03 March 2022: Clinical Research
Alteration of Serum Metabolites in Women of Reproductive Age with Chronic Constipation
Shuai Liu1ABCEF, Chuanli Yang1ACD, Hongxia Li1BF, Xinshu Bai1B, Tianjiao Hu1B, Xin Xue1B, Jie An1F, Yan Zhang1F, Xiushan Dong1AG*DOI: 10.12659/MSM.934117
Med Sci Monit 2022; 28:e934117
Abstract
BACKGROUND: Chronic constipation is a common gastrointestinal disease. Our previous studies confirmed that there are differences in the composition and function of gut microbiota between women of reproductive age with chronic constipation and healthy controls. However, little is known about the differences in the metabolic profile of the 2 groups. The aim of this study was to observe changes in serum metabolites and identify potential metabolic pathways in the development of chronic constipation.
MATERIAL AND METHODS: A total of 50 participants were included in this study: 25 female patients of childbearing age with chronic constipation who met the inclusion and exclusion criteria and 25 healthy participants as a control group. Serum samples of these participants were collected; 1 portion of the serum sample was used for clinical biochemical analysis, and the other was used for non-targeted metabolomic testing.
RESULTS: Compared with the control group, serum 2-hydroxyphenylacetic acid levels were higher (P<0.05) and DL-phenylalanine levels were lower (P<0.05) in the constipation group. Other amino acids, such as 5-hydroxy-l-lysine and l-pipecolic acid, were upregulated, and L-valine, glycine, L-leucyl-L-proline, and N-formylmethionine were downregulated in the constipation group. In addition, levels of the bile acid, 3b-hydroxy-5-cholenoic acid, were higher in the constipation group than in the control group. Pathway analysis showed that the significantly altered pathways were phenylalanine metabolism and glycine, serine, and threonine metabolism.
CONCLUSIONS: These results strongly suggest that serum metabolites and pathways are significantly altered in women of reproductive age with chronic constipation.
Keywords: Constipation, Energy Metabolism, Metabolomics, Women, Adult, Chronic Disease, Female, Gastrointestinal Microbiome, Humans, Male, Metabolome, young adult
Background
Chronic constipation, which affects approximately 11% to 12% of the adult population worldwide annually, is a syndrome that indicates various, persistent, complex, and symptom-based gastrointestinal disorders [1,2]. The prevalence of constipation ranges from 2.6% to 26.9%, with a median prevalence of 16% in all adults in the United States [2]. Due to changes in lifestyle and increased social pressure, the prevalence of chronic constipation in adults seems to be increasing [3], and its incidence in women is twice that of in men [4]. Camilleri et al found that patients with chronic constipation tended to be younger [5]. The pathogenesis of chronic constipation is extremely complex and involves multiple factors, such as the central and peripheral nervous system, intestinal motility and secretion, and the balance of intestinal microecology, with complex interactions between these factors. Recent research has also shown that microbial treatment, such as fecal microbiota transplantation, can be an effective way to cure constipation because of its effect on the human body [6,7].
The human gut microbiota is a complex ecosystem that inhabits and critically maintains homeostasis of gastrointestinal physiological function [8,9]. Many studies reported that the gut microbiota is significantly associated with constipation [10–12]. In our previous study, we also found that compared with healthy controls, the level of
The traditional treatment for chronic constipation is mainly the use of various laxatives. Owing to the strong stimulating effect of drugs on the enteric nervous system, it is easy for these drugs to cause disturbances in the enteric nervous system of patients and affect gastrointestinal motility to aggravate the symptoms of constipation. Chronic constipation is a multifactorial disorder with a strong female prevalence, and the crosstalk between gut microbiota, metabolites, and the host is exceedingly complex and dynamic. Therefore, it is extremely important to further explore the mechanism of constipation through the gut microbiota and its metabolites.
The composition and diversity of the gut microbiota are strongly correlated with age and sex, and the balance between the microbiota and host is easily disrupted [20,21]. Therefore, we recruited female patients of reproductive age with chronic constipation to exclude the effects of hormones, age, sex, and other factors on the study. We compared serum metabolites between the women with constipation with those of healthy controls using liquid chromatography tandem-mass spectrometry (LC-MS/MS). Most importantly, we observed typical serum metabolite changes in women with constipation.
Material and Methods
STUDY POPULATION:
To reduce the effects of age, sex, work, and hormone levels on the microbiome, we enrolled patients from Shanxi Bethune Hospital, which is one of the functional gastrointestinal disease centers in Shanxi, China. Most of the patients were women who worked in this institution. A total of 25 patients with chronic constipation and 25 healthy controls were included in this study (age: 33.63±6.584 years vs 32.07±6.968 years; body mass index (BMI): 21.82±2.868 kg·m2 vs 22.25±2.010 kg·m2,
The patients were included if they were (a) diagnosed with chronic constipation according to the Rome IV criteria, (b) were women of reproductive age (15–49 years), and (c) the BMI was within the normal range of 18.5 to 24 kg·m2 [22]. Participants with the following diseases were excluded: (a) systemic diseases such as metabolic disorders, neuropsychiatric disorders, diabetes, Parkinson disease, and cancer; (b) infectious diseases such as hepatitis, tuberculosis, and acquired immunodeficiency syndrome; (c) other autoimmune diseases such as rheumatoid arthritis, ankylosing spondylitis, and inflammatory bowel disease; and (d) a history of medications such as laxatives, antibiotics, probiotics, prebiotics, non-steroidal anti-inflammatory drugs, opioid drugs, proton pump inhibitors, traditional Chinese medicine, and histamine receptor antagonists in the past 3 months.
After collecting blood samples, we performed several routine examinations, including BMI, fasting blood glucose (FBG), triglyceride (TG), high- and low-density lipoprotein (HDL/LDL), and liver function tests, such as alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), direct bilirubin (DBIL), indirect bilirubin (IBIL), albumin (ALB), total bile acid (TBA), and cholesterol (CHO). Blood samples were collected at random times throughout the day, without any dietary intervention.
Before recruitment, all patients and healthy controls signed an informed consent form. This study was approved by the Ethics Committee of Shanxi Bethune Hospital (Taiyuan, China; certificate no. XYLL-2019-124).
