23 May 2022: Clinical Research
Microalbuminuria Complicated with Low Estimated Glomerular Filtration Rate: Early Risk Factors for Contrast-Induced Acute Kidney Injury After Coronary Intervention
Meijuan Hu12ABCDEF, Erfei Luo2E, Gaoliang Yan3D, Chengchun Tang3BCE, Lei Wang1BCF, Qigao Zhang1ABE, Jianbin Gong1AE*DOI: 10.12659/MSM.935455
Med Sci Monit 2022; 28:e935455
Abstract
BACKGROUND: We aimed to investigate the impact of microalbuminuria complicated with low estimate glomerular filtration rate (eGFR) on the incidence and prognosis of contrast-induced acute kidney injury (CI-AKI) in patients with coronary artery disease after coronary intervention.
MATERIAL AND METHODS: A total of 943 patients were enrolled in the study. Based on microalbumin/creatinine (ACR) measurements, the patients were divided into a microalbuminuria cohort (MA; 222 patients) and a normal albuminuria cohort (NA; 721 patients). According to eGFR levels, the cohorts were further subdivided into normal, mild, moderate, and severe renal dysfunction groups. The basic data and indicators of all enrolled patients were collected. The patients were followed up at 30 days, 6 months, 1 year, and 3 years after surgery.
RESULTS: The overall incidence of CI-AKI in the MA cohort was higher than that in the NA cohort (17.6% vs 8.2%, P<0.001). The incidence of CI-AKI in different eGFR groups in the MA cohort was significantly different among the normal renal function group (3.3%), mild renal dysfunction group (9.2%), moderate renal dysfunction group (24.6%), and severe renal dysfunction group (36%) (P<0.001). When microalbuminuria was complicated with eGFR <45 mL/min/1.73 m², the risk of CI-AKI increased (P<0.001). During the 3-year follow-up, the rehospitalization rate and incidence of major adverse cardiovascular events in patients with low eGFR levels complicated with microalbuminuria were significantly higher than those with normal albuminuria (42.8% vs 19.9%, 16.3% vs 3.5%, P<0.001).
CONCLUSIONS: Microalbuminuria complicated with low eGFR levels may increase the risk of CI-AKI and adverse cardiovascular events in patients after coronary intervention.
Keywords: Acute Kidney Injury, Contrast Media, Prognosis, Albuminuria, Creatinine, Glomerular Filtration Rate, Humans, Risk Factors
Background
With the increasingly wide application of coronary intervention in the diagnosis, evaluation, and treatment of diseases, renal injury caused by contrast medium continues to occur and is called contrast-induced acute kidney injury (CI-AKI). Parfrey and Barret proposed the concept of contrast-induced nephropathy in 1994 as follows: within 48 to 72 h after the application of contrast medium, the serum creatinine level increases by 0.5 mg/dL (44.2 μmol/L) from the baseline value or the relative value increases by 25%; other possible causes of renal injury were excluded [1]
In their latest study, the American College of Radiology recommended that CI-AKI should be used to define contrast-induced nephropathy when the serum creatinine level (26.5 μmol/L) is 0.3 mg/dL higher than the baseline within 48 h after the application of contrast medium, 50% higher than the baseline within 7 days after the application of contrast medium, or the urine volume drops below 0.5 mL/kg/h for more than 6 h [2]. Studies have shown that the incidence of CI-AKI is approximately 1% to 30%; most courses of CI-AKI are self-limited, and patients with acute kidney injury can recover within 10 days. However, the natural development of the disease will increase the mortality, hospital stay, and the incidence of adverse renal and cardiovascular events [3]. According to the class I recommendation of the European Society of Cardiology and European Association for Cardio-Thoracic Surgery Guidelines 2018, (1) perioperative risk stratification should be performed in all patients undergoing coronary angiography; (2) all patients undergoing coronary angiography should be fully hydrated; (3) for patients with moderate or severe chronic kidney disease, the dosage of contrast medium should be controlled, with the total dosage of contrast medium/GFR <3.7; and (4) isotonic or hypotonic contrast medium can be used in patients with moderate or severe chronic kidney disease [4].
Approximately 700 000 patients undergo coronary interventions in China every year [5], including coronary angiography and percutaneous coronary intervention. Most of these patients have high-risk factors for contrast-induced kidney injury, such as existing chronic kidney disease, geriatric issues, diabetes, metabolic syndrome, hypertension, progressive heart failure, anemia, hypoproteinemia, dehydration, recent hypotension, hyperuricemia, history of vascular disease, and administration of nephrotoxic drugs [6,7]. Risk score models are used to predict contrast-induced nephropathy after percutaneous coronary intervention [8]. The Mehran score is the prediction scoring system of contrast-induced kidney injury calculated according to a total score of risk factors for renal injuries, low blood pressure, treatment of intra-aortic balloon counterpulsation, congestive heart failure, age greater than 75 years, anemia, diabetes, and contrast agent, by which patients are divided into low-risk, moderate, high-risk groups [9,10]. Among the above-mentioned risk factors, chronic kidney disease is the most important and common risk factor for contrast-induced nephropathy [11,12].
The Chinese Heart Survey showed that over 75% of patients with coronary artery disease have high blood glucose and that approximately 50% of patients have the complication of known or newly diagnosed diabetes [13], while microalbuminuria is one of the indicators of early damage to renal function in diabetic nephropathy [14]. Microalbuminuria is defined as a persistent elevation of albumin in the urine, of 30 to 300 mg/day. The use of the urine albumin to urine creatinine ratio (ACR) is recommended as the preferred screening strategy for detecting microalbuminuria [15]. Since the 1980s, the leakage of small amounts of proteins in urine has been considered a crucial sign of early kidney disease, especially in patients with diabetes. An increasing interest in microalbuminuria as a marker of cardiovascular risk has been more recently considered [16]. Currently, reduced eGFR is considered the most important risk factor for CI-AKI [17].Therefore, the preoperative assessment of renal injury and renal function in patients is particularly important for the prevention of CK-AKI after coronary intervention [18,19].
There is no large-sample study on the impact of microalbuminuria, an early kidney damage indicator, and chronic renal dysfunction on the incidence and long-term prognosis of CI-AKI in China. We aimed to investigate the relationship between pre-existing microalbuminuria and low eGFR levels on the incidence and prognosis of CI-AKI to determine whether microalbuminuria complicated with a low eGFR level could be an early risk factor for CI-AKI after coronary intervention.
Material and Methods
PATIENT ENROLLMENT:
The study enrolled a total of 1048 patients who underwent coronary angiography or percutaneous coronary intervention at Jinling Hospital from September 2015 to February 2019. The cardiac catheterization procedures and interventions were performed according to practice standards for our center, using the radial or femoral approach (Siemens DSA, Germany).
INCLUSION CRITERIA:
Patients who voluntarily participated in the trial, signed informed consent, were aged ≥18 years, intended to receive coronary intervention, and were without contraindications were included in the study.
EXCLUSION CRITERIA:
Patients who were lactating or pregnant, used contrast medium or nephrotoxic drugs in the previous week, had albuminuria with an microalbumin/creatinine ratio (ACR) of 300 mg/g, had an allergy to iodine contrast medium, used an intra-aortic balloon pump, or had acute heart failure or liver failure, connective tissue diseases, tumors, severe infectious diseases, cardiogenic shock in the hospital, or sudden cardiac death in the hospital were excluded from the study.
