30 January 2024: Clinical Research
Risk Factors and Clinical Outcomes of COVID-19 Infection in Multiple Sclerosis Patients: A Retrospective Study from a Single Center in Kosovo
Edmond Komoni1ABCDEFG, Fisnik Jashari1ACD, Dren Boshnjaku1DF, Blerim Myftiu2BF, Melihate Pushka1BF, Afrim Blyta1A, Rajmonda Nallbani-KomoniDOI: 10.12659/MSM.942992
Med Sci Monit 2024; 30:e942992
Abstract
BACKGROUND: Multiple sclerosis (MS) is treated with disease-modifying therapies (DMTs) that can increase susceptibility to viral infections. This retrospective study aimed to evaluate the presentation, management, and outcomes of patients with MS on DMTs admitted with symptoms of COVID-19 to a single center in Prishtina, Kosovo between March 2020 and April 2022.
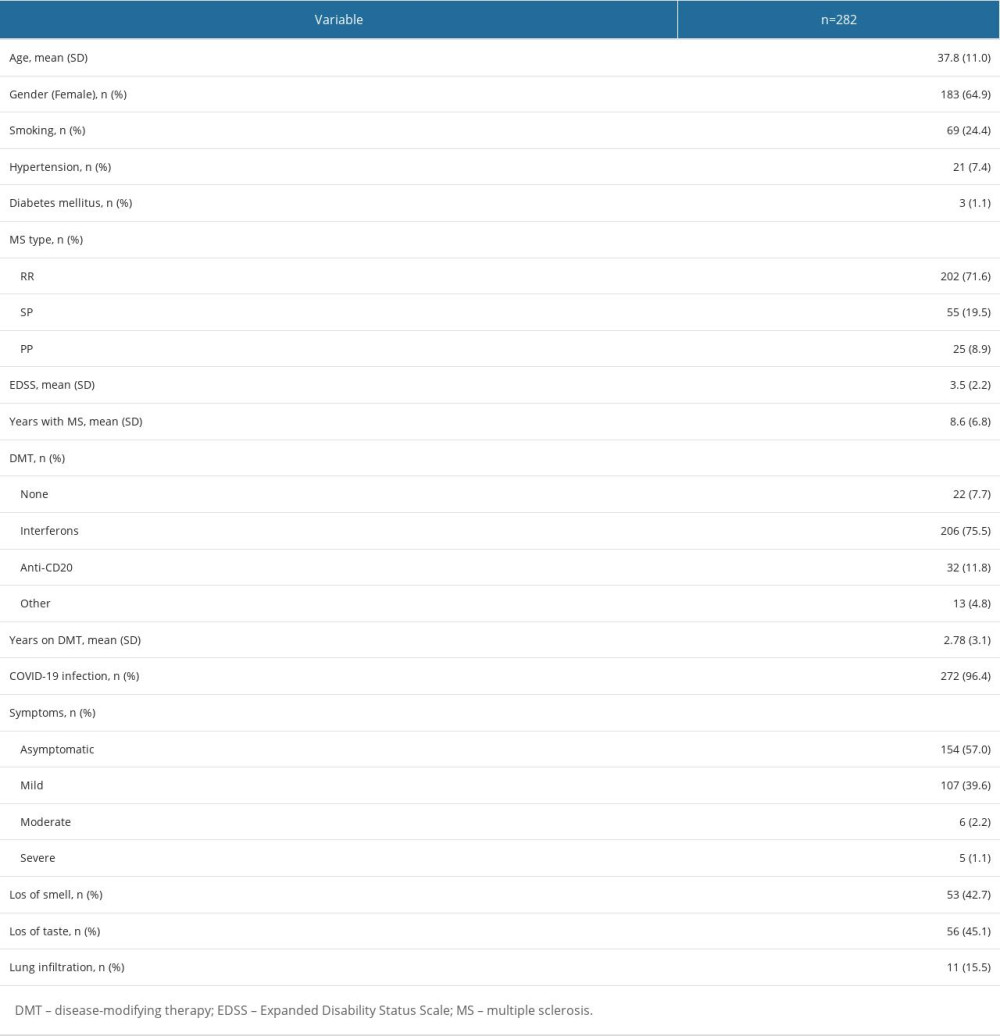
MATERIAL AND METHODS: In this observational, single-center study, we included 282 patients with MS (mean age 37.8±11, 64.9% females), of whom 272 (96.4%) had confirmed COVID-19 infection, either through the presence of antibodies in the serum or a positive PCR test.
RESULTS: Most patients with COVID-19 infection were either asymptomatic or mildly symptomatic, while 11 patients were hospitalized due to moderate to severe symptoms. Among those with severe infection, 2 patients have died. Patients with moderate and severe COVID-19 had more advanced MS disease (P=0.001) and higher disability scales (P<0.001). In a logistic regression analysis, advanced MS remained significantly associated with worse symptoms, even after adjusting for other risk factors, with a relative risk (RR) of 2.8 (95% CI=1.1-6.6, P=0.018). MS patients on anti-CD20 DMTs more frequently experienced moderate and severe symptoms (RR=2.1, 95% CI=1.1-4.0, P=0.012). Anti-SARS-CoV-2 IgG was also lower in patients treated with anti-CD20. Notably, patients receiving vitamin D supplementation experienced a lower frequency of moderate to severe symptoms (P=0.007).