SAMPLE PROCESSING:
Blood samples were collected from the antebrachial vein of patients and healthy controls. The samples were collected in standard 3-mL vacutainer vials/heparin anticoagulation tubes (Suzhou Bidi Medical Instruments, #9176983), placed on ice, and transported to the laboratory for immediate processing. A portion of the blood sample was used for the analysis of serum biochemical indicators; the remaining sample was used for non-targeted metabolomic analysis. The blood was centrifuged at 3000 rpm for 10 min within 1 h of collection. The supernatant (serum) was then extracted 0.2 mL/tube was distributed into 1.5-mL centrifuge tubes. The serum samples were then transferred to a freezer kept at −80°C within 30 min until metabolomic analyses were performed [23,24].
BIOCHEMICAL ANALYSIS OF SERUM SAMPLES:
BMI was calculated by dividing the patient’s weight by the height squared. After blood collection, FBG levels were measured using the glucose oxidase method. Liver function parameters (ALT, AST, TBIL, DBIL, IBIL, ALB, and TBA) and lipid metabolism parameters (CHO, TG, HDL, and LDL) were measured quantitatively by biochemical analysis.
LC-MS/MS ANALYSIS:
Ultra-high performance liquid chromatography (UHPLC) separation was carried out using a 1290 Infinity series UHPLC System (Agilent Technologies) equipped with a UPLC BEH Amide column (2.1×100 mm, 1.7 μm, Waters). The mobile phase consisted of 25 mmol/L ammonium acetate and 25 mmol/L ammonia hydroxide in water (pH = 9.75) (A) and acetonitrile (B). The analysis was performed with an elution gradient as follows: 0–0.5 min, 95% B; 0.5–7.0 min, 95–65% B; 7.0–8.0 min, 65–40% B; 8.0–9.0 min, 40% B; 9.0–9.1 min, 40–95% B; 9.1–12.0 min, 95% B. The column temperature was maintained at 25°C. The auto-sampler temperature was 4 °C, and the injection volume was 2 μL (positive and negative) [25,26].
TripleTOF 6600 mass spectrometry (AB Sciex) was used for its ability to acquire MS/MS spectra on an information-dependent basis during an LC/MS experiment. In this mode, the acquisition software (Analyst TF 1.7, AB Sciex) continuously evaluates the full scan survey MS data as it collects and triggers the acquisition of MS/MS spectra, depending on preselected criteria. In each cycle, the 12 most intensive precursor ions with intensities above 100 were chosen for MS/MS at a collision energy of 30 eV. The cycle time was 0.56 s. ESI source conditions were set as follows: gas 1, 60 psi; gas 2, 60 psi; curtain gas, 35 psi; source temperature, 600 °C; declustering potential, 60 V; and ion spray voltage floating (ISVF), 5000 V or −4000 V for positive and negative modes, respectively [27,28].
STATISTICAL ANALYSIS:
The characteristics of the constipation group are described as mean±SD, median, or percentage. Statistical analysis of group differences in clinical biochemical variables was performed using the t test. Statistical significance was set at P<0.05. R software was used for graphing. In detail, the algorithms for principal component analysis (PCA), orthogonal projections to latent structures-discriminant analysis (OPLS-DA), permutation test, and volcano plot were based on SIMCA software (version 15.0.2, Sartorius Stedim Data Analytics AB, Umea, Sweden). Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis was obtained from the KEGG website (https://www.kegg.jp/). The bubble figures in pathway analysis were obtained from the MetaboAnalyst website (https://www.metaboanalyst.ca/). The statistical analyses were performed using SPSS version 25.0 (IBM Corp, Armonk, NY, USA). MS raw data files were converted to the mzXML format using ProteoWizard and processed using the R package, XCMS (version 3.2). The process included peak deconvolution, alignment, and integration. Minfrac and cut-off were set as 0.5, and 0.3, respectively. An in-house MS2 database was used for metabolite identification [29,30].
Results
DIFFERENCES IN BMI AND BLOOD BIOCHEMICAL INDICATORS BETWEEN PATIENTS WITH CONSTIPATION AND HEALTHY CONTROLS:
The characteristics of all participants are summarized in Table 1. Unfortunately, there were no significant differences in FBG, BMI, serum ALT, AST, TBIL, DBIL, IBIL, ALB, TBA, CHO, TG, HDL, or LDL between the 2 groups (P>0.05).
DIFFERENCES IN METABOLIC PROFILES BETWEEN PATIENTS WITH CONSTIPATION AND HEALTHY CONTROLS:
Our previous research found that the composition of the gut microbiota in women of reproductive age with constipation was different from that in healthy controls [13]. To further investigate differences in metabolites between the 2 groups, we performed a serum metabolomic analysis. The differences following PCA of the serum profiles of patients with constipation and healthy controls are shown in Figure 1A. The PCA results in the constipation group were not significantly different from those in the healthy control group. Supervised multivariate statistical analysis was used to further examine metabolic changes. An OPLS-DA model was established to compare serum samples from the 2 groups (Figure 1B). The scattered shape and color in Figure 1 represent the disparate studied groups. As shown in Figure 1, according to the results of PCA and OPLS-DA plot scores, there were clear differences in serum metabolism between the 2 groups. These differences were significant, based on Hotelling’s T-squared elliptic analysis, within a 95% confidence interval. Permutation tests were performed to prevent overfitting of the models (Figure 1C). R2 (cumulative) and Q2 (cumulative) represent the interpretability and predictability of the models, respectively. R2 (cumulative) and Q2 (cumulative) were 0.931 and 0.419 in the constipated patient and healthy control groups, respectively, indicating that the model had good predictive value, and there was no overfitting.
The purpose of this study was to gain a more comprehensive understanding of serum metabolic changes in women of reproductive age with chronic constipation. A total of 50 differential metabolites, which contained 20 upregulated metabolites and 30 downregulated metabolites, were selected based on multivariate statistical analysis, with a value of importance projection <1 and P<0.05. Serum 2-hydroxyphenylacetic acid levels were significantly higher (P<0.05), whereas DL-phenylalanine levels were significantly lower (P<0.05) in the chronic constipation group than in the control group. These results suggest that the phenylalanine metabolism pathway is significantly altered in women of reproductive age with chronic constipation. Other amino acids, such as 5-hydroxy-l-lysine and l-pipecolic acid, were upregulated (P<0.05). In addition to changes in amino acid metabolism pathways, levels of bile acids, such as 3b-hydroxy-5-cholenoic acid, were higher in the constipation group. Tables 2 and 3 show the detailed results for distinct metabolites.