WITHDRAWAL CRITERIA:
Patients were withdrawn from the study for the following reasons: the patient requested withdrawal from the trial; the investigator considered it necessary to terminate the trial following a serious adverse event; the patient was found to not meet the inclusion criteria during the trial; the patient’s records were incomplete or the patient was unable to complete follow-up evaluation; and the patient seriously violated or deviated from the trial protocol.
This study was approved by the Ethics Committee of Jinling Hospital, and all enrolled patients signed informed consent agreements.
STUDY DESIGN:
This was a prospective study, and the test formula for 2 independent proportions was used to estimate the required sample size in each group. At a 2-tailed significance level of α=0.05, with 80% power and 5% loss to follow-up rate, at least 138 patients were required in each group. After eliminating confounding factors, a total of 943 patients were included in the study, and all were hospitalized prior to enrollment. Based on the urine ACR measurements, they were divided into 2 cohorts: the microalbuminuria cohort (MA) with 222 patients (ACR 30–299 mg/g), and the normal albuminuria cohort (NA) with 721 patients (ACR <30 mg/g). According to eGFR levels, each cohort was further subdivided into the following groups: normal renal function group (eGFR ≥60 mL/min/1.73 m2), mild renal dysfunction group (45≤ eGFR <60 mL/min/1.73 m2), moderate renal dysfunction group (30≤ eGFR <45 mL/min/1.73 m2), and severe renal dysfunction group (eGFR <30 mL/min/1.73 m2). During follow-up, 44 patients in the MA cohort were lost to follow-up, and 165 patients in the NA cohort were lost to follow-up (Figure 1).
STUDY ENDPOINTS:
The basic data and indicators of all enrolled patients were collected, including serum creatinine (SCr) 24 h before, 48 h after, and 7 days after coronary angiography, preoperative ACR, urinary neutrophil gelatinase-associated lipocalin level 4 h after surgery, eGFR level, and urine volume 6 h after surgery. The patients were followed up for 30 days, 6 months, 1 year, and 3 years after surgery for adverse renal and cardiovascular and cerebrovascular events. All SCr and microalbuminuria levels were determined by laboratory personnel using an autoanalyzer in the Department of Laboratory Medicine in the Jinling Hospital.
CI-AKI was defined as a SCr level within 48 h after administration of contrast medium of 0.3 mg/dL (26.5 μmol/L) higher than the baseline, an SCr level within 7 days of 50% higher than the baseline, or a urine volume of below 0.5 mL/kg/h for more than 6 h [2]. The definition of albuminuria was a urine ACR of 30 to 299 mg/g. Coronary intervention was defined as coronary angiography or percutaneous coronary intervention. The eGFR level based on the SCr level is a modified modification of diet in renal disease formula suitable for the Chinese population: eGFR (mL/min/1.73 m2)=175×SCr (mg/dL)−1.234× age−0.179×(0.79 if female) [20]. Hypotonic contrast medium was chosen during coronary intervention for all enrolled patients, and the dosage of contrast medium was controlled at <4 mL/kg, or the dosage of contrast medium/glomerular filtration rate was controlled at <3.7. The standard hydration protocol was as follows: normal saline 1 to 1.5 mL/kg/h was given before surgery for 12 h and after surgery for 24 h. For patients with capacity-limited heart failure, physiological saline 3 mL/kg/h was chosen and given before surgery for 1 h, and physiological saline was administered at 1 mL/kg/h after surgery for 6 h [20].
STATISTICAL ANALYSIS:
The measurement data are expressed by mean±standard deviation, and the enumeration data are expressed as percentages. Continuous variables were compared using 1-way ANOVA or the 2-sided
Results
BASIC CLINICAL CHARACTERISTICS:
A total of 943 patients were enrolled in this study. Among the 222 patients in the MA cohort, 61 had normal renal function, 54 had mild renal dysfunction, 57 had moderate renal dysfunction, and 50 had severe renal dysfunction. Among the 721 patients in the NA cohort, 208 had normal renal function, 195 had mild renal dysfunction, 178 had moderate renal dysfunction, and 140 had severe renal dysfunction. The basic clinical conditions of the 2 cohorts showed statistically significant differences in fasting blood glucose (6.7±1.2 vs 5.5±1.3 mmol/L, P<0.001), diabetes (66.7% vs 24.5, P<0.001), and ACR (93.5±53.9 vs 11.8±6.2 mg/g, P<0.001), while there were no significant differences between the 2 cohorts in age (74.5±8.7 vs 75.3±7.9, P=0.157), male sex (74.8% vs 79.2%, P=0.163), heart failure (10.6% vs 8.8%, P=0.351), hypertension (72.1% vs 70.7%, P=0.701), hyperlipidemia (29.3% vs 23.4%, P=0.078), myocardial infarction (10.4% vs 9.2%, P=0.591), proportion of percutaneous coronary intervention treatment (49.1% vs 43.1%, P=0.118), and dosage of contrast medium (125.8±68.5 vs 126.5±65.1 mL, P=0.891) (Table 1).
CI-AKI INCIDENCE:
The incidence of CI-AKI in all patients was 10.4%. The incidence of CI-AKI in the MA cohort was higher than that in the NA cohort (17.6% vs 8.2%, P<0.001). The incidence of CI-AKI in patients in the MA cohort complicated with eGFR <45 mL/min/1.73 m2 was higher than that in the control cohort (24.6% vs 6.2%, 36.0% vs 9.3%, P<0.001) (Table 2). The incidence of CI-AKI in different eGFR groups was significantly different and increased with the decline in renal function, as follows: normal renal function group, mild renal dysfunction group, moderate renal dysfunction group, and severe renal dysfunction group had CI-AKI incidences of 8.6%, 7.6%, 10.6%, and 6.3%, respectively (P=0.017). The incidence of CI-AKI in different eGFR groups in the MA cohort was significantly different, with rates in the normal renal function group, mild renal dysfunction group, moderate renal dysfunction group, and severe renal dysfunction group of 3.3%. 9.6%, 24.6%, and 36.0%, respectively (P<0.001). There was no significant difference in the incidence of CI-AKI among the different eGFR groups in the NA cohort (10.1% vs 7.2% vs 6.2% vs 9.3%, P=0.484) (Figure 2).
EARLY RISK FACTORS AND PREDICTIVE INDICATORS:
Low eGFR levels in the MA cohort were correlated with the incidence of CI-AKI, and when the MA cohort had 30≤ eGFR <45 mL/min/1.73 m2, the incidence risk of CI-AKI increased (odds ratio [OR], 1.356; 95% confidence interval [CI], 1.125–1.633; P<0.001). When the MA cohort had eGFR <30 mL/min/1.73 m2, the incidence risk of CI-AKI increased (OR, 1.505; 95% CI, 1.210–1.872; P<0.001) (Table 3). The ROC curve showed that the eGFR cut-off value in the MA cohort was 36.7 mL/min/1.73 m2 (sensitivity, 43.8%; specificity, 86.7%; AUC, 0.639; 95% CI, 0.520–0.757; P=0.023) (Figure 3).
