29 January 2021: Database Analysis
Network Pharmacology-Based Analysis on the Mechanism of Action of Ephedrae Herba-Cinnamomi Ramulus Couplet Medicines in the Treatment for Psoriasis
Shun Guo12ACG*, Jin-Yong Zhou3BF, Cheng Tan12CE, Le Shi4D, Yue Shi12B, Jianxin Shi12EFDOI: 10.12659/MSM.927421
Med Sci Monit 2021; 27:e927421
Abstract
BACKGROUND: This study explored the mechanism of action of Ephedrae Herba-Cinnamomi Ramulus couplet medicine (MGCM) at the pharmacological level in the treatment of psoriasis.
MATERIAL AND METHODS: The active ingredients in MGCM were mined through literature retrieval and the BATMAN-TCM database, and potential targets were predicted. In addition, targets associated with psoriasis were acquired using multiple disease-related databases. Thereafter, an interaction network between candidate MGCM targets and the known psoriasis-associated targets was constructed based on the protein–protein interaction (PPI) data, using the STRING database. Then, the topological parameter degree was determined for mining the core targets for MGCM in the treatment of psoriasis, which also represented the major hubs within the PPI network. In addition, the core networks of targets and ingredients were constructed using Cytoscape software to apply MGCM in the treatment for psoriasis. These core targets were then analyzed for Gene Ontology biological processes and Kyoto Encyclopedia of Genes and Genomes pathway enrichment using OmicShare.
RESULTS: The ingredient-target core network of MGCM for treating psoriasis was constructed; it contained 52 active ingredients and corresponded to 19 core targets. In addition, based on enrichment analysis, these core targets were majorly enriched for several biological processes (immuno-inflammatory responses, leukocyte differentiation, energy metabolism, angiogenesis, and programmed cell death) together with the relevant pathways (Janus kinase-signal transducer and activator of transcription, toll-like receptors, nuclear factor κB, vascular endothelial growth factor, and peroxisome proliferator-activated receptor), thus identifying the possible mechanism of action of MGCM in treating psoriasis.
CONCLUSIONS: The present network pharmacology study indicated that MGCM alleviates various pathological factors of psoriasis through multiple compounds, multiple targets, and multiple pathways.
Keywords: Ethnopharmacology, Psoriasis, Databases, Factual, Databases, Genetic, Drugs, Chinese Herbal, Ephedra sinica, gene ontology, Molecular Docking Simulation, Protein Interaction Maps, Software
Background
Psoriasis, a common skin disorder, is easily diagnosed but refractory to treatment and prone to recurrence [1]. According to an epidemiological survey, psoriasis has an incidence of about 0.5% in the Chinese population [2]. However, the underlying pathogenesis of psoriasis remains incompletely understood. Existing studies suggest that aberrant psoriasis-susceptible gene expression, autoimmune disorders, obesity, and abnormalities in multiple inflammatory signaling pathways contribute to the development of psoriasis [3]. However, the pathogenesis remains largely unclear, which hinders specific treatment. Currently, there is no curative treatment for psoriasis. Traditional Chinese medicine (TCM) has a distinct effect in the treatment of psoriasis, and it has a therapeutic impact via multiple targets and pathways that correspond to the diverse pathways that are dysregulated in psoriasis [4–6]. Yet the underlying mechanism of action of TCM in psoriasis treatment remains unclear, thus restricting its internationalization and standardization in treating psoriasis.
In the TCM clinical treatment of psoriasis, the heat-clearing and blood-cooling method is usually adopted, but no satisfactory effect is consistently achieved [7]. Based on the treatment idea of promoting the expulsion of exogenous pathogenic evils, numerous TCM experts have proposed that the additional application of the sweating method could significantly increase the therapeutic effect of TCM on psoriasis [8]. Ephedrae Herba-Cinnamomi Ramulus couplet medicine (MGCM) contains the 2 most representative traditional Chinese herbal medicines for inducing sweating and dispelling exogenous evils: Ephedrae Herba (Mahuang, MH) and Cinnamomi Ramulus (Guizhi, GZ). This combination represents the empirical couplet medicines adopted in the Department of Dermatology in our hospital to treat psoriasis. Numerous preclinical and clinical studies indicate that these 2 herbal medicines are effective for treating psoriasis [9,10]. In addition, years of clinical practice show that MGCM is effective against psoriasis. MGCM was shown in our prior research to suppress abnormal keratinocyte proliferation and chemokine release and thus to inhibit infiltration of multiple immunocytes [11–13]. Nonetheless, the scientific foundation and exact molecular mechanisms of MGCM remain unknown, so more research is warranted.
In traditional studies that examine the TCM mechanism, the “one drug, one target, one disease” model is adopted, but it cannot reveal the “multiple components, multiple targets, and multiple pathways” of TCM. In the present study, several algorithm- and network-based computational methods were adopted in combination to predict active ingredients, mine various drug targets, and construct core networks of targets and ingredients of MGCM for treating psoriasis. Macroscopic network analysis was then performed to illustrate the possible mechanisms of MGCM and provide a basis for future research.
Material and Methods
SELECTION OF CANDIDATE MGCM ACTIVE INGREDIENTS AND TARGETS:
The BATMAN-TCM database (http://bionet.ncpsb.org.cn/batman-tcm/) has been developed as the bioinformatics analytical approach to analyze the active ingredients in TCM [14,15]. To obtain information on MGCM ingredients, “Ephedrae Herba” and “Cinnamomi Ramulus” were used as keywords to search the BATMAN database. A total of 116 compounds were identified, and their names and code numbers are shown in Table 1.
The BATMAN-TCM database also predicts the candidate compound targets according to their similarities to known drug-target interactions (Target score cutoff ≥20 and P value cutoff <0.05) [16]. In addition, the Traditional Chinese Medicine System Pharmacology Database (TCMSP, http://lsp.nwu.edu.cn/tcmsp.php) [17] was used to predict the potential targets of medicinal components.
SCREENING OF THE KNOWN PSORIASIS-ASSOCIATED TARGETS:
To obtain the known targets related to psoriasis, “psoriasis” was used as the keyword in searches of the DisGeNet platform [18], MalaCards database [19], DrugBank database [20], and Therapeutic Target Database [21]. The DisGeNet database results were classified according to the disease specificity index (DSI), and targets with a DSI value lower than the median value of genes known to be related to psoriasis were removed. In addition, relevant targets were removed if the drugs had aberrant status in DrugBank and the Therapeutic Target Database. Supplementary Table 1 summarizes more details of the known targets related to psoriasis following redundancy deletion.
MINING OF CORE TARGETS OF MGCM IN THE TREATMENT OF PSORIASIS AND ESTABLISHMENT OF CORE ACTIVE INGREDIENT-TARGET NETWORK:
First, Homo sapiens was selected as the species to standardize targets that were acquired in the aforementioned 2 steps (including candidate MGCM targets and the known targets related to psoriasis) based on the UniProt database [22], to obtain the names of individual universal genes. Thereafter, the candidate MGCM targets and known targets related to psoriasis were imported into the Wayne diagram online tool (http://bioinformatics.psb.ugent.be/webtools/Venn/) for mapping. In other words, targets obtained via the 2 sets were intersected for acquiring potential MGCM targets in psoriasis treatment.
