compared with patients with DILI, patients with AIH had higher alkaline phosphatase, globulin, and total bile acid levels. Multivariate analyses of risk factors for AIH-associated advanced liver fibrosis in Chinese patients revealed an estimated adjusted odds ratio (AOR) (95% CI) of 1.609 (1.028–2.517) in patients with higher immunoglobulin A (IgA) levels. Patients with higher gamma-glutamyl transpeptidase (GGT)-to-platelet ratio (GPR) values had a significantly higher risk of serious liver fibrosis than patients with lower GPR values. Advanced fibrosis risk was higher in patients with higher RPR values than in patients with lower RPR values [AOR (95% CI): 25.507 (2.934–221.784)]. The result for area under the curve (0.821) analysis for lnRPR levels indicated this variable had high diagnostic performance for predicting advanced AIH-related fibrosis.
CONCLUSIONS: The degree of histological liver fibrosis in patients with AIH was significantly associated with an increased red blood cell distribution width-to-platelet ratio, GPR, and increased serum levels of IgA.
Keywords: drug-induced liver injury, Erythrocyte Indices, Hepatitis, Autoimmune, Liver Cirrhosis, Multivariate Analysis, ROC Curve, Severity of Illness Index
Background
Children and adults worldwide are affected by autoimmune hepatitis (AIH). For the most part, the etiology of this severe liver disease is unknown. The diagnosis of AIH is made based on blood test and pathology results, including increased serum transaminase and immunoglobulin G (IgG) levels, autoantibodies, and interface hepatitis [1,2]. However, liver autoantibodies can also be detected in patients with infectious hepatitis. This finding suggests these indicators are nonspecific and must be used carefully for the diagnosis of AIH. There are also reports of drug-induced hepatotoxicity accompanied by an autoimmune response [1,3]. Therefore, because the clinical signs of both conditions overlap [4], diagnosis of idiopathic AIH versus drug-induced liver injury (DILI) can be challenging [1,4–7]. The American Association for the Study of Liver Disease [8] gives a conditional recommendation (low certainty) for the use of budesonide and azathioprine or prednisone/prednisolone and azathioprine as initial treatments for patients who present with AIH without cirrhosis, if it is not acute severe AIH. Therefore, liver cirrhosis or fibrosis stage are key factors that contribute to successful treatment of AIH patients.
Liver fibrosis severity is typically assessed using liver biopsy [9]. However, this criterion-standard method is costly and invasive. It cannot be used in some groups of patients because of associated contraindications and complications [10]. Many of the noninvasive parameters used to indicate liver abnormalities [11–14] are also expensive and are unsuitable for use in daily clinical practice. Most were developed to assess patients infected with hepatitis C and hepatitis B viruses [13,15–18].
Other noninvasive methods to detect fibrosis have been examined, including aspartate aminotransferase (AST)-platelet (PLT) index (APRI), the Fibrosis-4 Index (FIB-4) [19–23], gamma-glutamyl transpeptidase (GGT)-to-PLT ratio (GPR), AST-to-alanine aminotransferase (ALT) ratio (AAR), and the red blood cell volume distribution width (RDW) [24]. The red blood cell volume distribution width (RDW)-to-platelet ratio (RPR) can successfully detect significant fibrosis and cirrhosis in patients with chronic hepatitis B (CHB) [18]. Recently, some studies have been published on associations between RPR values and degree of severity of AIH-related fibrosis, showing that RPR value is a simple predictor of liver fibrosis in AIH patients [24,25].
The study objectives were to investigate clinical characteristics of AIH and DILI in the Chinese population. This retrospective study at a single center aimed to evaluate the role of RPR and other laboratory indices associated with the severity of histological hepatic fibrosis in liver biopsy in patients with AIH.
Material and Methods
PATIENTS:
For this retrospective study, we reviewed data from 2097 patients who underwent standard laboratory tests and liver biopsies (January 1, 2010 through December 31, 2019; the First Hospital of Jilin University, China) for inclusion. Test and biopsy results indicated that 74 patients were diagnosed with AIH and 187 patients were diagnosed with DILI. The data from 25 patients with incomplete medical information were excluded from the analysis. In total, data from 236 patients with complete laboratory information and medical histories were used in our study. Of these patients, the 72 with AIH were the case group and the 164 with DILI were the control group. All AIH patients were definitely diagnosed according to relevant guidelines of the International Autoimmune Hepatitis Group (IAIHG) [26]. The diagnosis of DILI was defined according to the 2015 Chinese Guideline for Diagnosis and Treatment of DILI [27].
The study protocol and the use of data from human subjects were approved by the First Hospital of Jilin University Independent Institutional Review Board.
LIVER BIOPSY:
The Menghini technique [28] was used to perform each ultrasound-guided percutaneous liver biopsy, which was performed using a 18-gauge disposable needle. All liver samples were preserved in phosphate-buffered formalin and then paraffin-embedded and sectioned and stained with hematoxylin-eosin for histology. All liver specimens were scored by pathologists blinded to patient clinical characteristics. They used the Metavir system to score the degree of liver fibrosis [24,29] because some studies found Metavir system is superior to complex scoring systems (such as Ishak histological scoring systems) for an individual patient’s disease severity [28]: F0 means no fibrosis, F1 means portal fibrosis without septa, F2 means portal fibrosis with few septa, F3 means numerous septa without cirrhosis, and F4 means cirrhosis. Each patient with AIH was assigned to 1 of 2 groups based on stage of fibrosis (i.e., “no or minimal fibrosis” group had grade F0, F1, or F2 fibrosis; “advanced fibrosis” group had grade F3 or F4 fibrosis). We excluded tissue sections with fewer than 3 portal tracts (i.e., poor quality).
RDW-TO-LYMPHOCYTE RATIO, RDW-TO-PLT COUNT RATIO, AND AST-TO-ALT RATIO:
RLR was calculated using RLR=RDW (%)/lymphocyte (109/L), RPR was calculated using RPR=RDW (%)/PLT (109/L), and AAR was calculated using AAR=AST (IU/L)/ALT (IU/L).
FIB-4 SCORE AND AST-TO-PLATELET RATIO INDEX (APRI):
The FIB-4 score was calculated using [30] FIB-4=(age(years)×AST (U/L))/(platelet count(PLT)(109/L)×ALT(U/L)1/2) and APRI was calculated using [19] APRI=(AST/upper limit of normal)/PLT(109/L)×100. Upper limit of AST=40 (range, 7–40 U/L).