3-YEAR FOLLOW-UP:
During the 3-year follow up, 178 patients were followed up in the MA cohort, and there were 19 major adverse cardiovascular events (MACEs); 556 patients were followed up in the NA cohort, which had 27 MACEs. The incidence of MACEs was significantly different (10.7% vs 4.9%, P=0.005). There were a total of 107 high-risk patients with eGFR <45 mL/min/1.73 m2 in the MA cohort, of which 98 were followed up, and there were 318 patients in the control cohort, of which 282 were followed up. Between the 2 cohorts, there were statistically significant differences in rehospitalization rate (42.8% vs 19.9%, P<0.001) and MACEs (16.3% vs 3.5%, P<0.001) (Table 4). With MACEs as the endpoint event, Kaplan-Meier survival curves were compared between the MA cohort and the NA cohort when eGFR <45 mL/min/1.73 m2, indicating that the risk of MACEs increased in patients with moderate renal dysfunction (eGFR <45 mL/min/1.73 m2) in the MA cohort (P<0.001) (Figure 4).
Discussion
With the widespread application of coronary interventions, the incidence of CI-AKI has continued to increase. In recent years, many scholars have conducted research on this topic, and the high-risk population vulnerable to diseases has attracted increased attention from clinicians. Therefore, it is important to identify the early risk factors related to CI-AKI.
In the K/DOQI clinical practice guidelines for chronic kidney disease, eGFR <60 mL/min, is one of the most vital diagnostic criterion for chronic kidney disease (CKD), and the eGFR level is used for the stratified classification of CKD [21]. Moreover, microalbuminuria is used for the diagnosis of CKD. We found that the incidence of CI-AKI in patients with pre-existing microalbuminuria was higher than those with normal albuminuria. As described in the guidelines, microalbuminuria is a sensitive marker of CKD resulting from diabetes, hypertension, and glomerular disease [16]. Additionally, eGFR is not sensitive to the diagnosis and evaluation of CKD, because even if 50% of the nephrons of both kidneys are lost, eGFR cannot lead to a reduction in GFR. Nevertheless, microalbuminuria can always be detected in the early stage of kidney injury [22]. Therefore, eGFR alone is insufficient to detect kidney injury but is important to monitor microalbuminuria.
The China Heart Survey showed that over 25% of patients undergoing coronary intervention had the complication of confirmed diabetes [13], and patients with diabetes were positive for microalbuminuria when kidney injury appeared. In the present study, we found that the clinical characteristics of the microalbuminuria cohort were not completely matched with the normal albuminuria cohort before angiography, as a larger proportion of patients in the microalbuminuria cohort had diabetes mellitus and higher fasting blood glucose levels than the normal albuminuria cohort. Thus, microalbuminuria is not rare in patients with coronary artery disease. Our findings are consistent with the China Heart Study [13]. Recent studies have confirmed that microalbuminuria and low eGFR are independent risk factors for cardiovascular diseases [23–25]. A positive microalbumin test is one of the prominent markers of early kidney injury in patients with diabetes, and existing kidney injury increases the risk of CI-AKI after coronary intervention [26,27].
Almost every report describing risk factors for CI-AKI lists abnormal baseline SCr levels and reduced eGFR levels as risk factors. To the best of our knowledge, our study is the first aimed to investigate the effect of microalbuminuria complicated with low eGFR on the incidence and prognosis of CI-AKI in patients with coronary artery disease after coronary intervention. We found that in all patients, SCr levels increased and eGFR levels decreased before and after coronary angiography, indicating the existence of the effect on renal function. With the decrease of eGFR, the incidence of CI-AKI increased; however, in patients without albuminuria, the decrease of eGFR did not affect the incidence of CI-AKI. The incidence of CI-AKI increased with the deterioration of eGFR and occurred only in patients with albuminuria. The changes of serum creatinine and kidney injury markers in high-risk populations with renal dysfunction (eGFR <45 ml/min/1.73 m2) after coronary angiography showed that patients with microalbuminuria had a higher risk of developing CI-AKI. This may be due to the following 3 factors: First, patients with microalbuminuria often have diabetes or hypertension [28], for which the important pathological features are high glomeruli pressure and high filtration of the kidney. Long-term high pressure and high filtration causes fibrosis in glomeruli, and the filtration function can be impaired. Due to the strong compensatory renal function, the loss of less than 50% of the nephrons does not cause a significant decrease in GFR [29], and therefore, the glomeruli of patients with microalbuminuria have already been damaged, although the GFR is normal. Second, because the functions of renal tubules include secretion and reabsorption and the glomerular filtration function of patients with microalbuminuria is impaired, a large number of macromolecular substances in the plasma, including albumin, are filtered into the renal capsule, which is bound to increase the reabsorption burden of renal tubules. The detection of microalbuminuria in urine indicates that the filtered albumin has exceeded the reabsorption function of renal tubules and that the renal tubules are in a high-load working state. At that time, the renal tubules will be more susceptible to the toxic damage of contrast media [30], and one of the important mechanisms of contrast-induced nephropathy is the direct toxic effect of contrast media on renal tubules. Third, microalbuminuria is an important sign of damage to systemic vascular endothelial cells. Microalbuminuria can cause not only microvascular lesions of glomeruli and retina, but also macrovascular lesions [16,31]. Hence, the blood vessels of patients with microalbuminuria, including renal blood vessels, are more susceptible to contrast media damage, which in turn leads to the constriction of renal blood vessels and ultimately results in the decline in intrarenal blood flow and renal dysfunction [32]. As a result, glomerular injury, renal tubular overload, and renal vascular endothelial dysfunction jointly lead to an increase in the incidence of CI-AKI in patients with microalbuminuria.
The results of follow-up for an average of 3 years revealed that the incidence of adverse cardiovascular events in patients with microalbuminuria complicated with eGFR <45 mL/min/1.73 m2 was higher than that in patients without albuminuria, and the long-term prognosis of the microalbuminuria cohort was poorer. Preventive intervention is recommended for high-risk patients with microalbuminuria, especially for those with preoperative eGFR <45 mL/min/1.73 m2.
Our findings have several potentially crucial clinical implications. First, low eGFR is a well-known predictor of CI-AKI, yet microalbuminuria complicated with low eGFR has not been regarded as a risk factor for CI-AKI. Our results suggest that this group of patients should be considered as a high-risk population for CI-AKI who may need to be closely monitored for albuminuria and renal function during the perioperative period, and active interventions should be taken for the purpose of prevention. Second, microalbuminuria may be a modifiable intervention target for the prevention of CI-AKI.
There are several potential limitations in this study. First, this was a single-center study with a small sample size, and large multi-center randomized controlled trials are needed. Second, clinical studies have not proven that hydration or other CI-AKI prevention measures have a definite effect on patients with microalbuminuria and renal dysfunction, which needs to be confirmed in future investigations.
Conclusions
Microalbuminuria complicated with low eGFR may increase the risk of CI-AKI and adverse cardiovascular events in patients after coronary intervention. Preventive intervention is recommended for high-risk patients with microalbuminuria, especially for those with preoperative eGFR <45 mL/min/1.73 m2.
