22 August 2020: Animal Study
Role of Sirtuin-1 in Neonatal Hypoxic-Ischemic Encephalopathy and Its Underlying Mechanism
Zhen Zhang1ABCDEFG, Xin Chen1ABCDEFG, Sichen Liu2BCEFG*DOI: 10.12659/MSM.924544
Med Sci Monit 2020; 26:e924544
Abstract
BACKGROUND: Neonatal hypoxic-ischemic encephalopathy (HIE) is a dreaded disease and one of the leading causes of severe neurological dysfunction in neonates. The present study explored the functions of Sirtuin-1 (SIRT1) in neonatal HIE.
MATERIAL AND METHODS: A HIE neonatal rat model was generated to determine SIRT1 levels in brain tissues. Cell apoptosis and cell viability were analyzed by flow cytometry and MTT assay. qRT-PCR and Western blot analysis were used to assess gene mRNA and protein levels. Subsequently, the effect of SIRT1 on HIE was investigated in vitro by constructing an oxygen-glucose deprivation (OGD) cell model.
RESULTS: The effective construction of the HIE rat model was confirmed by the enhanced brain cell apoptosis and the increased expression of HIE-related molecular markers, including S100 calcium-binding protein B (S100B) and neuron-specific enolase (NSE). SIRT1 expression was downregulated in HIE rat brain tissues. These findings indicated that SIRT1 was downregulated in neuronal cells subjected to OGD. In addition, enhanced cell viability and reduced cell apoptosis were observed, suggesting that SIRT1 overexpression relieved OGD-induced neuronal cell injury. Transfection with SIRT1-siRNA further increased OGD-induced neuronal cell injury, evidenced by decreased cell viability and enhanced cell apoptosis. Finally, SIRT1 overexpression significantly downregulated p-p65 protein expression.
CONCLUSIONS: Our findings revealed that SIRT1 may be a novel and promising therapy target for HIE treatment.
Keywords: Hypoxia-Ischemia, Brain, Neurons, Sirtuin 1, Animals, Newborn, Brain, Down-Regulation, Glucose, Oxygen, RNA, Messenger, RNA, Small Interfering, Transfection
Background
HIE is a common type of brain injury caused by various perinatal factors of cerebral hypoxia or ischemia [1,2]. HIE mainly causes damage to neurons and white matter, thus adversely affecting the brain of a developing baby [3]. HIE is one of the primary causes of infant mortality and the most common cause of neonatal seizures [4–6]. In addition, HIE has a high mortality rate, long-term incidence, poor prognosis, and complex pathogenesis. Previous research reported HIE incidence rates of 1.5 cases per 1000 live births in developed countries and 10–20 per 1000 live births in low- and middle-income countries [7,8]. About 24% of HIE patients die in the neonatal intensive care unit (NICU). Among the surviving newborns, cerebral palsy (10–20%), auditory and visual problems (about 40%), and motor and behavioral impairments such as overall developmental delay, epilepsy, and autism [9–11] are diagnosed. The advancement of medical standards has provided a deeper understanding of fetal pathophysiology. However, HIE remains a critical complication of mature infants, and further investigation is needed to improve HIE treatment.
Sirtuin-1 (SIRT1), a NAD-dependent lysine deacetylase, is stimulated under stress in various cell types [12]. SIRT1 protects the heart from aging, hypertrophy, and myocardial infarction [13–15]. Sun et al. indicated that SIRT1 is involved in the vascular system by regulating cell proliferation and cell cycle [16]. Nuclear factor-κB (NF-κB) is deacetylated by SIRT1. In addition, many studies have reported that SIRT1 is a vital regulator in various biological processes, including cisplatin (CDDP)-induced oxidative stress and inflammatory response via the activation of downstream genes at transcriptional levels, such as nuclear-related factor 2 (Nrf2) and NF-κB [17, 18]. Nevertheless, whether SIRT1 exhibits functions in neonatal HIE remains unclear.
We designed this research to assess the roles of SIRT1 in neonatal HIE and to explore the underlying mechanism.
Material and Methods
ANIMALS:
A total of 10 male Sprague-Dawley rats (7 days old, weighing 18–20 g) were provided by the Experimental Animal Center of Kunming Medical University (China). All animals were kept in a controlled environment (22–25°C, 40–50% humidity) and a 12-h light/dark cycle with
RAT HIE MODEL CONDUCTION:
We anesthetized 7-day-old rats with isoflurane (4% induction and 1.5% maintenance) before surgery. The left common carotid artery was ligated at both ends using a 6-0 silk surgical suture, and cut along the middle. Following 1.5 h of recovery, the rats were placed in an anoxic sealed chamber (N2 and 8% O2 equilibrated) at 37°C for 2.5 h. The sham-operated rats were anesthetized and the left common carotid arteries were exposed, but not ligated. Rats were anesthetized with propylthiouracil injection (40 mg/kg) and then killed by cervical vertebra dislocation (rats with cardiac and respiratory arrest were confirmed as dead) at 72 h following hypoxia for further experiments. All efforts were made to alleviate the pain of the rats throughout the experiment. In addition, all experiments were stopped when the rats lost more than 15% of their body weight.
CELL CULTURE AND TRANSFECTION:
Primary neuronal cells were isolated from newborn rats. Briefly, the rats were anesthetized and then killed by cervical dislocation. The hippocampal tissue was collected, digested with 0.05% trypsin (Gibco; Thermo Fisher Scientific, Inc.) and then centrifuged. Subsequently, the deposits were re-suspended in DMEM medium (Gibco; Thermo Fisher Scientific, Inc) supplemented with 5% serum (Gibco; Thermo Fisher Scientific, Inc.).
Primary rat neurons were transfected with 0.2 μM SIRT1-siRNA, 0.2 μM control-siRNA, 1 μg SIRT1-plasmid, or 1 μg control-plasmid for 48 h using lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) in accordance with the manufacturer’s instructions. Transfection efficiency was determined using qRT-PCR and Western blot assay 48 h following cell transfection. Experiments were repeated 3 times.
OGD MODEL ESTABLISHMENT:
Primary rat neurons were grown in glucose-free DMEM (without FBS and glucose) and then cultured in a humidified atmosphere containing 95% N2 and 5% CO2, at 37°C for 2 h. Subsequently, the cell culture medium was replaced with complete medium prior the completion of the OGD. Cells were then placed into a normal incubator for an additional 24 h. The normal medium under normoxia served as the control.
MTT ASSAY:
Primary rat neurons were transfected with SIRT1-siRNA, control-siRNA, SIRT1-plasmid, or control-plasmid for 48 h and subsequently underwent OGD treatment. Next, cells were stimulated with 20 μl MTT reagent (Solarbio) and incubated for an additional 4 h at 37°C. DMSO (150 μl) was added into each well and shaken for 15 min. The optical density (OD) was detected at 570 nm by a micro-plate reader. Experiments were repeated 3 times.
CELL APOPTOSIS ASSAY:
Neuronal cell apoptosis was analyzed using an Annexin V-FITC/propidium iodide (PI) Apoptosis Detection kit (MultiSciences Biotech Co.) according to the manufacturer’s instructions. In brief, Annexin V-FITC and PI were used to dye neuronal cells for 30 min in the dark. The apoptotic cells were measured using flow cytometry (BD Biosciences) and the data were analyzed using WinMDI software (version 2.5; Purdue University Cytometry Laboratories). Experiments were repeated 3 times.
Rat brain tissues were mechanically dissociated into single-cell suspension using a 200-mesh sieve to investigate cell apoptosis in HIE rats. Subsequently, the cell suspension was centrifuged (670.8×g) at 4°C for 5 min and the supernatant was discarded. The cell apoptosis was analyzed as previously described.
QRT-PCR ASSAY:
Total RNA from brain tissue and neuronal cells were treated with Trizol (Takara Biotechnology Co.) following the manufacturer’s protocol. Then, cDNA was synthesized from total RNA using a reverse transcription kit (Vazyme) according to the manufacturer’s protocol. The reaction volume was 20 μl and the reverse transcription reaction conditions were 70°C for 5 min, 37°C for 5 min, and 42°C for 60 min. qRT-PCR was performed using a SYBR kit (Vazyme). The qPCR reaction was conducted with the following parameters: 95°C for 3 min, 40 cycles of 95°C for 30 s, 56°C for 30 s, and 72°C for 30 s. Gene expressions were performed by 2−ΔΔCt method.