For every interaction, the STRING server [23] produced a “combined score” of 0–1, with a higher score indicating more confidence that an interaction exists. In STRING, the interactions >0.4 and >0.7 indicate medium and high confidence, respectively. The potential targets were uploaded to the STRING database to obtain PPIs, with a minimum interaction score of 0.4 and Homo sapiens as the species. For every target (node) within the network, the topological factor degree, which means the number of edges shared with other nodes, was determined through the plug-in cytoHubba [24]. Then, twice the median of degrees for all targets served as the screening criterion. Nodes in which the degree values were greater than twice the median were selected to be the pivotal hubs within that PPI network; that is, they were the core MGCM targets for treating psoriasis. Finally, the core active ingredient-target network was constructed using Cytoscape.
CORE TARGET ENRICHMENT ANALYSIS:
Core targets were assessed in Gene Ontology (GO) biological process (BP) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses using OmicShare software [25] with the species of Homo. The adjusted P value of ≤0.01 was utilized as the criterion in enrichment analysis.
Results
SELECTION OF CANDIDATE ACTIVE INGREDIENTS AS WELL AS TARGETS OF MGCM:
The BATMAN-TCM database was retrieved comprehensively, and altogether 116 MGCM compounds were identified. Of these compounds, 104 and 14 were the active ingredients of MH and GZ, respectively. Several compounds were extensively distributed in these 2 herbs, including camphor and nerolidol. Table 1 presents the basic MGCM ingredients.
Thereafter, the potential targets of MGCM components were identified, and altogether 1338 targets were identified (
To acquire comprehensive understanding of the network of candidate ingredients and targets of MGCM, we established a network map with the Cytoscape software, which included 1384 nodes and 8464 edges (Figure 1). Specifically, the node degree indicated the number of targets or edges correlated with the node based on topological analysis. Altogether, 58 ingredients with the degree of ≥33 were discovered in our established network, including piperitone, ephedrine, and cinnamic acid, which acted on 266, 219, and 56 targets, respectively. These compounds have been proven to have a wide range of pharmacological activities (e.g., anti-inflammatory, antioxidant, immune regulation) [26,27], which were subsequently considered to be the active ingredients of MGCM.
MINING OF MGCM CORE TARGETS IN THE TREATMENT OF PSORIASIS:
Psoriasis is considered a polygenic disease. Investigating the association of genes with the environment might contribute to revealing the pathogenesis of psoriasis. Targets that had an aberrant status from DrugBank and Therapeutic Target Database or with the median DSI of <0.535 based on the DisGeNet database were removed, and a total of 605 psoriasis-related targets (Supplementary Table 1) were obtained from those 4 sources. In addition, 117 recognized candidate MGCM targets were also targets related to psoriasis (or therapeutics) (Supplementary Table 3, Figure 2A) and were identified as potential MGCM targets in psoriasis treatment.
Later, to further select the MGCM core targets in psoriasis treatment, the PPI network was established based on the above-mentioned targets using the STRING database (Figure 2B). Then, topological parameters (Degree) for all nodes within the network were calculated by the plug-in cytoHubba (Supplementary Table 4). Afterwards, twice the median number of degrees for all targets was utilized as the selection criteria. Any node in which the degree values were greater than twice the median (=29) was identified as a pivotal hub that played a vital part within the PPI network. Consequently, 19 targets (Table 2) were selected according to the topological parameter values (Figure 2C), and they were selected as the MGCM core targets for the treatment of psoriasis.
ESTABLISHMENT OF THE CORE NETWORK OF ACTIVE INGREDIENTS-TARGETS FOR MGCM IN THE TREATMENT OF PSORIASIS:
To better understanding the “multiple target and multiple ingredient” mechanism of MGCM in the treatment of psoriasis, the candidate MGCM ingredients affecting 19 core targets were identified according to the association of ingredients with corresponding targets (Table 3). Thereafter, a core network was established regarding the active ingredients and targets (Figure 3A) by Cytoscape, and degree value of each node within the network was analyzed statistically. As shown in Figure 3B, the degree values of active ingredients within the core network were between 1 and 11, and the median was 2, which suggested that over half of the compounds had at least 2 targets. In addition, the degree value of targets was between 1 and 23 (Figure 3C), and the median was 9. The top 3 active ingredients with the highest degrees were ephedrine, pseudoephedrine, and coumarinic acid. The top 3 targets with the highest degrees were tumor necrosis factor (TNF), interleukin (IL)-10, and IL-1B, which all play vital roles in psoriasis pathogenesis and are involved in activities such as aberrant keratinocyte differentiation, inflammatory reactions, and immune cell infiltration [28–30].
MGCM CORE TARGET ENRICHMENT ANALYSIS IN THE TREATMENT OF PSORIASIS:
The multiple-target and multiple-pathway mechanism of MGCM in the treatment of psoriasis was further explored through performing GO-BP and KEGG enrichment analyses of the core targets using the OmicShare platform. In addition, MGCM-regulated BPs and related signal transduction pathways in psoriasis treatment were mined. The above-mentioned 19 core targets participated in some BPs, which mainly included the cell responses to multiple stimuli (such as nutrient substances and oxidative stress), immuno-inflammatory responses, leukocyte differentiation, cellular energy metabolism, angiogenesis, and programmed cell death (Figure 4A). In addition, the 5 most significant signaling pathways, namely, the JAK-STAT pathway and pathways involving toll-like receptors (TLRs), nuclear factor (NF)-κB, vascular endothelial growth factor (VEGF), and peroxisome proliferator-activated receptor (PPAR), were selected based on the P values of the enriched pathways as well as the corresponding psoriasis correlations (Figure 4B).
Discussion
MGCM has been developed as an empirical prescription to treat psoriasis in the Department of Dermatology in our hospital. MGCM has achieved significant clinical efficacy in treating psoriasis; however, its core targets and active ingredients remain largely unknown, which has blocked the development and further clinical application of MGCM. Network pharmacology represents a novel drug design and development approach that is based on rapidly developing multidirectional pharmacology and systemic biology. Initially put forward by Hopkins [31] in 2007, this concept has led to a change from the established “disease, single target, single drug” model to the “disease, multiple targets, multiple drugs” model for the development of new drugs. This concept coincides with the TCM “holistic view” of patient care [32]. As a result, applying the network pharmacology approach provides some insight into the MGCM mechanism in the treatment of psoriasis.