Figures
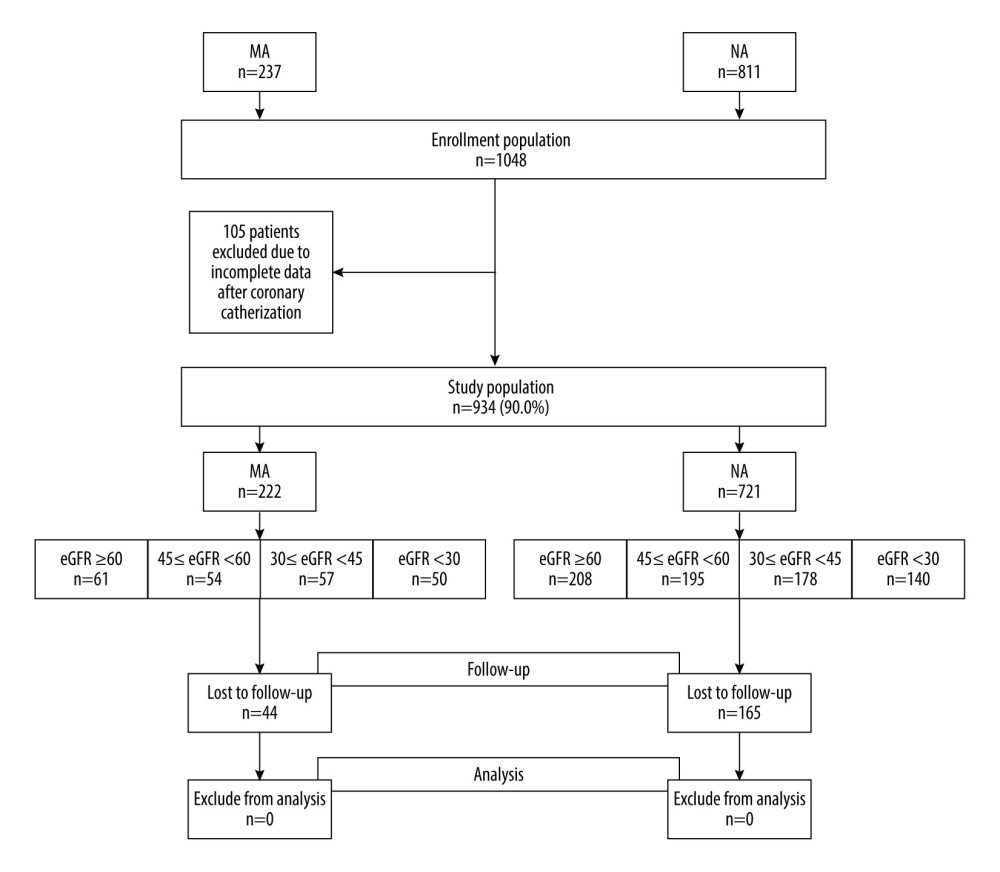 Figure 1. Study flow diagram. eGFR – estimate glomerular filtration rate; MA – microalbuminuria; NA – normal albuminuria. (Microsoft Office, 2016, Microsoft).
Figure 1. Study flow diagram. eGFR – estimate glomerular filtration rate; MA – microalbuminuria; NA – normal albuminuria. (Microsoft Office, 2016, Microsoft). 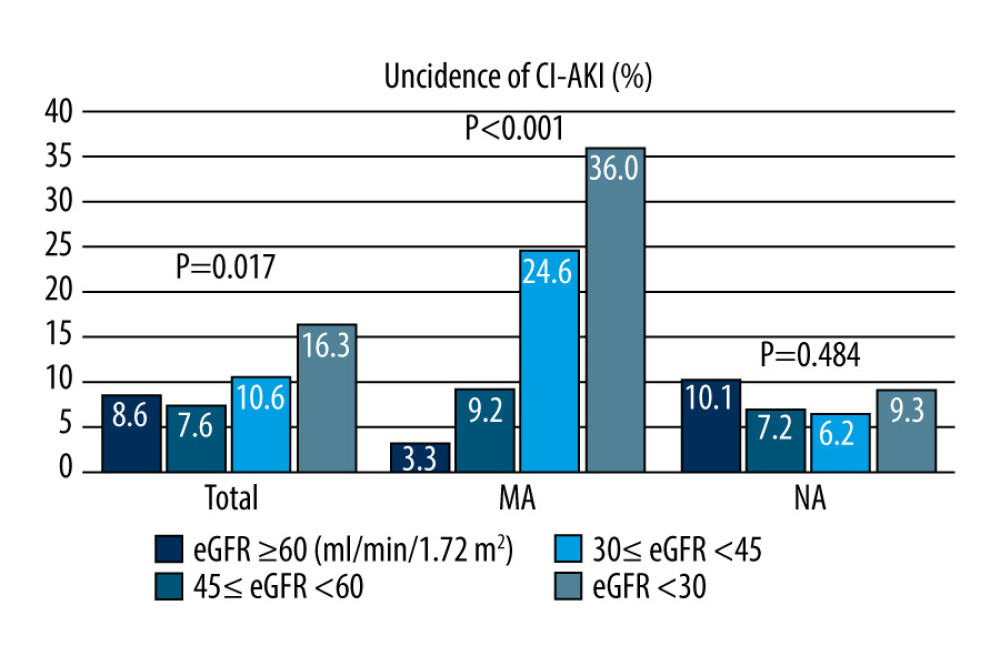 Figure 2. Incidence of contrast-induced acute kidney injury (CI-AKI).The incidence of CI-AKI increased with a decreasing estimate glomerular filtration rate (eGFR) in all patients (P=0.017) and the patients with microalbuminuria (P<0.001). There was no difference in the incidence of CI-AKI between different eGFR groups in patients with normal microalbuminuria (P=0.484). CI-AKI – contrast-induced acute kidney injury; eGFR – estimate glomerular filtration rate; MA – microalbuminuria; NA – normal-albuminuria. (Microsoft office, 2016, Microsoft).