WESTERN BLOT ANALYSIS:
RIPA lysis buffer (Beyotime, China) and protease inhibitors were used to isolate total proteins from neuronal cells or brain tissues. Cells were incubated for 30 min and then re-suspended with lysate. Cells suspension was centrifuged and the supernatants containing proteins were stored prior to use. The protein concentration was determined by a BCA assay kit. Subsequently, 30 μg of protein extracts were subjected to 12% SDS-PAGE electrophoresis and transferred to PVDF membranes (EMD Millipore). The membranes were incubated with 5% no-fat milk in TBS supplemented with 0.1% Tween at room temperature for 1.5 h. Finally, the membranes were cultivated in primary antibody at 4°C overnight, then the membranes were incubated with an HRP-conjugated secondary antibody at room temperature for 1 h. Protein bands were quantified by the enhanced chemiluminescence method (ECL, EMD Millipore). GAPDH protein expression levels served as the loading control for normalization.
Primary antibodies anti-Bcl-2, anti-Bax, anti-SIRT1, anti-p65, anti-p-p65, anti-GAPDH and the secondary goat anti-rabbit antibody were obtained from Cell Signaling Technology.
STATISTICAL ANALYSIS:
The results are depicted as the mean±SD from triplicate experiments. GraphPad 6.0 software was used for the statistical analysis. The
Results
EXPRESSION LEVEL OF SIRT1 IN HIE RATS BRAIN TISSUES:
Three days after establishing the neonatal HIE rat model, the brain tissue was isolated and the brain cell apoptosis was assessed using flow cytometry. We found more apoptotic cells in rat brain tissues of the HIE group than in the control group (Figure 1A, 1B). Subsequently, the levels of related molecular markers S100B and NSE, which participate in the progression of HIE [19–22], were detected. Western blot assay revealed that S100B and NSE protein levels were significantly higher in the HIE group than in the control group (Figure 1C). Western blot assay and qRT-PCR were conducted to detect the expression of SIRT1 in HIE neonatal rats brain tissues. As indicated in Figure 1D and 1E, the levels of SIRT1 were distinctly lower in the rat brain tissue of the HIE group compared to the control group.
LEVELS OF SIRT1 IN THE OGD MODEL:
The OGD cell model was established in vitro. Primary rat neuronal cells were exposed to OGD (95% N2 and 5% CO2) for 48 h. Western blot assay showed that OGD treatment markedly enhanced S100B and NSE protein levels in cultured neurons (Figure 2A). Western blot assay and qRT-PCR were performed to investigate the SIRT1 expression levels. SIRT1 mRNA levels and protein expression were obviously lower in the OGD group than in the control group (Figure 2B, 2C).
TRANSFECTION EFFICIENCY OF SIRT1-PLASMID/SIRT1-SIRNA IN PRIMARY RAT NEURONAL CELLS:
To assess the roles of SIRT1 in HIE, we first determined the transfection efficiency of SIRT1 overexpression/silencing in primary rat neuronal cells. SIRT1-siRNA, control-siRNA, SIRT1-plasmid, or control-plasmid were transfected in primary rat neuronal cells for 48 h and SIRT1 levels were analyzed using qRT-PCR and Western blot assays. The results showed that transfection of the primary rat neuronal cells with the SIRT1-plasmid (Figure 3A, 3B) and SIRT1-siRNA (Figure 3C, 3D) increased and decreased the expression levels of SIRT1, respectively, compared with the control group.
SIRT1 OVEREXPRESSION RELIEVED OGD-TREATED NEURONAL CELL DAMAGE:
To further explore the effect of SIRT1 overexpression (SIRT1-plasmid) on OGD-induced neuronal cell injury, MTT and flow cytometry were performed to evaluate cell viability and apoptosis, respectively. Our data demonstrated that cell viability in the OGD group was notably lower than in the control group. In addition, SIRT1 overexpression increased neuronal cells viability compared with the OGD group (Figure 4A). Compared with the control group, cell apoptosis in the OGD group was higher, whereas SIRT1 overexpression decreased neuronal cell apoptosis (Figure 4B, 4C). In addition, Western blot assay showed that Bax and Bcl-2 expression levels were increased and decreased in the OGD group, respectively. Compared with the OGD group, SIRT1 overexpression inhibited Bax and induced Bcl-2 protein expression (Figure 4D–4F). These results indicated that SIRT1 overexpression reduced the OGD-induced cell injury.
SIRT1 SILENCING FURTHER AGGRAVATED OGD-INDUCED NEURONAL CELL INJURY:
Subsequently, the effect of SIRT1 silencing (SIRT1-siRNA) on OGD-induced neuronal cell injury was investigated. MTT assay revealed a decreased cell viability rate in the OGD group compared to control. In addition, compared with the OGD group, SIRT1 silencing further decreased the viability rate of neuronal cells (Figure 5A). OGD treatment promoted more apoptotic cells than that in the control group, whereas SIRT1 downregulation further promoted neuronal cell apoptosis (Figure 5B, 5C). Finally, Western blot assay showed that Bax expression was increased and Bcl-2 expression was decreased in the OGD group. However, SIRT1 silencing obviously increased and reduced the expression of Bax and Bcl-2, respectively (Figure 5D). These results indicated that SIRT1 downregulation further aggravated OGD-induced neuronal cell injury.
THE EFFECT OF SIRT1 EXPRESSION ON NF-κB SIGNALING PATHWAY IN OGD-INDUCED NEURONAL CELLS:
Finally, we investigated the specific potential mechanisms of SIRT1 on OGD-induced neuronal cells. NF-κB signaling pathway activity was analyzed by measuring the protein levels of p-p65 and p65. Compared with the control group, p-p65 protein expression and the ratio of p-p65/p65 were significantly increased in the OGD group. In addition, SIRT1 overexpression (Figure 6A, 6B) and silencing (Figure 6C, 6D) significantly decreased and increased, respectively, the p-p65 protein expression and the p-p65/p65 ratio.
Discussion
In this research we constructed a neonatal HIE rat model to determine SIRT1 expression. On the third day, the rats were killed and brain tissue was collected. The results revealed that the cell apoptosis rate and the expression of S100B and NSE were remarkably amplified, whereas SIRT1 expression was obviously reduced in the HIE group compared to the control group. Subsequently, an OGD cell model was established to elucidate the effect of SIRT1 expression on HIE
Levene et al. demonstrated that 3–5/1000 newborn babies have HIE [23]. Previous studies suggested that HIE is the main cause of high morbidity and mortality [24]. The pathophysiology of HIE involves several different events (mitochondrial dysfunction, calcium surge, excitotoxicity, reactive oxygen species accumulation, and inflammation) that are strictly interlinked [25]. In the pathogenesis of hypoxia-ischemia, time of injury and timing of treatment play critical roles [26]. Therapeutic hypothermia (TH) has become the standard treatment method of HIE, resulting in reduced infant mortality and neurodevelopmental disability [27–31]. Previous reports have shown that TH can promote the survival and reduce the disability rate of neonates with HIE, but the mortality and disability of HIE neonates remains high (40–50%) [32–34]. In recent years, adjuvant therapies used with TH have been studied, including erythropoietin and stem cell therapy [35,36]. Also, some novel neuroprotectants have been shown to be of great value in HIE therapy by regulating oxidative stress, inflammatory response, glutaminergic excitotoxicity, and apoptosis [37,38]. After an HI event, there is increased regeneration of pathways, which may be enhanced by novel treatments [39]. Recently, stem cell-based therapy has been revealed to have the potential to rescue and replace the ischemic tissue caused by HI and may facilitate endogenous brain repair [40]. However, there are still some gaps in our knowledge of the pathophysiology and timing of endogenous neuroprotection and nerve regeneration mechanisms. Exploring the mechanism and treatment strategy of HIE is still urgent.