GAMMA-GLUTAMYL TRANSPEPTIDASE (GGT)-TO-PLT COUNT RATIO (GPR) AND NEUTROPHIL-TO-LYMPHOCYTE RATIO (NLR):
GPR was calculated using GPR=gamma-glutamyl transpeptidase level (GGT) (IU/L)/platelet count (PLT) (109/L) and NLR was calculated using NLR=neutrophil (109/L)/lymphocyte (109/L).
STUDY VARIABLES:
Demographic characteristics (e.g., age, sex, drinking, smoking) and variables associated with clinical presentation (e.g., histories of medication use, autoimmune disease, and diabetes mellitus) were included in the analysis.
Fasting blood samples were obtained when the liver biopsies were performed. We obtained data on patient white blood cell (WBC), neutrophil, and lymphocyte counts, and on hemoglobin (HGB), RDW, PLT, serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), GGT, alkaline phosphatase (ALP), total bilirubin (TBIL), total bile acids (TBAs), albumin (ALB), globulin (GLO), and prothrombin time (PT) levels from patient medical records.
STATISTICAL ANALYSIS:
The results for continuous variables were calculated as median, 25th, and 75th percentile values. These variables were examined using 2-tailed independent-sample
Receiver operating characteristic (ROC) curves and area under the ROC (AUROC) curve values were used to evaluate and compare the accuracy of AAR, lnRPR, RLR, APRI, FIB-4, GPR, NLR, and RDW for the diagnosis of AIH fibrosis severity. ROC curve analysis and Z tests were used to compute and compare AUROCs, respectively (MedCalc Statistical Software v. 16.1, MedCalc Software bvba, Ostend, Belgium). Maximizing the sum of sensitivity and specificity or optimizing a specificity of at least 95% were used to obtain cut-off values.
Results
DEMOGRAPHIC AND PATIENT CHARACTERISTICS:
Demographic information for patients included in the study are summarized in Table 1A. The AIH patient group consisted of 11.1% males, and the median age was 54.00 (48.25, 62.75) years. The DILI group consisted of 24.4% males and the median age was 50.00 (42.00, 56.00) years. The prevalence of history of autoimmune disease was significantly higher in the patients with AIH than in the patients with DILI (20.8% versus 0.0%; P<0.001). We found that 37.5% of the patients with AIH had medication histories. However, the between-group differences in the values for prevalence of smoking, drinking, and diabetes mellitus were not significant.
AIH group patients had lower levels of ALB, HGB, and PLT compared with the DILI group patients. ALP, GLO, TBA, and PT levels were higher in the AIH group compared with the DILI group. The between-group differences in AST, ALT, TBIL, GGT, WBC, neutrophil, lymphocyte, and RDW levels were not significant.
Clinical characteristics are presented in Table 1B. The initial symptom of pruritis occurred in 6 (8.3%) patients in the AIH group and in 1 (0.61%) patient in the DILI group; this difference was statistically significant (P=0.001). Other initial symptoms and clinical signs were similar between the 2 groups (i.e., fatigue, abdominal distension, jaundice, nausea, fever, and no symptoms or clinical signs). The most prevalent initial clinical manifestations were fatigue (43.1%) in the AIH group and jaundice (42.1%) in the DILI group.
UNIVARIATE AND MULTIVARIATE ANALYSES:
We evaluated risk factors for severity of AIH-related fibrosis in the 72 patients with this condition (Table 2). Univariate analyses revealed significantly different levels of immunoglobulin A (IgA) levels, RDW levels, AAR levels, FIB-4 levels, RLR levels, and RPR levels between patients with advanced fibrosis (F ≥3) and those with no or minimal fibrosis (F=0–2). Sex, age, smoking, drinking, history of medication, history of autoimmune disease, presence of diabetes mellitus, GLO, IgG, IgA, RDW, AAR, APRI, FIB-4, GPR, NLR, RLR, and RPR were included in the multivariate analysis. The AOR for patients with higher IgA levels was 1.609 (95% CI: 1.028–2.517; P=0.037), compared with patients with lower IgA values. Compared with patients with lower GPR levels, patients with higher GPR levels had a higher risk for development of advanced fibrosis (1.638 [95% CI: 1.014–2.647], P=0.044). Patients with higher RPR values (ln) had a higher risk for development of severe fibrosis (25.507 [95% CI: 2.934–221.784], P=0.003). We found no statistically significant associations between levels of IgG, RDW, AAR, APRI, FIB-4, NLR, or RLR and advanced fibrosis in the AIH patients.
DIAGNOSTIC PERFORMANCE AND THRESHOLDS OF SERUM MODELS FOR ADVANCED FIBROSIS IN PATIENTS WITH AUTOIMMUNE HEPATITIS:
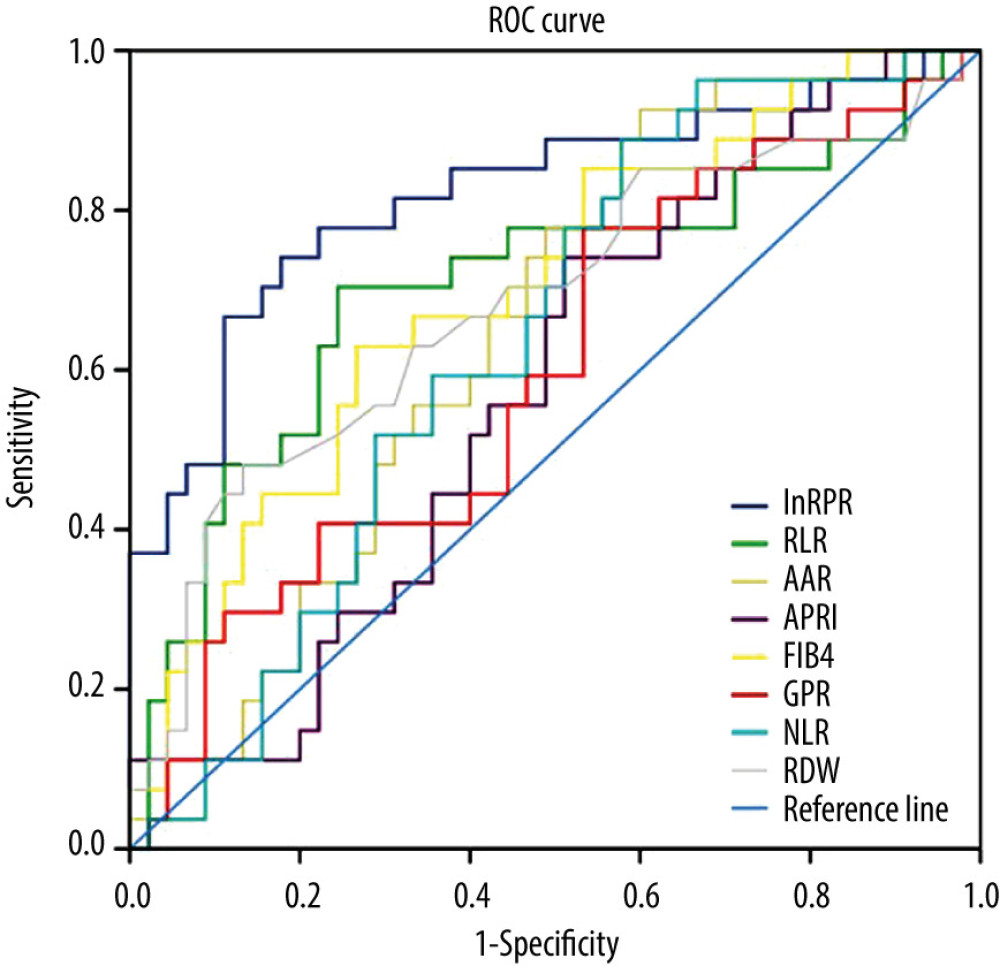
Maximizing the sum of sensitivity and specificity, the optimal cut-off for lnRPR was −2.313, with a sensitivity of 77.8% and a specificity of 77.8% for diagnosis of advanced fibrosis. The AUROC for lnRPR in advanced liver fibrosis was 0.821 (Table 3, Figure 1). The percent correctly classified was 77.8%. The AUROC value for RLR in predicting significant liver fibrosis was 0.705 (95% CI: 0.571–0.839), and the optimal cut-off value was 10.747, with a sensitivity of 70.4% and a specificity of 75.6%. The optimal cut-off for FIB-4 was 5.104 for diagnosis of severe fibrosis; the sensitivity was 63.0% and the specificity was 73.3%. The AUROC (95% CI) values for AAR, APRI, GPR, NLR, and RDW were 0.646 (0.520, 0.772), 0.579 (0.446, 0.711), 0.599 (0.463, 0.735), 0.637 (0.510, 0.764), and 0.682 (0.549, 0.816), respectively.