Figure 2. Incidence of contrast-induced acute kidney injury (CI-AKI).The incidence of CI-AKI increased with a decreasing estimate glomerular filtration rate (eGFR) in all patients (P=0.017) and the patients with microalbuminuria (P<0.001). There was no difference in the incidence of CI-AKI between different eGFR groups in patients with normal microalbuminuria (P=0.484). CI-AKI – contrast-induced acute kidney injury; eGFR – estimate glomerular filtration rate; MA – microalbuminuria; NA – normal-albuminuria. (Microsoft office, 2016, Microsoft). 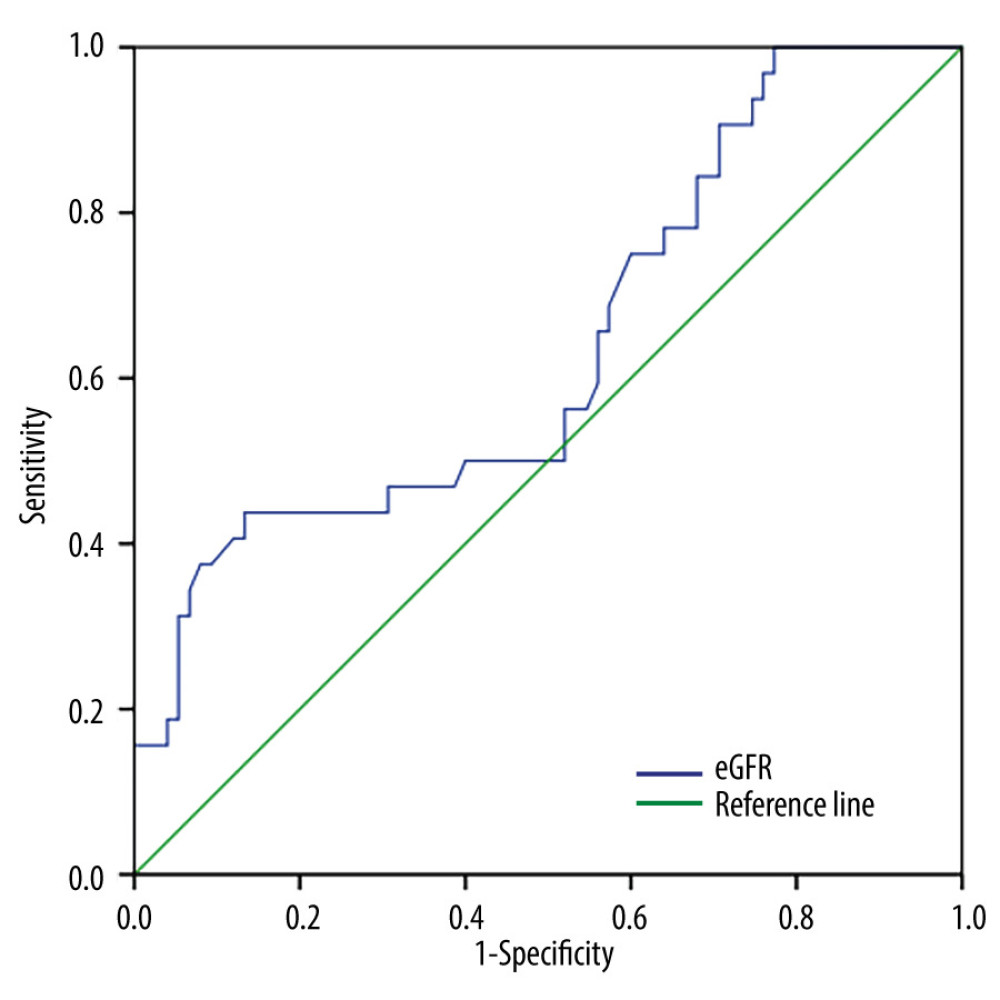 Figure 3. Receiver operating characteristic curve analysis showing the prognostic value of the estimate glomerular filtration rate in predicting contrast-induced acute kidney injury in patients with microalbuminuria. CI-AKI – contrast-induced acute kidney injury; eGFR – estimate glomerular filtration rate; MA – microalbuminuria; NA – normal albuminuria. (SPSS, 24.0, IBM).
Figure 3. Receiver operating characteristic curve analysis showing the prognostic value of the estimate glomerular filtration rate in predicting contrast-induced acute kidney injury in patients with microalbuminuria. CI-AKI – contrast-induced acute kidney injury; eGFR – estimate glomerular filtration rate; MA – microalbuminuria; NA – normal albuminuria. (SPSS, 24.0, IBM). 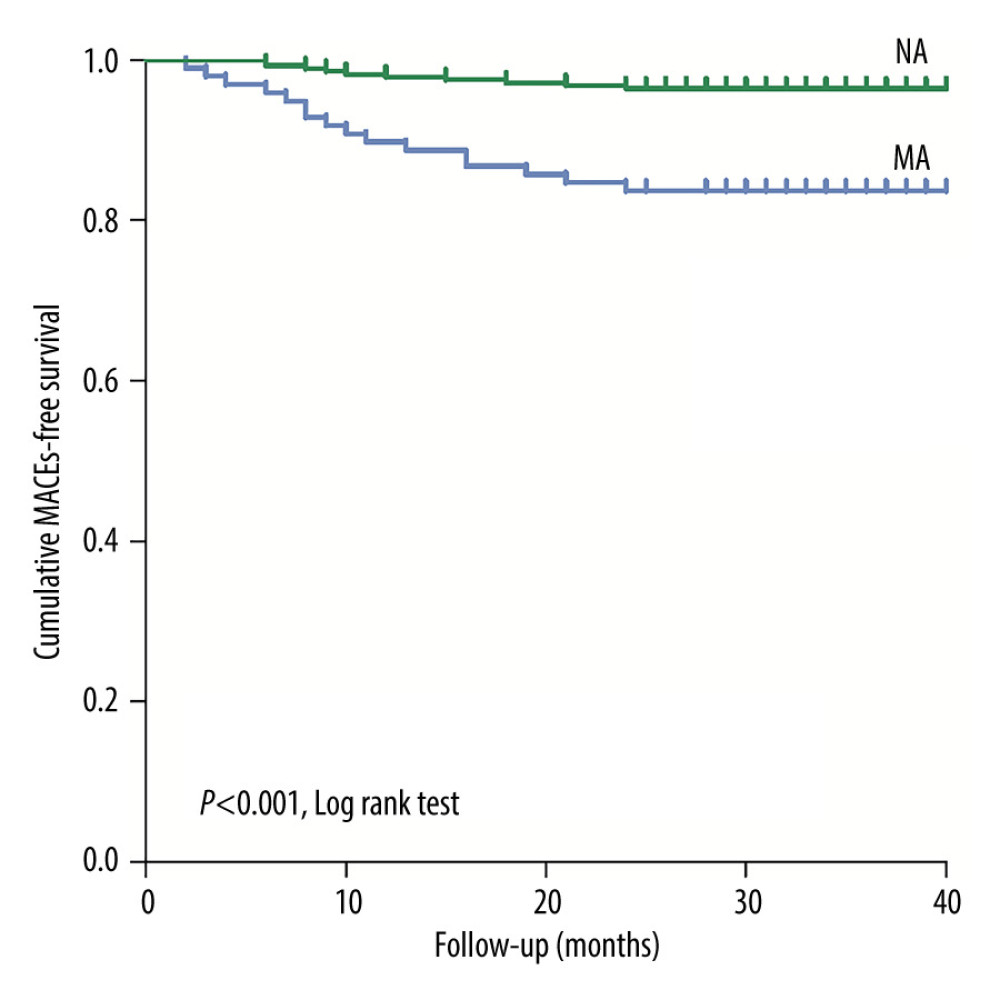 Figure 4. Kaplan-Meier curves of major adverse cardiovascular events in the patients with estimate glomerular filtration rates <45 mL/min/1.73 m2 during the 3-year follow-up after coronary angiography or intervention. The log rank test shows that these endpoints are significantly increased with the presence of microalbuminuria (P<0.001). eGFR – estimate glomerular filtration rate; MA – microalbuminuria; MACEs – major adverse cardiovascular events; NA – normal albuminuria. (SPSS, 24.0, IBM).