SIRT is a class III histone deacetylase (HDAC), including SIRT1-SIRT7. SIRT depends on NAD+, indicating that sirtuin activity acts as a regulator for the solute ratio of NAD+/NADH cells, thus directly linking sirtuin activity to cellular metabolism and energy status [41,42]. SIRT1, a member of the silent information regulator 2 (Sir2) family, was reported to be involved in the occurrence and development of tumors [43,44]. In addition, whether SIRT1 acts as a tumor-suppressor or a tumor-promoter protein depends on cell type [45]. Increasing evidence shows that SIRT1 is related to hypoxia. SIRT1-regulated HMGB1 production is related to TLR4 signaling and is a possible anti-neuroinflammatory mechanism of resveratrol in neonatal hypoxic-ischemic brain damage [46]. SIRT1 was reported to be involved in the role of ghrelin in modulating oxidative stress and neuronal apoptosis in a neonatal HIE rat model [47]. Licochalcone A has neuroprotective effects on OGD/R-treated rat cortical neurons through the SIRT1/Nrf2 pathway [48]. In this research, we generated a HIE rat model to investigate the roles of SIRT1 in the neonatal HIE and to illustrate the underlying mechanisms. However, our study has certain limitations. The expression levels of the related molecular markers S100B and NSE during the development of the HIE were confirmed by Western blot assay, but results from HE staining would be more convincing. The levels of SIRT1 in the brain tissue of HIE rats was assessed using Western blot and qRT-PCR assay, but not IHC.
We found that SIRT1 was downregulated in the HIE group. Laemmle et al. indicated that SIRT1 was obviously upregulated in hypoxic cells [48]. Furthermore, it has been reported that SIRT1 is overexpressed in various tumor cells and tissues [49–51]. Thereafter,
Conclusions
SIRT1 can protect neurons from ischemia and hypoxia by inhibiting activation of the NF-κB signaling pathway, thus exhibiting a protective effect on HIE. Our observations indicate that SIRT1 may serve as a novel and effective therapeutic target for HIE treatment. However, this research is only a preliminary study of the role of SIRT1 in HIE. To clarify the role and mechanism of SIRT1 in HIE, further in-depth investigations are needed. For example, whether SIRT1 directly or indirectly regulates NF-κB in hypoxic-ischemic encephalopathy has not been studied to date and needs further investigation. The expression of SIRT1 in HIE patients needs to be confirmed, and the relationship between SIRT1 expression and clinicopathological features of patients with HIE should be investigated. We plan to address all of these topics in the future.
Figures
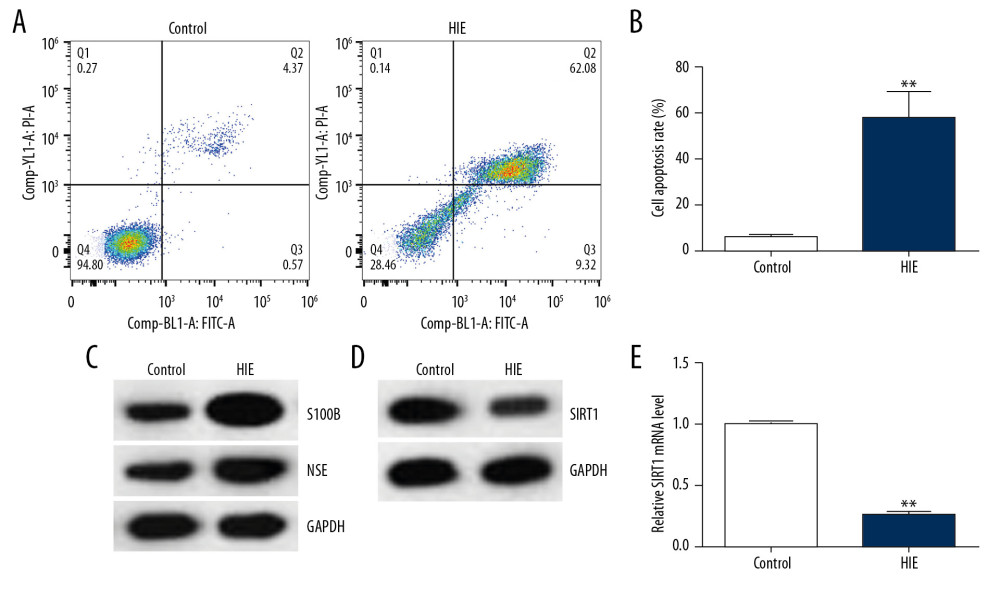 Figure 1. SIRT1 expression was downregulated in the HIE rat brain tissues. (A) Cell apoptosis of rat brain tissues was analyzed using flow cytometry. (B) Cell apoptosis rate was calculated and presented (n=5). (C) The levels of the related molecular markers S100B and NSE during the development of HIE were measured by Western blot. (D) Western blot analysis of SIRT1 levels. (E) qRT-PCR analysis of SIRT1 mRNA levels (n=5). The results showed as the mean ± SD. ** P<0.01 vs. control group. SIRT1 – sirtuin-1; HIE – hypoxic-ischemic encephalopathy; NSE – neuron-specific enolase; S100B – S100 calcium binding protein B.
Figure 1. SIRT1 expression was downregulated in the HIE rat brain tissues. (A) Cell apoptosis of rat brain tissues was analyzed using flow cytometry. (B) Cell apoptosis rate was calculated and presented (n=5). (C) The levels of the related molecular markers S100B and NSE during the development of HIE were measured by Western blot. (D) Western blot analysis of SIRT1 levels. (E) qRT-PCR analysis of SIRT1 mRNA levels (n=5). The results showed as the mean ± SD. ** P<0.01 vs. control group. SIRT1 – sirtuin-1; HIE – hypoxic-ischemic encephalopathy; NSE – neuron-specific enolase; S100B – S100 calcium binding protein B. 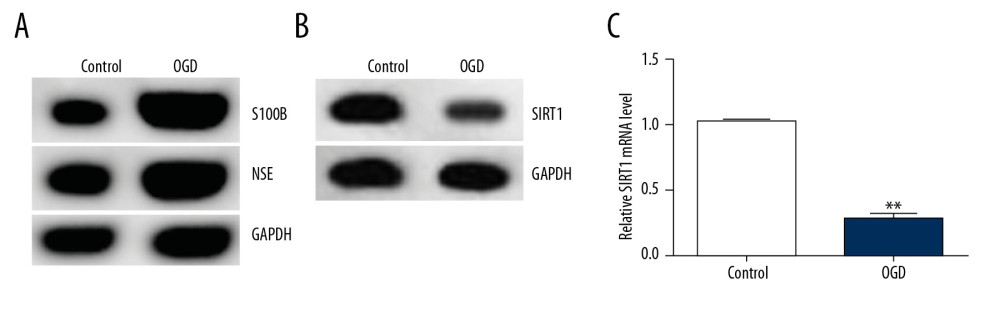 Figure 2. SIRT1 expression was downregulated in the OGD-induced primary rat neurons. (A) The protein expression levels of the related molecular markers S100B and NSE during the development of HIE were determined by Western blot assay. (B) SIRT1 protein levels were determined by Western blot assay. (C) SIRT1 mRNA gene expression was determined by qRT-PCR. Control: cells without any treatment; OGD: cells were stimulated with OGD. The results showed as the mean±SD. ** P<0.01 vs. control group. SIRT1 – sirtuin-1; OGD – oxygen and glucose deprivation.