Discussion
There are relatively few studies on the relationship between RPR values and the degree of liver cirrhosis in AIH patients. Liu et al. [24] indicated that RPR had the highest accuracy compared to other noninvasive tests for predicting advanced liver fibrosis. Wang et al. [31] also found that RPR could predict significant fibrosis and liver cirrhosis with relatively high accuracy. In the present study, we further determined that RPR was significantly correlated with the results of histology staging in patients with AIH. At a cut-off of −2.313, lnRPR predicted severe fibrosis (sensitivity 77.8%, specificity 77.8%). The percent correctly classified was as high as 77.8%, with an AUROC of 0.821. This finding provides a new clinical application for RPR. The 1 or more mechanisms via which RPR interacts with AIH remain to be determined.
Variability in red blood cell (RBC) size can be measured using RDW. RDW measurement is typically included in complete blood cell counts [32–34]. RDW indicates the variability in circulating RBC size and is used to differentiate types of anemia [35]. The results presented here suggest that RDW has potential to predict the prognosis of diseases, including renal diseases, cardiovascular diseases, pulmonary hypertension, lung cancer, and sepsis [36–42]. Significant associations have been found between RDW and chronic liver disease severity. In patients with chronic hepatic B, HGB, RDW, and PLT are independent predictors of liver fibrosis stage [18]. In patients with nonalcoholic fatty liver disease and alcoholic cirrhosis, higher RDW values are associated with disease severity [43–45]. Elevated RDW levels occur in patients with autoimmune liver disease (e.g., AIH and primary biliary cholangitis) [25,46,47].
The mechanism linking RDW and progression of fibrosis is poorly understood. Abnormalities (e.g., inflammation, erythrocyte fragmentation, oxidative stress, poor nutritional condition, and abnormality of erythropoietin function) can cause significant variations in RDW [20,32,48–51]. Because these disorders and anemia are correlated with liver disease severity, elevated RDW values might also be associated with liver disease severity. Inflammation results in impairment of erythrocyte maturation and entry of immature erythrocytes into the systemic circulation, which results in elevated RDW values [25,52]. Study results suggest that inflammatory cytokines (e.g., tumor necrosis factor-α, interleukin (IL)-1β, and IL-6) inhibit iron metabolism and erythropoietin production. This process results in disorders of RBC synthesis and abnormal erythropoietin production [52–54]. Impairment of the balance between oxidant and antioxidant defenses are characteristic of the oxidative stress that occurs in liver disease. Erythrocyte homeostasis and survival are strongly affected by oxidative stress, and low serum antioxidant concentrations are correlated with increased RDW levels [55,56]. Thus, our results suggest oxidative stress is a mechanism that results in increased RDW levels in patients with liver disease. A poor liver disease-associated nutritional status (e.g., iron deficiency, folate deficiency, vitamin B12 deficiency) can also result in abnormal RBC production and increased RDW levels [57]. Patients with chronic liver disease can experience the common complication of portal hypertension. This condition can cause splenomegaly with associated increases in the rate of RBC destruction, release of immature RBCs to the systemic circulation, and consequent increases in RDW values [55,56].
Thrombocytopenia is a common hematological complication in patients with liver fibrosis [58]. In patients with chronic liver disease, the mechanisms associated with decreased PLT numbers include hypersplenism due to portal hypertension [59]. Reduced PLT production or sequestration or increased PLT destruction results in thrombocytopenia [58]. Reduced PLT production can result from reduced thrombopoietin production in the liver, bone marrow suppression from some medications, alcohol consumption, viruses, and iron deficiency [60]. Portal hypertension associated with liver fibrosis can result in PLT sequestration in the spleen [61]. Stress, production of antiplatelet antibodies, bacterial translocation, hyperfibrinolysis, and sepsis are other proposed mechanisms of increased platelet destruction [58].
Development of liver fibrosis is a complex process that results from excess accumulation of extracellular matrix components (e.g., collagens) [62]. PLTs have key roles in both hepatic regeneration and fibrosis pathophysiology [63, 64]. PLTs are essential for liver regeneration (e.g., platelet-derived serotonin). PLTs can also worsen liver damage (e.g., immune-mediated injury). Animal models of liver fibrosis are used to examine the positive and negative effects of PLTs. Profibrogenic mediators (e.g., CXC chemokine ligand 4) essential for the progression of liver fibrosis are released by platelets. On the other hand, platelet-derived hepatocyte growth factor-associated downregulation of hepatic stellate cell collagen production results in a thrombocytopenia-associated increase in liver fibrosis severity [63].
