28 November 2020: Clinical Research
Use of Palpation Imaging in Diagnosis of Breast Diseases: A Way to Improve the Detection Rate
Yihan Ding12ABE, Chenyu Sun3CDEF, Qin Zhou4CD, Ce Cheng5DF, Cunye Yan6AF, Benzhong Wang12AEG*DOI: 10.12659/MSM.927553
Med Sci Monit 2020; 26:e927553
Abstract
BACKGROUND: Breast diseases pose increasing threat to women health as peoples lifestyle changes. The aim of this study was to investigate the clinical application value of Palpation Imaging (PI) in the diagnosis of breast diseases.
MATERIAL AND METHODS: From October 2019 to February 2020, 184 patients with 225 breast lesions were examined by using PI, ultrasound, and mammography in the department of Breast Surgery, the First Affiliated Hospital of Anhui Medical University. All cases were confirmed pathologically by core-needle biopsy or excisional biopsy. The cut-off value of the PI tests was determined by receiver operating characteristic (ROC) curve. We compared the examination results of PI with ultrasound and mammography to analyze the diagnostic value of PI.
RESULTS: Pathological examination revealed that 186/225(82.67%) lesions were benign, while 39 were malignant. All 8 parameters of PI were significantly correlated with pathological findings (P<0.05). The best cut-off value for the PI score was 19.5 and the area under the curve (AUC) for the PI was 0.921 (95% CI: 0.874–0.968, P<0.001) with 89.7% sensitivity and 86.0% specificity. PI showed greater sensitivity (89.7%) and its specificity (86.0% vs. 86.4%, P=0.931) and accuracy (86.7% vs. 84.6%, P=0.604) were similar to those of mammography. The combination of 3 types of test is superior to a single examination. The sensitivity was 100% and the specificity was 98.8%.
CONCLUSIONS: PI has high clinical value in differentiation of benign and malignant breast lesions. Combination examination has the potential to improve the detection of breast cancer in screening and diagnostic capacities and can be used as a supplement to ultrasound and mammography.
Keywords: Breast Neoplasms, Mammography, Palpation, Ultrasonography, Adolescent, Aged, 80 and over, Breast Diseases, Child, Diagnostic Imaging, ROC Curve, Sensitivity and Specificity, young adult
Background
Breast diseases remain a major health concern for women, and breast cancer is the most commonly diagnosed malignant tumor in female patients globally according to the World Health Organization (WHO) [1], and its incidence in China has been increasing by approximately 3% annually [2]. Over the years, a number of preventive measures and risk factors have been studied to reduce the incidence of breast cancer, such as risk assessment [3], epigenetic changes [4], and selective estrogen receptors [5]. But these strategies cannot eliminate the majority of breast cancers in low- and middle-income countries, where breast cancer usually is diagnosed at a very late stage. Therefore, early detection to improve breast cancer outcome and survival has played a significant role in breast cancer control and surveillance.
Clinical trials over the last 2 decades have suggested that early diagnosis and treatment are important in improving the survival rate of patients with cancer [6,7]. The current methods for breast examinations include clinical breast examination (CBE), ultrasound, mammography, and magnetic resonance imaging (MRI). Each method for the screening and diagnosis of breast cancer has its own advantages and disadvantages. Currently, the most widely used modality for breast cancer screening is mammography, which is recommended by the WHO as the only proven method because of its high specificity [1], but the sensitivity of mammography is affected by breast density and it decreases with increasing parenchymal density [8]. Thus, mammography is not very effective for young women under 40 years of age and women with dense breasts [9]. At present, the capability of ultrasound as a screening method for breast cancer remains controversial as it is highly operator-dependent and also has other limitations [10]. In addition, the high false-positive rates of MRI, as well as its high cost, limits its application in breast cancer screening [11]. Based on elasticity theory, palpation imaging (PI) was first proposed for breast cancer screening in 2006 [12]. PI records the pressure and location data of breast lesions by sensors and translates palpation findings into a visual record. A colorful image in both two-dimensional (2-D) and three-dimensional (3-D) formats reflects morphological characterization data of a tumor, including size, shape, hardness, and other parameters. Malignant and benign lesions can be categorized according to these parameters [12]. The present study reports the results of PI, ultrasound, and mammography performed in 184 patients, which aimed to assess the value of PI in the diagnosis of breast diseases.
Material and Methods
STUDY POPULATION:
A total of 187 participants with breast diseases were selected from outpatients who visited the Department of Breast Surgery, the First Affiliated Hospital of Anhui Medical University (Anhui, China) from October 2019 to February 2020. The patients were 12–80 years of age, with a median age of 42 years. All patients were subjected to PI, ultrasound, and mammography, and final diagnosis was confirmed by core-needle biopsy or excisional biopsy and pathological reports. Of these, 67 patients were excluded from mammography due to age restriction. Altogether, 187 patients with 225 lesions were finally included. All research subjects signed a written informed consent to participate in the study.
METHODS:
Breast ultrasound was performed using a Mindray 7S ultrasound machine (Mindray Resona7S; Shenzhen Mindray Bio-Medical Electronics Corp., Shenzhen, China). The patients were examined in supine position with both arms above the head to adequately expose the breast. Each quadrant of the breast to the bilateral axilla was detected. If a lump was found by ultrasound, further ultrasonic scanning of the lesion was performed in longitudinal and transverse sections. A GE Senograph DS mammography machine (GE Healthcare, Milwaukee, WI, USA) was used for molybdenum target X-ray examination, and bilateral breast images were obtained in the craniocaudal and mediolateral oblique views. The examinations and diagnoses were performed independently by 2 expert radiologists.
PI was performed using a breast palpation imaging machine (BT9; Beijing Xiantongkangqiao Medical Technology Corp., Beijing, China), which consists of a probe with pressure sensors, an electronic unit, and a touch screen laptop (Figure 1). The patients were in supine position. The pressure response acquired by the probe sensors while scanning target lesions were converted to images shown on the screen in real time. The color image in both 2-D and 3-D formats records the 8 parameters of the lesion, including 2-D color, 2-D shape, 2-D dynamic, 3-D peak profile, 3-D peak height, 3-D summit shape, 3-D base, and 3-D dynamic. PI examination of the women with breast diseases were performed by 2 clinical operators and also reviewed by an independent specialist.