Figure 2. SIRT1 expression was downregulated in the OGD-induced primary rat neurons. (A) The protein expression levels of the related molecular markers S100B and NSE during the development of HIE were determined by Western blot assay. (B) SIRT1 protein levels were determined by Western blot assay. (C) SIRT1 mRNA gene expression was determined by qRT-PCR. Control: cells without any treatment; OGD: cells were stimulated with OGD. The results showed as the mean±SD. ** P<0.01 vs. control group. SIRT1 – sirtuin-1; OGD – oxygen and glucose deprivation. 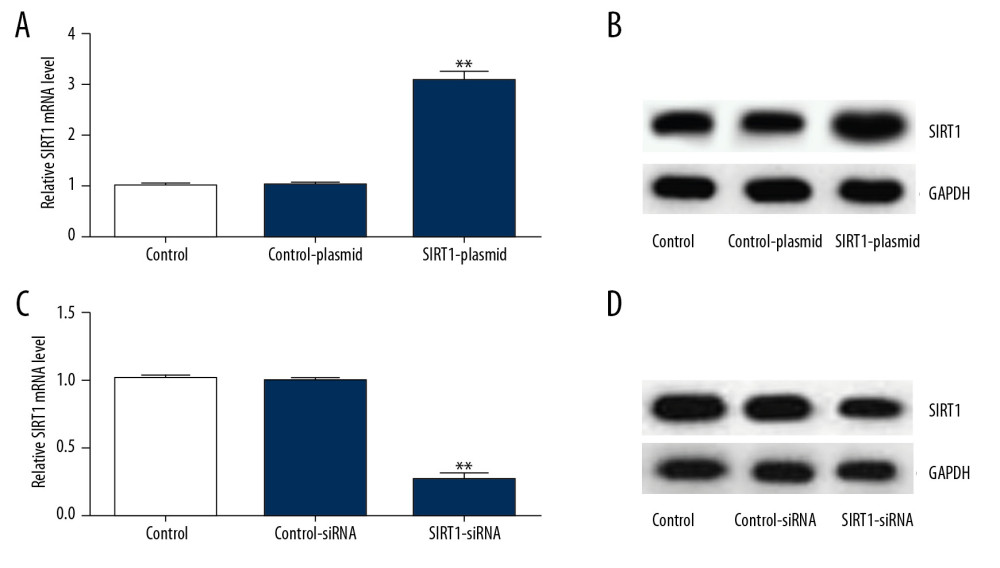 Figure 3. Transfection efficiency of SIRT1 overexpression (SIRT1-plasmid) or silencing (SIRT1-siRNA) in primary rat neuronal cells. SIRT1 protein levels and mRNA gene expression in primary rat neuronal cells transfected with SIRT1-plasmid or control-plasmid for 48 h were determined by (A) Western blot and (B) qRT-PCR assays, respectively. SIRT1 protein levels and mRNA gene expression in primary rat neuronal cells transfected with SIRT1-siRNA or control-siRNA for 48 h were determined by (C) Western blot and (D) qRT-PCR assays, respectively. Control – cells without any treatment; Control-plasmid: cells were transfected with control-plasmid; SIRT1-plasmid: cells were transfected with SIRT1-plasmid; Control-siRNA: cells were transfected with control-siRNA; SIRT1-siRNA: cells were transfected with SIRT1-siRNA. The results showed as the mean±SD. ** P<0.01 vs. control group. SIRT1 – sirtuin-1.
Figure 3. Transfection efficiency of SIRT1 overexpression (SIRT1-plasmid) or silencing (SIRT1-siRNA) in primary rat neuronal cells. SIRT1 protein levels and mRNA gene expression in primary rat neuronal cells transfected with SIRT1-plasmid or control-plasmid for 48 h were determined by (A) Western blot and (B) qRT-PCR assays, respectively. SIRT1 protein levels and mRNA gene expression in primary rat neuronal cells transfected with SIRT1-siRNA or control-siRNA for 48 h were determined by (C) Western blot and (D) qRT-PCR assays, respectively. Control – cells without any treatment; Control-plasmid: cells were transfected with control-plasmid; SIRT1-plasmid: cells were transfected with SIRT1-plasmid; Control-siRNA: cells were transfected with control-siRNA; SIRT1-siRNA: cells were transfected with SIRT1-siRNA. The results showed as the mean±SD. ** P<0.01 vs. control group. SIRT1 – sirtuin-1. 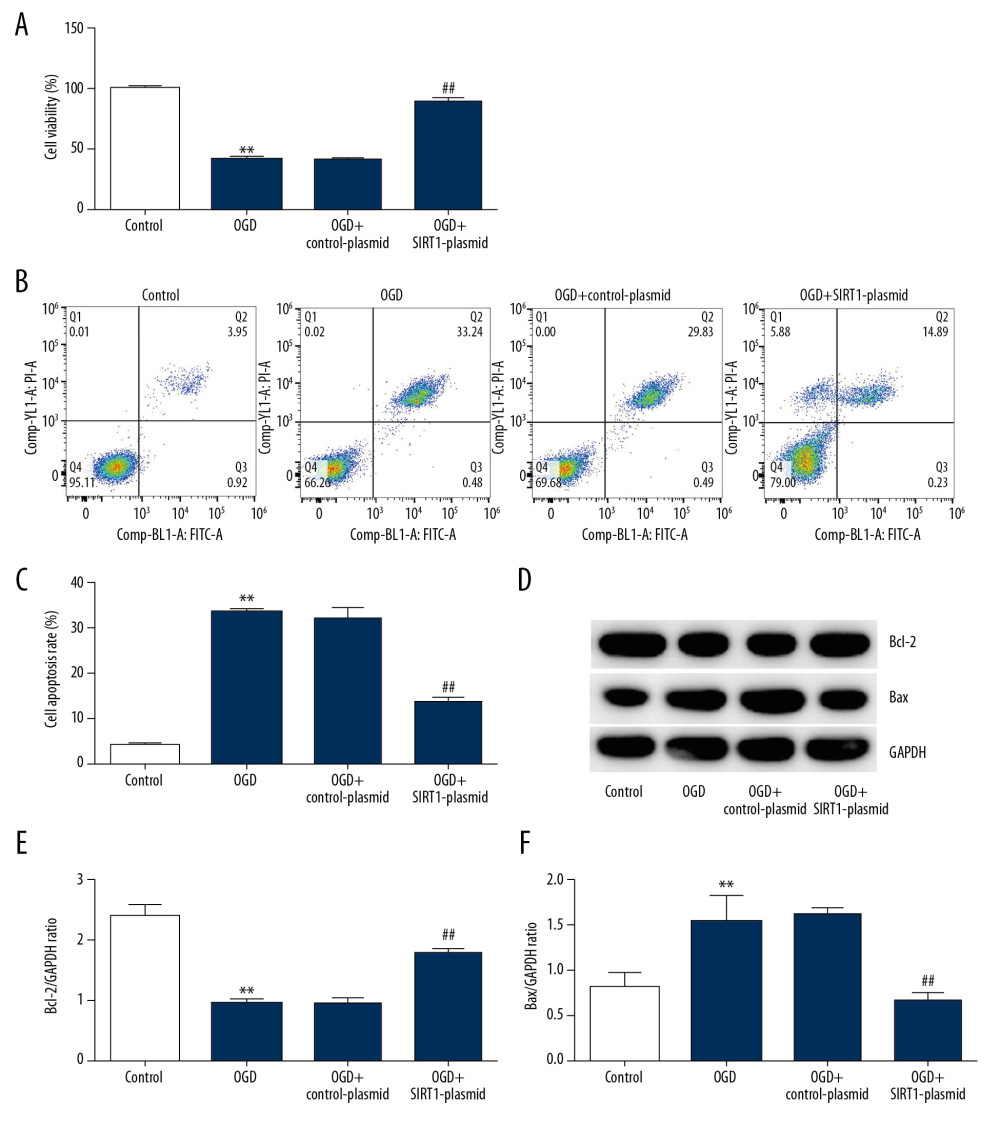 Figure 4. SIRT1 overexpression increased cell viability and decreased cell apoptosis in OGD-induced primary rat neuronal cells. Primary rat neuronal cells were transfected with SIRT1-plasmid or control-plasmid for 48 h, then the cells were exposed to OGD for 48 h. (A) Cell proliferation was assessed using MTT assay. (B) Flow cytometry was used to determine apoptotic cells. (C) Quantification of apoptotic cells. (D) Bax and Bcl-2 protein levels were determined by Western blot assay. (E, F) The ratio of Bcl-2/GAPDH and Bax/GAPDH was calculated and presented. Control: cells without any treatment; OGD: cells were subjected to OGD treatment; OGD+control-plasmid: cells were transfected with control-plasmid and then subjected to OGD treatment; OGD+SIRT1-plasmid: cells were transfected with SIRT1-plasmid and then subjected to OGD treatment. The results showed as the mean±SD. ** P<0.01 vs. control group; ## P<0.01 vs. OGD group. OGD – oxygen and glucose deprivation; SIRT1 – sirtuin-1.
