18 November 2021: Clinical Research
A Prospective Single-Center Study of the Effects of Repetitive Transcranial Magnetic Stimulation at 2-Week Follow-Up in 17 Patients with Chronic Orofacial Pain Diagnosed by Infrared Thermography
Jitka Fricova1ABDEG*, Markéta Janatova2ABDF, Jakub Albrecht3BD, Tadeas Mares3BD, Richard Rokyta4ADEG, Vaclav Masopust5ADF, Martin AndersDOI: 10.12659/MSM.933017
Med Sci Monit 2021; 27:e933017
Abstract
BACKGROUND: Infrared thermography is a diagnostic method used to monitor acute and chronic orofacial pain syndrome. Repetitive transcranial magnetic stimulation (rTMS) is a form of non-invasive brain stimulation. This prospective study from a single center aimed to investigate the effects of rTMS and used infrared thermography as a confirmatory test of orofacial pain.
MATERIAL AND METHODS: We used infrared thermography to examine the incidence of inflammatory changes as orofacial pain triggers. During the analysis of rTMS effects on patients with orofacial pain, we compared the decrease in pain and the thermal difference in the study group (n=17) and in the research group (n=13).
RESULTS: In the control group (n=13), there were no statistically significant changes. Both groups showed a significant decrease in self-reported pain. Numerical pain rating scores were significantly lower after S2 stimulation than after S1/M1 (P=0.0071) or sham (P=0.0187) stimulation. The Brief Pain Inventory scores were also lower 3 to 5 days after S2 stimulation than at the pretreatment baseline (P=0.0127 for the intensity of pain and p=0.0074 for the interference of pain), and after S1/M1 (P=0.001 and P=0.0001) and sham (P=0.0491 and P=0.0359) stimulations.
CONCLUSIONS: The findings from this study support the role of infrared thermography for the diagnosis of chronic orofacial pain, and showed that on the first and fifth days of rTMS therapy in the study group there was a significant reduction of the thermography findings when compared with the control group without rTMS therapy.
Keywords: facial pain, Magnetic Resonance Imaging, Thermography, Transcranial Magnetic Stimulation, chronic pain, Female, Follow-Up Studies, Humans, Male, Prospective Studies, Retreatment
Background
Few clinical studies on the diagnosis of orofacial pain have been published. The diagnosis and management of facial pain below the eye can be very different depending on whether the patient visits a dentist or medical practitioner. A structure for accurate diagnosis is proposed, beginning with a very careful history-taking [1]. The Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) is intended for use within any clinical setting and supports the full range of diagnostic activities, from screening to definitive evaluation and diagnosis. The new protocol provides a common language for all clinicians while providing the researcher with the methods for valid phenotyping of their subjects, especially for pain-related problems [2]. According to the new classification of pain in The Classification of Chronic Pain for ICD-11, 2019 (The International Classification of Diseases) [3], orofacial pain is defined as a headache occurring on at least 50% of days within the last 3 months and lasting for a minimum of 2 h per occurrence [4,5]. Usually, it is classified as atypical odontalgia. Chronic unilateral or bilateral orofacial pain can be defined as a localized primary headache [3]. This localization is very common in trigeminal autonomic cephalalgias, less common in migraines, and rare in tension-type headaches. The temporal definition of ‘chronic’ has been removed from the general definition of chronic headache [6]. Another paradigm that is more mechanistic suggests that orofacial pain may be nociceptive, inflammatory, neuropathic, or pain without clear evidence of tissue damage [6,7].
Insufficient knowledge of the etiopathology of pain and the neurobiological and pathophysiological mechanisms underlying persistent pain can lead to unclear diagnoses as well as suboptimal or unsafe treatment [8]. This study was based on the hypothesis that pain can have an inflammatory origin. Our aim was to objectify the pain, contribute to more precise diagnoses, and determine the source of the inflammatory-inducing processes mentioned above. To achieve this, we used thermography to acquire thermal images of the face. Therefore, this prospective study from a single center aimed to investigate effects of rTMS at 2-week follow-up in patients with chronic orofacial pain diagnosed by infrared thermography.
Infrared thermography is a contactless method based on the detection of infrared radiation, which is emitted by the body surface [9]. During a thermographic examination, changes in the spatial distribution of temperature and differences between symmetrical sides of the examined area (the left and right parts of the body) are observed under the same circumstances [10–12].