CONCLUSIONS: Patients with advanced MS exhibiting higher disability scales and those on anti-CD20 therapy faced an increased risk of experiencing more pronounced symptoms after COVID-19 infection. Patients on vitamin D supplementation had better clinical outcomes.
Keywords: COVID-19, Multiple Sclerosis, SARS-CoV-2, Spike Protein, SARS-CoV-2
Background
Several studies have evaluated the clinical course of coronavirus disease-19 (COVID-19) in patients with multiple sclerosis (MS). From the onset of the pandemic, individuals with chronic conditions, including those with MS, were perceived to face an elevated risk of infection and an increased likelihood of experiencing a more severe course of COVID-19 compared to the general population [1]. This risk was particularly pronounced among those undergoing disease-modifying therapies (DMTs). Patients with MS, particularly those with more severe phenotypes and greater disability are in general more prone to infections [1].
Multiple sclerosis (MS) is an autoimmune disorder affecting the central nervous system (CNS), marked by persistent inflammation, demyelination, gliosis, and neuronal loss [2]. The disease can manifest as a relapsing-remitting (RR) or progressive course. The primary approach to managing MS, particularly the RR phenotype, involves the use of disease-modifying therapies. Among the notable options are glatiramer acetate, dimethyl fumarate, fingolimod, interferon-beta preparations, natalizumab, and mitoxantrone [3]. Patients with MS generally have increased vulnerability to infections, and this risk is influenced by the selected treatment. The lowest incidence of infections was observed with interferon-beta and glatiramer acetate (GA), while among newer treatments, the off-label use of rituximab was associated with the highest rate of serious infections [1]. These findings have been corroborated by other studies [4,5].
Among patients with MS, there are several identified risk factors that might predict more severe outcomes of SARS-CoV-2 infection, including male sex, older age, higher disability scale, progressive types of disease, and preexisting comorbidities [6–8]. The most discussed question in many studies was the impact of DMTs on COVID-19 course, as about 70% of MS patients are on DMTs. While several studies have shown an association between anti-CD20 DMTs and severe COVID-19, including higher rates of hospitalization, ICU admission, and death, others have suggested that patients treated with interferon-β or gletiramer acetate experience less severe COVID-19 symptoms, suggesting a potential benefit from this category of DMTs [6–8]. Piñar Morale et al [9] reported an unfavorable prognosis in COVID-19 linked to older age and elevated disability scores. However, the use of disease-modifying therapy (DMT) and lymphocytopenia did not affect the clinical course of the disease.
Considering that MS shows regional differences in prevalence, and the same is observed in SARS-CoV-2 infection, it is important to have information from different countries on the rate of COVID-19 infections and outcomes in MS patients. Therefore, this retrospective study aimed to evaluate the presentation, management, and outcomes of 282 patients with MS on DMTs admitted with symptoms of COVID-19 to a single center in Prishtina, Kosovo between March 2020 and April 2022.
Material and Methods
DATA COLLECTION:
This study received ethics approval from the Ethics Commission for Clinical Studies at the University Clinical Center of Kosovo. All participants in the study were duly informed, and their participation was confirmed through the signing of a consent information letter. In this observational study, we evaluated COVID-19 infection among patients diagnosed with MS. Data on demographics, MS type, Expanded Disability Scale (EDSS), disease-modifying therapies (DMTs), comorbidities, and the other MS clinical and radiological features were collected from the MS registry in Kosovo. There are 356 patients with confirmed MS diagnosis in our national MS registry. In this study, we included only MS patients who presented in our MS unit for routine follow-up disease evaluation and treatment and from the Clinic of Infectious Disease and other hospitals that were adopted to admit patients infected with COVID-19 starting from March 13, 2020 through April 30, 2022. Inclusion criteria were any patients with MS with confirmed diagnosis of COVID-19 with SARS-CoV-2-PCR or positive serum anti-SARS-CoV-2 antibody titer.
MS DIAGNOSIS AND CLINICAL PHENOTYPING:
The diagnosis of MS was performed using the McDonald criteria [10]. MS phenotype was categorized as relapsing-remitting MS (RRMS), secondary progressive MS (SPMS), and primary progressive MS (PPMS). Disability was assessed using the Expanded Disability Status Scale (EDSS) [11,12]. Participants were sked about their current smoking status. Current DMTs use included interferon-β (interferon-β 1a or interferon-β 1b), fingolimod, ocrelizumab, rituximab, dimethyl fumarate and teriflunomide, categorized as none (without DMTs), interferons (interferon-β 1a, interferon-β 1b), anti-CD20 (ocrelizumab, rituximab), and other (fingolimod, teriflunomide, and dimethyl fumarate).