Figure 4. SIRT1 overexpression increased cell viability and decreased cell apoptosis in OGD-induced primary rat neuronal cells. Primary rat neuronal cells were transfected with SIRT1-plasmid or control-plasmid for 48 h, then the cells were exposed to OGD for 48 h. (A) Cell proliferation was assessed using MTT assay. (B) Flow cytometry was used to determine apoptotic cells. (C) Quantification of apoptotic cells. (D) Bax and Bcl-2 protein levels were determined by Western blot assay. (E, F) The ratio of Bcl-2/GAPDH and Bax/GAPDH was calculated and presented. Control: cells without any treatment; OGD: cells were subjected to OGD treatment; OGD+control-plasmid: cells were transfected with control-plasmid and then subjected to OGD treatment; OGD+SIRT1-plasmid: cells were transfected with SIRT1-plasmid and then subjected to OGD treatment. The results showed as the mean±SD. ** P<0.01 vs. control group; ## P<0.01 vs. OGD group. OGD – oxygen and glucose deprivation; SIRT1 – sirtuin-1. 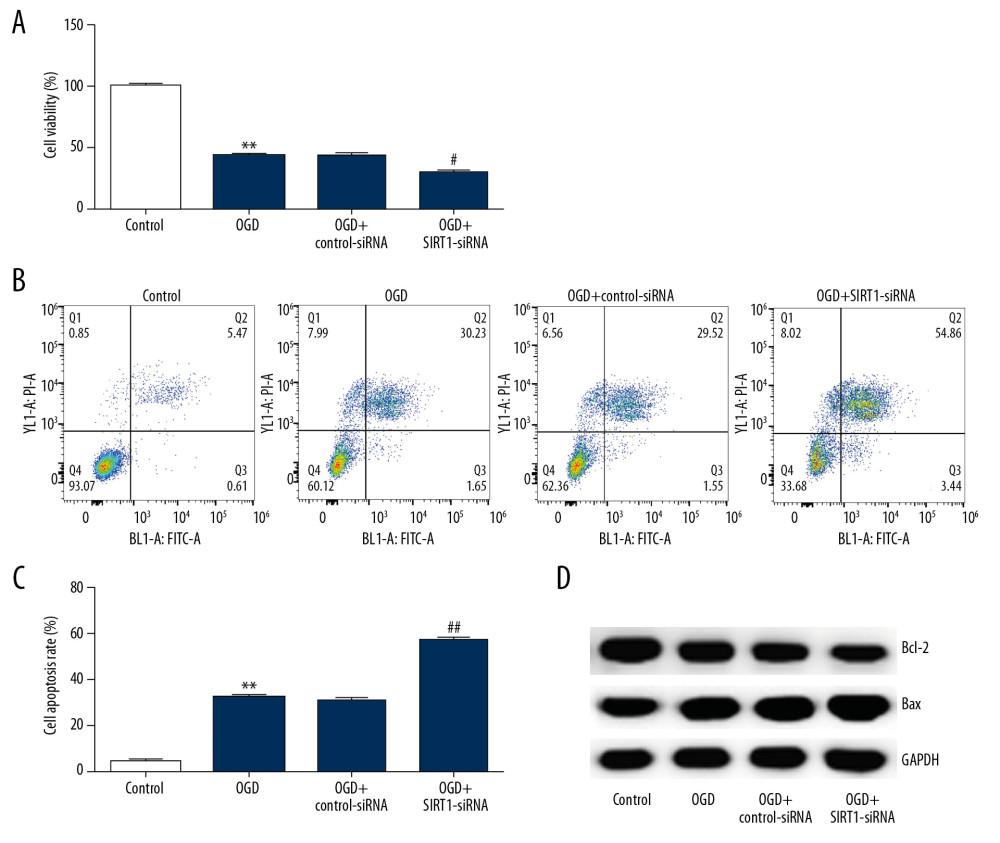 Figure 5. SIRT1 silencing decreased the viability and increased cell apoptosis in OGD-induced primary rat neuronal cells. Primary rat neuronal cells were transfected with SIRT1-siRNA or control-siRNA for 48 h, then the cells were treated with OGD for 48 h. (A) Cell viability was determined by MTT assay. (B, C) Cell apoptosis was determined by flow cytometry. (D) Bax and Bcl-2 protein levels were determined by Western blot assay. Control: cells without any treatment; OGD: cells were subjected to OGD treatment; OGD+control-siRNA: cells were transfected with control-siRNA and then subjected to OGD treatment; OGD+SIRT1-siRNA: cells were transfected with SIRT1-siRNA and then subjected to OGD treatment. The results are presented as the mean±SD. ** P<0.01 vs. control group; ## P<0.01 vs. OGD group. OGD – oxygen and glucose deprivation; SIRT1 – sirtuin-1.
Figure 5. SIRT1 silencing decreased the viability and increased cell apoptosis in OGD-induced primary rat neuronal cells. Primary rat neuronal cells were transfected with SIRT1-siRNA or control-siRNA for 48 h, then the cells were treated with OGD for 48 h. (A) Cell viability was determined by MTT assay. (B, C) Cell apoptosis was determined by flow cytometry. (D) Bax and Bcl-2 protein levels were determined by Western blot assay. Control: cells without any treatment; OGD: cells were subjected to OGD treatment; OGD+control-siRNA: cells were transfected with control-siRNA and then subjected to OGD treatment; OGD+SIRT1-siRNA: cells were transfected with SIRT1-siRNA and then subjected to OGD treatment. The results are presented as the mean±SD. ** P<0.01 vs. control group; ## P<0.01 vs. OGD group. OGD – oxygen and glucose deprivation; SIRT1 – sirtuin-1. 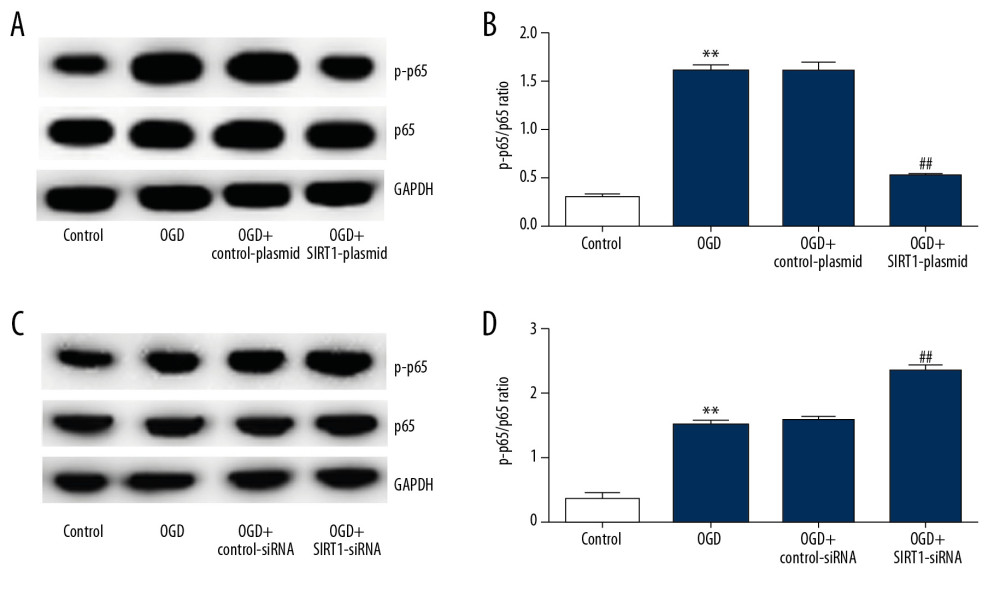 Figure 6. Effect of SIRT1 on NF-κB signaling pathway in OGD-induced neuronal cells. Primary rat neuronal cells were transfected with SIRT1-plasmid/SIRT1-siRNA or control-plasmid/control-siRNA for 48 h, then the cells were treated with OGD for 48 h. (A) p-p65 and p65 protein levels in neurons transfected with SIRT1-plasmid or control-plasmid for 48 h were determined by Western blot assay. (B) The ratio of p-p65/p65 in neurons transfected with SIRT1-plasmid or control-plasmid for 48 h was calculated and presented. (C) p-p65 and p65 protein levels in neurons transfected with SIRT1-siRNA or control-siRNA for 48 h were determined by Western blot assay. (D) The ratio of p-p65/p65 in neurons transfected with SIRT1-siRNA or control-siRNA for 48 h was calculated and presented. Control: cells without any treatment; OGD: cells were subjected to OGD treatment; OGD+control-plasmid: cells were transfected with control-plasmid and then subjected to OGD treatment; OGD+SIRT1-plasmid: cells were transfected with SIRT1-plasmid and then subjected to OGD treatment; OGD+control-siRNA: cells were transfected with control-siRNA and then subjected to OGD treatment; OGD+SIRT1-siRNA: cells were transfected with SIRT1-siRNA and then subjected to OGD treatment. The results are presented as the mean±SD. ** P<0.01 vs. control group; # P<0.05, ## P<0.01 vs. OGD group. OGD – oxygen and glucose deprivation; SIRT1 – sirtuin-1.