All serum metabolites were used in hierarchical cluster analysis among the constipation and healthy control groups. The heatmap (Figure 2A) and volcano plot (Figure 2B) showed the differences in plasma metabolism between the constipation group and healthy control group, which is consistent with the result we mentioned before. We then mapped these metabolites to their biochemical pathways based on the KEGG database. As shown in Figure 3, the significantly altered pathways were phenylalanine metabolism; pantothenate and CoA biosynthesis; glycerophospholipid metabolism; riboflavin metabolism; caffeine metabolism; ascorbate and aldarate metabolism; and glycine, serine, and threonine metabolism.
Discussion
Constipation is a common functional disorder of the digestive system and can occur secondary to metabolic disease, gastrointestinal obstruction, neurological disease, endocrine disease, or mental disorders [5]. Due to changes in dietary structure and work pressure, the incidence of functional constipation has been higher in younger patients in recent years. In addition, chronic constipation has an insidious onset and prolonged course, and patients often abuse laxatives, which can easily lead to dilatation of the colonic cavity, decreased contractility, and peristaltic function or loss. The Rome IV diagnostic criteria in 2016 define functional gastrointestinal disorders as “disorders of gut-brain interaction”; it is a group of disorders classified by gastrointestinal symptoms related to any combination of the following: motility disturbance, visceral hypersensitivity, altered mucosal and immune function, altered gut microbiota, and altered central nervous system processing [31]. The gastrointestinal tract is inhabited by a complex and dynamic collection of microbiota and their metabolites, and their composition and function are associated with most aspects of host physiology and disease [32]. Currently, many gut microbial metabolites, such as short-chain fatty acids and secondary bile acids, have been identified as signaling molecules in host-microbiome crosstalk [33,34]. Yano et al reported that the gut microbiota can influence intestinal motility by regulating host 5-HT levels [17]. A study found that bacteria-derived tryptamine, a ligand for the 5-HT4 receptor, modulates gut function via G-protein coupled receptors [35]. Therefore, chronic constipation is more likely to be a systemic chronic metabolic disease, rather than being limited to the intestine itself. Because of the important role of microbiota and their metabolites in the gut-brain-microbiota axis, the study of chronic constipation from the perspective of intestinal microbiota and its metabolites may provide a theoretical basis for the study of chronic constipation. In the present study, we found 50 metabolites in women of reproductive age with constipation. Based on the KEGG database, we further performed hierarchical clustering and metabolic pathway analysis for the 50 metabolites. Eventually, we found that phenylalanine and the phenylalanine metabolic pathway may be involved in the occurrence and development of chronic constipation.
Phenylalanine is not only an essential amino acid, but also an aromatic amino acid. Most proteins and peptides of dietary origin are digested and broken down in the small intestine to produce a variety of amino acids, which then enter the circulation via active transport. It has been found that intestinal bacteria metabolize phenylalanine in 2 main ways. The first is the production of various trace amines catalyzed by phenylalanine decarboxylase, which can be further transformed into phenylethylamine, tyramine, and tryptamine. Phenylethylamine and tyramine lose their biological activity in the liver through monoamine oxidase when entering the body [36]. The second pathway is the trans amino pathway mediated by AAA aminotransaminase. Phenylalanine, tyrosine, and tryptophan are converted to phenylpyruvic acid, 4-hydroxyphenylpyruvic acid, and indole-3-pyruvic acid, respectively. These pyruvic acids can be partially oxidized and reduced to acetic acid derivatives and propionic acid derivatives, such as phenylacetic acid, 4-hydroxyphenylacetic acid, indole-3-acetic acid, phenylpropionic acid, 4-hydroxyphenylpropionic acid, and indole-3-propionic acid [37]. Interestingly, we found that serum phenylalanine levels were significantly lower and 2-hydroxyphenylacetic acid levels were significantly higher in women of reproductive age with chronic constipation. Hierarchical clustering analysis showed consistent results, and pathway analysis indicated that phenylalanine metabolism was the most affected metabolic pathway.
There are many factors that regulate intestinal motility, such as the enteric nervous system, autonomic nervous system, and central nervous system. In terms of its role, the gut microbiota has been likened to a previously unknown organ; it has extensive metabolic capabilities and carries 150 times more genes than are contained in the human genome. Microorganisms provide the host with a range of metabolic capabilities that are otherwise unattainable [38]. As early as 1994, the tryptophan metabolite, 5-HT, a neurotransmitter, was shown to affect normal intestinal physiological function [39]. 5-HT stimulates local enteric nervous reflexes to initiate secretion and propulsive motility and acts on vagal afferents to modulate contractile activities [40]. Furthermore, metabolic products from gastrointestinal microbiota fermentation, such as short-chain fatty acids or peptides, can stimulate the enteric nervous system and affect gut transit [41]. Although our results did not detect short-chain fatty acids and their derivatives, we found that other metabolites, such as 5-hydroxy-l-lysine and l-pipecolic acid, were upregulated, while L-valine, glycine, L-leucyl-L-proline, N-formylmethionine, and D-alanyl-D-alanine were downregulated in patients with constipation.
In addition, we observed that levels of the bile acid, 3b-hydroxy-5-cholenoic acid, were higher in patients with constipation. 3B-hydroxy-5-cholenoic acid is an endogenous monohydroxy isochoric acid and an intermediate product in the metabolism of bile acids [42]. Bile acids are another key metabolic molecule in intestinal microbiota-host interactions and are endogenous metabolites synthesized from cholesterol in the liver and further metabolized by the flora after entering the intestine [43]. By shaping the host’s intestinal immune landscape and some intrinsic antimicrobial properties, bile acids influence gut microbiota composition [44]. Conversely, alterations in gut microbiota composition affect microbial bile acid metabolism and, in turn, alter signaling via bile acid receptors [45,46]. Owing to their role as mediators of gut-liver communication and regulators of host metabolism and inflammation, they represent attractive therapeutic targets for various liver diseases. However, their effects on the gastrointestinal tract are ambiguous.