Ultrasound and mammograms were evaluated using the criteria given by the American College of Radiology Breast Imaging Reporting and Data System (BI-RADS) [13,14]. Images containing lesions with BI-RADS of 4B or greater were considered as malignant [15]. For the PI examination, statistical analysis was performed to assess the correlation between PI parameters and pathologic findings. ROC curves were plotted and AUC was calculated to get the optimal cut-off of PI scores as the criterion to differentiate benign vs. malignant lesions.
The collected data were entered into Excel 2019 and statistical analysis was performed with SPSS 23.0 software. Associations were tested using the Pearson chi-square test or Fisher exact test.
Results
GENERAL DATA:
Malignant or benign breast lesions were confirmed by pathological examination as the criterion standard, and 225 lesions were analyzed in this study. In total, 186 benign lesions were diagnosed (82.67%), including 102 fibroadenomas (45.3%), 43 adenoses (19.1%), 16 intraductal papillomas (7.1%), 9 inflammations (4.0%), 7 phyllodes tumors (3.1%), 5 atypical ductal hyperplasias (2.2%), 2 duct ectasias (0.9%), and 2 breast tissues (0.9%). Among 39 malignant breast lesions, the diagnoses included 34 invasive ductal carcinomas (15.1%), 2 intraductal papillary carcinomas (0.9%), 2 ductal carcinomas in situ (0.9%, 1 high-grade ductal carcinoma in situ and 1 moderate-to-high-grade ductal carcinoma in situ with microinvasion and Paget’s disease), and 1 encapsulated papillary carcinoma (0.4%). The patient and tumor characteristics are shown in Table 1.
PI RESULTS:
The results of PI examination are shown in Table 2. All 8 parameters (2-D color, 2-D shape, 2-D dynamic, 3-D peak profile, 3-D peak height, 3-D summit shape, 3-D base, and 3-D dynamic) of PI were significantly correlated with pathological findings. All P values were less than 0.05. Representative images of the benign and malignant lesions are shown in Figure 2. In the 2-D image, the peak of malignant mass lesions was in black or red and was shown as an irregular shape with inhomogeneous central pressure. In the 3-D image, malignant tumor was characterized by multiple peaks with higher peak heights. A burr sign of the peak summit and stationary base were also the features of breast malignancy in PI examination. Conversely, 2-D images shown in orange, yellow, or blue were mostly benign. Images with regular shape, sharp summit shape, and single peak are more likely to be benign lesions.
On the basis of the Breast Palpation Imaging Reporting and Data System (BPI-RADS) (Table 3) [16], we calculated the cumulative score of each lesion as the PI score. A higher score indicated a higher possibility of malignancy. The ROC curves were plotted according to the sensitivity and specificity data obtained from the actual diagnosis using PI (Figure 3) and the AUC was 0.921 (95% CI: 0.874–0.968, P<0.001). The best cut-off value for the PI score using the Youden index was 19.5 to differentiate between benign and malignant breast lesions.
COMPARISON OF DIFFERENT IMAGING STUDIES:
Using 19.5 score as the cut-off value in our study, the sensitivity of PI was 89.7%, the specificity was 86.0%, and the accuracy was 86.7%. For ultrasound and mammography, the sensitivities were 79.5% and 80.6%, the specificities were 96.8% and 86.4%, and the accuracies were 93.8% and 84.6%, respectively. The diagnostic performance of each method is shown in Table 4.
In comparison of the sensitivity of the 3 diagnostic methods, PI examination demonstrated higher sensitivity (89.7%) than ultrasound (79.5%) and mammography (80.6%). However, chi-square statistical analysis of the sensitivity results showed that the differences among the 3 methods were not statistically significant (P=0.413).
Ultrasound provided the highest specificity (96.8%), and the sensitivities of PI and mammography were similar (86.0% and 86.4%, respectively). Overall, the difference in specificity across the 3 methods was statistically significant (P=0.001). No significant difference in specificity was observed between PI and mammography (86.0% vs. 86.4%, P=0.931), but the specificities of PI and mammography were significantly (P<0.05) lower than that of ultrasound (96.8%) (Table 5).
The diagnostic accuracies of the 3 methods were compared, showing statistically significant (P=0.012) differences. The accuracy of PI was 86.7%, which was significantly lower than the accuracy of ultrasound (93.8%, P=0.011). The results for mammography (84.6%) were similar to those of PI examination (Table 5).
RESULTS OF COMBINATION TESTS:
Previous studies have confirmed that the sensitivity for detecting breast cancer by combined application of PI and ultrasound or PI and mammography was higher than that using a single modality [17]. Use of a combination test could improve the diagnostic sensitivity, specificity, and accuracy. In our study, we combined these 3 methods in parallel and serial tests to assess sensitivity and specificity.
In a parallel test of PI, ultrasound, and mammography, a malignant result with any of these 3 tests was defined as a positive result. When the 3 methods were combined, the sensitivity of breast cancer diagnosis increased to 100%. There was a statistically significant difference in sensitivity, with the combination test showing higher sensitivity than ultrasound (83.3%, P=0.025) and mammography (80.6%, P=0.011) (Table 6).
However, in the serial test, to be considered as positive, all 3 image tests must yield malignant results. As a result, the specificity increased to 98.8% when the 3 methods were combined. This was significantly higher than the results of PI (82.7%, P<0.001) or mammography (86.4%, P=0.003) (Table 7).