COVID-19 DIAGNOSIS AND SYMPTOMS CATEGORIZATION:
COVID-19 was diagnosed based on patients’ symptoms, PCR testing, and anti-SARS-COV-2 IgG assay was measured in the serum of the sampled blood, using electrochemiluminescence immunoassay (ECLIA). Symptoms severity was categorized into 4 groups: a) asymptomatic: Individuals with positive SARS-CoV-2 test or positive antibodies in the serum but without symptoms consistent with COVID-19; b) Mild illness: Individuals with and symptoms of COVID-19 but without shortness of breath, dyspnea, or abnormal chest imaging; and c) Moderate illness: Individuals with evidence of lower respiratory disease with an oxygen saturation ≥94% on room. Severe Illness: Individuals with SpO2 <94% on room or with infiltrates [13,14].
STATISTICAL ANALYSIS:
Descriptive statistics were employed to summarize the demographic and clinical data of the patients. Continuous variables were presented as means and standard deviations (SD), while categorical variables were expressed as counts and percentages. Comparisons between patients’ symptoms and continuous variables were conducted using ANOVA with post hoc Bonferroni analysis, and for non-parametric variables, the analysis involved Kruskal-Wallis and Mann-Whitney tests after group selection. Chi-square tests were used for the comparison of binary and ordinary variables. The results derived from the analyses are presented in tables and figures. Binary logistic regression analysis was applied to determine the risk of mild and moderate COVID-19 symptoms in patients with various types of MS, while adjusting for other risk factors such as age, sex, EDSS, and MS types. Data analysis was performed using SPSS version 21.
Results
PATIENTS’ DATA:
We included 282 patients with confirmed MS diagnosis; their mean age was 37.8±11 and 64.9% were females. Most patients – 272 (96.4%) – had a positive IgG titer and in 112 patients COVID-19 was confirmed by PCR at the time of infection. Regarding treatment, 92.5% were DMTs; 206 (75.7%) were on interferons, 32 (11.8%) were on anti-CD20, and 13 (4.8%) were receiving other DMTs. Participants were predominantly of RRMS phenotype (72.1%) and the EDSS was 3.6±2.5. Most of the patients (n=154) were asymptomatic, and 39.6% (n=107) had only mild symptoms. Moderate symptoms were present in 6 patients and severe illness in 5 patients (Table 1). None of the patients in this cohort were fully vaccinated at the time of COVID-19 infection.
SEVERITY OF COVID-19 IN MS PATIENTS AND IMMUNITY RESPONSE:
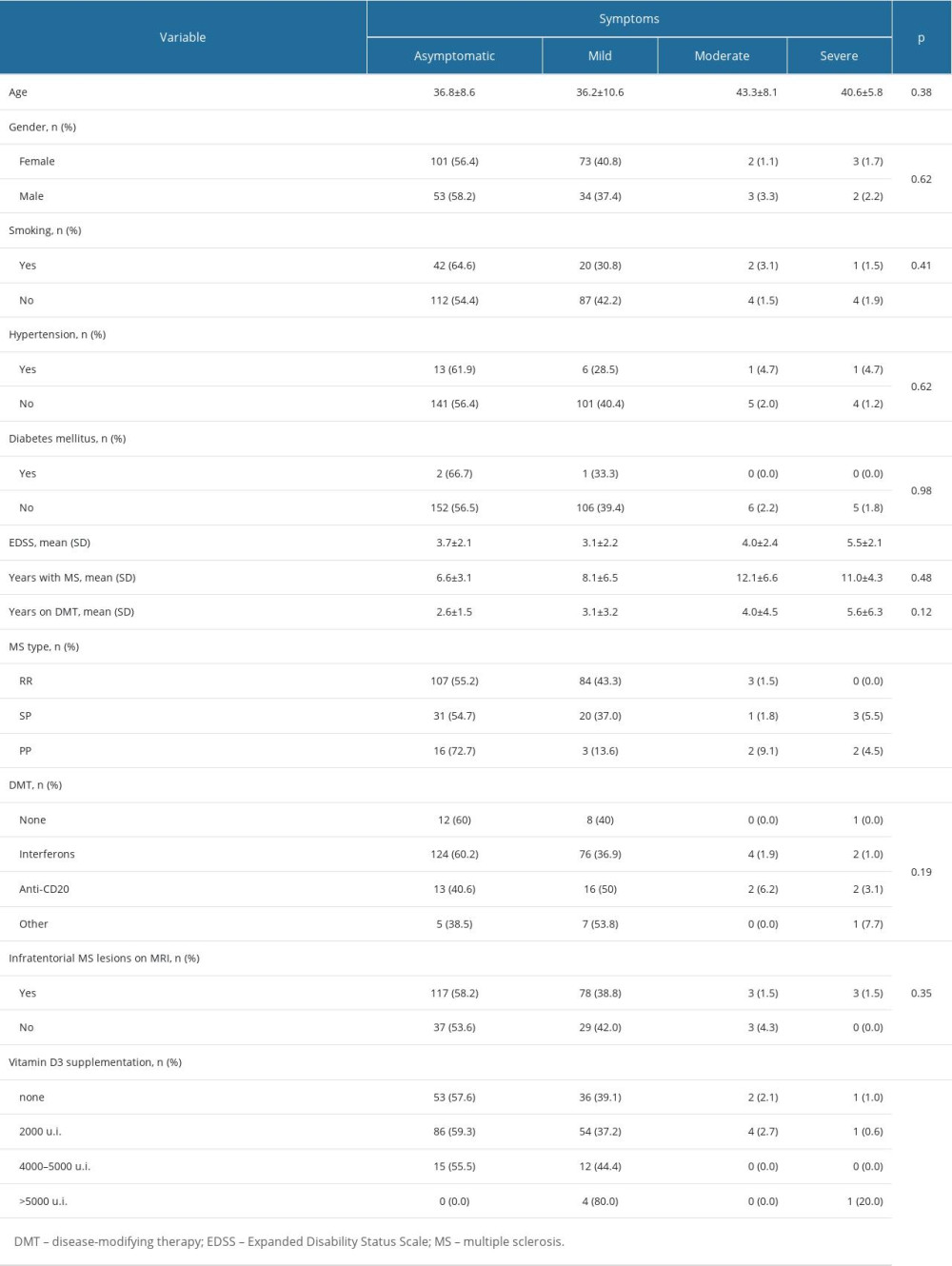
Patients with SPMS and PPMS more frequently had moderate and severe COVID-19 symptoms compared to patients with RRMS (P=0.005) (Table 2, Figure 1). The mean age of the patients with moderate and severe symptoms tended to be older compared to asymptomatic and patients with mild symptoms, but the difference was not significant (Table 2). Compared to asymptomatic patients and patients with mild symptoms, EDSS was higher in patients with moderate and severe symptoms (3.7±2.1 vs 3.1±2.2 vs 4.0±2.4 vs 5.5±2.1, P<0.001) (Figure 2). In a binary logistic regression analysis, advanced types of MS (SPMS and PPMS) remained