Figure 6. Effect of SIRT1 on NF-κB signaling pathway in OGD-induced neuronal cells. Primary rat neuronal cells were transfected with SIRT1-plasmid/SIRT1-siRNA or control-plasmid/control-siRNA for 48 h, then the cells were treated with OGD for 48 h. (A) p-p65 and p65 protein levels in neurons transfected with SIRT1-plasmid or control-plasmid for 48 h were determined by Western blot assay. (B) The ratio of p-p65/p65 in neurons transfected with SIRT1-plasmid or control-plasmid for 48 h was calculated and presented. (C) p-p65 and p65 protein levels in neurons transfected with SIRT1-siRNA or control-siRNA for 48 h were determined by Western blot assay. (D) The ratio of p-p65/p65 in neurons transfected with SIRT1-siRNA or control-siRNA for 48 h was calculated and presented. Control: cells without any treatment; OGD: cells were subjected to OGD treatment; OGD+control-plasmid: cells were transfected with control-plasmid and then subjected to OGD treatment; OGD+SIRT1-plasmid: cells were transfected with SIRT1-plasmid and then subjected to OGD treatment; OGD+control-siRNA: cells were transfected with control-siRNA and then subjected to OGD treatment; OGD+SIRT1-siRNA: cells were transfected with SIRT1-siRNA and then subjected to OGD treatment. The results are presented as the mean±SD. ** P<0.01 vs. control group; # P<0.05, ## P<0.01 vs. OGD group. OGD – oxygen and glucose deprivation; SIRT1 – sirtuin-1. References
1. Charriaut-Marlangue C, Nguyen T, Bonnin P, Sildenafil mediates blood-flow redistribution and neuroprotection after neonatal hypoxia-ischemia: Stroke, 2014; 45; 850-56
2. Threlkeld SW, Lim YP, La Rue M, Immuno-modulator inter-alpha inhibitor proteins ameliorate complex auditory processing deficits in rats with neonatal hypoxic-ischemic brain injury: Brain Behav Immun, 2017; 64; 173-79
3. Brandon D, Cesar R, Wing H, Neuroprotective strategies after neonatal hypoxic, ischemic encephalopathy: Int J Mol Sci, 2015; 16; 22368-401
4. Shetty J, Neonatal seizures in hypoxic-ischaemic encephalopathy-risks and benefits of anticonvulsant therapy: Dev Med Child Neurol, 2015; 57; 40-43
5. Volpe JJ, Perinatal brain injury: From pathogenesis to neuroprotection: Ment Retard Dev Disabil Res Rev, 2001; 7(1); 56-64
6. Doycheva D, Shih G, Chen H, Granulocyte-colony stimulating factor in combination with stem cell factor confers greater neuroprotection after hypoxic–ischemic brain damage in the neonatal rats than a solitary treatment: Transl Stroke Res, 2013; 4; 171-78
7. Lawn J, Shibuya K, Stein C, No cry at birth: Global estimates of intrapartum stillbirths and intrapartum-related neonatal deaths: Bull World Health Organ, 2005; 83; 409-17
8. Montaldo P, Pauliah SS, Lally PJ, Cooling in a low-resource environment: lost in translation: Semin Fetal Neonatal Med, 2015; 20; 72-79
9. Barnett A, Mercuri E, Rutherford M, Neurological and perceptual-motor outcome at 5–6 years of age in children with neonatal encephalopathy: Relationship with neonatal brain MRI: Neuropediatrics, 2002; 33; 242-48
10. Douglas-Escobar M, Weiss MD, Hypoxic-ischemic encephalopathy: A review for the clinician: JAMA Pediatr, 2015; 169; 397-403
11. Al-Macki N, Miller SP, Hall N, Shevell M, The spectrum of abnormal neurologic outcomes subsequent to term intrapartum asphyxia: Pediatr Neurol, 2009; 41; 399-405
12. Haigis MC, Sinclair DA, Mammalian sirtuins: Biological insights and disease relevance: Annu Rev Pathol, 2010; 5; 253-95
13. Alcendor RR, Gao S, Zhai P, Sirt1 regulates aging and resistance to oxidative stress in the heart: Circ Res, 2007; 100; 1512-21
14. Hsu CP, Zhai P, Yamamoto T, Silent information regulator 1 protects the heart from ischemia/reperfusion: Circulation, 2010; 122; 2170-82
15. Planavila A, Iglesias R, Giralt M, Villarroya F, Sirt1 acts in association with PPARalpha to protect the heart from hypertrophy, metabolic dysregulation, and inflammation: Cardiovasc Res, 2011; 90; 276-84
16. Sun Q, Zhang X, Fang K, Phenotype of vascular smooth muscle cells (VSMCs) is regulated by miR-29b by targeting Sirtuin 1: Med Sci Monit, 2018; 24; 6599-607
17. Zhang X, Wu Q, Lu Y, Cerebroprotection by salvianolic acid b after experimental subarachnoid hemorrhage occurs via nrf2- and sirt1-dependent pathways: Free Radic Biol Med, 2018; 124; 504-16
18. Zhang Y, Tao X, Yin L, Protective effects of dioscin against cisplatin-induced nephrotoxicity via the microrna-34a/sirtuin 1 signalling pathway: Br J Pharmacol, 2017; 174; 2512-27
19. Okumus N, Turkyilmaz C, Onal EE, Tau and S100B proteins as biochemical markers of bilirubin-induced neurotoxicity in term neonates: Pediatric Neurol, 2008; 39; 245-52
20. Douglas-Escobar M, Weiss MD, Biomarkers of hypoxic-ischemic encephalopathy in newborns: Front Neurol, 2012; 3; 144
21. Azuma J, Nabatame S, Nakano S, Prognostic factors for acute encephalopathy with bright tree appearance: Brain Dev, 2015; 37; 191-99
22. Wang Z, Liu Y, Shao M, Combined prediction of miR-210 and miR-374a for severity and prognosis of hypoxic-ischemic encephalopathy: Brain Behav, 2017; 8; e00835
23. Levene MI, Sands C, Grindulis H, Comparison of two methods of predicting outcome in perinatal asphyxia: Lancet, 1986; 1; 67-69
24. Vannucci RC, Current and potentially new management strategies for perinatal hypoxic-ischemic encephalopathy: Pediatrics, 1990; 85; 961-68
25. Yıldız EP, Ekici B, Tatlı B, Neonatal hypoxic ischemic encephalopathy: An update on disease pathogenesis and treatment: Expert Rev Neurother, 2017; 17; 449-59
26. Jacobs SE, Berg M, Hunt R, Cooling for newborns with hypoxic ischaemic encephalopathy: Cochrane database Syst Rev, 2007; 1; CD003311
27. Azzopardi D, Strohm B, Marlow N, Effects of hypothermia for perinatal asphyxia on childhood outcomes: N Engl J Med, 2014; 371; 140-49
28. Azzopardi DV, Strohm B, Edwards AD, Moderate hypothermia to treat perinatal asphyxial encephalopathy: N Engl J Med, 2009; 361; 1349-58
29. Gluckman PD, Wyatt JS, Azzopardi D, Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: Multicentre randomised trial: Lancet, 2005; 365; 663-70
30. Simbruner G, Mittal RA, Rohlmann F, Muche Rneo.nEURO.network Trial Participants, Systemic hypothermia after neonatal encephalopathy: Outcomes of neo.nEURO.network RCT: Pediatrics, 2010; 126; e771-78
31. Shankaran S, Laptook AR, Ehrenkranz RA, Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy: N Engl J Med, 2005; 353; 1574-84
32. Chiang MC, Jong YJ, Lin CH, Therapeutic hypothermia for neonates with hypoxic ischemic encephalopathy: Pediatr Neonatol, 2017; 58; 475-83
33. Tagin MA, Woolcott CG, Vincer MJ, Hypothermia for neonatal hypoxic ischemic encephalopathy: An updated systematic review and meta-analysis: Arch Pediatr Adolesc Med, 2012; 166; 558-66