Discussion
In recent years, as morbidity and mortality rates are increasing annually, breast cancer has become a major threat to women’s health globally [18]. Numerous studies have reported that high-quality screening, early diagnosis, and early treatment could reduce mortality rates of cancer [19,20]. The current methods for breast cancer screening include CBE, ultrasound, mammography, and MRI, among which, mammography is the most widely used and is recommended by the WHO as the only proven method [1]. Because of its high specificity, US National Comprehensive Cancer Network (NCCN) guidelines have suggested that women aged 40 years and older should get mammograms every 1–2 years [21]. However, some studies found its sensitivity, especially in dense breasts, was relatively low [9]. A large study conducted in the United States and Canada found that the diagnostic accuracy of mammography for breast cancer was 78% [22]. Furthermore, the results of a study with 55 350 patients in Denmark suggested a decreasing sensitivity of mammography, with increasing parenchymal density [8]. Thus, mammography is not very effective for young women under 40 years of age and women with dense breasts. Ultrasound can discover breast disease characterized by solid or cystic tumors and help to determine the nature of the lesions. However, the accuracy and effectiveness of ultrasound are closely related to operator experience and techniques and the ability to interpret images [10]. At present, the utility of ultrasound as a screening method for breast cancer remains controversial [23]. MRI offers high sensitivity in detecting breast cancer, superior to mammography [24]. However, due to high false-positive rates and high cost, MRI is often recommended to screen high-risk patients with a family history of breast cancer [11]. Clinical breast palpation is a simple and brief screening test for breast cancer [25]. However, for obese women and patients with very large breasts, the sensitivity of breast palpation is greatly reduced because of operational difficulties. A cross-sectional study in France did not recommend clinical breast palpation as routine screening for breast cancer due to the lack of unified diagnostic criteria and examination reporting [26]. The variability and subjectivity of clinical breast palpation have led to this method falling out of favor. For a public health screening intervention, the sensitivity and specificity of clinical breast examination are too low and variable to use as a screening test [27]. Therefore, an alternative screening method that is more cost-effective and less operator-dependent in an office setting is needed.
Elastography is an ultrasound imaging method first suggested by Ophir et al. in 1991 [28]. In a subsequent study, Krouskop et al. [29] investigated the difference in elastic coefficient between different tissues and found that the larger the elastic coefficient, the greater the hardness of the tissue. This has been called the theory of tissue elasticity. A small elastic coefficient indicates low hardness of normal breast tissue. Conversely, a larger elastic coefficient represents greater hardness and greater likelihood of malignant tissue. Based on the elasticity theory, PI use a highly sensitive probe consisting of 192 pressure sensors [30], and under the pressure application to the breast issue, reaction force from breast lesions can be converted to digital signals. Tumor characterization data, including size, shape, hardness, and other parameters, are presented in color images in 2-D and 3-D formats. PI was first reported by Kaufman et al. in 2006 in a study of 110 patients with breast lesions [12]. Pathology examination showed the positive predictive value and diagnostic accuracy of PI were both 94% in 36 cases of breast cancer. Meanwhile, the diagnostic accuracy of clinical palpation was 86% and the positive predictive value was only 78% [12]. In another multicenter study, 179 cases collected from 4 different clinical sites demonstrated that PI had a sensitivity of 91.4% and specificity of 86.8% [30]. Egorov et al. [30] suggested that PI might be expected to become an early screening method for breast cancer.
In the present study, we demonstrated that all 8 parameters (2-D color, 2-D shape, 2-D dynamic, 3-D peak profile, 3-D peak height, 3-D summit shape, 3-D base, and 3-D dynamic) of PI were significantly correlated with pathological findings. All
Before this study, the hardness of detected lesions was intended to be used as a single indicator to distinguish benign and malignant mass lesions [12]. Our study complements the other 8 parameters of PI to provide a more comprehensive assessment of breast lesions. We demonstrated that all 8 parameters of PI were significantly correlated with pathological results. All these indicators were included to the analysis, supporting the high sensitivity of PI and assisting in the detection of malignancy. Malignant tumors are characterized by hard texture, unclear boundary, and weak activity upon clinical palpation. However, owing to the lack of objective criteria or systems, it is sometimes difficult to exactly confirm a mass by means of breast palpation, even for an experienced clinician. In this study, we used a semi-quantitative index to describe the hardness, shape, size, homogeneity, and other characteristics. This digital palpation of breast tissues of PI also makes the examination and diagnosis more quantitative, objective, and tenable.
As a screening test, PI has a high sensitivity and negative predictive value (NPV). For a PI score cut-off of 19.5, the sensitivity and NPV were 89.7% and 97.6%, respectively. Compared with other diagnostic modalities, PI possess several advantages, such as lower cost, operation convenience, and applicability to all age groups. The present results demonstrate that PI can be widely used in breast cancer detection. In our results, its specificity and accuracy were somewhat lower than those of ultrasound and mammography. One of the reasons could be the broad diagnostic criteria of PI. Some benign breast lesions with irregular shape, multiple peaks, or burr sign characteristics were mistaken for malignancy, which lead to reduced specificity and accuracy. There may be another reason for this. By analyzing the histopathological types of misdiagnosis, these included 6 patients with intraductal papilloma, 3 patients with inflammation, 3 patients with phyllodes tumors, and 3 with granulomatous mastitis. Also, most of these exhibited rich dotted and strip blood flow and calcification in terms of radiological features. In summary, PI also has its own deficiencies when examining patients with atypical breast mass and we need additional studies and more data to improve diagnostic approaches. In further work, we will explore more diagnostic parameters of PI to improve specificity and accuracy through big data analysis.
The innovative feature of our study is the combination of PI, ultrasound, and mammography. When these 3 methods were combined, the sensitivity of the parallel test and the specificity of the serial test were 100% and 98.8%, respectively, which were better than that of either method alone. From a clinical point of view, a combination test may improve the efficiency of early diagnosis for patients with breast cancer and capture cases that cannot be diagnosed by a single test. Mammography, which is the most commonly applied modality for breast screening, has a high false-positive rate, which limits its use in low-risk patients. This problem is common among Asian women who have relatively dense breasts, and false-positive results of mammography lead to many unnecessary biopsies. In this case, bionic palpation devices, including PI, could be a potential alternative to mammography for patients in particular groups, such as those with dense breasts, and provide better performance in diagnosis. Regarding ultrasound, which has been traditionally used to differentiate cysts and solid masses, there are substantial overlaps between benign and malignant image features, especially for small lesions. PI could be help compensate for this deficiency of ultrasound by comprehensively analyzing 2-D and 3-D formats of tumors. PI examination may have difficulty in diagnosing intraductal lesions and calcification of breast masses, but ultrasound and mammography perform well in diagnosing these pathologies. Therefore, the combination of PI, ultrasound, and mammography could be used to analyze ambiguous clinical cases and improve the diagnosis of breast lesions.
Conclusions
PI is a noninvasive, convenient screening tool, especially when used with another diagnostic modality, and its use can significantly improve early detection rates of tumors. PI could be particularly helpful for health care providers to screen breast cancer at early stage, given its easiness to apply in office-based settings.
Figures
 Figure 1. Palpation imaging machine (BT9; Beijing Xiantongkangqiao Medical Technology Corp., Beijing, China).