In some instances, a thermal decrease in the affected area and observation of an adaptation reaction have been used to increase diagnostic sensitivity [13,14]. Our studies have found that the results of thermographic measurements correlate with results from other methods, such as X-ray examination [15]. Thermographic measurements help detect abnormalities in vascular adaptation resulting from dysfunction of the autonomic nervous system and delayed response to thermal changes in patients with complex regional pain syndrome [13,14]. In patients with postherpetic neuralgia, the subjectively most painful spot has been associated with increased temperature [12]. In patients with orofacial pain, temperature increases or decreases have been recorded depending on the underlying etiology. Thermography is a method that can be beneficial in the differential diagnosis of the origin of pain [10]. In our study, patients were referred initially for non-invasive brain stimulation therapy (rTMS), which lasted for 5 consecutive days. Before rTMS, functional magnetic resonance imaging was performed to localize the painful region. We attempted to measure the effectiveness and/or response of rTMS using thermography. With thermography, it is also possible to monitor the influence of rTMS application using thermal images of the painful region. Nevertheless, in patients with chronic neuropathic pain, there is a high incidence of positive responses due to the placebo effect [16].
Material and Methods
SUBJECTS AND STUDY DESIGN:
The study followed the principles of the Declaration of Helsinki and was approved by the Ethics Committee of the Third Faculty of Medicine, Charles University. Written informed consent was obtained from all participants prior to them taking part in the study.
In our study, we prospectively included subjects with chronic orofacial pain who met the following inclusion criteria: (a) orofacial pain syndrome with a duration of at least 6 months, intractable pharmacotherapy-resistant pain (defined as the longer duration of pain despite at least 2 attempts at a pharmacological treatment in the past, both of sufficient dose and time); (b) stable analgesic medication for at least 1 month before the start of the study and throughout the course of the study and the 2-week follow-up evaluation; (c) aged 18–71 years; (d) absence of severe organic brain damage or other serious diseases that could be affected by rTMS (eg, epilepsy), and the absence of any metallic implants in the body (restrictions similar to those for magnetic resonance imaging [MRI]). This single-center study contained a 2-arm double-blind, placebo-controlled, randomized single-session study with a 2-week follow-up. Patients were randomly selected according to a permuted block design in a 1: 1 ratio (no stratification) to either active (A) or sham (S) TMS sessions. The patients and raters were blinded to the treatment received. Only the clinician administering the rTMS was aware of the treatment group allocated to each participant. Two groups were formed with a total of 30 subjects. The research group consisted of 17 subjects, 9 women and 8 men, aged 38–71 years (mean age 52.9±11.1 years); the control group consisted of 13 subjects, 9 women and 4 men, aged 30–71 years (mean age 51.9±12.2 years; Table 1).
The sham coils generated clicks that were very similar to the active coils, which were also sensed on the scalp. The subjects were unable to distinguish which session was the sham (control) and which was the rTMS session (research group).
THERMOGRAPHY:
Measurements using a thermal camera were performed at the Department of Psychiatry, First Faculty of Medicine and General University Hospital in Prague, Czech Republic, always using the same technical conditions, in the same department, with a constant ambient temperature of 23.4–23.7°C. A thermal camera (ThermaCAM™ E300, FLIR systems, Wilsonville, OR, USA) was used for measurements, with a resolution of 320×240 pixels, an imaging frequency of 50 Hz, and a sensitivity of 0.1°C. All patients followed pre-measurement instructions. No complications occurred during any of the measurements. Differences in measured temperature between duplicate pictures from the same region and on the same side of the face were in the order of 0.1±0.1°C. Airflow was kept to a minimum. The room was darkened, without direct sunlight. A thermal camera was placed on a stand so that no reflective surfaces were present in the field of the camera, and no heat sources were close enough to affect the results. Patients were informed about the course of the study in advance, signed a written consent, and obtained an information leaflet with instructions regarding precautionary measures before measurements were taken. Thermal camera measurements were performed under the following conditions: no food, no drink, and no use of a cell phone 1 h prior to the examination; no smoking for 3 h before the examination; no alcohol consumption for 12 h before the examination; avoidance of analgesics, non-steroidal anti-inflammatory drugs, and face shaving for 24 h before the examination; no acupuncture or manual manipulation therapy for 72 h before the examination; and avoidance of sunbathing for 10 days before the examination. During the measurements, it was necessary to have clean, dry skin, without cosmetics. Before the examination, patients were seated for 20 min in a quiet room with a stable temperature between 20°C and 24°C. Before the beginning of each measurement, localization and pain grade were recorded using the Pain Intensity Scale. During thermography imaging, patients were seated in an armchair. The distance between the thermal camera and the tested subject and its height and position were adjusted for each patient. A rotating stand was adapted so that the position of the thermal camera could be optimally adjusted in all imaging directions.