associated with moderate and severe symptoms, even after adjusting for age, smoking, hypertension, diabetes mellitus, and DMTs, with RR=2.8 (95% CI=1.1–6.6, P=0.018). Although we did not find significant differences in symptoms severity comparing all DMTs together (Table 2), between-group comparison showed that patients on anti-CD20 were more likely to acquire moderate or severe infection compared to patients on interferons (12.1% vs 2.9%) or other DMTs (12.1% vs 7.7%), (Figures 1, 3). The association of anti-CD20 treatment with symptoms remained significant even after adjusting for age, sex, obesity, and MS type with RR=2.1 (95% CI=1.1–4.0, P=0.012). Compared to patients treated with interferons and fingolimod, patients on anti-CD20 had lower titer of serum anti-SARS-CoV-2 IgG, with median and interquartile rates of 6.9 (0.6–33.2) vs 11.8 (0.8–37.3) vs 0.9 (0.3–8.1), respectively (Figure 4). Among patients with severe symptoms, 2 patients have died: a man aged 34 years old with PPMS, diagnosed 13 years ago, with EDSS=5.0 who was not on treatment with DMTs, and a 55-year-old woman with SPMS, diagnosed 30 years ago, with EDSS=8, on treatment with interferon-β 1b.
VITAMIN D SUPPLEMENTATION AND COVID-19 SYMPTOMS SEVERITY:
Patients on vitamin D supplementation 2000–5000 U.I. were less likely to have moderate and severe symptoms compared to patients without vitamin D supplementation and those with >5000 U.I daily dose (
Discussion
OUR FINDINGS:
In our cohort of patients with MS, 96.4% had COVID-19. Patients were mainly asymptomatic or manifested mild symptoms. Patients with more aggressive types of MS presented more frequently with moderate and severe symptoms. Also, MS patients on anti-CD20 presented more frequently with moderate and severe COVID-19 symptoms. Two patients, a 34-year-old man diagnosed with PPMS, and a 55-year-old woman diagnosed with SPMS have died of COVID-19. In addition, MS patients on 2000–5000 U.I. daily doses of vitamin D supplementation had better outcomes.
DATA INTERPRETATION:
Several studies have reported on the COVID-19 outcomes in patients with MS and risk factors associated with more severe presentation of COVID-19, including age, higher disability scale, and comorbidities [9,15]. Other studies derived from national registries reported associations between certain type of DMTs, especially B-cell-depleting therapies and increased risk of severe COVID-19 illness [1,16,17]. In accordance with the current evidence, we found that COVID-19 severity was associated with more aggressive MS phenotypes and higher disability. Patients with PPMS and SPMS more frequently had moderate and severe COVID-19. In addition, patients on second-line DMTs more frequently presented with severe COVID-19 symptoms and lung infiltration on chest imaging compared to patient treated with first-line DMTs. The severity of COVID-19 symptoms in patients on second-line DMTs might be explained by the immunosuppressive effect of the therapy or by fact that most patients on second-line DMTs have also greater disability. This study adds to the body of literature that focuses on COVID-19 symptoms and outcomes in MS patients.