We also found a significant association between IgA levels, but not GLO levels, and severity of AIH fibrosis. Patients with cirrhosis of various etiologies often have hypergammaglobulinemia [65–68]. The portal venous system delivers antigens that are cleared by Kupffer cells. Reduced Kupffer cell activity results in increased antigen exposure to antibody-producing sites via the systemic circulation [66,69]. Therefore, the elevated serum immunoglobulin levels are likely the result rather than the cause of cirrhosis [65,66,70]. We found no association between GLO and fibrosis severity in AIH patients. The reason might be because relatively few cirrhosis patients (S4) were included in our study and there was little difference in GLO levels between fibrosis patients.
The risks of some non-inflammatory fibrotic diseases (e.g., idiopathic pulmonary fibrosis, cystic fibrosis, retroperitoneal fibrosis, endomyocardial fibrosis, and non-cirrhotic portal fibrosis) are associated with elevated serum immunoglobulin levels [69,71–74]. Our previous study results indicate that hepatic fibrogenesis is directly affected by immunoglobulins [75]. Ethanol increases serum IgA levels in humans and is a strong promoter of hepatic fibrogenesis, so the correlation between elevated IgA levels and fibrosis was predictable [76]. Studies of the role of IgA in immune-based chronic liver diseases (e.g., AIH, primary biliary cirrhosis, and primary sclerosing cholangitis) have been performed [77–79]. Yokoyama et al. [80] administered ethanol to adult guinea pigs and found that it has limited fibrogenic properties, but extensive hepatic fibrosis occurred when the ethanol was administered with IgA immunoglobulins. This result may indicate liver injury mediated by IgA or that IgA has a direct effect on hepatic fibrosis, or both. Our results and the results of these other studies suggest that IgA is a valid biomarker for advanced fibrosis in patients with AIH.
ALP and TBA levels were significantly higher in patients with AIH than in patients with DILI, based on assessing patient demographics and information about symptoms. Patients with AIH were significantly more likely to experience pruritus than were patients with DILI. We found no associations between advanced AIH-related fibrosis and APRI or FIB-4, although they can predict significant liver fibrosis in patients with chronic hepatitis C and hepatitis B [81,82]. However, GPR levels were significantly higher in patients with advanced fibrosis than in patients with no or minimal fibrosis. Taken together, these findings suggest that cholestasis has an important role during the development in AIH. However, studies with larger sample sizes are required to further test this hypothesis.
There were some limitations to our study. First, the retrospective design could have caused selection bias that resulted in underestimated sensitivity and overestimated specificity values [83]. Second, detailed information about the types of medication and medication duration in AIH patients was not available. More research is needed to understand the associations between drugs used and AIH development. Third, because the study did not include a large sample, subgroup analyses by type of AIH could not be performed. The number of cases was limited by the requirements to include patients with a diagnosis of AIH with liver biopsy and exclude patients without complete medical information.
Conclusions
In conclusion, this retrospective study evaluated laboratory indices associated with the severity of histological hepatic fibrosis on liver biopsy in patients with autoimmune hepatitis. The degree of histological liver fibrosis in patients with AIH was significantly associated with an increased red blood cell distribution width-to-platelet ratio, gamma-glutamyl transpeptidase-to-platelet ratio, and increased serum levels of IgA.
Tables
Table 1A. Demographic characteristics of autoimmune hepatitis group and drug-induced liver injury group.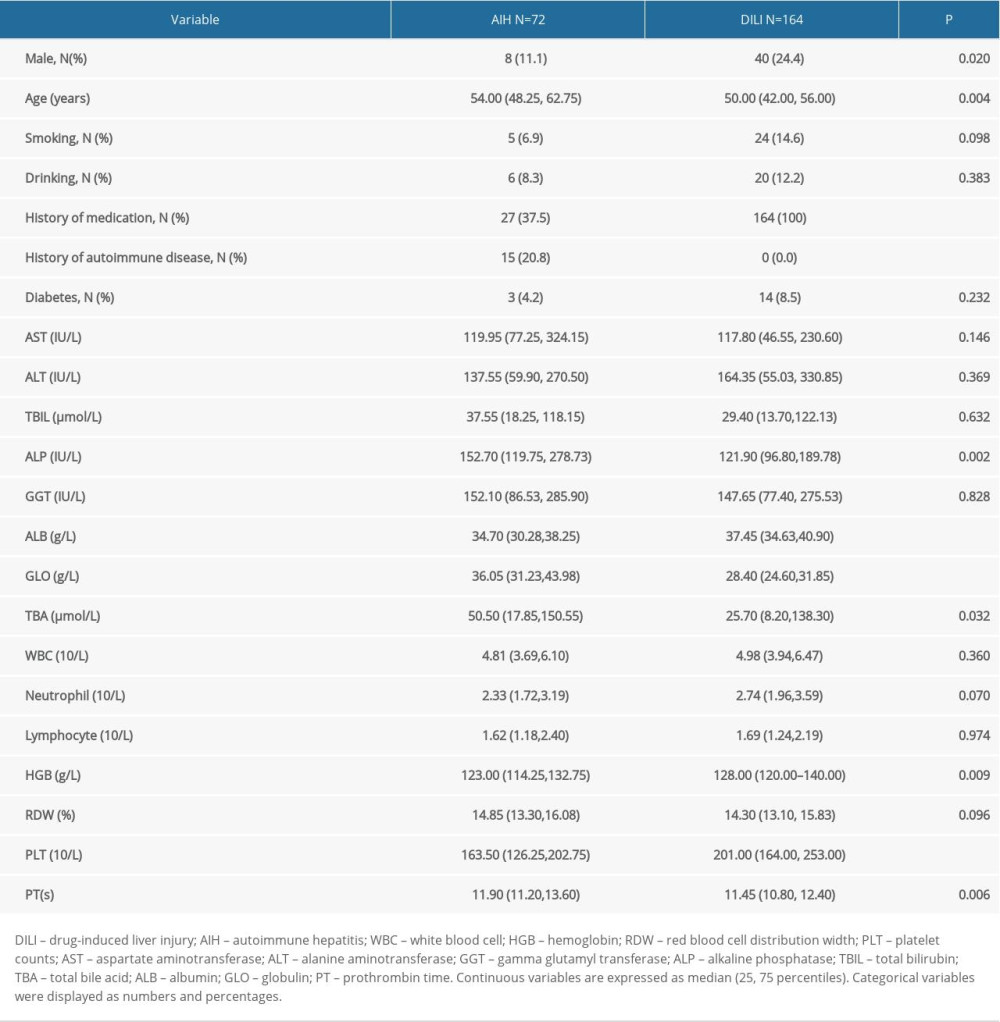 Table 1B. Initial symptoms of 72 patients with autoimmune hepatitis (AIH) and 164 patients with drug-induced liver injury (DILI).