This study had some limitations. We used a serum non-targeted metabolome to detect changes in metabolites, and the method mainly focuses on the breadth of detection. Our previous study found the typical microbiota changes between women with constipation and healthy controls. Therefore, in the follow-up study, we will further target metabolomics to analyze specific metabolite changes and combine them with the results of the microbiota changes in patients with constipation. In conclusion, our findings provide new insights into the composition of serum metabolites in women of reproductive age with constipation. The results of this study provide clues for further exploring the relationship between metabolic changes and constipation symptoms in women of reproductive age with constipation.
Conclusions
Previous studies and our previous publication indicated that patients with chronic constipation have a disorganized intestinal flora structure, and the present study found altered serum metabolites and metabolic pathways in women of reproductive age with chronic constipation. This study further explored the relationship between chronic constipation and microbiota from the perspective of serum metabolism.
Figures
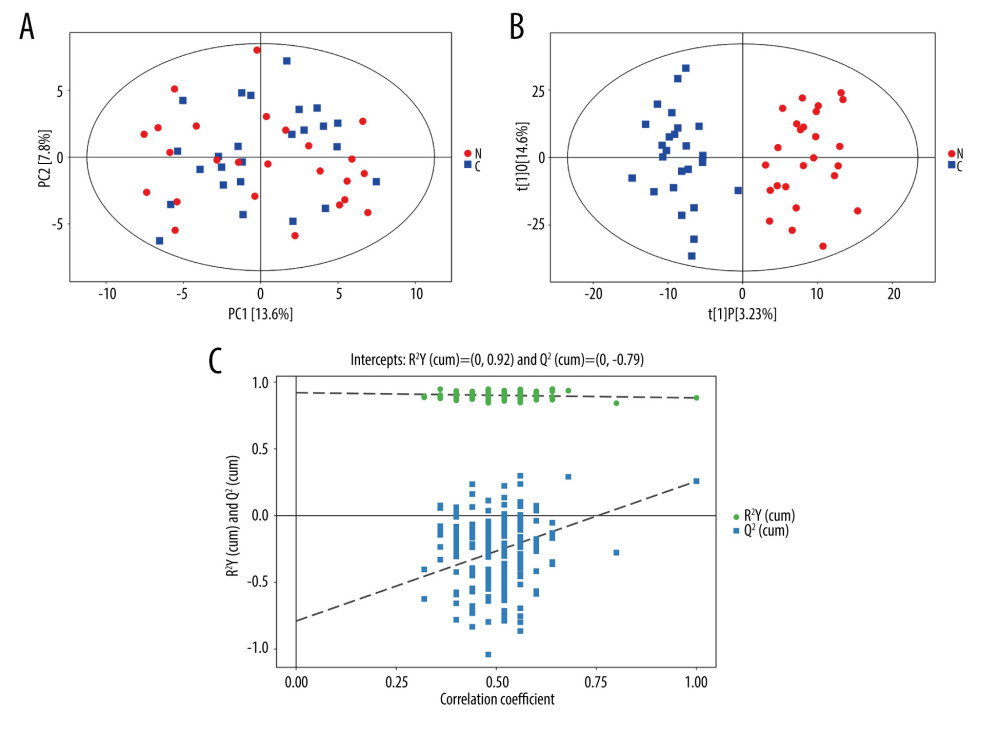 Figure 1. Principal component analysis (PCA) and orthogonal projections to latent structures-discriminant analysis (OPLS-DA) score plots of constipated patients and healthy controls. (A) PCA score plot for patients with constipation vs healthy controls (SIMCA, version 15.0.2, Sartorius Stedim Data Analytics AB, Umea, Sweden). (B) OPLS-DA score plot for patients with constipation vs healthy controls in integrated ion mode (SIMCA). (C) Permutation test of the OPLS-DA model (SIMCA). The slope of R2 is >0 and the Y-intercept of Q2 is <0.05, indicating a valid model.
Figure 1. Principal component analysis (PCA) and orthogonal projections to latent structures-discriminant analysis (OPLS-DA) score plots of constipated patients and healthy controls. (A) PCA score plot for patients with constipation vs healthy controls (SIMCA, version 15.0.2, Sartorius Stedim Data Analytics AB, Umea, Sweden). (B) OPLS-DA score plot for patients with constipation vs healthy controls in integrated ion mode (SIMCA). (C) Permutation test of the OPLS-DA model (SIMCA). The slope of R2 is >0 and the Y-intercept of Q2 is <0.05, indicating a valid model. 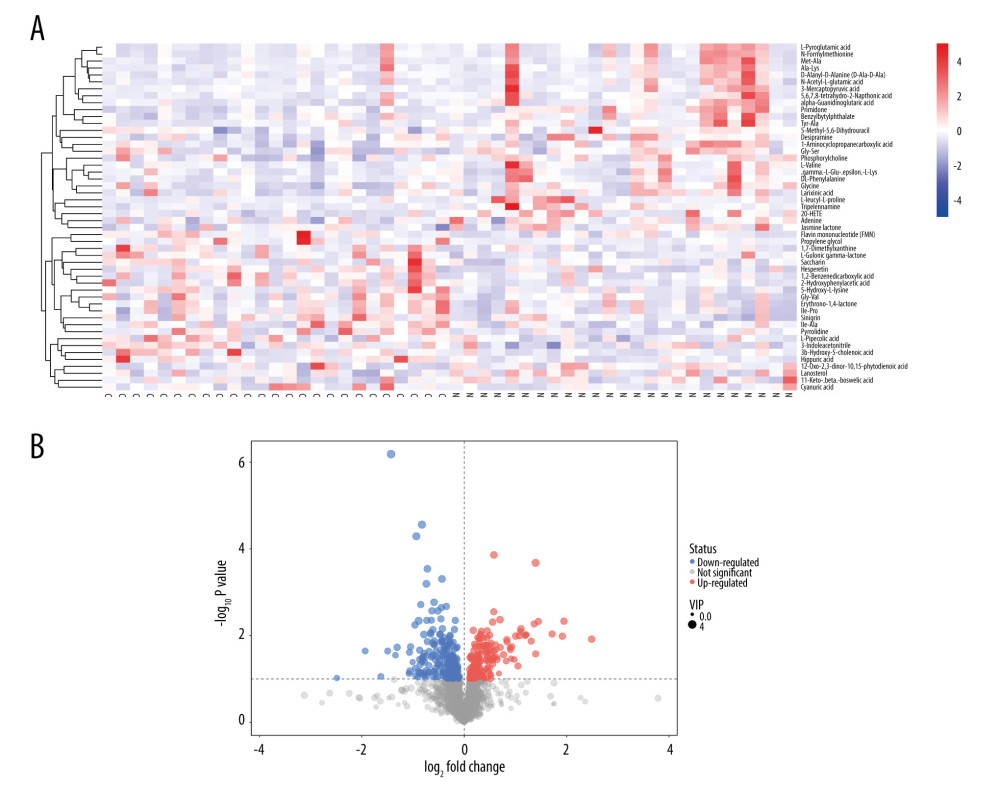 Figure 2. (A, B) Heatmap (R software & Kyoto Encyclopedia of Genes and Genomes (KEGG) website (https://www.kegg.jp/)) and volcano plot (SIMCA, version 15.0.2, Sartorius Stedim Data Analytics AB, Umea, Sweden) of metabolites with different levels between patients with constipation and healthy controls. Twenty metabolites had higher levels and 30 metabolites had lower levels in patients with constipation than in healthy controls.