MS patients who are being treated with interferon-β tend to be at lower risk of COVID-19 and at lower risk of severe symptoms. An Italian [18] cohort showed a decreased frequency of MS patients treated with interferon-β in the COVID-19 cohort as compared to the expected frequency in the general population, indicating a possible protective effect of interferons. In our cohort, patients on interferon-β were less likely to have severe COVID-19 symptoms compared to those treated with anti-CD20 (ocrelizumab and rituximab), similar to untreated MS patients. Anti-CD20 treatments have a direct impact on B-cell and T-cell responses, with an impact on the production of IL-6 and B-cell depletion [19,20]. Treatment with anti-CD20 may be associated with more severe COVID-19 [1]. An increased risk of severe forms of COVID-19 in MS patients treated with ocrelizumab was reported in Italian and French cohorts [8,18]. Patients in our cohort treated with ocrelizumab were also more likely to have moderate and severe COVID-19 symptoms. A possible beneficial effect of fingolimod in patients with COVID-19 was suggested in studies showing that its mechanisms of action are involved in potentiation of the pulmonary endothelial barrier [21], and data from MS patients treated with fingolimod suggested a decreased risk of severe symptomatic COVID-19 [18]. In agreement with this, none of the MS patients treated with fingolimod in our cohort had severe COVID-19. Teriflunomide and dimethyl fumarate are immunomodulatory drugs with some pleiotropic effect on the immune system [22]; there are conflicting data in the literature on their effect on COVID-19 prognosis among patients with MS, with studies mainly reporting low risk of severe outcome [8,23]. In our cohort, there was only 1 patient treated with teriflunomide who had severe COVID-19 symptoms and another patient treated with dimethyl fumarate who had mild symptoms. Dimethyl fumarate (DMF) is another immunomodulatory drug employed in the treatment of MS that effects activation of several intracellular pathways, including the antioxidant nuclear factor (erythroid-derived 2)-like 2 (Nrf2) pathway [24]. Experimental studies have shown that SARS-CoV2 inhibits the Nrf2 pathway and that treatment with DMF can affect SARS-CoV-2 replication [25]. Studies have reported that MS patients treated DMF did not have increased risk for COVID-19 symptoms [4,12]. In our cohort, only 1 patient was on DMF, and he experienced only mild symptoms.
While MS patients treated with ocrelizumab and fingolimod had an impaired humoral vaccine response with decreased secretion of SARS-CoV-2 anti-Spike IgG [26,27] after vaccination, an increased humoral vaccine response has been reported in MS patients treated with interferons [28]. We have reported an impaired immune response after infection in patients treated with anti-CD20 with low level of serum anti-SARS-CoV-2 antibodies compared to patients treated with interferons. Periera et al [29], in a recent systematic reviews and meta-analysis, suggested that vitamin D insufficiency is associated with increased hospitalization and mortality in patients with MS. To the best of our knowledge, there are no published studies reporting an association of vitamin D concentration or vitamin D supplementation with COVID-19 symptoms in MS patients. Our findings suggest a protective effect of 2000–5000 U.I. vitamin D supplementation in MS patients with COVID-19.
LIMITATIONS:
This retrospective study, prone to inherent limitations, relied primarily on patient-reported data for assessing the severity of COVID-19 disease rather than using objective examinations. This becomes particularly important when considering symptoms like dyspnea and hyposmia, where a disparity exists between the subjective perception reported by patients and the objective degree of impairment. Additionally, a constraint of the study is the absence of PCR testing confirmation for COVID-19 infection in all participants; instead, confirmation relied on patients’ symptoms and serological antibody screening. Anti-SARS-CoV-2 antibodies were measured during routine examinations at various intervals after infection. Furthermore, the study is limited by the relatively small number of patients without disease-modifying therapies (DMTs) in our cohort, and the inclusion of only a few patients on DMTs other than interferons and anti-CD20.
Conclusions
In this cohort of MS patients with COVID-19 from a single center in Kosovo, we found a very high rate of COVID-19 infection among patients with MS. Patients with advanced MS phenotype, those exhibiting greater disability, and those undergoing anti-CD20 therapy faced an increased risk of experiencing more severe symptoms after COVID-19. Patients on vitamin D supplementation tended to have better clinical outcomes. Patients on anti-CD20 exhibited a compromised humoral response after infection compared to those on other DMTs. Vitamin D supplementation seems to confer a protective effect on COVID-19 infection in patients with MS. Our study adds to the body of literature on COVID-19 symptoms and outcomes in MS patients.
Figures
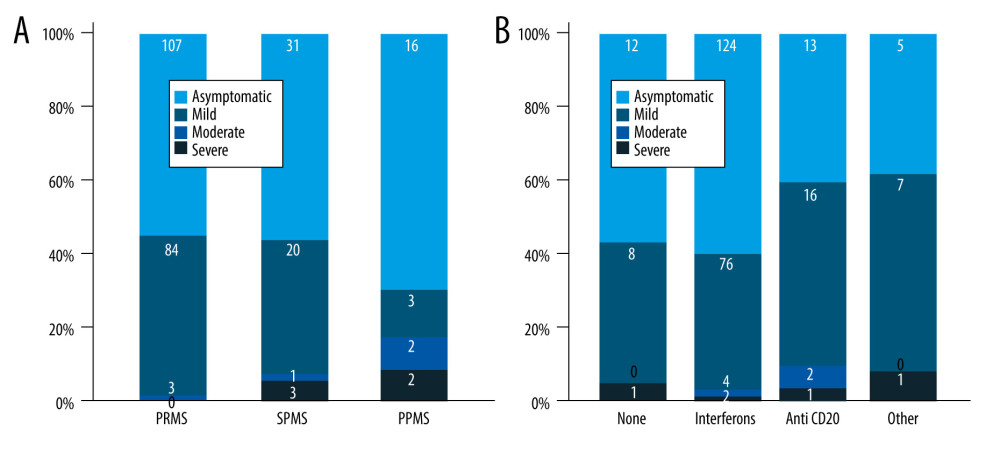 Figure 1. Severity of COVID-19 symptoms in patients with MS. (A) Patients categorized based on the type of MS; (B) Patients categorized based on DMTs. DMT – disease-modifying therapy; MS – multiple sclerosis. RRMS – relapsing-remitting MS; SPMS – second primary MS; PPMS – primary progressive MS.