The current study identified 116 candidate MGCM active ingredients for the treatment of psoriasis. The identifications were based on multiple network pharmacological approaches, and the active ingredients corresponded to 19 core targets. Psoriasis is currently associated with 4 well-recognized histopathological characteristics, including inflammatory infiltration in the epidermis and dermis, aberrant keratinocyte biological behaviors (apoptosis, hyperproliferation and differentiation), metabolic dysregulation, and the tortuously elevated formation of dermal capillaries and blood vessels [33–36]. First of all, a majority of the 19 core targets, including ILs, prostaglandin-endoperoxide synthase 2, TNF, C-C motif chemokine ligand 2, epidermal growth factor receptor, and interferon γ, were identified as participating in the aberrant inflammatory infiltration. These targets modulate lymphocyte chemotaxis and differentiation, generate cytokines, and control immunological inflammatory responses in the epidermis and dermis [29,37–39]. Second, MAPKs, TP53, and JUN exhibit abnormal keratinocyte biological behaviors in the context of psoriasis [40–42]. Third, abnormalities in insulin and albumin metabolism usually occur in patients with psoriasis [43,44]. Finally, intercellular adhesion molecule 1 is tightly correlated with the proliferation, adhesion, and migration of endothelial cells, which are correlated with the tortuously elevated dermal capillaries and blood vessels [45].
According to results of GO-BP and KEGG enrichment analyses of core targets, MGCM intervened with psoriasis via several BPs and some signal transduction pathways, including JAK-STAT, TLRs, NF-κB, VEGF, and PPAR. These 5 signal transduction pathways had cross-talk effects within this network. The VEGF signaling pathway is suggested to cause pathological angiogenesis within psoriatic lesions through modulating endothelial cell differentiation and proliferation. It induces inflammatory response through enhancing the vascular permeability, which promotes the infiltration of inflammatory cells [46]. Additionally, psoriasis represents a T-lymphocyte-mediated inflammatory disorder, in which aberrant differentiation of T lymphocytes (particularly Th1 and Th17 cells) and excessive secretion of pro-inflammatory factors (e.g., ILs) are closely correlated with disease progression [47–49]. Findings in the present study indicated that some key signal transduction pathways were correlated with the MGCM-mediated differentiation of T lymphocytes and the production of pro-inflammatory factors.
In this study, our network pharmacological analysis supports that the ephedrine alkaloids in MGCM (including ephedrine and pseudoephedrine) may be the core pharmacodynamic active compounds exerting the most critical effects against psoriasis. The ephedrine alkaloids have been verified in previous research to activate the α and β receptors, which can directly activate the adrenergic receptor in the body and indirectly promote the release of noradrenaline neurotransmitter to excite the sympathetic nerve, thus promoting perspiration and dispelling the internal pathogenic evils. In GZ, the cinnamic acid and cinnamaldehyde can dilate blood vessels, promote blood circulation, accelerate blood flow to the body surface, and reinforce the perspiration caused by MH. Both MH and GZ represent drugs for inducing sweat. The combined application of these 2 drugs facilitates expulsion of the internal pathogenic evils, thus producing a therapeutic effect [11]. Moreover, our previous research suggests that ephedrine and pseudoephedrine can suppress the β-adrenergic receptor on the keratinocyte membrane surface, induce the intracellular cAMP level, and regulate cell proliferation. In addition, previous research also indicated that ephedrine and pseudoephedrine can regulate the immune inflammatory response in the body and suppress the release of inflammatory factors at lesion sites [12,13].
According to our previous study, using MGCM to treat psoriasis is safe and effective based on clinical observations. Findings in the present work revealed that MGCM exerts a non-unilateral regulatory effect on the treatment of psoriasis; instead, it has indirect or direct effects on the integrated treatment of those 4 main pathological parameters via several signal transduction pathways related to metabolism, immune and inflammatory responses, and aberrant angiogenesis. Nonetheless, certain limitations should be noted despite the significant findings. First of all, several compounds in MGCM herbal medicines were not taken into account due to insufficient laboratory results or available data. Second, we have already treated the quality control components (such as ephedrine, pseudoephedrine, and cinnamaldehyde) in MH and GZ in the current standard from Chinese pharmacopoeia or the components with relatively high contents as the candidate pharmacodynamic compounds for research. Meanwhile, these components have also been predicted and screened as the core active ingredients in MGCM against psoriasis in this study. However, this study may treat all components equally to some extent and thus ignore the influence of the absolute content of each compound in MGCM and the serum and skin tissue distribution concentrations. Third, this study only predicts the drug-target interactions through network pharmacological means; it does not illustrate the type of effect on targets (e.g., activation or suppression, upregulation or downregulation). As a result, in future research, we aim to extensively examine (1) the enrichment degrees and contents of screened core active ingredients in the blood or skin tissues of experimental animals or patients through UPLC-MS to further confirm the core active ingredients in MGCM against psoriasis; (2) the regulatory effect of MGCM and the core active ingredients on the screened core targets and signaling pathways in patients, animal models, and
Conclusions
In this work, we successfully systematically illuminated the possible “multiple compounds, multiple targets” therapeutic action of MGCM on psoriasis, and predicted, screened, and analyzed the genes, proteins, and pathways that might play a vital role in the biological process. However, because this study was based on data mining and data analysis, further studies should be undertaken to validate the findings.
Figures
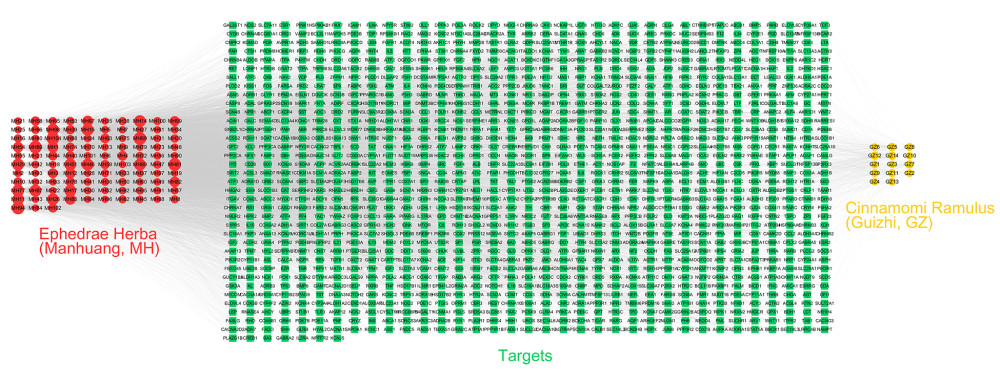 Figure 1. Construction of the network of Ephedrae Herba-Cinnamomi Ramulus couplet medicine (MGCM) compounds and their potential targets. The active compounds (compounds ID) collected from diverse herbal medicines were linked with corresponding potential targets to construct the compound-target network, with a node indicating an active compound (the diverse colors of circle stand for diverse herbal medicines) and the target (green square).