For precise adjustment of the required angle, a protractor affixed to the diagnostic armchair in the subcranial region was used. The patient’s face and head were free of obstruction, including glasses, hats, and hair. Imaging was performed on the first and the fifth day before and after rTMS and always from a constant distance and in the direction toward the examined region. Two pictures were taken from the front, from the side, and from an angle of 45° from the left and right sides of the face.
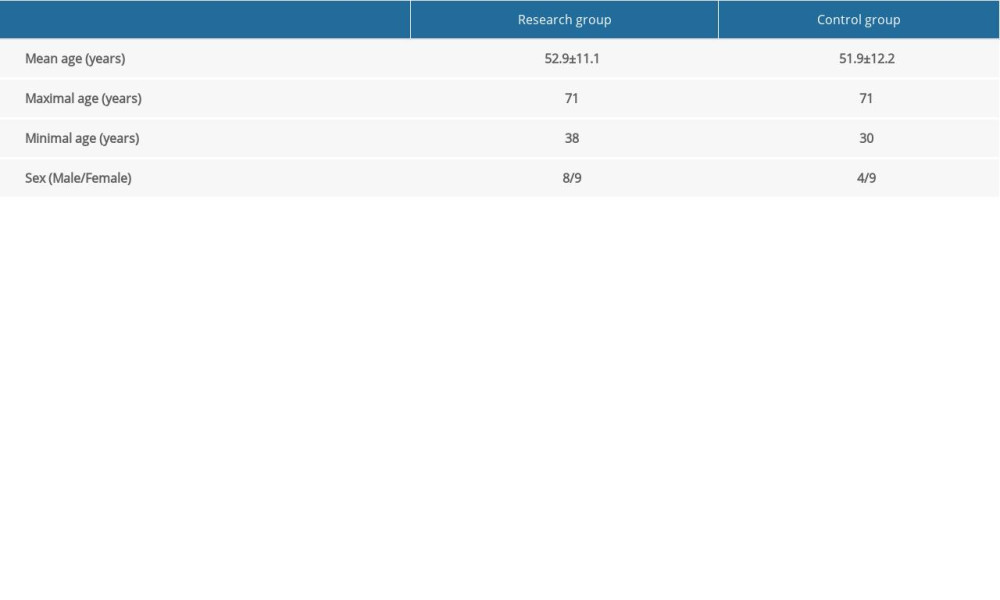
Differences in the thermal maximum, minimum, and average of the regions on the right and left sides of the face were compared before and after the first therapy (Figure 1), before and after the last therapy (Figure 2), and before and after each individual therapy. Similarly, the results were compared with the pain grade indicated by the patient on the day of the examination.
TRANSCRANIAL MAGNETIC STIMULATION (RTMS):
rTMS stimulation was applied using a Magstim® Rapid2 with an AirFilm® Coil (Rapid version) air-cooled, 70-mm coil, creating a magnetic field of 1–2 Tesla in a time interval of 100–200 ms using a Magstim Super Rapid stimulator (Magstim, Whitland, UK). rTMS stimulation was administered to patients over 5 consecutive days. We used increasing stimulus intensity of 85%, 90%, and 95% of the motor threshold, and for a total of 600 pulses per session. The stimuli were applied during 5 sessions, several days apart, with the rTMS applied over the contralateral motor cortex at a point corresponding to the somatotopic localization of pain. The area in the motor cortex was identified using a validated method of functional site identification and stimulation of the motor cortex.