Figure 1. Palpation imaging machine (BT9; Beijing Xiantongkangqiao Medical Technology Corp., Beijing, China). 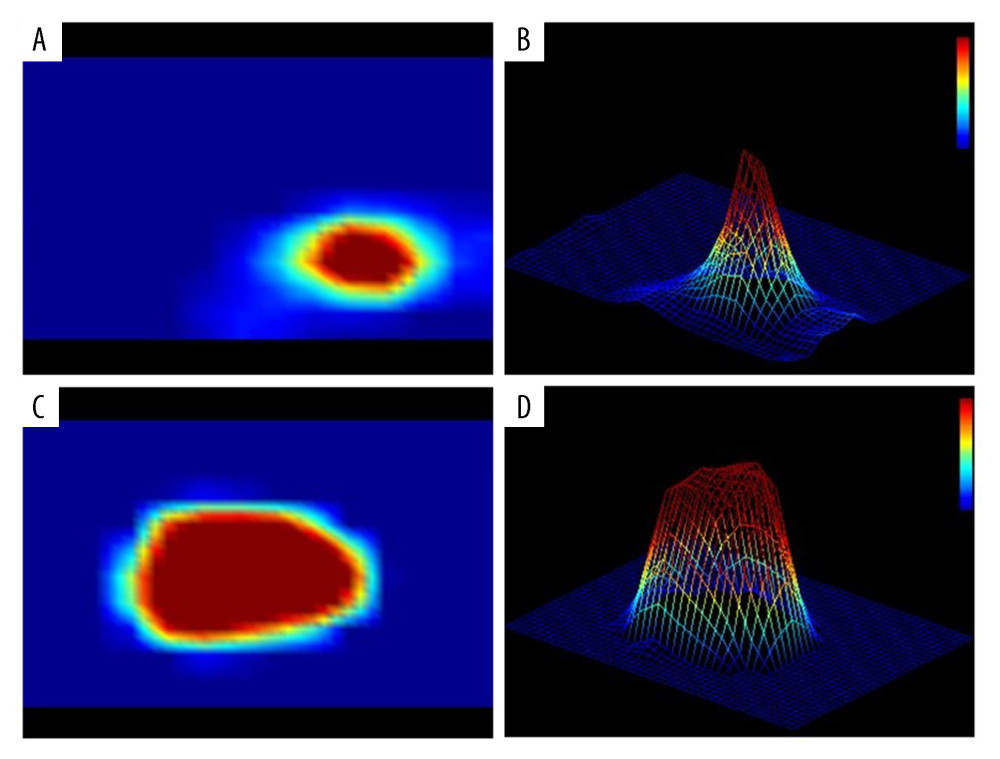 Figure 2. Representative images of the malignant lesions. (A) 2-D image of benign lesion. (B) 3-D image of benign lesion. (C) 2-D image of malignant lesion. (D) 3-D image of malignant lesion.
Figure 2. Representative images of the malignant lesions. (A) 2-D image of benign lesion. (B) 3-D image of benign lesion. (C) 2-D image of malignant lesion. (D) 3-D image of malignant lesion. 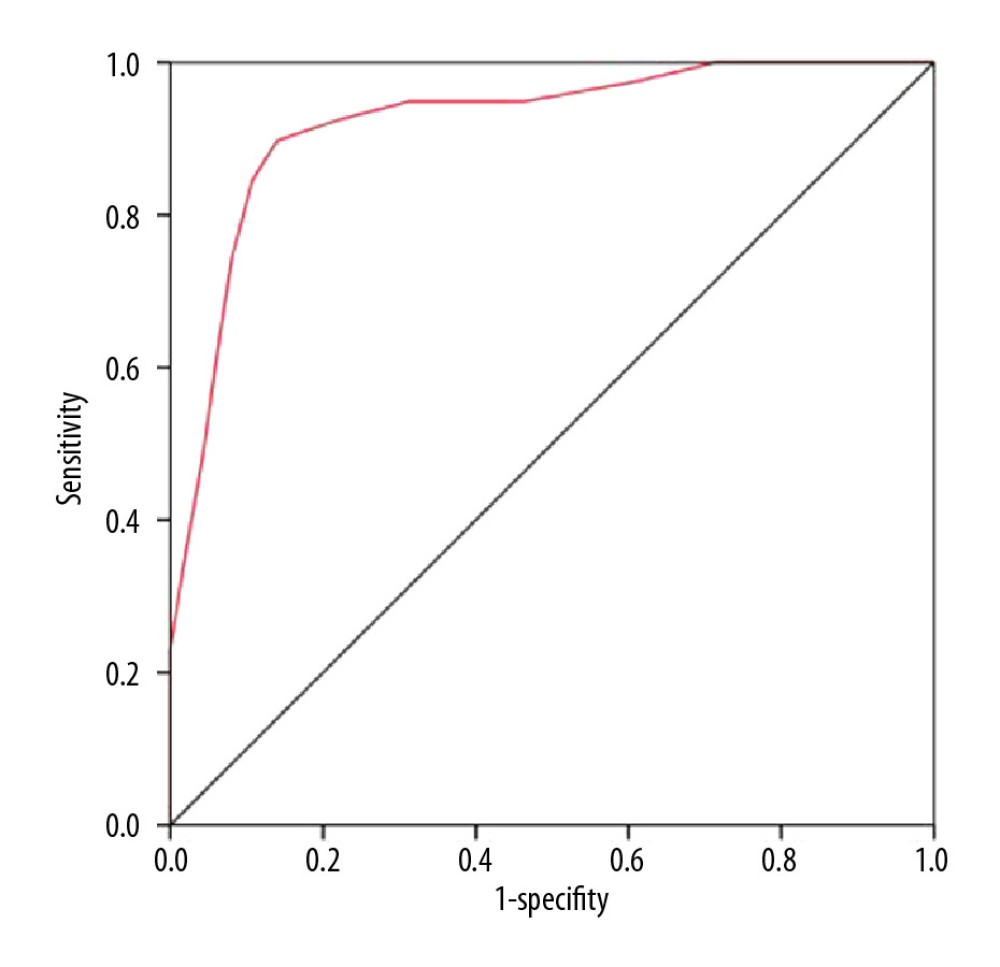 Figure 3. ROC curve of PI.