Figure 2. (A, B) Heatmap (R software & Kyoto Encyclopedia of Genes and Genomes (KEGG) website (https://www.kegg.jp/)) and volcano plot (SIMCA, version 15.0.2, Sartorius Stedim Data Analytics AB, Umea, Sweden) of metabolites with different levels between patients with constipation and healthy controls. Twenty metabolites had higher levels and 30 metabolites had lower levels in patients with constipation than in healthy controls. 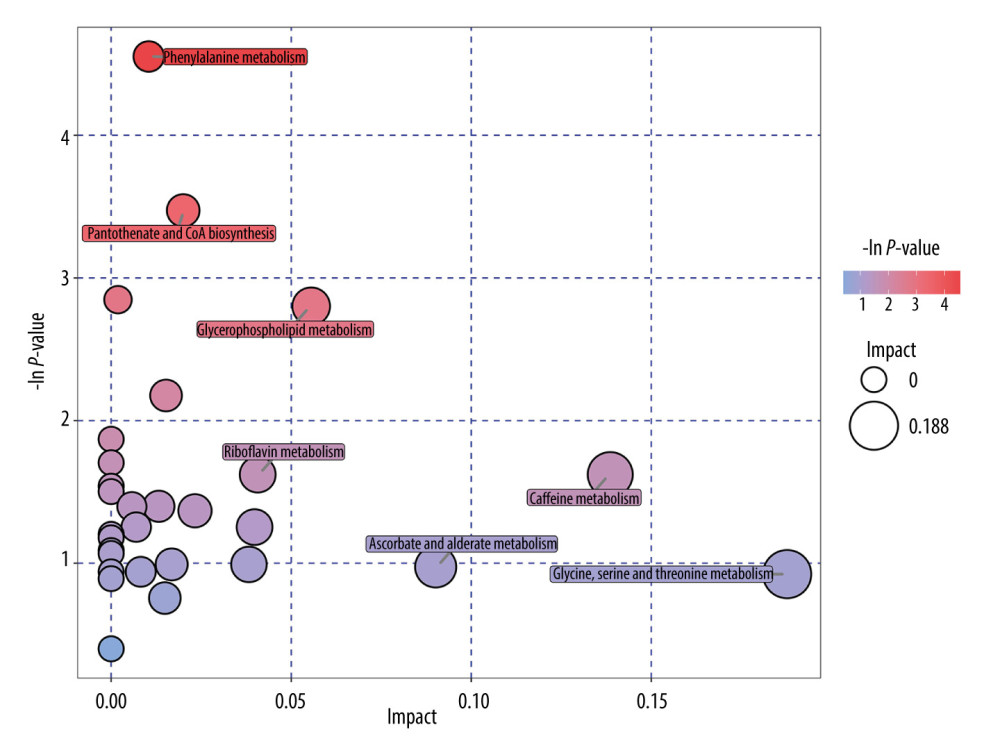 Figure 3. Pathway analysis of metabolites with differences between patients with constipation and healthy controls (R software & MetaboAnalyst website (https://www.metaboanalyst.ca/)). Seven significantly altered metabolic pathways were identified, including phenylalanine metabolism; caffeine metabolism; and glycine, serine, and threonine metabolism.
Figure 3. Pathway analysis of metabolites with differences between patients with constipation and healthy controls (R software & MetaboAnalyst website (https://www.metaboanalyst.ca/)). Seven significantly altered metabolic pathways were identified, including phenylalanine metabolism; caffeine metabolism; and glycine, serine, and threonine metabolism. Tables
Table 1. Differences in body mass index and blood biochemical indicators between patients with constipation and healthy controls.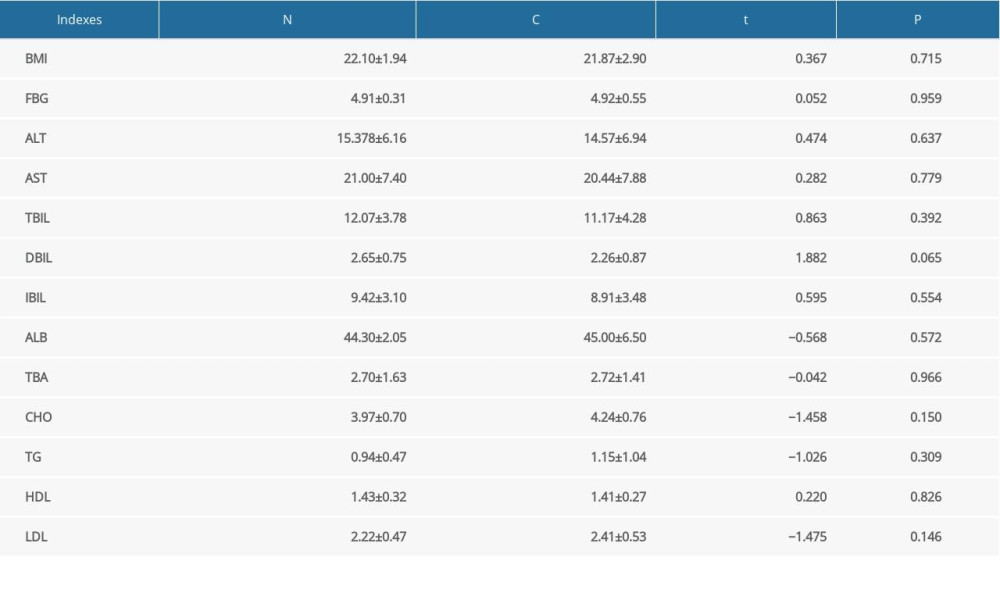 Table 2. Differences in upregulated metabolites between patients with constipation and healthy controls.