Figure 1. Severity of COVID-19 symptoms in patients with MS. (A) Patients categorized based on the type of MS; (B) Patients categorized based on DMTs. DMT – disease-modifying therapy; MS – multiple sclerosis. RRMS – relapsing-remitting MS; SPMS – second primary MS; PPMS – primary progressive MS. 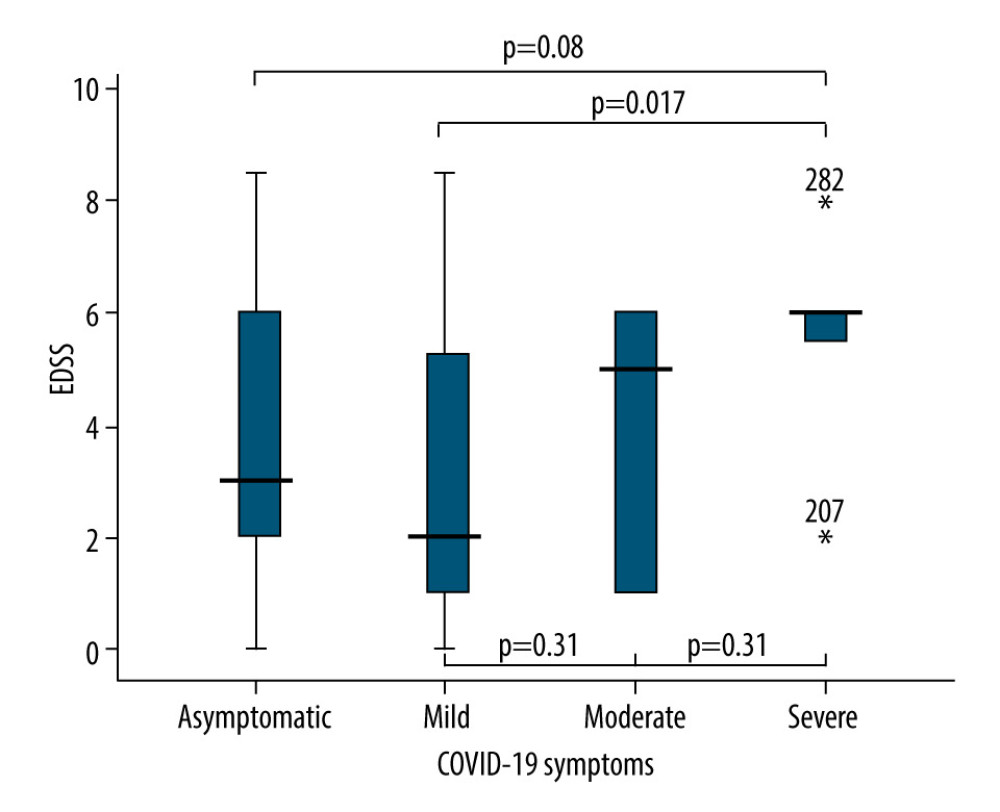 Figure 2. Comparison of EDSS means and SD in patients with different COVID-19 symptoms severity. EDSS – Expanded Disability Status Scale; SD – standard deviations.
Figure 2. Comparison of EDSS means and SD in patients with different COVID-19 symptoms severity. EDSS – Expanded Disability Status Scale; SD – standard deviations. 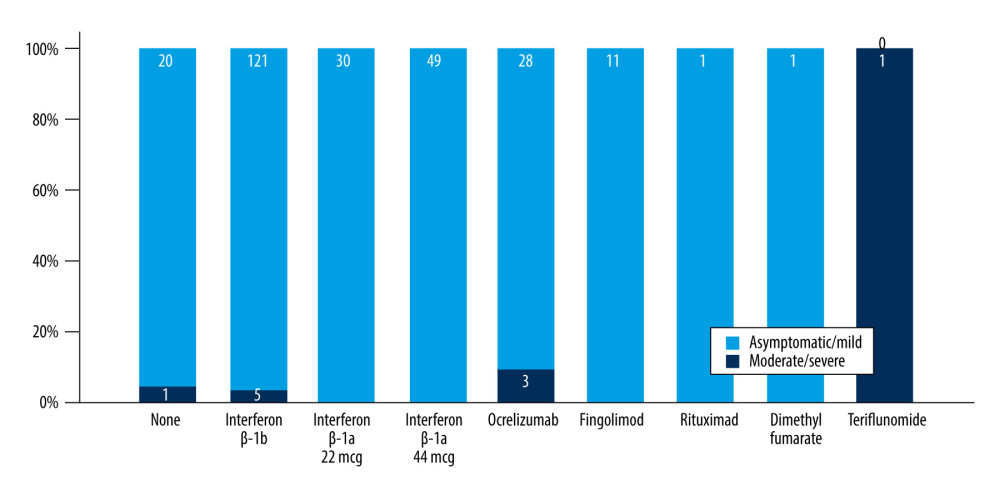 Figure 3. Frequency of moderate and severe COVID-19 symptoms in MS patients on different DMTs. MS – multiple sclerosis; DMT – disease-modifying therapy.