Figure 3. ROC curve of PI. Tables
Table 1. Patient and tumor characteristics.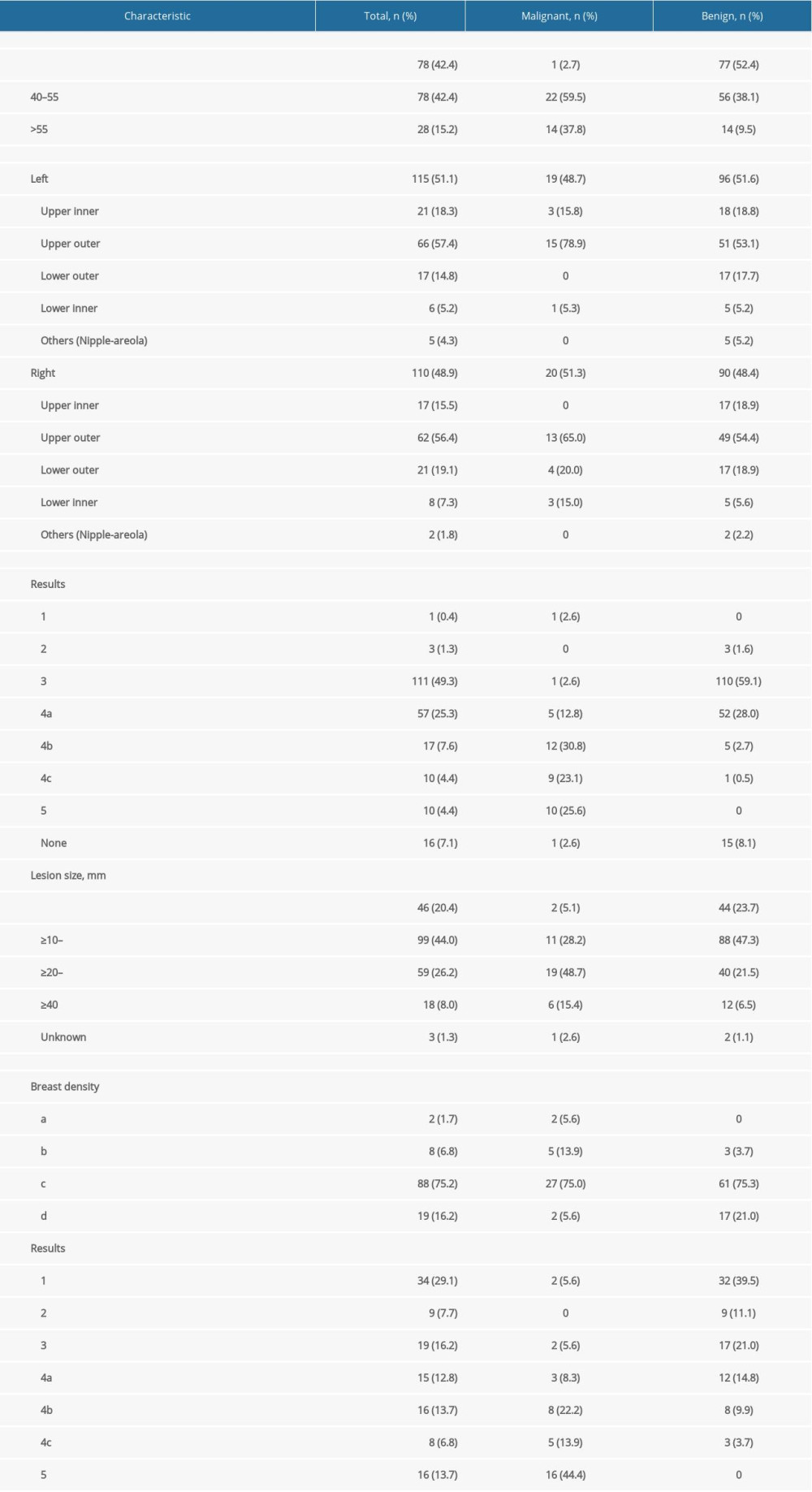 Table 2. Correlation between PI examination index and clinicopathological data of patients with breast cancer.
Table 2. Correlation between PI examination index and clinicopathological data of patients with breast cancer.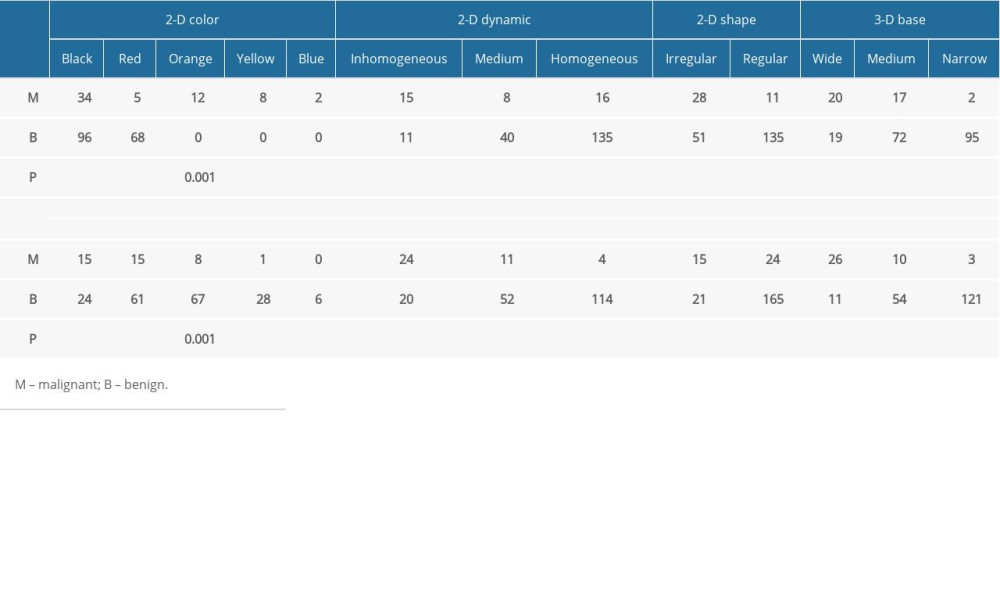 Table 3. BPI-RADS classification.