Figure 4. Kaplan-Meier curves of major adverse cardiovascular events in the patients with estimate glomerular filtration rates <45 mL/min/1.73 m2 during the 3-year follow-up after coronary angiography or intervention. The log rank test shows that these endpoints are significantly increased with the presence of microalbuminuria (P<0.001). eGFR – estimate glomerular filtration rate; MA – microalbuminuria; MACEs – major adverse cardiovascular events; NA – normal albuminuria. (SPSS, 24.0, IBM). Tables
Table 1. Baseline clinical characteristics.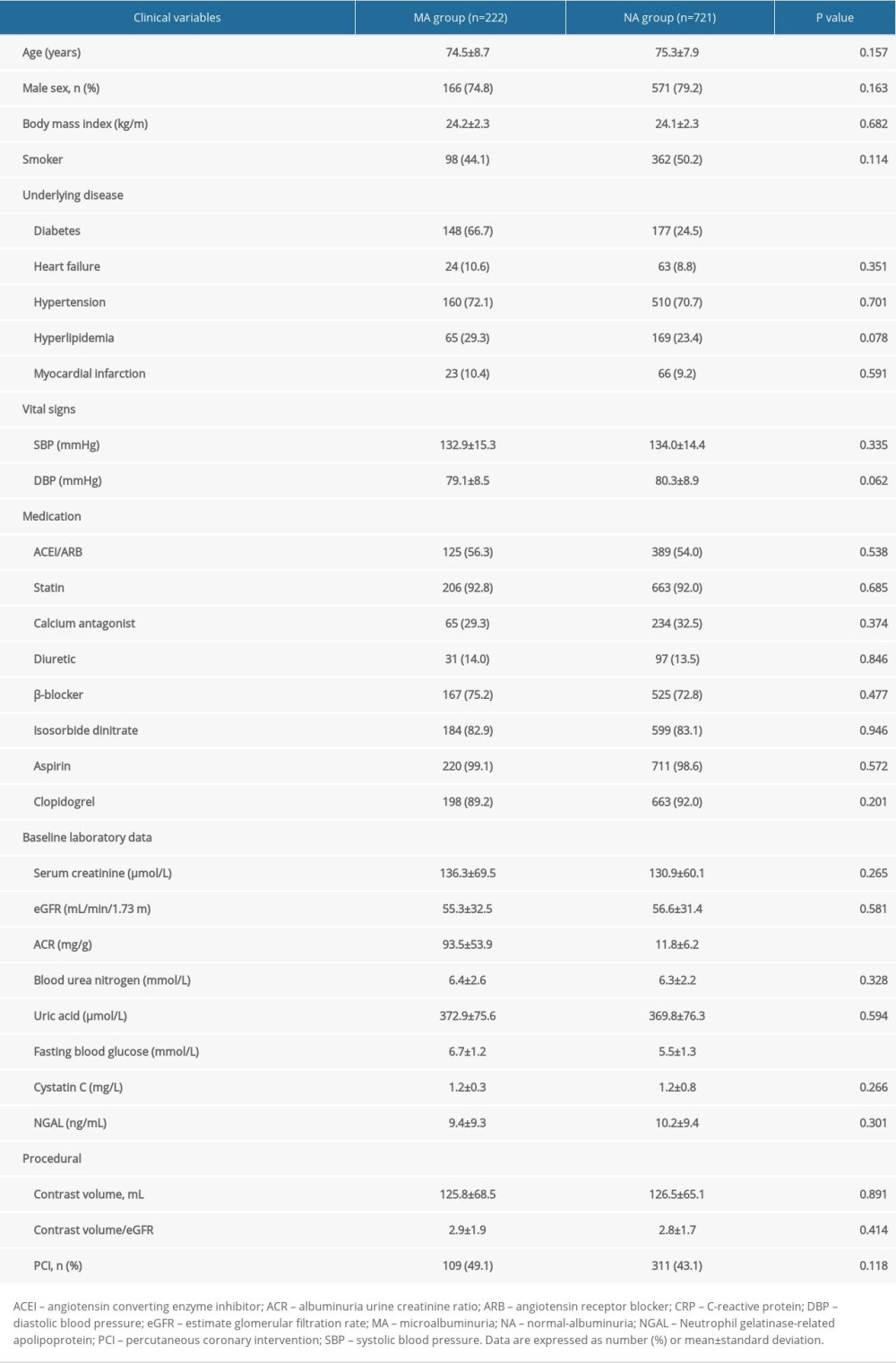 Table 2. The effect of microalbuminuria in patients with different estimate glomerular filtration rates.
Table 2. The effect of microalbuminuria in patients with different estimate glomerular filtration rates.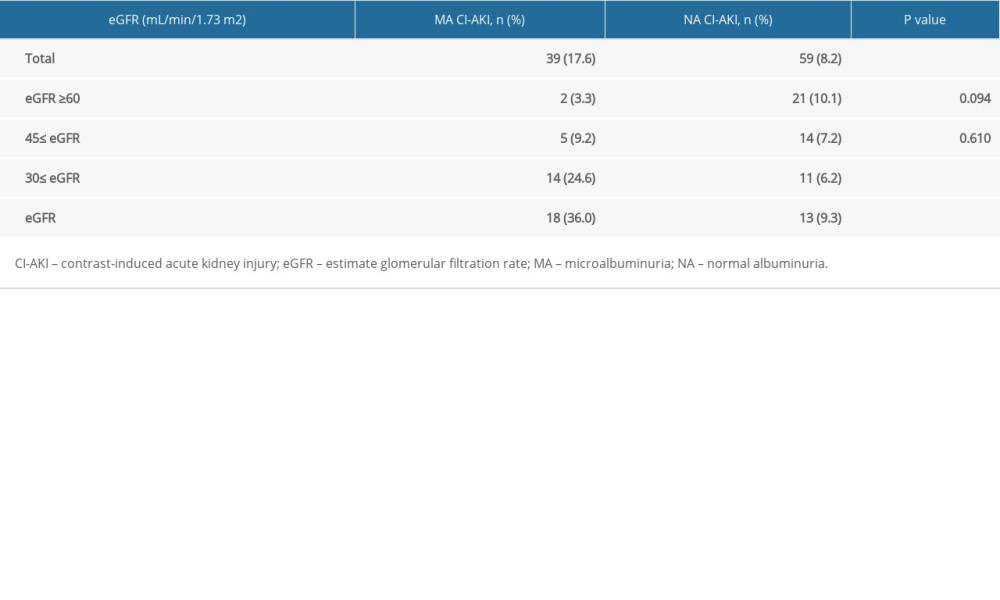 Table 3. Univariate logistic regression analysis for the identification of predictors of contrast-induced acute kidney injury.