Figure 3. Frequency of moderate and severe COVID-19 symptoms in MS patients on different DMTs. MS – multiple sclerosis; DMT – disease-modifying therapy. 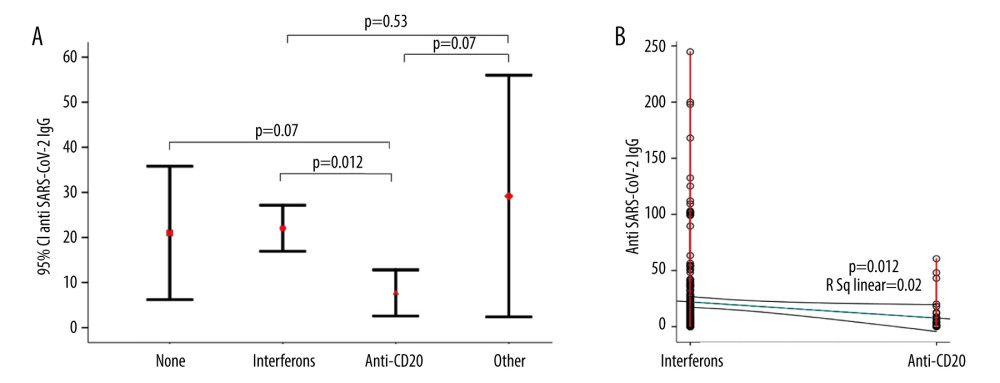 Figure 4. Comparison of the anti-SARS-CoV-2 antibodies between MS patients on different DMTs. (A) Error bars of anti-SARS-CoV-2 IgG titer in different DMTs. (B) Scatter plots of the anti-SARS-CoV-2 IgG titer between patients on interferons and Anti-CD20. MS – multiple sclerosis; DMT – disease-modifying therapy.
Figure 4. Comparison of the anti-SARS-CoV-2 antibodies between MS patients on different DMTs. (A) Error bars of anti-SARS-CoV-2 IgG titer in different DMTs. (B) Scatter plots of the anti-SARS-CoV-2 IgG titer between patients on interferons and Anti-CD20. MS – multiple sclerosis; DMT – disease-modifying therapy. References
1. Luna G, Alping P, Burman J, Infection risks among patients with multiple sclerosis treated with fingolimod, natalizumab, rituximab, and injectable therapies: Neurol, 2020; 77(2); 184-91
2. Noyes K, Weinstock-Guttman B, Impact of diagnosis and early treatment on the course of multiple sclerosis: Am J Manag Care, 2013; 19(17 Suppl); s321-s31
3. Cree BA, Gourraud PAUniversity of California, San Francisco MS-EPIC Team, Long-term evolution of multiple sclerosis disability in the treatment era: Ann Neurol, 2016; 80(4); 499-510
4. Yong KP, Kim HJ, Disease modifying therapies and infection risks in multiple sclerosis – a decision-making conundrum: Ann Transl Med, 2020; 8(11); 722
5. Reen GK, Silber E, Langdon DW, Multiple sclerosis patients’ understanding and preferences for risks and benefits of disease-modifying drugs: A systematic review: J Neurol Sci, 2017; 375; 107-22
6. Parrotta E, Kister I, Charvet L, COVID-19 outcomes in MS: Observational study of early experience from NYU Multiple Sclerosis Comprehensive Care Center: Neurol Neuroimmunol Neuroinflamm, 2020; 7(5); e835
7. Bsteh G, Bitschnau C, Hegen H, Multiple sclerosis and COVID-19: How many are at risk?: Eur J Neurol, 2021; 28(10); 3369-74
8. Louapre C, Collongues N, Stankoff B, Clinical characteristics and outcomes in patients with coronavirus disease 2019 and multiple sclerosis: JAMA Neurol, 2020; 77(9); 1079-88
9. Piñar Morales R, Ramírez Rivas MA, Barrero Hernández FJ, SARS-CoV-2 infection and seroprevalence in patients with multiple sclerosis: Neurologia (Engl Ed), 2021; 36(9); 698-703
10. Thompson AJ, Banwell BL, Barkhof F, Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria: Lancet Neurol, 2018; 17(2); 162-73
11. Kurtzke JF, Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS): Neurology, 1983; 33(11); 1444-52
12. D’Souza M, Yaldizli Ö, John R, Neurostatus e-Scoring improves consistency of Expanded Disability Status Scale assessments: A proof of concept study: Mult Scler, 2017; 23(4); 597-603
13. Brodin P, Immune determinants of COVID-19 disease presentation and severity: Nat Med, 2021; 27(1); 28-33
14. : Coronavirus disease 2019 (COVID-19) treatment guidelines April 21, 2021, Bethesda (MD), National Institutes of Health (US)
15. Kataria S, Tandon M, Melnic V, Sriwastava S, A case series and literature review of multiple sclerosis and COVID-19: Clinical characteristics, outcomes and a brief review of immunotherapies: eNeurologicalSci, 2020; 21; 100287
16. Salter A, Fox RJ, Newsome SD, Outcomes and risk factors associated with SARS-CoV-2 infection in a North American Registry of Patients with Multiple Sclerosis: JAMA Neurol, 2021; 78(6); 699-708
17. Simpson-Yap S, De Brouwer E, Kalincik T, Associations of disease-modifying therapies with COVID-19 severity in multiple sclerosis: Neurology, 2021; 97(19); e1870-e85
18. Sormani MP, De Rossi N, Schiavetti I, Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis: Ann Neurol, 2021; 89(4); 780-89
19. Barr TA, Shen P, Brown S, B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6-producing B cells: J Exp Med, 2012; 209(5); 1001-10