Table 2. Differences in upregulated metabolites between patients with constipation and healthy controls.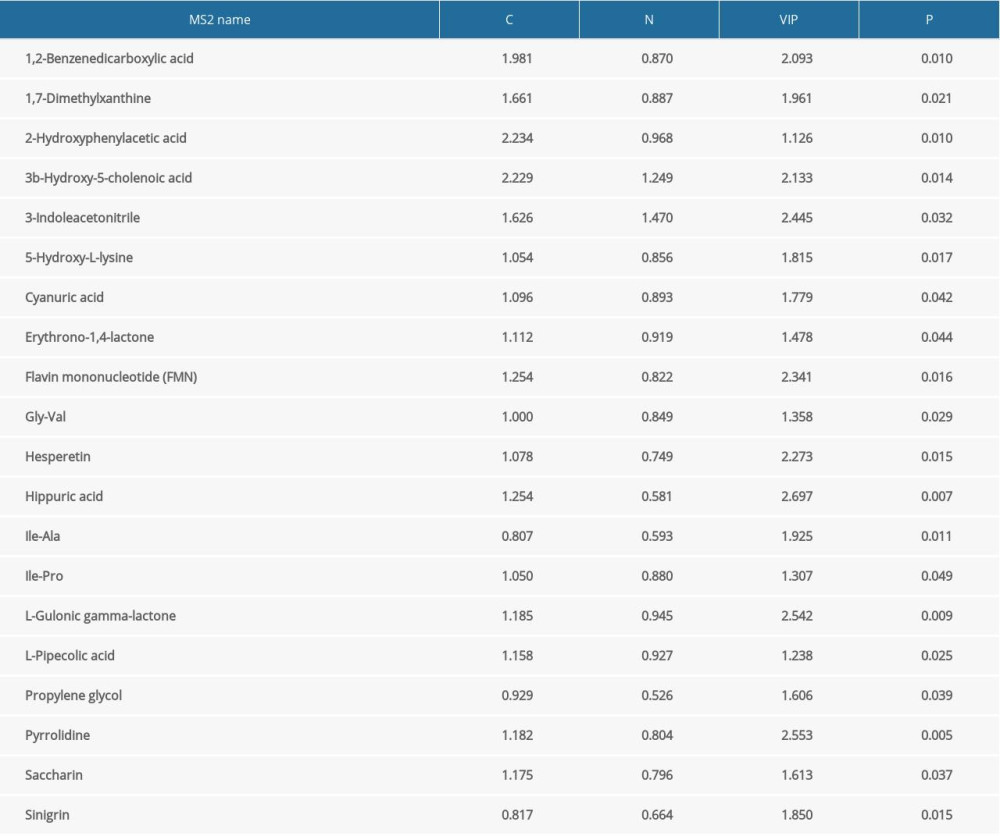 Table 3. Differences in downregulated metabolites between patients with constipation and healthy controls.
Table 3. Differences in downregulated metabolites between patients with constipation and healthy controls.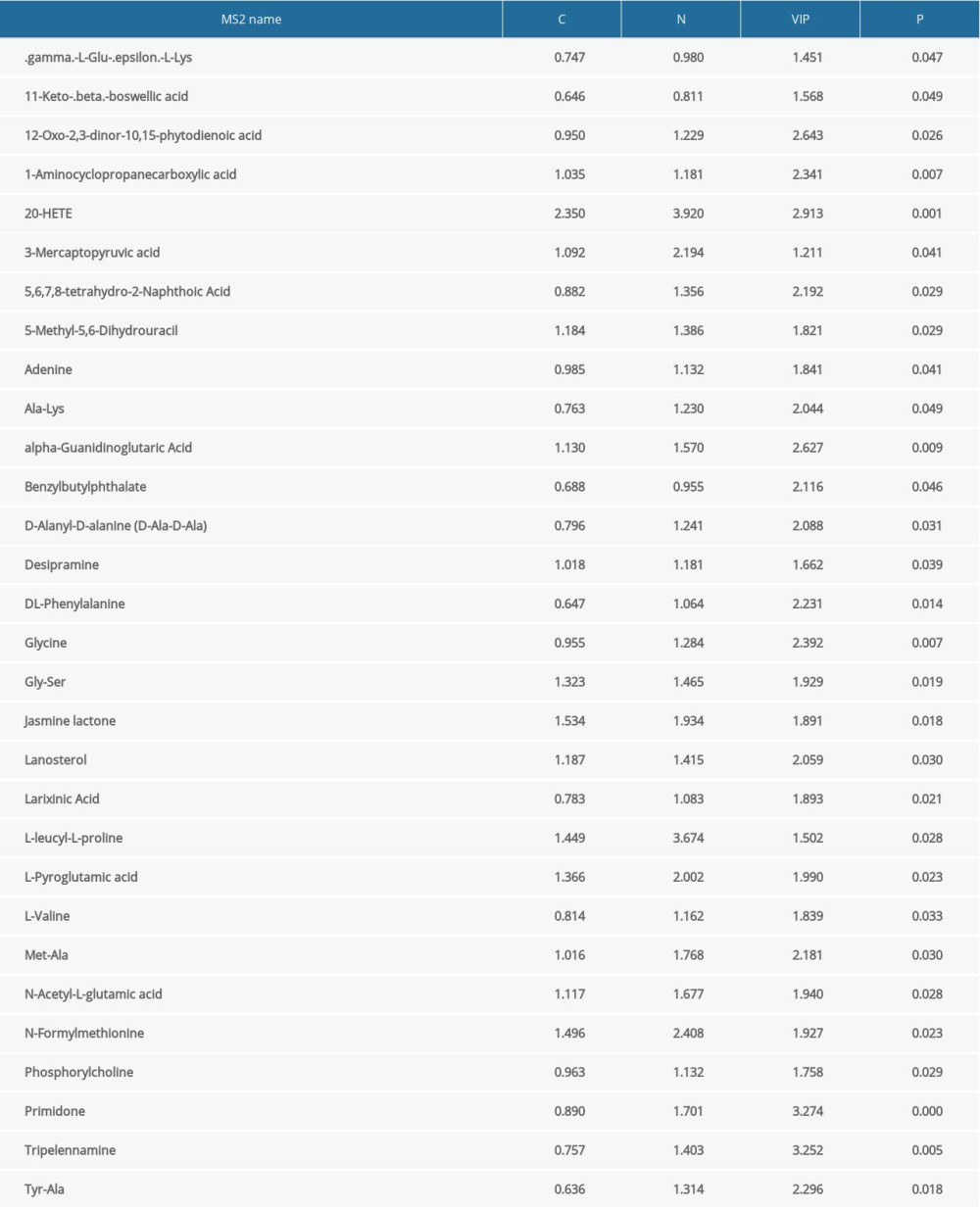
References
1. Schiller LR, Chronic constipation: New insights, better outcomes?: Lancet Gastroenterol Hepatol, 2019; 4; 873-82
2. Mugie SM, Benninga MA, Di Lorenzo C, Epidemiology of constipation in children and adults: A systematic review: Best Pract Res Clin Gastroenterol, 2011; 25; 3-18
3. Suares NC, Ford AC, Prevalence of, and risk factors for, chronic idiopathic constipation in the community: Systematic review and meta-analysis: Am J Gastroenterol, 2011; 106; 1582-91 quiz 1581, 1592
4. Baffy N, Foxx-Orenstein AE, Harris LA, Sterler S, Intractable constipation in the elderly: Curr Treat Options Gastroenterol, 2017; 15; 363-81
5. Camilleri M, Ford AC, Mawe GM, Chronic constipation: Nat Rev Dis Prim, 2017; 3; 17095
6. Huang L, Zhu Q, Qu X, Qin H, Microbial treatment in chronic constipation: Sci China Life Sci, 2018; 61; 744-52
7. Tian H, Ge X, Nie Y, Fecal microbiota transplantation in patients with slow-transit constipation: A randomized, clinical trial: PLoS One, 2017; 12; e0171308
8. Thursby E, Juge N, Introduction to the human gut microbiota: Biochem J, 2017; 474; 1823-36
9. Hillman ET, Lu H, Yao T, Nakatsu CH, Microbial ecology along the gastrointestinal tract: Microbes Environ, 2017; 32; 300-13
10. Baker JM, Al-Nakkash L, Herbst-Kralovetz MM, Estrogen-gut microbiome axis: Physiological and clinical implications: Maturitas, 2017; 103; 45-53
11. Vandeputte D, Falony G, Vieira-Silva S, Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates: Gut, 2016; 65; 57-62
12. Parthasarathy G, Chen J, Chen X, Relationship between microbiota of the colonic mucosa vs feces and symptoms, colonic transit, and methane production in female patients with chronic constipation: Gastroenterology, 2016; 150; 367-79e1
13. Li H, Chen J, Ren X, Gut microbiota composition changes in constipated women of reproductive age: Front Cell Infect Microbiol, 2021; 10; 557515
14. Li J, Jia H, Cai X, An integrated catalog of reference genes in the human gut microbiome: Nat Biotechnol, 2014; 32; 834-41
15. Consortium HMP, Structure, function and diversity of the healthy human microbiome: Nature, 2012; 486; 207-14
16. Wichmann A, Allahyar A, Greiner TU, Microbial modulation of energy availability in the colon regulates intestinal transit: Cell Host Microbe, 2013; 14; 582-90
17. Yano JM, Yu K, Donaldson GP, Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis: Cell, 2015; 161; 264-76
18. Shi Y, Chen Q, Huang Y, Function and clinical implications of short-chain fatty acids in patients with mixed refractory constipation: Colorectal Dis, 2016; 18; 803-10
19. Fukui H, Xu X, Miwa H, Role of gut microbiota-gut hormone axis in the pathophysiology of functional gastrointestinal disorders: J Neurogastroenterol Motil, 2018; 24; 367-86
20. Faith JJ, Guruge JL, Charbonneau M, The long-term stability of the human gut microbiota: Science, 2013; 341; 1237439
21. Kim S, Jazwinski SM, The gut microbiota and healthy aging: A mini-review: Gerontology, 2018; 64; 513-20
22. WHO Expert Consultation, Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies: Lancet, 2004; 363; 157-63
23. Yin P, Peter A, Franken H, Preanalytical aspects and sample quality assessment in metabolomics studies of human blood: Clin Chem, 2013; 59; 833-45
24. Dunn WB, Broadhurst D, Begley P, Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry: Nat Protoc, 2011; 6; 1060-83