Table 1B. Initial symptoms of 72 patients with autoimmune hepatitis (AIH) and 164 patients with drug-induced liver injury (DILI).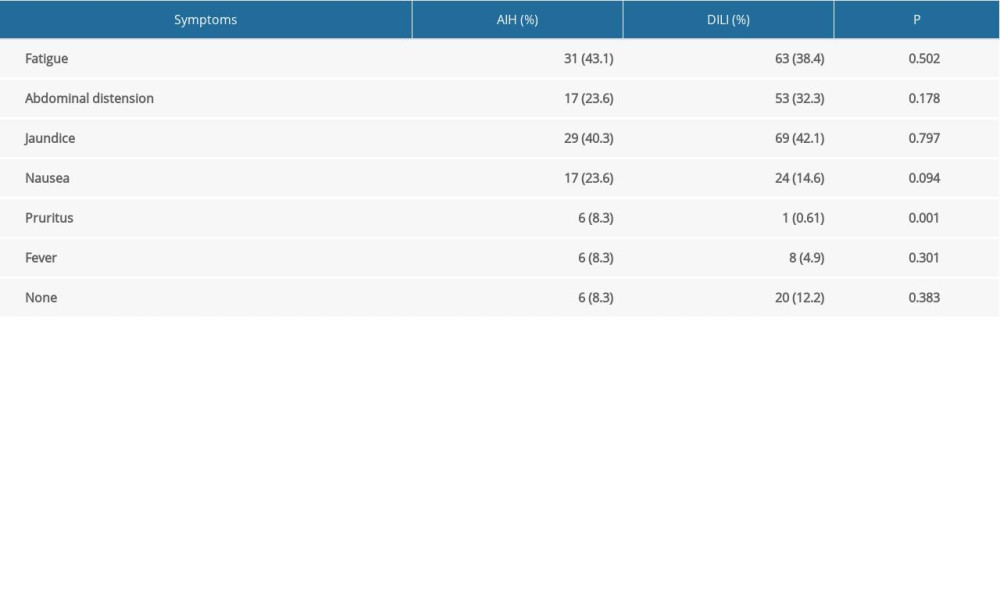 Table 2. Univariate and multivariate analyses of variables associated with autoimmune hepatitis-related liver fibrosis.
Table 2. Univariate and multivariate analyses of variables associated with autoimmune hepatitis-related liver fibrosis.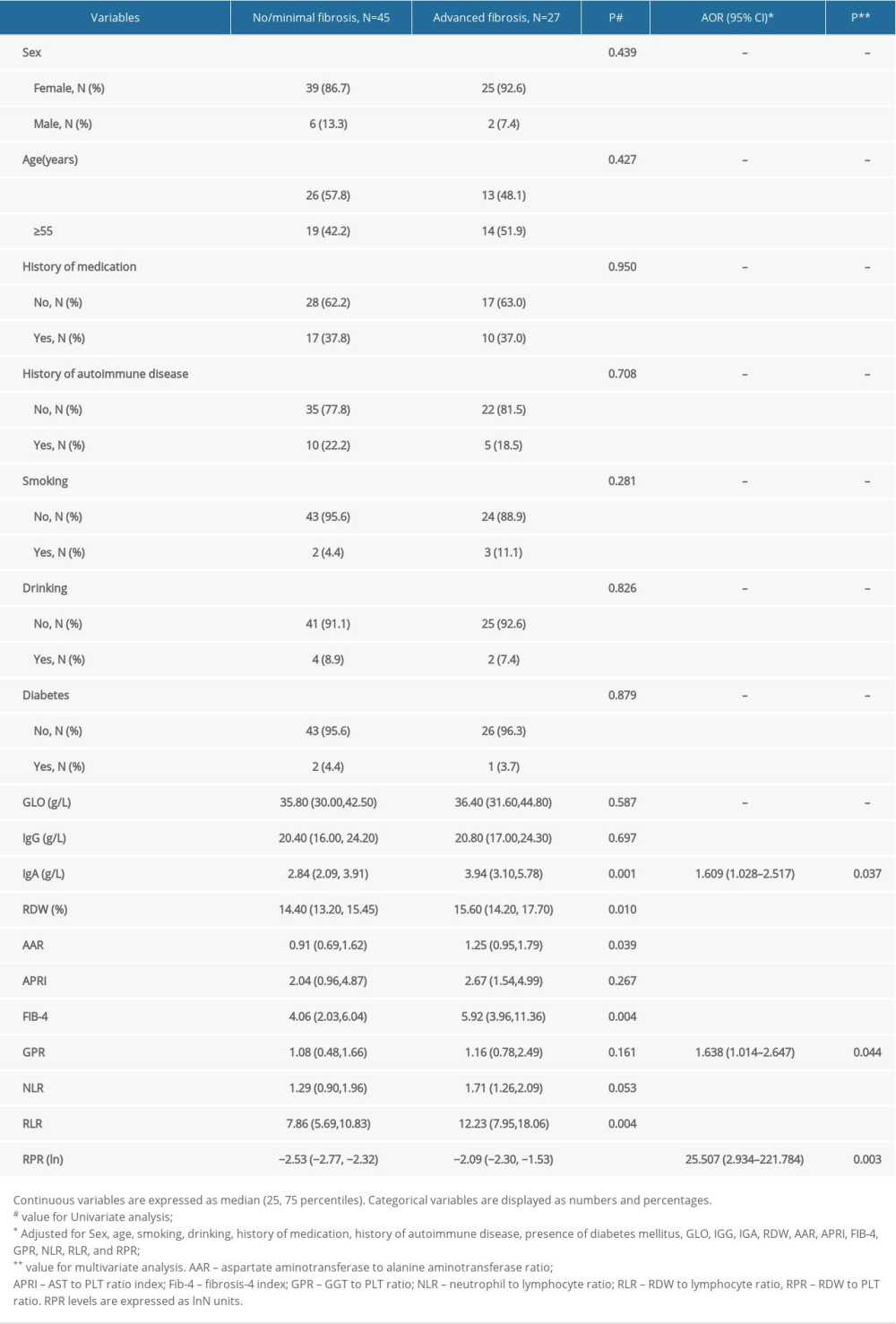 Table 3. Diagnostic performance of serum models of advanced autoimmune hepatitis-related fibrosis.