Figure 1. Construction of the network of Ephedrae Herba-Cinnamomi Ramulus couplet medicine (MGCM) compounds and their potential targets. The active compounds (compounds ID) collected from diverse herbal medicines were linked with corresponding potential targets to construct the compound-target network, with a node indicating an active compound (the diverse colors of circle stand for diverse herbal medicines) and the target (green square). 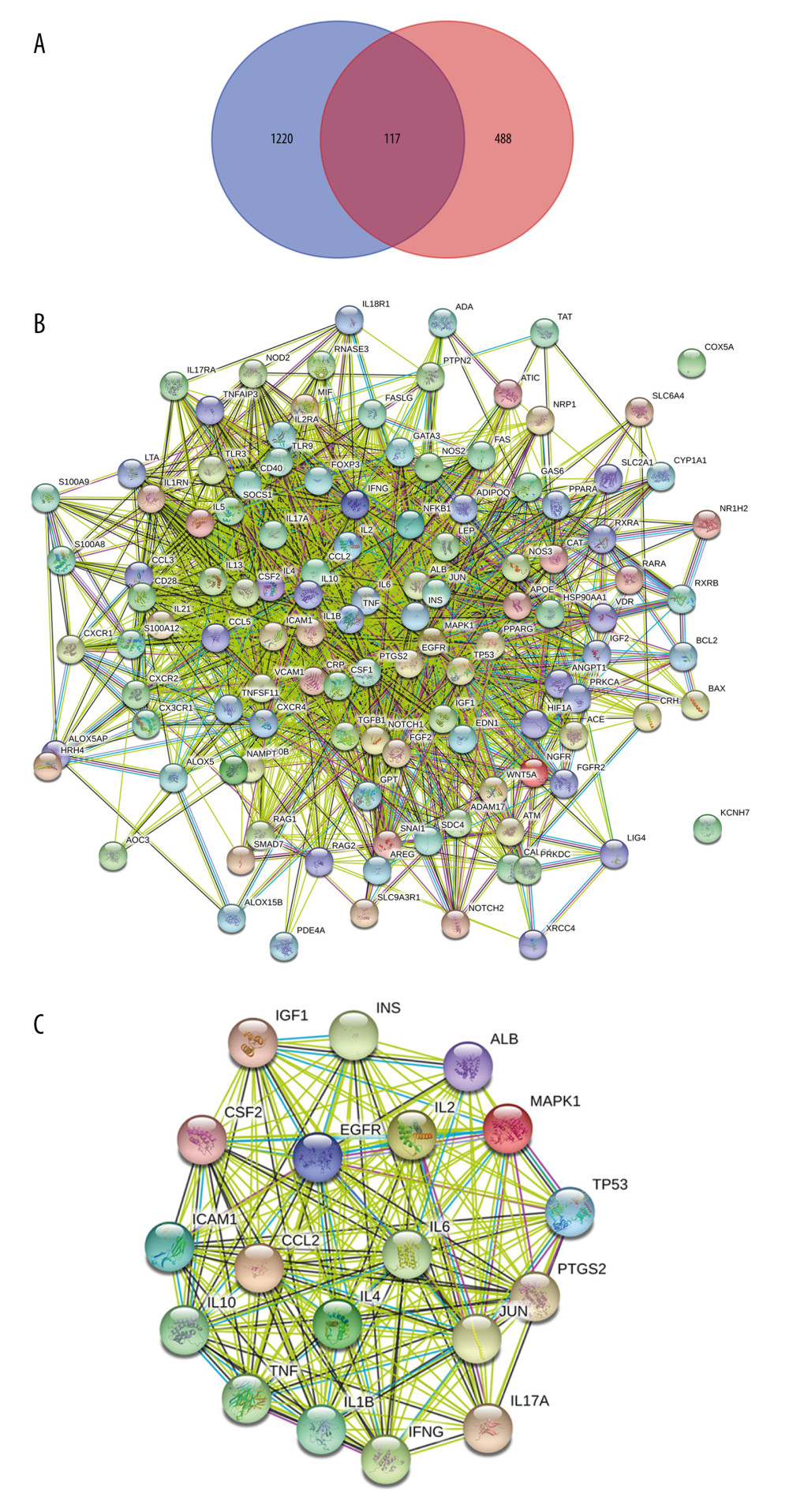 Figure 2. Identification of the core targets of Ephedrae Herba-Cinnamomi Ramulus couplet medicines (MGCM) in treating psoriasis. (A) The Venn diagram shows that MGCM shared 117 potential targets with known components of the pathological course related to psoriasis. (B) The protein–protein interaction (PPI) network of all 117 candidate targets of MGCM in treating psoriasis. (C) The PPI network of the core targets of MGCM in treating psoriasis.
Figure 2. Identification of the core targets of Ephedrae Herba-Cinnamomi Ramulus couplet medicines (MGCM) in treating psoriasis. (A) The Venn diagram shows that MGCM shared 117 potential targets with known components of the pathological course related to psoriasis. (B) The protein–protein interaction (PPI) network of all 117 candidate targets of MGCM in treating psoriasis. (C) The PPI network of the core targets of MGCM in treating psoriasis. 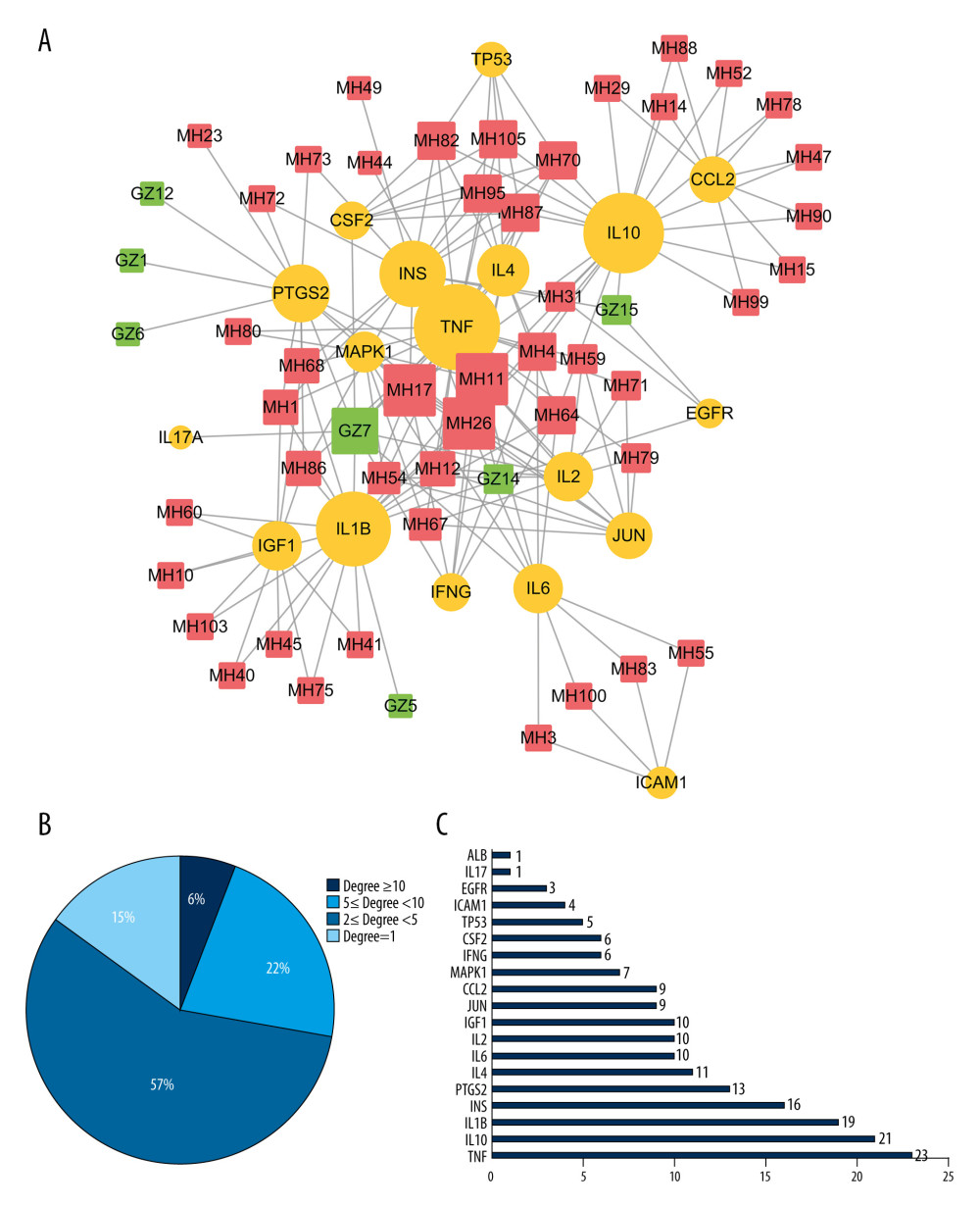 Figure 3. (A) Construction of the core network of active ingredients of Ephedrae Herba-Cinnamomi Ramulus couplet medicines (MGCM) and their targets in treating psoriasis, and the statistical analysis of the degree of each (B) ingredient and (C) target in the network. All nodes were sorted and calculated according to the degree of freedom, and the node size in the network was associated with the degree.