25. Wang J, Zhang T, Shen X, Serum metabolomics for early diagnosis of esophageal squamous cell carcinoma by UHPLC-QTOF/MS: Metabolomics, 2016; 12; 116
26. Saccenti E, Hoefsloot HCJ, Smilde AK, Reflections on univariate and multivariate analysis of metabolomics data: Metabolomics, 2014; 10; 361-74
27. Wiklund S, Johansson E, Sjöström L, Visualization of GC/TOF-MS-based metabolomics data for identification of biochemically interesting compounds using OPLS class models: Anal Chem, 2008; 80; 115-22
28. Trygg J, Wold S, Orthogonal projections to latent structures (O-PLS): J Chemom, 2002; 16; 119-28
29. Smith CA, Want EJ, O’Maille G, XCMS: Processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification: Anal Chem, 2006; 78; 779-87
30. Kuhl C, Tautenhahn R, Böttcher C, CAMERA: An integrated strategy for compound spectra extraction and annotation of liquid chromatography/mass spectrometry data sets: Anal Chem, 2012; 84; 283-89
31. Drossman DA, Functional gastrointestinal disorders: What’s new for Rome IV?: Lancet Gastroenterol Hepatol, 2016; 1; 6-8
32. Schroeder BO, Bäckhed F, Signals from the gut microbiota to distant organs in physiology and disease: Nat Med, 2016; 22; 1079-89
33. Jia W, Xie G, Jia W, Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis: Nat Rev Gastroenterol Hepatol, 2018; 15; 111-28
34. Sanna S, van Zuydam NR, Mahajan A, Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases: Nat Genet, 2019; 51; 600-5
35. Bhattarai Y, Williams BB, Battaglioli EJ, Gut microbiota-produced tryptamine activates an epithelial G-protein-coupled receptor to increase colonic secretion: Cell Host Microbe, 2018; 23; 775-85e5
36. Bortolato M, Chen K, Shih JC, Monoamine oxidase inactivation: from pathophysiology to therapeutics: Adv Drug Deliv Rev, 2008; 60; 1527-33
37. Dodd D, Spitzer MH, Van Treuren W, A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites: Nature, 2017; 551; 648-52
38. Qin J, Li R, Raes J, A human gut microbial gene catalogue established by metagenomic sequencing: Nature, 2010; 464; 59-65
39. Uribe A, Alam M, Johansson O, Microflora modulates endocrine cells in the gastrointestinal mucosa of the rat: Gastroenterology, 1994; 107; 1259-69
40. Spiller R, Recent advances in understanding the role of serotonin in gastrointestinal motility in functional bowel disorders: Alterations in 5-HT signalling and metabolism in human disease: Neurogastroenterol Motil, 2007; 19(Suppl 2); 25-31
41. Barbara G, Stanghellini V, Brandi G, Interactions between commensal bacteria and gut sensorimotor function in health and disease: Am J Gastroenterol, 2005; 100; 2560-68
42. Back P, Ross K, Identification of 3 beta-hydroxy-5-cholenoic acid in human meconium: Hoppe Seylers Z Physiol Chem, 1973; 354; 83-89
43. de Aguiar Vallim TQ, Tarling EJ, Edwards PA, Pleiotropic roles of bile acids in metabolism: Cell Metab, 2013; 17; 657-69
44. Begley M, Gahan CGM, Hill C, The interaction between bacteria and bile: FEMS Microbiol Rev, 2005; 29; 625-51
45. Modica S, Petruzzelli M, Bellafante E, Selective activation of nuclear bile acid receptor FXR in the intestine protects mice against cholestasis: Gastroenterology, 2012; 142; 355-65e1
46. Sayin SI, Wahlström A, Felin J, Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist: Cell Metab, 2013; 17; 225-35
Figures
 Figure 1. Principal component analysis (PCA) and orthogonal projections to latent structures-discriminant analysis (OPLS-DA) score plots of constipated patients and healthy controls. (A) PCA score plot for patients with constipation vs healthy controls (SIMCA, version 15.0.2, Sartorius Stedim Data Analytics AB, Umea, Sweden). (B) OPLS-DA score plot for patients with constipation vs healthy controls in integrated ion mode (SIMCA). (C) Permutation test of the OPLS-DA model (SIMCA). The slope of R2 is >0 and the Y-intercept of Q2 is <0.05, indicating a valid model.