FMRI AND NEURONAVIGATION:
Neuronavigation was performed using Visor™2ST (with software-based 3D modeling of the native T1 MRI brain scan) and NIFTI (Neuroimaging Informatics Technology Initiative) coordinates based on functional MRI (fMRI). Initially, each patient underwent fMRI scanning. A region of interest (ROI) was specified from the scan (ie, the region in the brain that should be stimulated). Patients underwent fMRI before the application of rTMS, and during the examination a painful sensation was created using Von-Frey hairs. Before rTMS stimulation, the stimulation coil was positioned toward the head of the patient. Anatomical images from the MRI were transformed by segmentation into anatomically accurate numerical models of each patient’s head together with models of the stimulation coil, and models of stimulation regions were coregistered in an electromagnetic field numerical simulator. The accuracy of rTMS applications was assessed based on calculations of the distribution of intensity of electric fields and their intersection with ROIs.
STATISTICAL ANALYSIS:
Datasets were acquired in the Department of Psychiatry of the General University Hospital, Prague. The Kolmogorov-Smirnov test was used to test the normality of the data (with a significance level
Results
Spearman’s correlation coefficients did not show a correlation between the level of pain and the difference in temperature in the localizations of the self-reported pain compared to corresponding areas on the opposite side of the face (Spearman: −0.1401,
We found a significant difference in temperature between the regions of self-reported pain compared to the reference areas on the opposite side of the face on the first and fifth therapy days in the research group (Figures 1, 2, 5)
For the control group, no significant change was seen. As shown in Figure 6, the difference in temperature was significantly lower in the research group on the fifth therapy day.
We found a significantly lower level of pain on the fifth day of therapy compared to the first day of therapy in both the control group (Figure 7) and the research group (Figure 8).
Spearman’s correlation coefficients did not show any association between the difference in temperature and the difference in pain and the change in pain before the first therapy and before the fifth therapy (control group: Spearman: 0.08595,
Discussion
Infrared thermography can be used as a supplementary diagnostic method in patients with orofacial pain [9,10,17]. According to new ICD 11 criteria, orofacial pain is defined as a headache occurring on at least 50% of days within the last 3 months and lasting a minimum of 2 h per occurrence.
Orofacial pain, especially chronic orofacial pain, is challenging to detect because it is often associated with bone structures in the mouth and face, and less often with the surrounding soft tissues [18]. There were no adverse effects among the patients in our study who underwent thermography. Testing burdens were not excessive during the measurements, and patients had to follow specific guidelines prior to thermography examination [9]. The indicated accuracy of the measuring device was 0.1°C. The variation between the measured values on duplicated pictures on the same side of the face was 0.1±0.1°C. In the diagnosis, a comparison of the thermal difference between the examined region and symmetrical references on the other side of the patient’s body were used [8,10,12]. Chronic orofacial pain, post-traumatic trigeminal neuropathic pain [19], persistent idiopathic orofacial pain, and burning mouth syndrome are usually referred to as primary chronic pain and neuropathic pain.
The difference in maximum temperature in the painful region corresponding to the same painful localization on the other side of the body was assessed in pictures taken from the frontal view, from the lateral view, and from an angle of 45°. The advantage of a thermal camera is most pronounced in patients where other methods have failed. After 1 therapy unit of rTMS, no statistically significant thermal change between the painful side and the same region on the other side of the face was found. A meta-analysis focused on the use of rTMS in patients with neuropathic pain found that the effectiveness of rTMS increases during a series of therapies [11,18,20]. The previous case study demonstrates the usefulness of thermal imaging in localizing inflammation associated with orofacial pain. In this pilot project, the use of infrared thermography, as an auxiliary aid in the diagnosis of orofacial pain, was demonstrated. In our patients, we found significantly elevated average, maximum, and minimum temperatures in the facial region of the self-reported pain compared to the corresponding region on the opposite side of the face. In the final measurement (ie, after 5 consecutive day rTMS treatments), the pronounced thermal asymmetry between the right and left sides of the face was gone [18].