Table 3. BPI-RADS classification.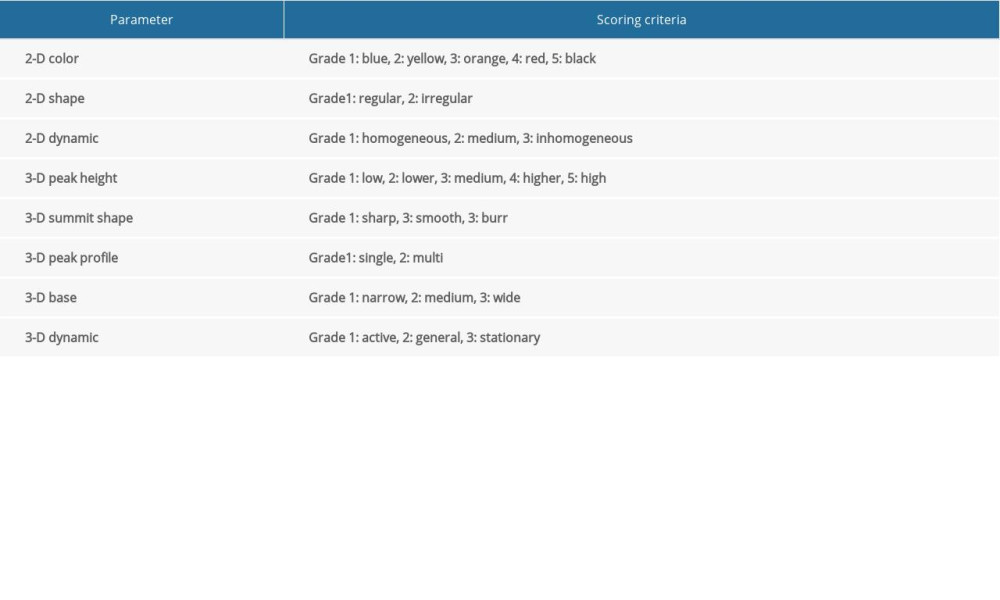 Table 4. Comparison of examination results by different methods.
Table 4. Comparison of examination results by different methods.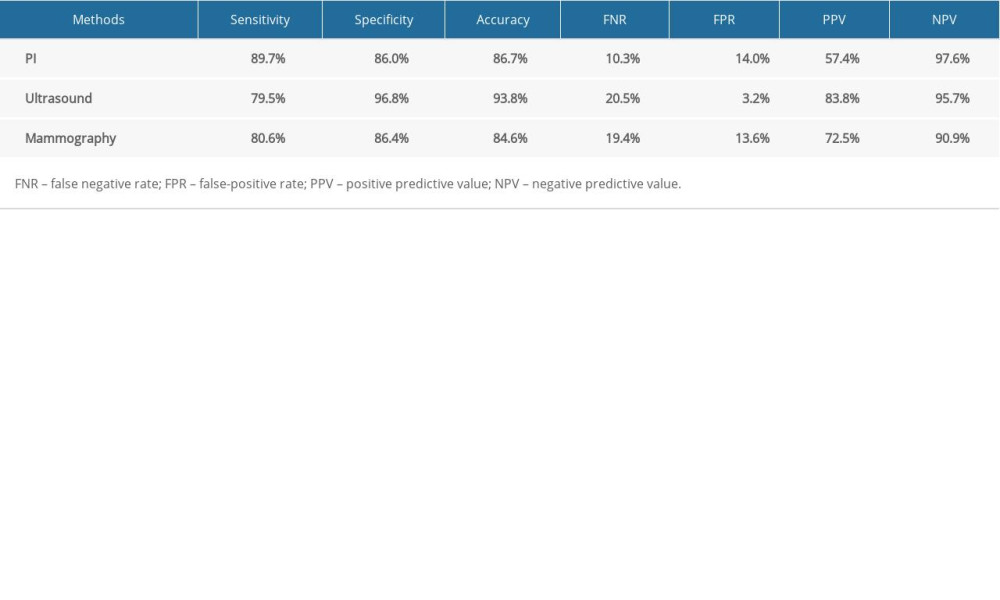 Table 5. Comparison of specificity and accuracy of different methods.
Table 5. Comparison of specificity and accuracy of different methods.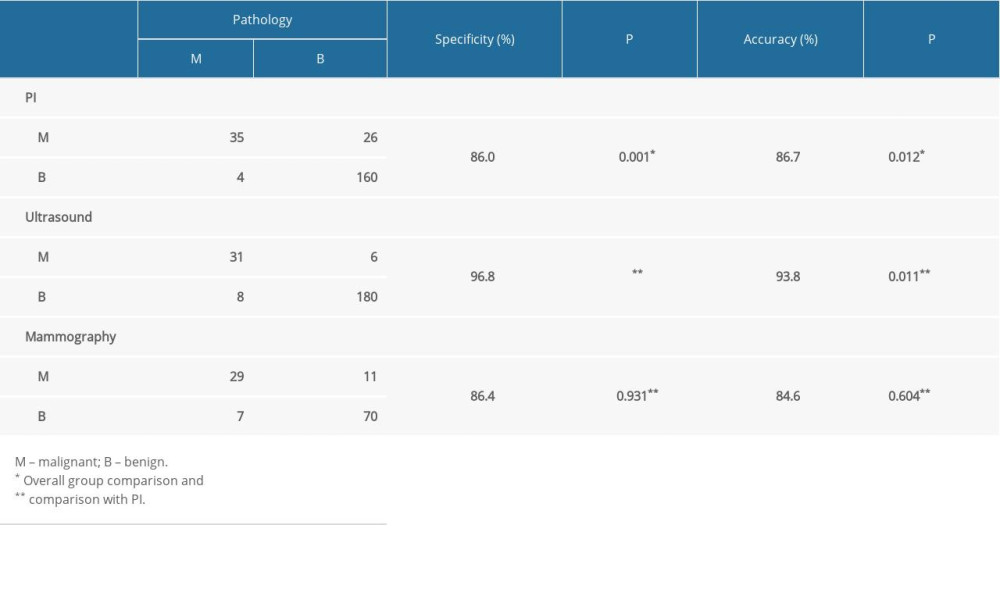 Table 6. Sensitivity of combination tests (parallel test).