34. Johnston MV, Fatemi A, Wilson MA, Northington F, Treatment advances in neonatal neuroprotection and neurointensive care: Lancet Neurol, 2011; 10; 372-82
35. Davidson JO, Wassink G, van den Heuij LG, Therapeutic hypothermia for neonatal hypoxic-ischemic encephalopathy – where to from here?: Front Neurol, 2015; 6; 198
36. Lan XB, Wang Q, Yang JM, Neuroprotective effect of Vanillin on hypoxic-ischemic brain damage in neonatal rats: Biomed Pharmacother, 2019; 118; 109196
37. Cardinali DP, An assessment of melatonin’s therapeutic value in the hypoxic-ischemic encephalopathy of the newborn: Front Synaptic Neurosci, 2019; 11; 34
38. Imai S, Armstrong CM, Kaeberlein M, Guarente L, Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase: Nature, 2000; 403; 795-800
39. Greco P, Nencini G, Piva I, Pathophysiology of hypoxic-ischemic encephalopathy: A review of the past and a view on the future: Acta Neurol Belg, 2020; 120; 277-88
40. Marcel MD, Alexis SD, Ahmet A, Human neural stem cell grafts modify microglial response and enhance axonal sprouting in neonatal hypoxic-ischemic brain injury: Stroke, 2010; 41; 516-23
41. Tanner KG, Landry J, Sternglanz R, Denu JM, Silent information regulator 2 family of NAD-dependent histone/protein deacetylases generates a unique product, 1-O-acetyl-ADP-ribose: Proc Natl Acad Sci, 2000; 97; 14178-82
42. Xu Y, Qinghong Q, Rushi C, SIRT1 promotes proliferation, migration, and invasion of breast cancer cell line MCF-7 by upregulating DNA polymerase delta1 (POLD1): Biochem Biophys Res Commun, 2018; 502; 351-57
43. Huffman DM, Grizzle WE, Bamman MM, SIRT1 is significantly elevated in mouse and human prostate cancer: Cancer Res, 2007; 67; 6612-18
44. Fang Y, Nicholl MB, Sirtuin 1 in malignant transformation: Friend or foe?: Cancer Lett, 2011; 306; 10-14
45. Le K, Chibaatar Daliv E, Wu S, SIRT1-regulated HMGB1 release is partially involved in TLR4 signal transduction: A possible anti-neuroinflammatory mechanism of resveratrol in neonatal hypoxic-ischemic brain injury: Int Immunopharmacol, 2019; 75; 105779
46. Huang J, Liu W, Doycheva DM, Ghrelin attenuates oxidative stress and neuronal apoptosis via GHSR-1α/AMPK/Sirt1/PGC-1α/UCP2 pathway in a rat model of neonatal HIE: Free Radic Biol Med, 2019; 141; 322-37
47. Liu X, Ma Y, Wei X, Neuroprotective effect of licochalcone A against oxygen-glucose deprivation/reperfusion in rat primary cortical neurons by attenuating oxidative stress injury and inflammatory response via the SIRT1/Nrf2 pathway: J Cell Biochem, 2018; 119; 3210-19
48. Laemmle A, Lechleiter A, Roh V, Inhibition of SIRT1 impairs the accumulation and transcriptional activity of HIF-1α protein under hypoxic conditions: PLoS One, 2012; 7; e33433
49. Hao C, Zhu P-X, Yang X, Overexpression of SIRT1 promotes metastasis through epithelial-mesenchymal transition in hepatocellular carcinoma: BMC Cancer, 2014; 14; 978
50. Deppert W, SIRT1 protein levels in cancer: Tuning SIRT1 to the needs of a cancer cell: Cell Cycle, 2008; 7; 2947-48
51. Kriegl L, Vieth M, Kirchner T, Up-regulation of c-MYC and SIRT1 expression correlates with malignant transformation in the serrated route to colorectal cancer: Oncotarget, 2012; 3; 1182-93
52. Chen Y, Liu H, Zhang H, The sirt1/NF-κB signaling pathway is involved in regulation of endothelin type B receptors mediated by homocysteine in vascular smooth muscle cells: Biomed Pharmacother, 2016; 84; 1979-85
53. Huang W, Shang W, Wang H: Acta Pharmacol Sin, 2012; 33; 668-74
Figures
 Figure 1. SIRT1 expression was downregulated in the HIE rat brain tissues. (A) Cell apoptosis of rat brain tissues was analyzed using flow cytometry. (B) Cell apoptosis rate was calculated and presented (n=5). (C) The levels of the related molecular markers S100B and NSE during the development of HIE were measured by Western blot. (D) Western blot analysis of SIRT1 levels. (E) qRT-PCR analysis of SIRT1 mRNA levels (n=5). The results showed as the mean ± SD. ** P<0.01 vs. control group. SIRT1 – sirtuin-1; HIE – hypoxic-ischemic encephalopathy; NSE – neuron-specific enolase; S100B – S100 calcium binding protein B.
Figure 1. SIRT1 expression was downregulated in the HIE rat brain tissues. (A) Cell apoptosis of rat brain tissues was analyzed using flow cytometry. (B) Cell apoptosis rate was calculated and presented (n=5). (C) The levels of the related molecular markers S100B and NSE during the development of HIE were measured by Western blot. (D) Western blot analysis of SIRT1 levels. (E) qRT-PCR analysis of SIRT1 mRNA levels (n=5). The results showed as the mean ± SD. ** P<0.01 vs. control group. SIRT1 – sirtuin-1; HIE – hypoxic-ischemic encephalopathy; NSE – neuron-specific enolase; S100B – S100 calcium binding protein B. Figure 2. SIRT1 expression was downregulated in the OGD-induced primary rat neurons. (A) The protein expression levels of the related molecular markers S100B and NSE during the development of HIE were determined by Western blot assay. (B) SIRT1 protein levels were determined by Western blot assay. (C) SIRT1 mRNA gene expression was determined by qRT-PCR. Control: cells without any treatment; OGD: cells were stimulated with OGD. The results showed as the mean±SD. ** P<0.01 vs. control group. SIRT1 – sirtuin-1; OGD – oxygen and glucose deprivation.
Figure 2. SIRT1 expression was downregulated in the OGD-induced primary rat neurons. (A) The protein expression levels of the related molecular markers S100B and NSE during the development of HIE were determined by Western blot assay. (B) SIRT1 protein levels were determined by Western blot assay. (C) SIRT1 mRNA gene expression was determined by qRT-PCR. Control: cells without any treatment; OGD: cells were stimulated with OGD. The results showed as the mean±SD. ** P<0.01 vs. control group. SIRT1 – sirtuin-1; OGD – oxygen and glucose deprivation. Figure 3. Transfection efficiency of SIRT1 overexpression (SIRT1-plasmid) or silencing (SIRT1-siRNA) in primary rat neuronal cells. SIRT1 protein levels and mRNA gene expression in primary rat neuronal cells transfected with SIRT1-plasmid or control-plasmid for 48 h were determined by (A) Western blot and (B) qRT-PCR assays, respectively. SIRT1 protein levels and mRNA gene expression in primary rat neuronal cells transfected with SIRT1-siRNA or control-siRNA for 48 h were determined by (C) Western blot and (D) qRT-PCR assays, respectively. Control – cells without any treatment; Control-plasmid: cells were transfected with control-plasmid; SIRT1-plasmid: cells were transfected with SIRT1-plasmid; Control-siRNA: cells were transfected with control-siRNA; SIRT1-siRNA: cells were transfected with SIRT1-siRNA. The results showed as the mean±SD. ** P<0.01 vs. control group. SIRT1 – sirtuin-1.