While no change was seen immediately after 1 therapeutic unit, our results demonstrate the stability of the ambient temperature and that the acclimatization was of sufficient length before the examination. When comparing the difference in the maximum temperature of the painful region compared to the same region on the other side of the face in a research group, a statistically significant decrease was detected on the fifth day (Figures 2, 5). In the control group, there was no statistically significant decrease in thermal difference. When assessing self-assessed pain on the Pain Intensity Scale, pain was reduced in both the research and control group. In patients with chronic pain, this might also be caused by the placebo effect [16]. Pain relief in the control group could have been caused by the manipulation of the patient during the sham procedure. No correlation was found between the decrease in pain grade and the thermal difference. With regard to the subjective sensation of pain, pain relief was detected in both the research and control groups; however, using thermography, the effect was only seen in the research group. While the subjective pain scale is based on a patient’s assessment, thermography offers an objective diagnosis of changes in a physical quantity, and it is not influenced by the subjective sensation of the patient. The method showed itself to be acceptably sensitive, even without the application of provocation mechanisms used to increase diagnostic sensitivity [1,3]. As such, it is a suitable instrument that offers new diagnostic tools for use in complex analyses of the effectiveness of rTMS therapy, which uses a series of treatments for patients with chronic orofacial pain. Additionally, thermography makes it possible to monitor changes in the painful region during rTMS applications. A meta-analysis of studies focused on this therapy found that the effectiveness of rTMS increases with the number of intervention units [14,21]. However, in patients with chronic neuropathic pain, a high incidence of positive responses may be associated with the placebo effect [9].
rTMS stimulation in patients with chronic orofacial pain has been used several times previously; authors from Finland [22] used high-frequency rTMS directed at the sensorimotor (S1/M1) and the right secondary somatosensory (S2) cortices. Numerical pain rating scores were significantly lower after S2 stimulation than after S1/M1 (