Figure 1. Principal component analysis (PCA) and orthogonal projections to latent structures-discriminant analysis (OPLS-DA) score plots of constipated patients and healthy controls. (A) PCA score plot for patients with constipation vs healthy controls (SIMCA, version 15.0.2, Sartorius Stedim Data Analytics AB, Umea, Sweden). (B) OPLS-DA score plot for patients with constipation vs healthy controls in integrated ion mode (SIMCA). (C) Permutation test of the OPLS-DA model (SIMCA). The slope of R2 is >0 and the Y-intercept of Q2 is <0.05, indicating a valid model. Figure 2. (A, B) Heatmap (R software & Kyoto Encyclopedia of Genes and Genomes (KEGG) website (https://www.kegg.jp/)) and volcano plot (SIMCA, version 15.0.2, Sartorius Stedim Data Analytics AB, Umea, Sweden) of metabolites with different levels between patients with constipation and healthy controls. Twenty metabolites had higher levels and 30 metabolites had lower levels in patients with constipation than in healthy controls.
Figure 2. (A, B) Heatmap (R software & Kyoto Encyclopedia of Genes and Genomes (KEGG) website (https://www.kegg.jp/)) and volcano plot (SIMCA, version 15.0.2, Sartorius Stedim Data Analytics AB, Umea, Sweden) of metabolites with different levels between patients with constipation and healthy controls. Twenty metabolites had higher levels and 30 metabolites had lower levels in patients with constipation than in healthy controls. Figure 3. Pathway analysis of metabolites with differences between patients with constipation and healthy controls (R software & MetaboAnalyst website (https://www.metaboanalyst.ca/)). Seven significantly altered metabolic pathways were identified, including phenylalanine metabolism; caffeine metabolism; and glycine, serine, and threonine metabolism.
Figure 3. Pathway analysis of metabolites with differences between patients with constipation and healthy controls (R software & MetaboAnalyst website (https://www.metaboanalyst.ca/)). Seven significantly altered metabolic pathways were identified, including phenylalanine metabolism; caffeine metabolism; and glycine, serine, and threonine metabolism. Tables
 Table 1. Differences in body mass index and blood biochemical indicators between patients with constipation and healthy controls.
Table 1. Differences in body mass index and blood biochemical indicators between patients with constipation and healthy controls. Table 2. Differences in upregulated metabolites between patients with constipation and healthy controls.
Table 2. Differences in upregulated metabolites between patients with constipation and healthy controls. Table 3. Differences in downregulated metabolites between patients with constipation and healthy controls.
Table 3. Differences in downregulated metabolites between patients with constipation and healthy controls. Table 1. Differences in body mass index and blood biochemical indicators between patients with constipation and healthy controls.
Table 1. Differences in body mass index and blood biochemical indicators between patients with constipation and healthy controls. Table 2. Differences in upregulated metabolites between patients with constipation and healthy controls.
Table 2. Differences in upregulated metabolites between patients with constipation and healthy controls. Table 3. Differences in downregulated metabolites between patients with constipation and healthy controls.
Table 3. Differences in downregulated metabolites between patients with constipation and healthy controls. In Press
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