Figure 3. (A) Construction of the core network of active ingredients of Ephedrae Herba-Cinnamomi Ramulus couplet medicines (MGCM) and their targets in treating psoriasis, and the statistical analysis of the degree of each (B) ingredient and (C) target in the network. All nodes were sorted and calculated according to the degree of freedom, and the node size in the network was associated with the degree. 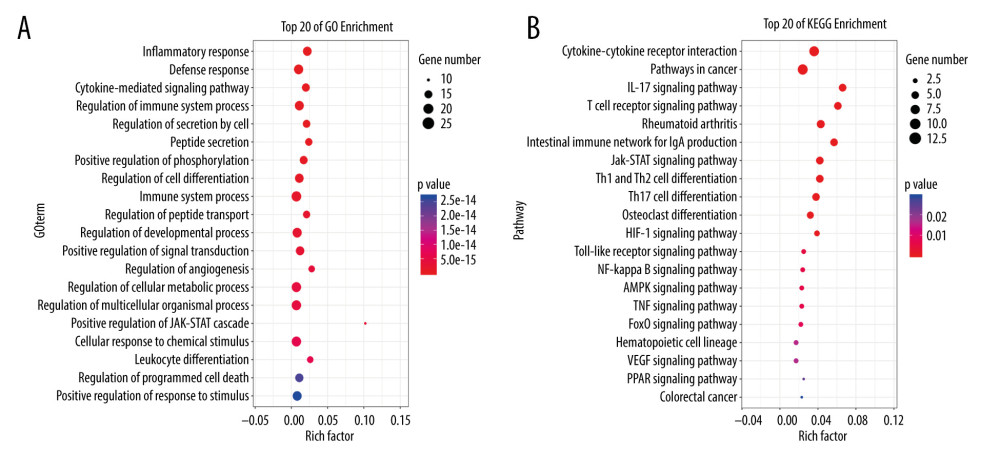 Figure 4. Enrichment analysis of core targets of Ephedrae Herba-Cinnamomi Ramulus couplet medicines (MGCM) in treating psoriasis based on OmicShare. We considered a P value cutoff of ≤0.05 as significant and applied hypergeometric tests to identify (A) enriched Gene Ontology biological processes (GO-BP) and (B) Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways. The chart shows an overview of the analysis with up to 20 significantly enriched processes and pathways.
Figure 4. Enrichment analysis of core targets of Ephedrae Herba-Cinnamomi Ramulus couplet medicines (MGCM) in treating psoriasis based on OmicShare. We considered a P value cutoff of ≤0.05 as significant and applied hypergeometric tests to identify (A) enriched Gene Ontology biological processes (GO-BP) and (B) Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways. The chart shows an overview of the analysis with up to 20 significantly enriched processes and pathways. Tables
Table 1. All the candidate ingredients of Ephedrae Herba-Cinnamomi Ramulus couplet medicines (MGCM).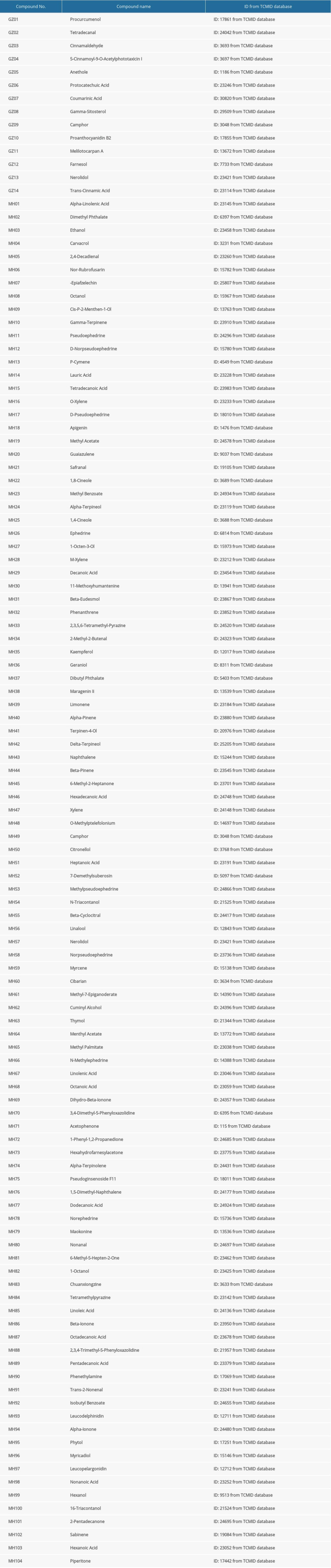 Table 2. Degree values of core targets for Ephedrae Herba-Cinnamomi Ramulus couplet medicines (MGCM) against psoriasis.