Table 6. Sensitivity of combination tests (parallel test).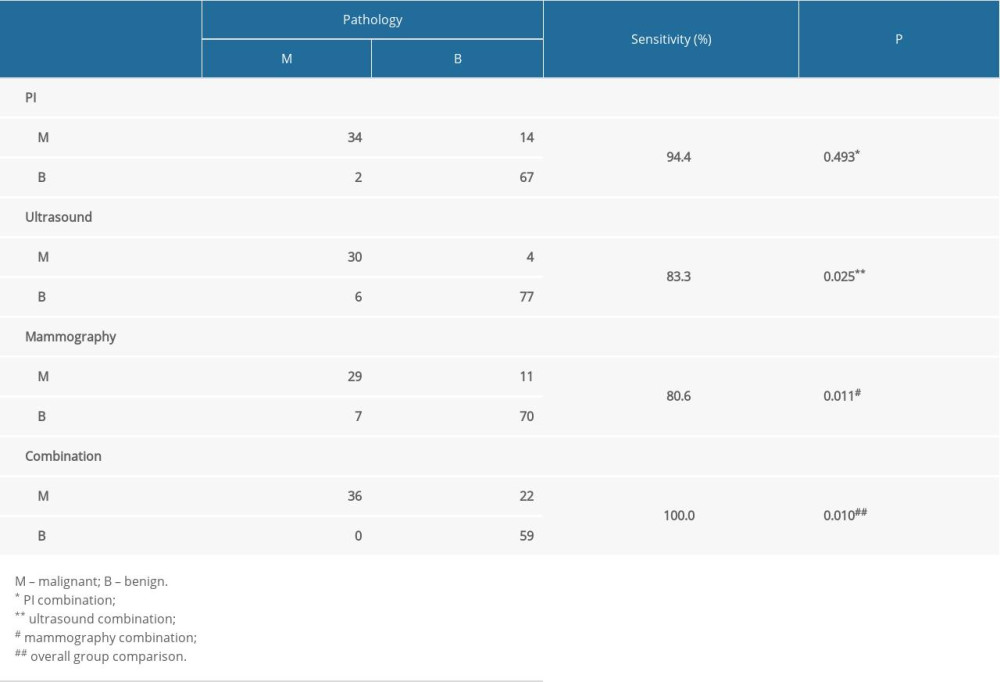 Table 7. Specificity of combination tests (serial test).
Table 7. Specificity of combination tests (serial test).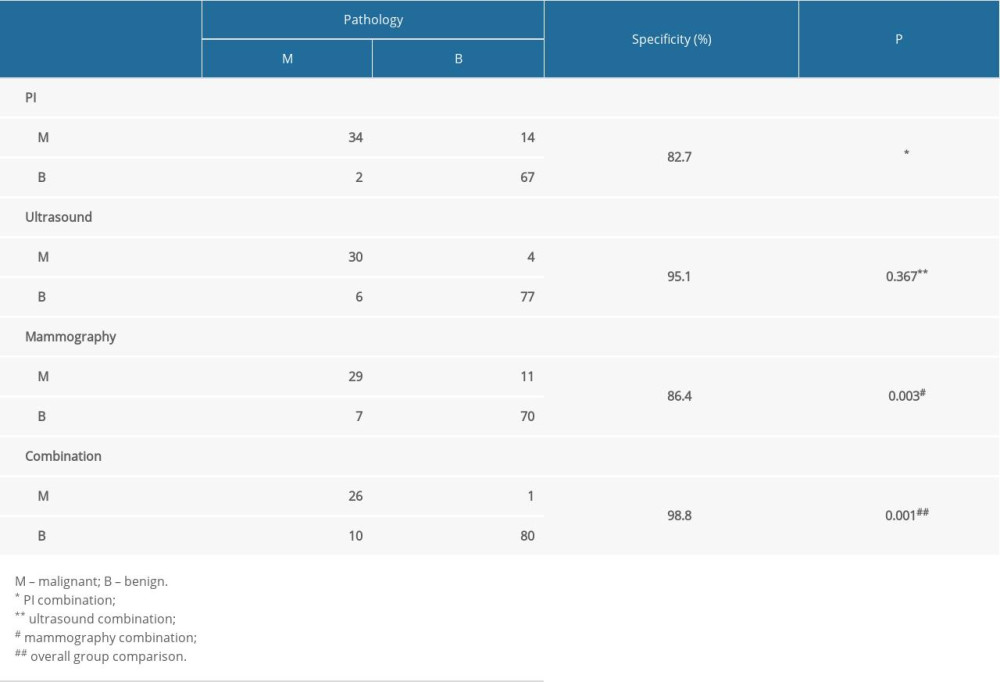
References
1. : World Health Organization (WHO) Breast Cancer Prevention and Control http://www.who.int/cancer/detection/breastcancer/en/
2. Hong W, Dong E, The past, present and future of breast cancer research in China: Cancer Lett, 2014; 351(1); 1-5
3. Gastounioti A, Hsieh M-K, Cohen E, Incorporating breast anatomy in computational phenotyping of mammographic parenchymal patterns for breast cancer risk estimation: Sci Rep, 2018; 8(1); 17489
4. Callahan CL, Wang Y, Marian C, DNA methylation and breast tumor clinicopathological features: The Western New York Exposures and Breast Cancer (WEB) Study: Epigenetics, 2016; 11(9); 643-52
5. Anuradha S, Su-Hyeong K, Andreas V, Singh SV, Suppression of FOXQ1 in benzyl isothiocyanate-mediated inhibition of epithelial-mesenchymal transition in human breast cancer cells: Carcinogenesis, 2013; 34(4); 864-73
6. An YY, Kim SH, Kang BJ, Characteristic features and usefulness of MRI in breast cancer in patients under 40 years old: Correlations with conventional imaging and prognostic factors: Breast Cancer, 2012; 21(3); 302-15
7. Ganz PA, Goodwin PJ, Breast Cancer Survivorship: Where are we today?: Adv Exp Med Biol, 2015; 862; 1-8
8. von Euler-Chelpin M, Abstract P2-02-05: Sensitivity of screening mammography by density and texture: A cohort study from a population based screening programme in Denmark: Cancer Res, 2020; 80(4 Suppl) P2-02-05
9. Checka CM, Chun JE, Schnabel FR, The relationship of mammographic density and age: implications for breast cancer screening: Am J Roentgenol, 2012; 198(3); W292-95
10. Lu B, Wei W, Sparse representation based multi-instance learning for breast ultrasound image classification: Comput Math Methods Med, 2017; 2017 7894705
11. Kuhl CK, Schrading S, Leutner CC, Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer: J Clin Oncol, 2005; 23(33); 8469-76
12. Kaufman CS, Jacobson L, Bachman BA, Kaufman LB, Digital documentation of the physical examination: Moving the clinical breast exam to the electronic medical record: Am J Surg, 2006; 192(4); 444-49
13. Mendelson E, Böhm-Vélez M, Berg W: ACR BI-RADS ultrasound ACR BI-RADS Atlas, Breast Imaging Reporting and Data System, 2013; 1-173, Reston, VA, American College of Radiology
14. Sickles E, d’Orsi C, Bassett L, 2013; 5
15. Wu JY, Chen WG, Mei ZYThe value of palpation imaging in the diagnosis of breast diseases: Chinese Journal of Practical Surgery, 2012; 32; 390-94 [in Chinese]
16. Wang B, Fu J, 2016, Beijing, China, Science Press [in Chinese]
17. Guo L, Tang LJ, Mao JDiagnostic value of sure-touch tactile breast imaging for breast cancer: Cancer Res Prev Treat, 2012; 39(6); 645-48 [in Chinese]
18. Ghoncheh M, Mahdavifar N, Darvishi E, Salehiniya H, Epidemiology, incidence and mortality of breast cancer in Asia: Asian Pac J Cancer Prev, 2016; 17(S3); 47-52
19. Swedish Organised Service Screening Evaluation Group, Reduction in breast cancer mortality from organized service screening with mammography: 1. Further confirmation with extended data: Cancer Epidemiol Biomarkers Prev, 2006; 15(1); 45-51
20. Aberle DR, Chiles C, Gatsonis C, Imaging and cancer: Research strategy of the American College of Radiology Imaging Network: Radiology, 2005; 235(3); 741-51
21. Bevers TB, Helvie M, Bonaccio E, Breast cancer screening and diagnosis, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology: J Natl Compr Cancer Netw, 2018; 16(11); 1362-89
22. Pisano ED, Gatsonis C, Hendrick E, Diagnostic performance of digital versus film mammography for breast-cancer screening: N Engl J Med, 2005; 353(17); 1773-83
23. Zanello P, Robim A, Oliveira TMG, Breast ultrasound diagnostic performance and outcomes for mass lesions using Breast Imaging Reporting and Data System category 0 mammogram: Clinics, 2011; 66(3); 443-48