Figure 3. Transfection efficiency of SIRT1 overexpression (SIRT1-plasmid) or silencing (SIRT1-siRNA) in primary rat neuronal cells. SIRT1 protein levels and mRNA gene expression in primary rat neuronal cells transfected with SIRT1-plasmid or control-plasmid for 48 h were determined by (A) Western blot and (B) qRT-PCR assays, respectively. SIRT1 protein levels and mRNA gene expression in primary rat neuronal cells transfected with SIRT1-siRNA or control-siRNA for 48 h were determined by (C) Western blot and (D) qRT-PCR assays, respectively. Control – cells without any treatment; Control-plasmid: cells were transfected with control-plasmid; SIRT1-plasmid: cells were transfected with SIRT1-plasmid; Control-siRNA: cells were transfected with control-siRNA; SIRT1-siRNA: cells were transfected with SIRT1-siRNA. The results showed as the mean±SD. ** P<0.01 vs. control group. SIRT1 – sirtuin-1. Figure 4. SIRT1 overexpression increased cell viability and decreased cell apoptosis in OGD-induced primary rat neuronal cells. Primary rat neuronal cells were transfected with SIRT1-plasmid or control-plasmid for 48 h, then the cells were exposed to OGD for 48 h. (A) Cell proliferation was assessed using MTT assay. (B) Flow cytometry was used to determine apoptotic cells. (C) Quantification of apoptotic cells. (D) Bax and Bcl-2 protein levels were determined by Western blot assay. (E, F) The ratio of Bcl-2/GAPDH and Bax/GAPDH was calculated and presented. Control: cells without any treatment; OGD: cells were subjected to OGD treatment; OGD+control-plasmid: cells were transfected with control-plasmid and then subjected to OGD treatment; OGD+SIRT1-plasmid: cells were transfected with SIRT1-plasmid and then subjected to OGD treatment. The results showed as the mean±SD. ** P<0.01 vs. control group; ## P<0.01 vs. OGD group. OGD – oxygen and glucose deprivation; SIRT1 – sirtuin-1.
Figure 4. SIRT1 overexpression increased cell viability and decreased cell apoptosis in OGD-induced primary rat neuronal cells. Primary rat neuronal cells were transfected with SIRT1-plasmid or control-plasmid for 48 h, then the cells were exposed to OGD for 48 h. (A) Cell proliferation was assessed using MTT assay. (B) Flow cytometry was used to determine apoptotic cells. (C) Quantification of apoptotic cells. (D) Bax and Bcl-2 protein levels were determined by Western blot assay. (E, F) The ratio of Bcl-2/GAPDH and Bax/GAPDH was calculated and presented. Control: cells without any treatment; OGD: cells were subjected to OGD treatment; OGD+control-plasmid: cells were transfected with control-plasmid and then subjected to OGD treatment; OGD+SIRT1-plasmid: cells were transfected with SIRT1-plasmid and then subjected to OGD treatment. The results showed as the mean±SD. ** P<0.01 vs. control group; ## P<0.01 vs. OGD group. OGD – oxygen and glucose deprivation; SIRT1 – sirtuin-1. Figure 5. SIRT1 silencing decreased the viability and increased cell apoptosis in OGD-induced primary rat neuronal cells. Primary rat neuronal cells were transfected with SIRT1-siRNA or control-siRNA for 48 h, then the cells were treated with OGD for 48 h. (A) Cell viability was determined by MTT assay. (B, C) Cell apoptosis was determined by flow cytometry. (D) Bax and Bcl-2 protein levels were determined by Western blot assay. Control: cells without any treatment; OGD: cells were subjected to OGD treatment; OGD+control-siRNA: cells were transfected with control-siRNA and then subjected to OGD treatment; OGD+SIRT1-siRNA: cells were transfected with SIRT1-siRNA and then subjected to OGD treatment. The results are presented as the mean±SD. ** P<0.01 vs. control group; ## P<0.01 vs. OGD group. OGD – oxygen and glucose deprivation; SIRT1 – sirtuin-1.
Figure 5. SIRT1 silencing decreased the viability and increased cell apoptosis in OGD-induced primary rat neuronal cells. Primary rat neuronal cells were transfected with SIRT1-siRNA or control-siRNA for 48 h, then the cells were treated with OGD for 48 h. (A) Cell viability was determined by MTT assay. (B, C) Cell apoptosis was determined by flow cytometry. (D) Bax and Bcl-2 protein levels were determined by Western blot assay. Control: cells without any treatment; OGD: cells were subjected to OGD treatment; OGD+control-siRNA: cells were transfected with control-siRNA and then subjected to OGD treatment; OGD+SIRT1-siRNA: cells were transfected with SIRT1-siRNA and then subjected to OGD treatment. The results are presented as the mean±SD. ** P<0.01 vs. control group; ## P<0.01 vs. OGD group. OGD – oxygen and glucose deprivation; SIRT1 – sirtuin-1. Figure 6. Effect of SIRT1 on NF-κB signaling pathway in OGD-induced neuronal cells. Primary rat neuronal cells were transfected with SIRT1-plasmid/SIRT1-siRNA or control-plasmid/control-siRNA for 48 h, then the cells were treated with OGD for 48 h. (A) p-p65 and p65 protein levels in neurons transfected with SIRT1-plasmid or control-plasmid for 48 h were determined by Western blot assay. (B) The ratio of p-p65/p65 in neurons transfected with SIRT1-plasmid or control-plasmid for 48 h was calculated and presented. (C) p-p65 and p65 protein levels in neurons transfected with SIRT1-siRNA or control-siRNA for 48 h were determined by Western blot assay. (D) The ratio of p-p65/p65 in neurons transfected with SIRT1-siRNA or control-siRNA for 48 h was calculated and presented. Control: cells without any treatment; OGD: cells were subjected to OGD treatment; OGD+control-plasmid: cells were transfected with control-plasmid and then subjected to OGD treatment; OGD+SIRT1-plasmid: cells were transfected with SIRT1-plasmid and then subjected to OGD treatment; OGD+control-siRNA: cells were transfected with control-siRNA and then subjected to OGD treatment; OGD+SIRT1-siRNA: cells were transfected with SIRT1-siRNA and then subjected to OGD treatment. The results are presented as the mean±SD. ** P<0.01 vs. control group; # P<0.05, ## P<0.01 vs. OGD group. OGD – oxygen and glucose deprivation; SIRT1 – sirtuin-1.
Figure 6. Effect of SIRT1 on NF-κB signaling pathway in OGD-induced neuronal cells. Primary rat neuronal cells were transfected with SIRT1-plasmid/SIRT1-siRNA or control-plasmid/control-siRNA for 48 h, then the cells were treated with OGD for 48 h. (A) p-p65 and p65 protein levels in neurons transfected with SIRT1-plasmid or control-plasmid for 48 h were determined by Western blot assay. (B) The ratio of p-p65/p65 in neurons transfected with SIRT1-plasmid or control-plasmid for 48 h was calculated and presented. (C) p-p65 and p65 protein levels in neurons transfected with SIRT1-siRNA or control-siRNA for 48 h were determined by Western blot assay. (D) The ratio of p-p65/p65 in neurons transfected with SIRT1-siRNA or control-siRNA for 48 h was calculated and presented. Control: cells without any treatment; OGD: cells were subjected to OGD treatment; OGD+control-plasmid: cells were transfected with control-plasmid and then subjected to OGD treatment; OGD+SIRT1-plasmid: cells were transfected with SIRT1-plasmid and then subjected to OGD treatment; OGD+control-siRNA: cells were transfected with control-siRNA and then subjected to OGD treatment; OGD+SIRT1-siRNA: cells were transfected with SIRT1-siRNA and then subjected to OGD treatment. The results are presented as the mean±SD. ** P<0.01 vs. control group; # P<0.05, ## P<0.01 vs. OGD group. OGD – oxygen and glucose deprivation; SIRT1 – sirtuin-1. In Press
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
06 Mar 2024 : Clinical Research
Prevalence and Variation of Medical Comorbidities in Oral Surgery Patients: A Retrospective Study at Jazan ...Med Sci Monit In Press; DOI: 10.12659/MSM.943884
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