Table 2. Degree values of core targets for Ephedrae Herba-Cinnamomi Ramulus couplet medicines (MGCM) against psoriasis.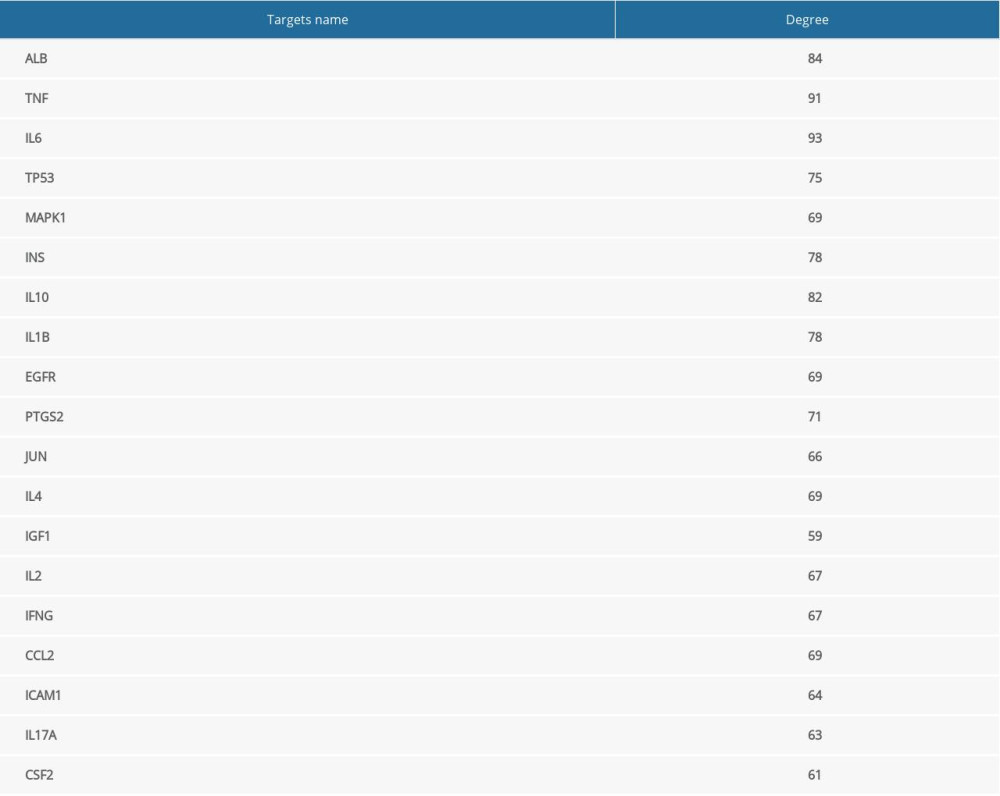 Table 3. Fifty-two core pharmacologically active ingredients of Ephedrae Herba-Cinnamomi Ramulus couplet medicines (MGCM) in the treatment of psoriasis.
Table 3. Fifty-two core pharmacologically active ingredients of Ephedrae Herba-Cinnamomi Ramulus couplet medicines (MGCM) in the treatment of psoriasis.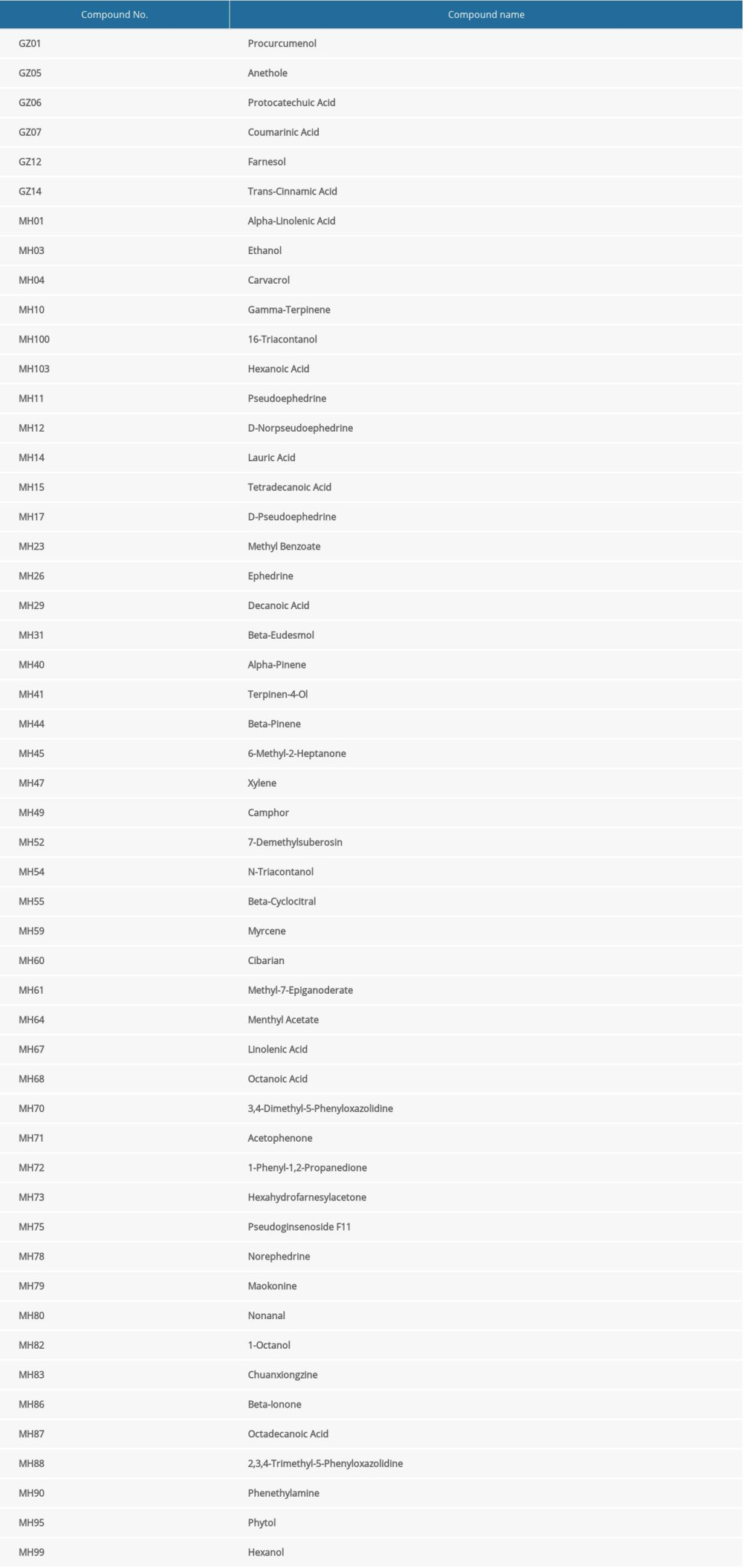
References
1. Micali G, Verzì AE, Giuffrida G, Inverse psoriasis: From diagnosis to current treatment options: Clin Cosmet Investig Dermatol, 2019; 12; 953-59
2. Luo Y, Ru Y, Sun X, Characteristics of psoriasis vulgaris in China: A prospective cohort study protocol: Ann Transl Med, 2019; 7; 694
3. Li J, Yu M, Wang YW, Prevalence of psoriasis and associated risk factors in China: protocol of a nationwide, population-based, cross-sectional study: BMJ Open, 2019; 9; e027685
4. Meng S, Lin Z, Wang Y, Psoriasis therapy by Chinese medicine and modern agents: Chin Med, 2018; 13; 16
5. Chiang CC, Cheng WJ, Lin CY, Kan-Lu-Hsiao-Tu-Tan, a traditional Chinese medicine formula, inhibits human neutrophil activation and ameliorates imiquimod-induced psoriasis-like skin inflammation: J Ethnopharmacol, 2020; 246; 112246
6. Coyle ME, Yu JJ, Zhang AL, Patient experiences of using Chinese herbal medicine for psoriasis vulgaris and chronic urticaria: a qualitative study: J Dermatolog Treat, 2020; 31(4); 352-58
7. Lin W, Yu Q, Qin Y, To explore the clinical efficacy of Traditional Chinese Medicine bath in the treatment of psoriasis vulgaris with blood-heat syndrome and its effect on related cytokines based on different temperature and different concentration: Medicine (Baltimore), 2020; 99; e20172
8. Xu PTreating psoriasis of the Xuere syndrome of the ‘winter severe-summer mild’ type by the Han theory: Clin J Chin Med, 2019; 11; 112-14 [in Chinese]
9. Li Z, Li J, Guo MClinical observation of Mahuang Zimei decoction on treating psoriasis: Chin Arch Trad Chin Med, 2013; 31; 51-52 [in Chinese]
10. Cui L, Liu AExperience of professor LIU Ai-min in treatment for ‘the yang deficiency combined with exogenous cold’ type of psoriasis by Mahuang Fuzi Xixin Decoction: Chin Arch Trad Chin Med, 2015; 29; 93-96 [in Chinese]
11. Zou Y, Guo S, Jiang YEffect of Ephedrae Herba-Cinnamomi Ramulus herb pair on proliferation and apoptosis of interleukin-22-mediated HaCaT cells: J Pract Med, 2016; 32; 3986-89 [in Chinese]
12. Guo S, Zhou X, Chen JEffect of β2-AR/cAMP pathway by “drug pair” of ephedra cassia twig on IL-22 induced HaCaT cells: Lishizhen Medicine and Materia Medica Research, 2019; 11; 2600-2 [in Chinese]
13. Jiang Y, Guo S, Zhou YEffect of ephedrine on CCL20 secretion of IL-17-induced immortalized human keratinocytes (HaCaT): Shandong Med J, 2017; 10; 24-27 [in Chinese]
14. Liu Z, Guo F, Wang Y, BATMAN-TCM: a Bioinformatics Analysis Tool for Molecular mechANism of Traditional Chinese Medicine: Sci Rep, 2016; 6; 21146
15. Zhang R, Zhu X, Bai H, Ning K, Network pharmacology databases for traditional Chinese medicine: Review and assessment: Front Pharmacol, 2019; 10; 123
16. Zhang G, Jiang X, Liu Y, Therapeutic efficiency of an external Chinese herbal formula of mammary precancerous lesions by BATMAN-TCM online bioinformatics analysis tool and experimental validation: Evid Based Complement Alternat Med, 2019; 2019; 2795010
17. Ru J, Li P, Wang J, TCMSP: A database of systems pharmacology for drug discovery from herbal medicines: J Cheminform, 2014; 6; 13
18. Piñero J, Ramírez-Anguita JM, Saüch-Pitarch J, The DisGeNET knowledge platform for disease genomics: 2019 update: Nucleic Acids Res, 2020; 48; D845-55
19. Rappaport N, Twik M, Plaschkes I, MalaCards: An amalgamated human disease compendium with diverse clinical and genetic annotation and structured search: Nucleic Acids Res, 2017; 45; D877-87
20. Wishart DS, Feunang YD, Guo AC, DrugBank 5.0: A major update to the DrugBank database for 2018: Nucleic Acids Res, 2018; 46; D1074-82
21. Wang Y, Zhang S, Li F, Therapeutic Target Database 2020: Enriched resource for facilitating research and early development of targeted therapeutics: Nucleic Acids Res, 2020; 48; D1031-41
22. The UniProt Consortium, UniProt: The universal protein knowledgebase: Nucleic Acids Res, 2017; 45; D158-69
23. Szklarczyk D, Morris JH, Cook H, The STRING database in 2017: Quality-controlled protein–protein association networks, made broadly accessible: Nucleic Acids Res, 2017; 45; D362-68