24. Warner E, Messersmith H, Causer P, Systematic review: Using magnetic resonance imaging to screen women at high risk for breast cancer: Ann Intern Med, 2008; 148(9); 671-79
25. Mahoney L, Csima A, Efficiency of palpation in clinical detection of breast cancer: Can Med Assoc J, 1982; 127(8); 729-30
26. Malmartel A, Tron A, Caulliez S, Accuracy of clinical breast examination’s abnormalities for breast cancer screening: Cross-sectional study: Eur J Obstet Gynecol Reprod Biol, 2019; 237; 1-6
27. Wolf CJD, Detection of breast cancer. Clinical breast examination is not an acceptable alternative to mammography: BMJ, 2001; 322(7289); 792
28. Ophir J, Cespedes I, Ponnekanti H, Elastography: A quantitative method for imaging the elasticity of biological tissues: Ultrason Imaging, 1991; 13(2); 111-34
29. Krouskop TA, Wheeler TM, Kallel F, Elastic moduli of breast and prostate tissues under compression: Ultrason Imaging, 1998; 20(4); 260-74
30. Egorov V, Kearney T, Pollak SB, Differentiation of benign and malignant breast lesions by mechanical imaging: Breast Cancer Res Treat, 2009; 118(1); 67
Figures
 Figure 1. Palpation imaging machine (BT9; Beijing Xiantongkangqiao Medical Technology Corp., Beijing, China).
Figure 1. Palpation imaging machine (BT9; Beijing Xiantongkangqiao Medical Technology Corp., Beijing, China). Figure 2. Representative images of the malignant lesions. (A) 2-D image of benign lesion. (B) 3-D image of benign lesion. (C) 2-D image of malignant lesion. (D) 3-D image of malignant lesion.
Figure 2. Representative images of the malignant lesions. (A) 2-D image of benign lesion. (B) 3-D image of benign lesion. (C) 2-D image of malignant lesion. (D) 3-D image of malignant lesion. Figure 3. ROC curve of PI.
Figure 3. ROC curve of PI. Tables
 Table 1. Patient and tumor characteristics.
Table 1. Patient and tumor characteristics. Table 2. Correlation between PI examination index and clinicopathological data of patients with breast cancer.
Table 2. Correlation between PI examination index and clinicopathological data of patients with breast cancer. Table 3. BPI-RADS classification.
Table 3. BPI-RADS classification. Table 4. Comparison of examination results by different methods.
Table 4. Comparison of examination results by different methods. Table 5. Comparison of specificity and accuracy of different methods.
Table 5. Comparison of specificity and accuracy of different methods. Table 6. Sensitivity of combination tests (parallel test).
Table 6. Sensitivity of combination tests (parallel test). Table 7. Specificity of combination tests (serial test).
Table 7. Specificity of combination tests (serial test). Table 1. Patient and tumor characteristics.
Table 1. Patient and tumor characteristics. Table 2. Correlation between PI examination index and clinicopathological data of patients with breast cancer.
Table 2. Correlation between PI examination index and clinicopathological data of patients with breast cancer. Table 3. BPI-RADS classification.
Table 3. BPI-RADS classification. Table 4. Comparison of examination results by different methods.
Table 4. Comparison of examination results by different methods. Table 5. Comparison of specificity and accuracy of different methods.
Table 5. Comparison of specificity and accuracy of different methods. Table 6. Sensitivity of combination tests (parallel test).
Table 6. Sensitivity of combination tests (parallel test). Table 7. Specificity of combination tests (serial test).
Table 7. Specificity of combination tests (serial test). In Press
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
06 Mar 2024 : Clinical Research
Prevalence and Variation of Medical Comorbidities in Oral Surgery Patients: A Retrospective Study at Jazan ...Med Sci Monit In Press; DOI: 10.12659/MSM.943884
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