Table 3. Diagnostic performance of serum models of advanced autoimmune hepatitis-related fibrosis.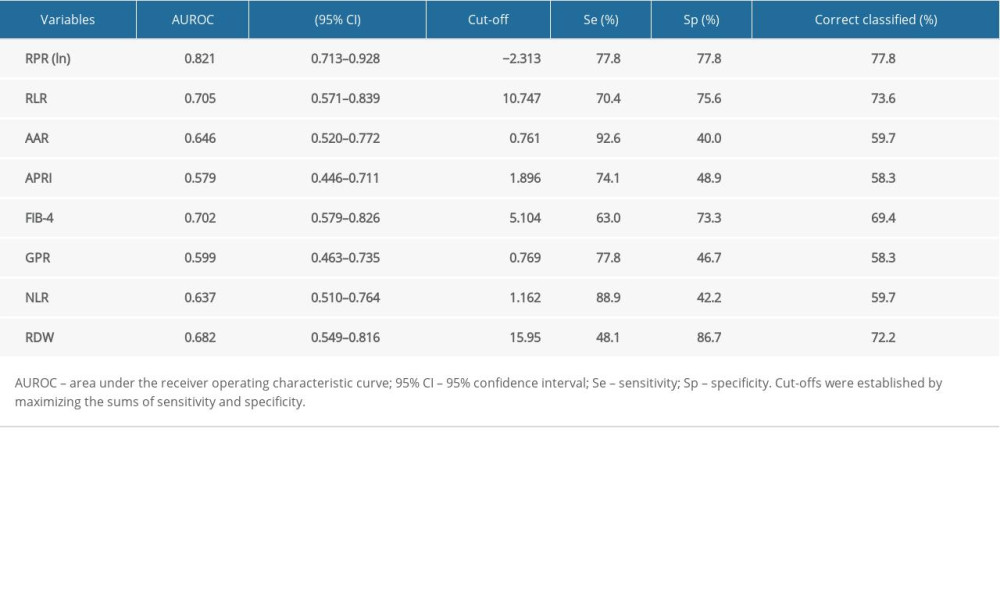
Referencs
1. Febres-Aldana CA, Alghamdi S, Krishnamurthy K, Poppiti RJ, Liver fibrosis helps to distinguish autoimmune hepatitis from DILI with autoimmune features: A review of twenty cases: J Clin Transl Hepatol, 2019; 7(1); 21-26
2. Manns MP, Czaja AJ, Gorham JD, Diagnosis and management of autoimmune hepatitis: Hepatology, 2010; 51(6); 2193-213
3. Mieli-Vergani G, Vergani D, Czaja AJ, Autoimmune hepatitis: Nat Rev Dis Primers, 2018; 4; 18017
4. Yu YC, Mao YM, Chen CW, CSH guidelines for the diagnosis and treatment of drug-induced liver injury: Hepatol Int, 2017; 11(3); 221-41
5. deLemos AS, Foureau DM, Jacobs C, Drug-induced liver injury with autoimmune features: Semin Liver Dis, 2014; 34(2); 194-204
6. Ghonaim M, Al-Ghamdi A, El-Bana H, Autoantibodies in chronic liver disease: Egypt J Immunol, 2005; 12(2); 101-11
7. , EASL Clinical Practice Guidelines: Autoimmune hepatitis: J Hepatol, 2015; 63(4); 971-1004
8. Mack CL, Adams D, Assis DN, Diagnosis and management of autoimmune hepatitis in adults and children: 2019 practice guidance and guidelines from the American Association for the study of liver diseases: Hepatology, 2020; 72(2); 671-722
9. Liaw YF, Leung N, Kao JH, Asian-Pacific consensus statement on the management of chronic hepatitis B: A 2008 update: Hepatol Int, 2008; 2(3); 263-83
10. Lee WS, Kim TY, Relation between red blood cell distribution width and inflammatory biomarkers in rheumatoid arthritis: Arch Pathol Lab Med, 2010; 134(4); 505-6
11. Kim DY, Kim SU, Ahn SH, Usefulness of FibroScan for detection of early compensated liver cirrhosis in chronic hepatitis B: Dig Dis Sci, 2009; 54(8); 1758-63
12. Degos F, Perez P, Roche B, Diagnostic accuracy of FibroScan and comparison to liver fibrosis biomarkers in chronic viral hepatitis: A multicenter prospective study (the FIBROSTIC study): J Hepatol, 2010; 53(6); 1013-21
13. Malekzadeh R, Poustchi H, Fibroscan for assessing liver fibrosis: An acceptable alternative for liver biopsy: Fibroscan: An acceptable alternative for liver biopsy: Hepat Mon, 2011; 11(3); 157-58
14. Udell JA, Wang CS, Tinmouth J, Does this patient with liver disease have cirrhosis?: JAMA, 2012; 307(8); 832-42
15. Maharaj B, Maharaj RJ, Leary WP, Sampling variability and its influence on the diagnostic yield of percutaneous needle biopsy of the liver: Lancet, 1986; 1(8480); 523-25
16. Regev A, Berho M, Jeffers LJ, Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection: Am J Gastroenterol, 2002; 97(10); 2614-18
17. World Health Organisation: Guidelines for the screening, care and treatment of persons with chronic hepatitis C infection Updated version April 2016, https://www.who.int/hepatitis/publications/hepatitis-c-guidelines-2016/en/
18. Chen B, Ye B, Zhang J, RDW to platelet ratio: a novel noninvasive index for predicting hepatic fibrosis and cirrhosis in chronic hepatitis B: PLoS One, 2013; 8(7); e68780
19. Wai CT, Greenson JK, Fontana RJ, A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C: Hepatology, 2003; 38(2); 518-26
20. Tekce H, Kin Tekce B, Aktas G, The evaluation of red cell distribution width in chronic hemodialysis patients: Int J Nephrol, 2014; 2014 754370
21. Xiao G, Yang J, Yan L, Comparison of diagnostic accuracy of aspartate aminotransferase to platelet ratio index and fibrosis-4 index for detecting liver fibrosis in adult patients with chronic hepatitis B virus infection: A systemic review and meta-analysis: Hepatology, 2015; 61(1); 292-302
22. Herrmann E, de Lédinghen V, Cassinotto C, Assessment of biopsy-proven liver fibrosis by two-dimensional shear wave elastography: An individual patient data-based meta-analysis: Hepatology, 2018; 67(1); 260-72
23. Jarcuska P, Bruha R, Horvath G, Evaluation of hepatic fibrosis – access to non-invasive methods, national practice/guidelines in Central Europe: Clin Exp Hepatol, 2016; 2(1); 12-15
24. Liu L, Cao J, Zhong Z, Noninvasive indicators predict advanced liver fibrosis in autoimmune hepatitis patients: J Clin Lab Anal, 2019; 33(7); e22922
25. Wang H, Wang J, Huang R, Red blood cell distribution width for predicting significant liver inflammation in patients with autoimmune hepatitis: Eur J Gastroenterol Hepatol, 2019; 31(12); 1527-32
26. Alvarez F, Berg PA, Bianchi FB, International Autoimmune Hepatitis Group Report: Review of criteria for diagnosis of autoimmune hepatitis: J Hepatol, 1999; 31(5); 929-38
27. Association Godilii CM, Chinese Guideline for diagnosis and treatment of DILI: J Clin Hepatol, 2015; 31(11); 1752-69
28. Rockey DC, Caldwell SH, Goodman ZD, Liver biopsy: Hepatology, 2009; 49(3); 1017-44
29. Bedossa P, Poynard T, An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group: Hepatology, 1996; 24(2); 289-93
30. Sterling RK, Lissen E, Clumeck N, Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection: Hepatology, 2006; 43(6); 1317-25
31. Zhou D, Wu Y, Lin Z, Prognostic value of combination of pretreatment red cell distribution width and neutrophil-to-lymphocyte ratio in patients with gastric cancer: Gastroenterol Res Pract, 2018; 2018 8042838
32. Lippi G, Dipalo M, Teti L, Cervellin G, Relationship between red blood cell distribution width and prognostic biomarkers in patients admitted to the emergency department with acute infections: Eur J Intern Med, 2013; 24(2); e15-16