24. Chin CH, Chen SH, Wu HH, cytoHubba: Identifying hub objects and sub-networks from complex interactome: BMC Syst Biol, 2014; 8(Suppl 4); S11
25. Su M, Guo C, Liu M, Liang X, Yang B, Therapeutic targets of vitamin C on liver injury and associated biological mechanisms: A study of network pharmacology: Int Immunopharmacol, 2019; 66; 383-87
26. Pontiki E, Hadjipavlou-Litina D, Multi-target cinnamic acids for oxidative stress and inflammation: Design, synthesis, biological evaluation and modeling studies: Molecules, 2018; 24(1); 12
27. Zheng Y, Guo Z, He W, Ephedrine hydrochloride protects mice from LPS challenge by promoting IL-10 secretion and inhibiting proinflammatory cytokines: Int Immunopharmacol, 2012; 13; 46-53
28. Furue K, Ito T, Tsuji G, Psoriasis and the TNF/IL23/IL17 axis: G Ital Dermatol Venereol, 2019; 154; 418-24
29. Chima M, Lebwohl M, TNF inhibitors for psoriasis: Semin Cutan Med Surg, 2018; 37; 134-42
30. Szabó K, Bata-Csörgő Z, Dallos A, Regulatory networks contributing to psoriasis susceptibility: Acta Derm Venereol, 2014; 94; 380-85
31. Hopkins AL, Network pharmacology: Nat Biotechnol, 2007; 25; 1110-11
32. Zhou Z, Chen B, Chen S, Applications of network pharmacology in traditional Chinese medicine research: Evid Based Complement Alternat Med, 2020; 2020; 1646905
33. Ni X, Lai Y, Keratinocyte: A trigger or an executor of psoriasis?: J Leukoc Biol, 2020; 108(2); 485-91
34. Ogawa E, Sato Y, Minagawa A, Okuyama R, Pathogenesis of psoriasis and development of treatment: J Dermatol, 2018; 45; 264-72
35. Sankar L, Arumugam D, Boj S, Pradeep P, Expression of angiogenic factors in psoriasis vulgaris: J Clin Diagn Res, 2017; 11; EC23-27
36. Hiebert P, Werner S, Targeting metabolism to treat psoriasis: Nat Med, 2018; 24; 537-39
37. Domala A, Bale S, Godugu C, Protective effects of nanoceria in imiquimod induced psoriasis by inhibiting the inflammatory responses: Nanomedicine (Lond), 2020; 15; 5-22
38. Grän F, Kerstan A, Serfling E, Current developments in the immunology of psoriasis: Yale J Biol Med, 2020; 93; 97-110
39. Behfar S, Hassanshahi G, Nazari A, Khorramdelazad H, A brief look at the role of monocyte chemoattractant protein-1 (CCL2) in the pathophysiology of psoriasis: Cytokine, 2018; 110; 226-31
40. Zenz R, Wagner EF, Jun signalling in the epidermis: From developmental defects to psoriasis and skin tumors: Int J Biochem Cell Biol, 2006; 38; 1043-49
41. Haase I, Hobbs RM, Romero MR, A role for mitogen-activated protein kinase activation by integrins in the pathogenesis of psoriasis: J Clin Invest, 2001; 108; 527-36
42. Raho G, Vena GA, Bizzoca A, Influence of infliximab on keratinocyte apoptosis in psoriasis patients: Immunopharmacol Immunotoxicol, 2011; 33; 227-31
43. Işik S, Kılıç S, Öğretmen Z, The correlation between the psoriasis area severity index and ischemia-modified albumin, mean platelet volume levels in patients with psoriasis: Postepy Dermatol Alergol, 2016; 33; 290-93
44. Polic MV, Miskulin M, Smolic M, Psoriasis severity – a risk factor of insulin resistance independent of metabolic syndrome: Int J Environ Res Public Health, 2018; 15(7); 1486
45. Wen J, Pei H, Wang X, Gambogic acid exhibits anti-psoriatic efficacy through inhibition of angiogenesis and inflammation: J Dermatol Sci, 2014; 74; 242-50
46. Marina ME, Roman II, Constantin A-M, VEGF involvement in psoriasis: Clujul Med, 2015; 88; 247-52
47. Picciani BLS, Domingos TA, Teixeira-Souza T, Evaluation of the Th17 pathway in psoriasis and geographic tongue: An Bras Dermatol, 2019; 94; 677-83
48. Shi Y, Chen Z, Zhao Z, IL-21 induces an imbalance of Th17/Treg cells in moderate-to-severe plaque psoriasis patients: Front Immunol, 2019; 10; 1865
49. Furiati SC, Catarino JS, Silva MV, Th1, Th17, and Treg responses are differently modulated by TNF-alpha inhibitors and methotrexate in psoriasis patients: Sci Rep, 2019; 9; 7526
Figures
 Figure 1. Construction of the network of Ephedrae Herba-Cinnamomi Ramulus couplet medicine (MGCM) compounds and their potential targets. The active compounds (compounds ID) collected from diverse herbal medicines were linked with corresponding potential targets to construct the compound-target network, with a node indicating an active compound (the diverse colors of circle stand for diverse herbal medicines) and the target (green square).