Table 3. Univariate logistic regression analysis for the identification of predictors of contrast-induced acute kidney injury.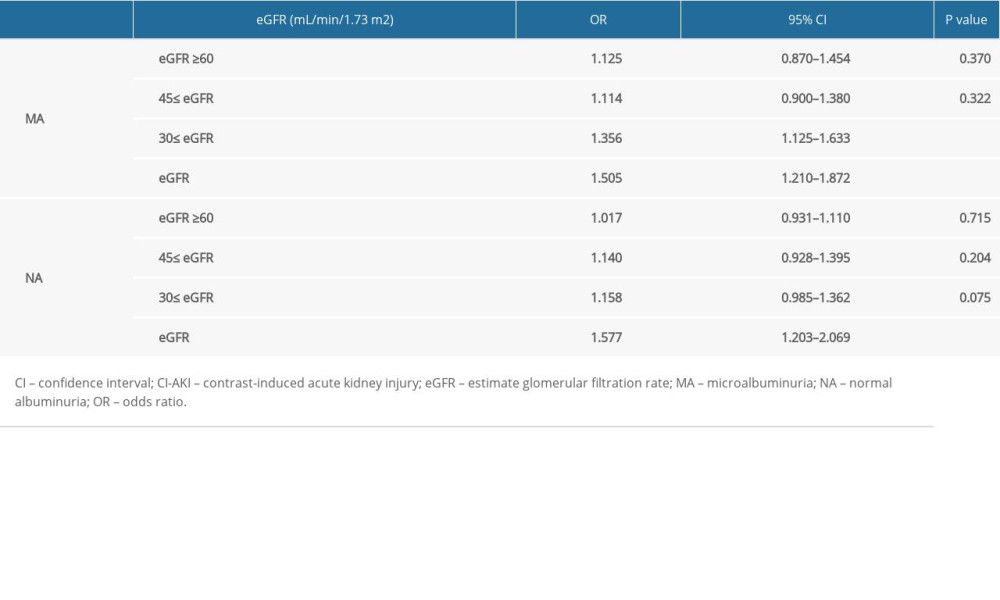 Table 4. The 3-year follow-up of patients with an estimate glomerular filtration rate <45 mL/min/1.73 m2.
Table 4. The 3-year follow-up of patients with an estimate glomerular filtration rate <45 mL/min/1.73 m2.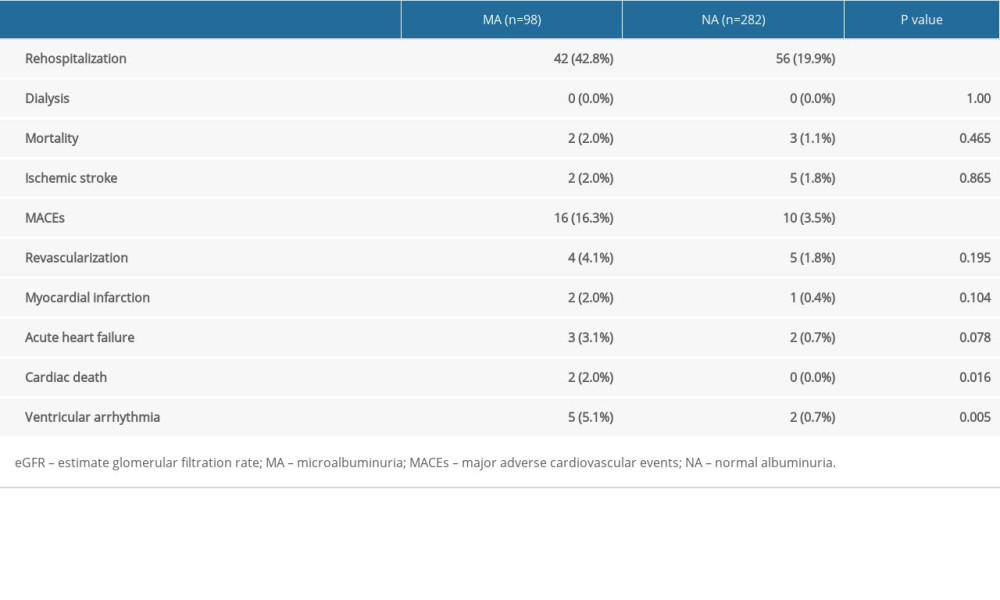
References
1. Makris K, Spanou L, Acute kidney injury: Diagnostic approaches and controversies: Clin Biochem Rev, 2016; 37(4); 153-75
2. Solomon R, Dauerman HL, Acute kidney injury: Circulation: Cardiovascular interventions, 2017; 10(1); e004742
3. Abe M, Morimoto T, Nakagawa Y, Impact of transient or persistent contrast-induced nephropathy on long-term mortality after elective percutaneous coronary intervention: Am J Cardiol, 2017; 120(12); 2146-53
4. Neumann F, Sousa-Uva M, Ahlsson A, 2018 ESC/EACTS Guidelines on myocardial revascularization: Eur Heart J, 2018; 40(2); 87-165
5. Zheng X, Curtis JP, Hu S, Coronary catheterization and percutaneous coronary intervention in China: 10-year results from the China PEACE-retrospective CathPCI study: JAMA Intern Med, 2016; 176(4); 512-21
6. Hossain MA, Costanzo E, Cosentino J, Contrast-induced nephropathy: Pathophysiology, risk factors, and prevention: Saudi J Kidney Dis Transpl, 2018; 29(1); 1-9
7. Liu Y, Liang X, Xin S, Risk factors for contrast-induced acute kidney injury (CI-AKI): protocol for systematic review and meta-analysis: BMJ Open, 2019; 9(8); e30048
8. Liu Y, Liu Y, Chen J, A simple pre-procedural risk score for contrast-induced nephropathy among patients with chronic total occlusion undergoing percutaneous coronary intervention: Int J Cardiol, 2015; 180; 69-71
9. Lin K, Zheng W, Bei W, A novel risk score model for prediction of contrast-induced nephropathy after emergent percutaneous coronary intervention: Int J Cardiol, 2017; 230; 402-12
10. Abellás-Sequeiros RA, Raposeiras-Roubín S, Abu-Assi E, Mehran contrast nephropathy risk score: Is it still useful 10 years later?: J Cardiol, 2016; 67(3); 262-67
11. Sato A, Aonuma K, Watanabe M, Association of contrast-induced nephropathy with risk of adverse clinical outcomes in patients with cardiac catheterization: From the CINC-J study: Int J Cardiol, 2017; 227; 424-29
12. Liu Y, Liu Y, Zhou Y, Comparison of different risk scores for predicting contrast induced nephropathy and outcomes after primary percutaneous coronary intervention in patients with ST elevation myocardial infarction: Am J Cardiol, 2016; 117(12); 1896-903
13. Hu DY, Pan CY, Yu JM, The relationship between coronary artery disease and abnormal glucose regulation in China: The China Heart Survey: Eur Heart J, 2006; 27(21); 2573-79
14. , KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease: Am J Kidney Dis, 2007; 49(2 Suppl 2); S12-154
15. Chugh A, Bakris GL, Microalbuminuria: What is it? Why is it important? What should be done about it? An update: J Clin Hypertens (Greenwich), 2007; 9(3); 196-200
16. Jarraya F, Lakhdar R, Kammoun K, Microalbuminuria: A useful marker of cardiovascular disease: Iran J Kidney Dis, 2013; 7(3); 178-86
17. Cronin RE, Contrast-induced nephropathy: Pathogenesis and prevention: Pediatr Nephrol, 2010; 25(2); 191-204
18. Nicola R, Shaqdan KW, Aran K, Contrast-induced nephropathy: identifying the risks, choosing the right agent, and reviewing effective prevention and management methods: Curr Probl Diagn Radiol, 2015; 44(6); 501-4
19. Watanabe M, Saito Y, Aonuma K, Prediction of contrast-induced nephropathy by the serum creatinine level on the day following cardiac catheterization: J Cardiol, 2016; 68(5); 412-18
20. Ma YC, Zuo L, Chen JH, Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease: J Am Soc Nephrol, 2006; 17(10); 2937-44
21. , K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification: Am J Kidney Dis, 2002; 39(2 Suppl 1); S1-266
22. Stehouwer CD, Smulders YM, Microalbuminuria and risk for cardiovascular disease: Analysis of potential mechanisms: J Am Soc Nephrol, 2006; 17(8); 2106-11
23. Saito Y, Watanabe M, Aonuma K, Proteinuria and reduced estimated glomerular filtration rate are independent risk factors for contrast-induced nephropathy after cardiac catheterization: Circ J, 2015; 79(7); 1624-30
24. Stehouwer CD, Smulders YM, Microalbuminuria and risk for cardiovascular disease: Analysis of potential mechanisms: J Am Soc Nephrol, 2006; 17(8); 2106-11
25. Adachi H, Microalbuminuria is an independent prognostic information for cardiovascular disease: Atherosclerosis, 2014; 237(1); 106-7
26. Meng H, Wu P, Zhao Y, Microalbuminuria in patients with preserved renal function as a risk factor for contrast-induced acute kidney injury following invasive coronary angiography: Eur J Radiol, 2016; 85(6); 1063-67
27. Saha TK, Bhattarai AM, Batra HS, Correlation of microalbuminuria with estimated GFR (eGFR) by Cockcroft-Gault and MDRD formula in type 2 diabetics and hypertensives: Indian J Clin Biochem, 2015; 30(3); 271-74
28. Gawandi S, Gangawane S, Chakrabarti A, A Study of microalbuminuria (MAU) and advanced glycation end products (AGEs) levels in diabetic and hypertensive subjects: Indian J Clin Biochem, 2018; 33(1); 81-85
29. Cirillo M, Lanti MP, Menotti A, Definition of kidney dysfunction as a cardiovascular risk factor: Use of urinary albumin excretion and estimated glomerular filtration rate: Arch Intern Med, 2008; 168(6); 617-24