33. Bessman JD, Hurley EL, Groves MR, Nondiscrete heterogeneity of human erythrocytes: comparison of Coulter-principle flow cytometry and Soret-hemoglobinometry image analysis: Cytometry, 1983; 3(4); 292-95
34. Salvagno GL, Sanchis-Gomar F, Picanza A, Lippi G, Red blood cell distribution width: A simple parameter with multiple clinical applications: Crit Rev Clin Lab Sci, 2015; 52(2); 86-105
35. Farkas N, Szabo A, Lorand V, Clinical usefulness of measuring red blood cell distribution width in patients with systemic sclerosis: Rheumatology, 2014; 53(8); 1439-45
36. Montagnana M, Cervellin G, Meschi T, Lippi G, The role of red blood cell distribution width in cardiovascular and thrombotic disorders: Clin Chem Lab Med, 2011; 50(4); 635-41
37. Arbel Y, Shacham Y, Finkelstein A, Red blood cell distribution width (RDW) and long-term survival in patients with ST elevation myocardial infarction: Thromb Res, 2014; 134(5); 976-79
38. Yoon HE, Kim SJ, Hwang HS, Progressive rise in red blood cell distribution width predicts mortality and cardiovascular events in end-stage renal disease patients: PLoS One, 2015; 10(5); e0126272
39. Jo YH, Kim K, Lee JH, Red cell distribution width is a prognostic factor in severe sepsis and septic shock: Am J Emerg Med, 2013; 31(3); 545-48
40. Lee JH, Yang DH, Jang SY, Incremental predictive value of red cell distribution width for 12-month clinical outcome after acute myocardial infarction: Clin Cardiol, 2013; 36(6); 336-41
41. Koma Y, Onishi A, Matsuoka H, Increased red blood cell distribution width associates with cancer stage and prognosis in patients with lung cancer: PLoS One, 2013; 8(11); e80240
42. Huang CF, Yeh ML, Tsai PC, Baseline gamma-glutamyl transferase levels strongly correlate with hepatocellular carcinoma development in non-cirrhotic patients with successful hepatitis C virus eradication: J Hepatol, 2014; 61(1); 67-74
43. Milic S, Mikolasevic I, Radic M, Clinical utility of red cell distribution width in alcoholic and non-alcoholic liver cirrhosis: Coll Antropol, 2011; 35(Suppl 2); 335-38
44. Lou Y, Wang M, Mao W, Clinical usefulness of measuring red blood cell distribution width in patients with hepatitis B: PLoS One, 2012; 7(5); e37644
45. Hu Z, Sun Y, Wang Q, Red blood cell distribution width is a potential prognostic index for liver disease: Clin Chem Lab Med, 2013; 51(7); 1403-8
46. Wang H, Xu H, Wang X, Red blood cell distribution width to platelet ratio is related to histologic severity of primary biliary cirrhosis: Medicine, 2016; 95(11); e3114
47. Zeng T, Yu J, Tan L, Noninvasive indices for monitoring disease course in Chinese patients with autoimmune hepatitis: Clin Chim Acta, 2018; 486; 135-41
48. Lippi G, Plebani M, Red blood cell distribution width (RDW) and human pathology. One size fits all: Clin Chem Lab Med, 2014; 52(9); 1247-49
49. Lee H, Kong SY, Sohn JY, Elevated red blood cell distribution width as a simple prognostic factor in patients with symptomatic multiple myeloma: Biomed Res Int, 2014; 2014 145619
50. Kim CH, Park JT, Kim EJ, An increase in red blood cell distribution width from baseline predicts mortality in patients with severe sepsis or septic shock: Crit Care, 2013; 17(6); R282
51. Senol K, Saylam B, Kocaay F, Tez M, Red cell distribution width as a predictor of mortality in acute pancreatitis: Am J Emerg Med, 2013; 31(4); 687-89
52. Lippi G, Targher G, Montagnana M, Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients: Arch Pathol Lab Med, 2009; 133(4); 628-32
53. Krintus M, Kozinski M, Kubica J, Sypniewska G, Critical appraisal of inflammatory markers in cardiovascular risk stratification: Crit Rev Clin Lab Sci, 2014; 51(5); 263-79
54. Allen LA, Felker GM, Mehra MR, Validation and potential mechanisms of red cell distribution width as a prognostic marker in heart failure: J Card Fail, 2010; 16(3); 230-38
55. Semba RD, Patel KV, Ferrucci L, Serum antioxidants and inflammation predict red cell distribution width in older women: The Women’s Health and Aging Study I: Clin Nutr, 2010; 29(5); 600-4
56. Friedman JS, Lopez MF, Fleming MD, SOD2-deficiency anemia: Protein oxidation and altered protein expression reveal targets of damage, stress response, and antioxidant responsiveness: Blood, 2004; 104(8); 2565-73
57. Mantovani A, Allavena P, Sica A, Balkwill F, Cancer-related inflammation: Nature, 2008; 454(7203); 436-44
58. Mitchell O, Feldman DM, Diakow M, Sigal SH, The pathophysiology of thrombocytopenia in chronic liver disease: Hepat Med, 2016; 8; 39-50
59. Kurokawa T, Ohkohchi N, Platelets in liver disease, cancer and regeneration: World J Gastroenterol, 2017; 23(18); 3228-39
60. Afdhal N, McHutchison J, Brown R, Thrombocytopenia associated with chronic liver disease: J Hepatol, 2008; 48(6); 1000-7
61. Peck-Radosavljevic M, Thrombocytopenia in liver disease: Can J Gastroenterol, 2000; 14(Suppl D); 60d-66d
62. Lin YL, Lin HW, Chen YC, Hepatoprotective effects of naturally fermented noni juice against thioacetamide-induced liver fibrosis in rats: J Chin Med Assoc, 2017; 80(4); 212-21
63. Ripoche J, Blood platelets and inflammation: Their relationship with liver and digestive diseases: Clin Res Hepatol Gastroenterol, 2011; 35(5); 353-57
64. Watanabe M, Murata S, Hashimoto I, Platelets contribute to the reduction of liver fibrosis in mice: J Gastroenterol Hepatol, 2009; 24(1); 78-89
65. Triger DR, Alp MH, Wright R, Bacterial and dietary antibodies in liver disease: Lancet, 1972; 1(7741); 60-63