Figure 1. Construction of the network of Ephedrae Herba-Cinnamomi Ramulus couplet medicine (MGCM) compounds and their potential targets. The active compounds (compounds ID) collected from diverse herbal medicines were linked with corresponding potential targets to construct the compound-target network, with a node indicating an active compound (the diverse colors of circle stand for diverse herbal medicines) and the target (green square). Figure 2. Identification of the core targets of Ephedrae Herba-Cinnamomi Ramulus couplet medicines (MGCM) in treating psoriasis. (A) The Venn diagram shows that MGCM shared 117 potential targets with known components of the pathological course related to psoriasis. (B) The protein–protein interaction (PPI) network of all 117 candidate targets of MGCM in treating psoriasis. (C) The PPI network of the core targets of MGCM in treating psoriasis.
Figure 2. Identification of the core targets of Ephedrae Herba-Cinnamomi Ramulus couplet medicines (MGCM) in treating psoriasis. (A) The Venn diagram shows that MGCM shared 117 potential targets with known components of the pathological course related to psoriasis. (B) The protein–protein interaction (PPI) network of all 117 candidate targets of MGCM in treating psoriasis. (C) The PPI network of the core targets of MGCM in treating psoriasis. Figure 3. (A) Construction of the core network of active ingredients of Ephedrae Herba-Cinnamomi Ramulus couplet medicines (MGCM) and their targets in treating psoriasis, and the statistical analysis of the degree of each (B) ingredient and (C) target in the network. All nodes were sorted and calculated according to the degree of freedom, and the node size in the network was associated with the degree.
Figure 3. (A) Construction of the core network of active ingredients of Ephedrae Herba-Cinnamomi Ramulus couplet medicines (MGCM) and their targets in treating psoriasis, and the statistical analysis of the degree of each (B) ingredient and (C) target in the network. All nodes were sorted and calculated according to the degree of freedom, and the node size in the network was associated with the degree. Figure 4. Enrichment analysis of core targets of Ephedrae Herba-Cinnamomi Ramulus couplet medicines (MGCM) in treating psoriasis based on OmicShare. We considered a P value cutoff of ≤0.05 as significant and applied hypergeometric tests to identify (A) enriched Gene Ontology biological processes (GO-BP) and (B) Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways. The chart shows an overview of the analysis with up to 20 significantly enriched processes and pathways.
Figure 4. Enrichment analysis of core targets of Ephedrae Herba-Cinnamomi Ramulus couplet medicines (MGCM) in treating psoriasis based on OmicShare. We considered a P value cutoff of ≤0.05 as significant and applied hypergeometric tests to identify (A) enriched Gene Ontology biological processes (GO-BP) and (B) Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways. The chart shows an overview of the analysis with up to 20 significantly enriched processes and pathways. Tables
 Table 1. All the candidate ingredients of Ephedrae Herba-Cinnamomi Ramulus couplet medicines (MGCM).
Table 1. All the candidate ingredients of Ephedrae Herba-Cinnamomi Ramulus couplet medicines (MGCM). Table 2. Degree values of core targets for Ephedrae Herba-Cinnamomi Ramulus couplet medicines (MGCM) against psoriasis.
Table 2. Degree values of core targets for Ephedrae Herba-Cinnamomi Ramulus couplet medicines (MGCM) against psoriasis. Table 3. Fifty-two core pharmacologically active ingredients of Ephedrae Herba-Cinnamomi Ramulus couplet medicines (MGCM) in the treatment of psoriasis.
Table 3. Fifty-two core pharmacologically active ingredients of Ephedrae Herba-Cinnamomi Ramulus couplet medicines (MGCM) in the treatment of psoriasis. Table 1. All the candidate ingredients of Ephedrae Herba-Cinnamomi Ramulus couplet medicines (MGCM).
Table 1. All the candidate ingredients of Ephedrae Herba-Cinnamomi Ramulus couplet medicines (MGCM). Table 2. Degree values of core targets for Ephedrae Herba-Cinnamomi Ramulus couplet medicines (MGCM) against psoriasis.
Table 2. Degree values of core targets for Ephedrae Herba-Cinnamomi Ramulus couplet medicines (MGCM) against psoriasis. Table 3. Fifty-two core pharmacologically active ingredients of Ephedrae Herba-Cinnamomi Ramulus couplet medicines (MGCM) in the treatment of psoriasis.
Table 3. Fifty-two core pharmacologically active ingredients of Ephedrae Herba-Cinnamomi Ramulus couplet medicines (MGCM) in the treatment of psoriasis. In Press
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