30. Chen YH, Chen HS, Tarng DC, More impact of microalbuminuria on retinopathy than moderately reduced GFR among type 2 diabetic patients: Diabetes Care, 2012; 35(4); 803-8
31. Zobel EH, von Scholten BJ, Reinhard H, Symmetric and asymmetric dimethylarginine as risk markers of cardiovascular disease, all-cause mortality and deterioration in kidney function in persons with type 2 diabetes and microalbuminuria: Cardiovasc Diabetol, 2017; 16(1); 88
32. Rifkin DE, Katz R, Chonchol M, Albuminuria, impaired kidney function and cardiovascular outcomes or mortality in the elderly: Nephrol Dial Transplant, 2010; 25(5); 1560-67
Figures
 Figure 1. Study flow diagram. eGFR – estimate glomerular filtration rate; MA – microalbuminuria; NA – normal albuminuria. (Microsoft Office, 2016, Microsoft).
Figure 1. Study flow diagram. eGFR – estimate glomerular filtration rate; MA – microalbuminuria; NA – normal albuminuria. (Microsoft Office, 2016, Microsoft). Figure 2. Incidence of contrast-induced acute kidney injury (CI-AKI).The incidence of CI-AKI increased with a decreasing estimate glomerular filtration rate (eGFR) in all patients (P=0.017) and the patients with microalbuminuria (P<0.001). There was no difference in the incidence of CI-AKI between different eGFR groups in patients with normal microalbuminuria (P=0.484). CI-AKI – contrast-induced acute kidney injury; eGFR – estimate glomerular filtration rate; MA – microalbuminuria; NA – normal-albuminuria. (Microsoft office, 2016, Microsoft).
Figure 2. Incidence of contrast-induced acute kidney injury (CI-AKI).The incidence of CI-AKI increased with a decreasing estimate glomerular filtration rate (eGFR) in all patients (P=0.017) and the patients with microalbuminuria (P<0.001). There was no difference in the incidence of CI-AKI between different eGFR groups in patients with normal microalbuminuria (P=0.484). CI-AKI – contrast-induced acute kidney injury; eGFR – estimate glomerular filtration rate; MA – microalbuminuria; NA – normal-albuminuria. (Microsoft office, 2016, Microsoft). Figure 3. Receiver operating characteristic curve analysis showing the prognostic value of the estimate glomerular filtration rate in predicting contrast-induced acute kidney injury in patients with microalbuminuria. CI-AKI – contrast-induced acute kidney injury; eGFR – estimate glomerular filtration rate; MA – microalbuminuria; NA – normal albuminuria. (SPSS, 24.0, IBM).
Figure 3. Receiver operating characteristic curve analysis showing the prognostic value of the estimate glomerular filtration rate in predicting contrast-induced acute kidney injury in patients with microalbuminuria. CI-AKI – contrast-induced acute kidney injury; eGFR – estimate glomerular filtration rate; MA – microalbuminuria; NA – normal albuminuria. (SPSS, 24.0, IBM). Figure 4. Kaplan-Meier curves of major adverse cardiovascular events in the patients with estimate glomerular filtration rates <45 mL/min/1.73 m2 during the 3-year follow-up after coronary angiography or intervention. The log rank test shows that these endpoints are significantly increased with the presence of microalbuminuria (P<0.001). eGFR – estimate glomerular filtration rate; MA – microalbuminuria; MACEs – major adverse cardiovascular events; NA – normal albuminuria. (SPSS, 24.0, IBM).
Figure 4. Kaplan-Meier curves of major adverse cardiovascular events in the patients with estimate glomerular filtration rates <45 mL/min/1.73 m2 during the 3-year follow-up after coronary angiography or intervention. The log rank test shows that these endpoints are significantly increased with the presence of microalbuminuria (P<0.001). eGFR – estimate glomerular filtration rate; MA – microalbuminuria; MACEs – major adverse cardiovascular events; NA – normal albuminuria. (SPSS, 24.0, IBM). Tables
 Table 1. Baseline clinical characteristics.
Table 1. Baseline clinical characteristics. Table 2. The effect of microalbuminuria in patients with different estimate glomerular filtration rates.
Table 2. The effect of microalbuminuria in patients with different estimate glomerular filtration rates. Table 3. Univariate logistic regression analysis for the identification of predictors of contrast-induced acute kidney injury.
Table 3. Univariate logistic regression analysis for the identification of predictors of contrast-induced acute kidney injury. Table 4. The 3-year follow-up of patients with an estimate glomerular filtration rate <45 mL/min/1.73 m2.
Table 4. The 3-year follow-up of patients with an estimate glomerular filtration rate <45 mL/min/1.73 m2. Table 1. Baseline clinical characteristics.
Table 1. Baseline clinical characteristics. Table 2. The effect of microalbuminuria in patients with different estimate glomerular filtration rates.
Table 2. The effect of microalbuminuria in patients with different estimate glomerular filtration rates. Table 3. Univariate logistic regression analysis for the identification of predictors of contrast-induced acute kidney injury.
Table 3. Univariate logistic regression analysis for the identification of predictors of contrast-induced acute kidney injury. Table 4. The 3-year follow-up of patients with an estimate glomerular filtration rate <45 mL/min/1.73 m2.
Table 4. The 3-year follow-up of patients with an estimate glomerular filtration rate <45 mL/min/1.73 m2. In Press
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