66. Triger DR, Wright R, Hyperglobulinaemia in liver disease: Lancet, 1973; 1(7818); 1494-96
67. Prytz H, Bjorneboe M, Christoffersen P: Gut, 1977; 18(1); 28-32
68. Husby G, Skrede S, Blomhoff JP, Serum immunoglobulins and organ non-specific antibodies in diseases of the liver: Scand J Gastroenterol, 1977; 12(3); 297-304
69. Hodson ME, Morris L, Batten JC, Serum immunoglobulins and immunoglobulin G subclasses in cystic fibrosis related to the clinical state of the patient: Eur Respir J, 1988; 1(8); 701-5
70. Thomas HC, McSween RN, White RG, Role of the liver in controlling the immunogenicity of commensal bacteria in the gut: Lancet, 1973; 1(7815); 1288-91
71. Reynolds HY, Fulmer JD, Kazmierowski JA, Analysis of cellular and protein content of broncho-alveolar lavage fluid from patients with idiopathic pulmonary fibrosis and chronic hypersensitivity pneumonitis: J Clin Invest, 1977; 59(1); 165-75
72. Datta DV, Ganguly NK, Mahajan RC, Datta U, Immunoglobulins in non-cirrhotic portal fibrosis: (Idiopathic portal hypertension): J Assoc Physicians India, 1978; 26(8); 683-86
73. Munro JM, van der Walt JD, Cox EL, A comparison of cytoplasmic immunoglobulins in retroperitoneal fibrosis and abdominal aortic aneurysms: Histopathology, 1986; 10(11); 1163-69
74. Mathai A, Kartha CC, Balakrishnan KG, Serum immunoglobulins in patients with endomyocardial fibrosis: Indian Heart J, 1986; 38(6); 470-72
75. Shen H, Huang G, Zhang M, Effects of immunoglobulin G on the proliferation and activation of sub-cultured rat hepatic stellate cells: Hepatology, 2001; 34; 403
76. Serfaty L, Chazouilleres O, Poujol-Robert A, Risk factors for cirrhosis in patients with chronic hepatitis C virus infection: Results of a case-control study: Hepatology, 1997; 26(3); 776-79
77. Berglin L, Bjorkstrom NK, Bergquist A, Primary sclerosing cholangitis is associated with autoreactive IgA antibodies against biliary epithelial cells: Scand J Gastroenterol, 2013; 48(6); 719-28
78. Gabeta S, Norman GL, Gatselis N, IgA anti-b2GPI antibodies in patients with autoimmune liver diseases: J Clin Immunol, 2008; 28(5); 501-11
79. Gabeta S, Norman GL, Liaskos C, Diagnostic relevance and clinical significance of the new enhanced performance M2 (MIT3) ELISA for the detection of IgA and IgG antimitochondrial antibodies in primary biliary cirrhosis: J Clin Immunol, 2007; 27(4); 378-87
80. Yokoyama H, Nagata S, Moriya S, Hepatic fibrosis produced in guinea pigs by chronic ethanol administration and immunization with acetaldehyde adducts: Hepatology, 1995; 21(5); 1438-42
81. Yosry A, Fouad R, Alem SA, FibroScan, APRI, FIB4, and GUCI: Role in prediction of fibrosis and response to therapy in Egyptian patients with HCV infection: Arab J Gastroenterol, 2016; 17(2); 78-83
82. Stasi C, Silvestri C, Voller F, Cipriani F, The epidemiological changes of HCV and HBV infections in the era of new antiviral therapies and the anti-HBV vaccine: J Infect Public Health, 2016; 9(4); 389-95
83. Choi BC, Sensitivity and specificity of a single diagnostic test in the presence of work-up bias: J Clin Epidemiol, 1992; 45(6); 581-86
Tables
 Table 1A. Demographic characteristics of autoimmune hepatitis group and drug-induced liver injury group.
Table 1A. Demographic characteristics of autoimmune hepatitis group and drug-induced liver injury group. Table 1B. Initial symptoms of 72 patients with autoimmune hepatitis (AIH) and 164 patients with drug-induced liver injury (DILI).
Table 1B. Initial symptoms of 72 patients with autoimmune hepatitis (AIH) and 164 patients with drug-induced liver injury (DILI). Table 2. Univariate and multivariate analyses of variables associated with autoimmune hepatitis-related liver fibrosis.
Table 2. Univariate and multivariate analyses of variables associated with autoimmune hepatitis-related liver fibrosis. Table 3. Diagnostic performance of serum models of advanced autoimmune hepatitis-related fibrosis.
Table 3. Diagnostic performance of serum models of advanced autoimmune hepatitis-related fibrosis. Table 1A. Demographic characteristics of autoimmune hepatitis group and drug-induced liver injury group.
Table 1A. Demographic characteristics of autoimmune hepatitis group and drug-induced liver injury group. Table 1B. Initial symptoms of 72 patients with autoimmune hepatitis (AIH) and 164 patients with drug-induced liver injury (DILI).
Table 1B. Initial symptoms of 72 patients with autoimmune hepatitis (AIH) and 164 patients with drug-induced liver injury (DILI). Table 2. Univariate and multivariate analyses of variables associated with autoimmune hepatitis-related liver fibrosis.
Table 2. Univariate and multivariate analyses of variables associated with autoimmune hepatitis-related liver fibrosis. Table 3. Diagnostic performance of serum models of advanced autoimmune hepatitis-related fibrosis.
Table 3. Diagnostic performance of serum models of advanced autoimmune hepatitis-related fibrosis. In Press
05 Mar 2024 : Clinical Research
Muscular Function Recovery from General Anesthesia in 132 Patients Undergoing Surgery with Acceleromyograph...Med Sci Monit In Press; DOI: 10.12659/MSM.942780
05 Mar 2024 : Clinical Research
Effects of Thermal Insulation on Recovery and Comfort of Patients Undergoing Holmium Laser LithotripsyMed Sci Monit In Press; DOI: 10.12659/MSM.942836
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952