20. Moreno Torres I, García-Merino A, Anti-CD20 monoclonal antibodies in multiple sclerosis: Expert Rev Neurother, 2017; 17(4); 359-71
21. Mohammed S, Harikumar KB, Corrigendum: Sphingosine 1-phosphate: A novel target for lung disorders: Front Immunol, 2018; 9; 1628
22. Gandoglia I, Ivaldi F, Laroni A, Teriflunomide treatment reduces B cells in patients with MS: Neurol Neuroimmunol Neuroinflamm, 2017; 4(6); e403
23. Bowen JD, Brink J, Brown TR, COVID-19 in MS: Initial observations from the Pacific Northwest: Neurol Neuroimmunol Neuroinflamm, 2020; 7(5); e783
24. Linker RA, Lee DH, Ryan S, Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway: Brain, 2011; 134(Pt 3); 678-92
25. Olagnier D, Farahani E, Thyrsted J, SARS-CoV2-mediated suppression of NRF2-signaling reveals potent antiviral and anti-inflammatory activity of 4-octyl-itaconate and dimethyl fumarate: Nat Commun, 2020; 11(1); 4938
26. Tortorella C, Aiello A, Gasperini C, Humoral- and T-cell-specific immune responses to SARS-CoV-2 mRNA vaccination in patients with MS using different disease-modifying therapies: Neurology, 2022; 98(5); e541-e54
27. Sormani MP, Inglese M, Schiavetti I, Effect of SARS-CoV-2 mRNA vaccination in MS patients treated with disease modifying therapies: EBioMedicine, 2021; 72; 103581
28. Maniscalco GT, Manzo V, Ferrara AL, Interferon Beta-1a treatment promotes SARS-CoV-2 mRNA vaccine response in multiple sclerosis subjects: Mult Scler Relat Disord, 2022; 58; 103455
29. Pereira M, Dantas Damascena A, Galvão Azevedo LM, Vitamin D deficiency aggravates COVID-19: Systematic review and meta-analysis: Crit Rev Food Sci Nutr, 2022; 62(5); 1308-16
Figures
 Figure 1. Severity of COVID-19 symptoms in patients with MS. (A) Patients categorized based on the type of MS; (B) Patients categorized based on DMTs. DMT – disease-modifying therapy; MS – multiple sclerosis. RRMS – relapsing-remitting MS; SPMS – second primary MS; PPMS – primary progressive MS.
Figure 1. Severity of COVID-19 symptoms in patients with MS. (A) Patients categorized based on the type of MS; (B) Patients categorized based on DMTs. DMT – disease-modifying therapy; MS – multiple sclerosis. RRMS – relapsing-remitting MS; SPMS – second primary MS; PPMS – primary progressive MS. Figure 2. Comparison of EDSS means and SD in patients with different COVID-19 symptoms severity. EDSS – Expanded Disability Status Scale; SD – standard deviations.
Figure 2. Comparison of EDSS means and SD in patients with different COVID-19 symptoms severity. EDSS – Expanded Disability Status Scale; SD – standard deviations. Figure 3. Frequency of moderate and severe COVID-19 symptoms in MS patients on different DMTs. MS – multiple sclerosis; DMT – disease-modifying therapy.
Figure 3. Frequency of moderate and severe COVID-19 symptoms in MS patients on different DMTs. MS – multiple sclerosis; DMT – disease-modifying therapy. Figure 4. Comparison of the anti-SARS-CoV-2 antibodies between MS patients on different DMTs. (A) Error bars of anti-SARS-CoV-2 IgG titer in different DMTs. (B) Scatter plots of the anti-SARS-CoV-2 IgG titer between patients on interferons and Anti-CD20. MS – multiple sclerosis; DMT – disease-modifying therapy.
Figure 4. Comparison of the anti-SARS-CoV-2 antibodies between MS patients on different DMTs. (A) Error bars of anti-SARS-CoV-2 IgG titer in different DMTs. (B) Scatter plots of the anti-SARS-CoV-2 IgG titer between patients on interferons and Anti-CD20. MS – multiple sclerosis; DMT – disease-modifying therapy. In Press
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
12 Mar 2024 : Clinical Research
Comparing Neuromuscular Blockade Measurement Between Upper Arm (TOF Cuff®) and Eyelid (TOF Scan®) Using Miv...Med Sci Monit In Press; DOI: 10.12659/MSM.943630
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952