Conclusions
Our hypothesis about the potential benefit of infrared thermography to analyze the effectiveness of rTMS in patients with chronic orofacial pain was confirmed. Equally, we showed that infrared thermography could be used to quantify and refine the diagnosis of orofacial pain. No correlation between the assessment of the pain grade and the maximum thermal difference between the painful region and its symmetrical counterpart on the other side of the face was found. Immediately after administration of 1 therapeutic unit, no thermal difference was detected. Comparing thermal differences on the first and fifth days of therapies showed a statistically significant lowering of the thermal difference in the research group, whereas in the control group no decrease in temperature was seen. In both groups (research and control), pain grades decreased. Thus, infrared thermography is recommended as a suitable supplementary method that can help quantify and refine the diagnosis of chronic orofacial pain. The findings from this study support the use of infrared thermography for the diagnosis of chronic orofacial pain. On the first and fifth days of rTMS therapy in the study group, there was a significant reduction of the thermography findings when compared with the control group without rTMS therapy.
Figures
 Figure 1. Thermography of orofacial area before therapy (orofacial pain on right side).
Figure 1. Thermography of orofacial area before therapy (orofacial pain on right side).  Figure 2. Thermography of orofacial area after therapy (orofacial pain on right side).
Figure 2. Thermography of orofacial area after therapy (orofacial pain on right side). 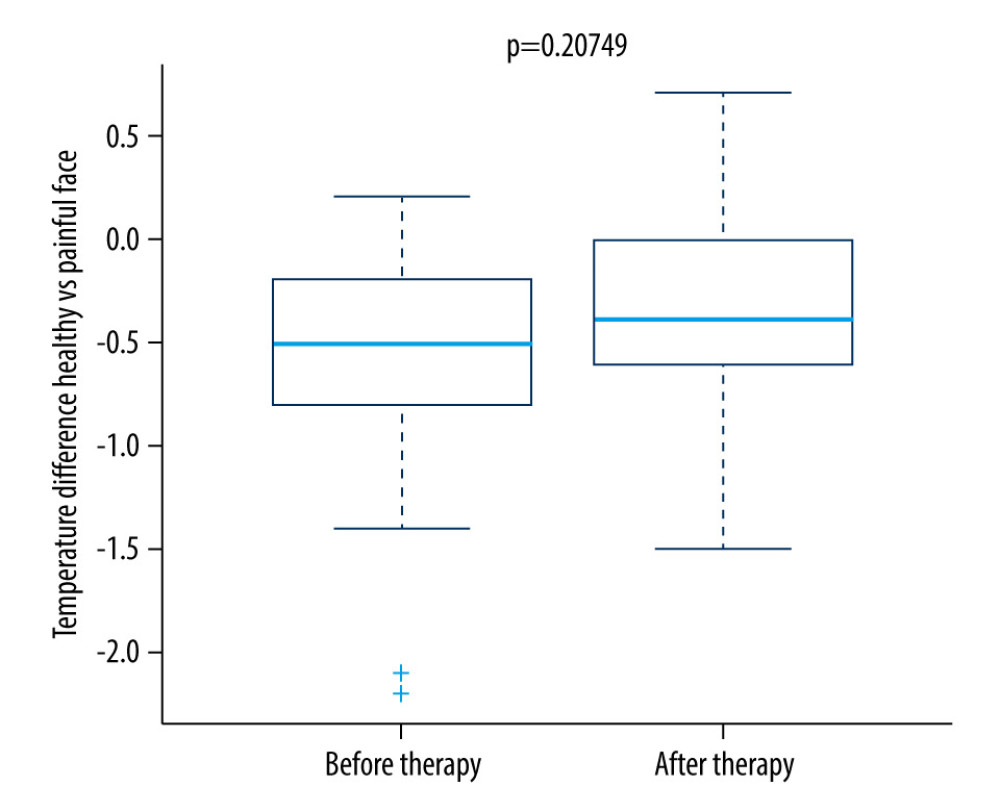 Figure 3. Difference in temperature after a single therapeutic session in the research group.
Figure 3. Difference in temperature after a single therapeutic session in the research group. 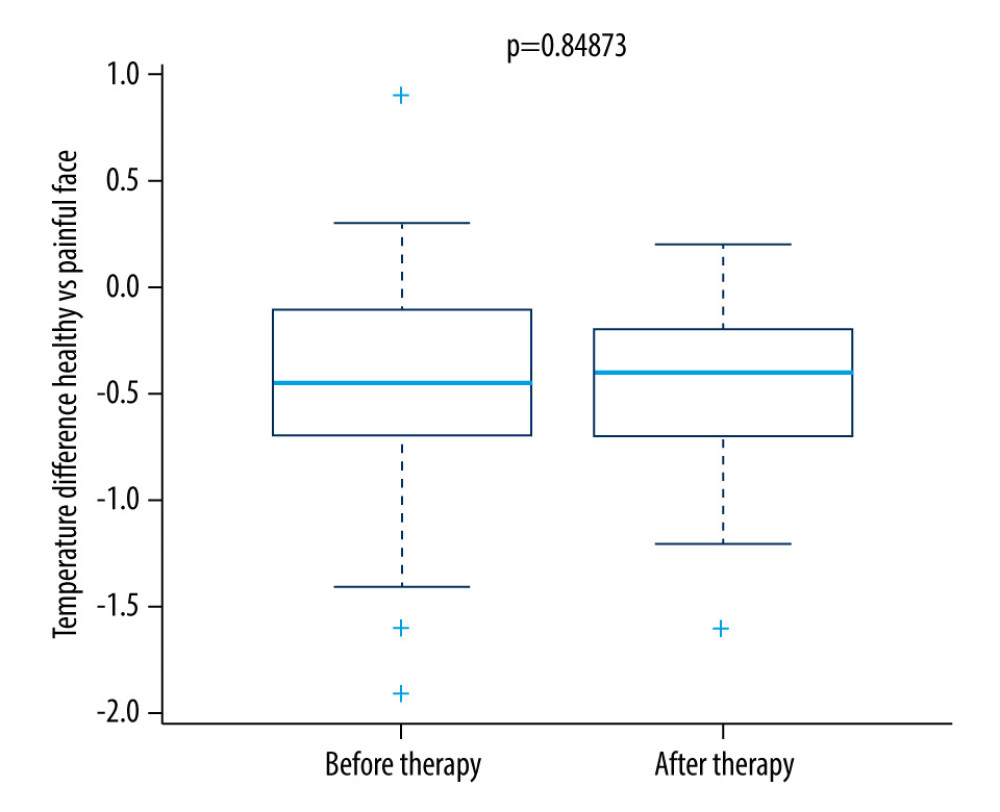 Figure 4. Difference in temperature after a single therapeutic session in the control group.
Figure 4. Difference in temperature after a single therapeutic session in the control group. 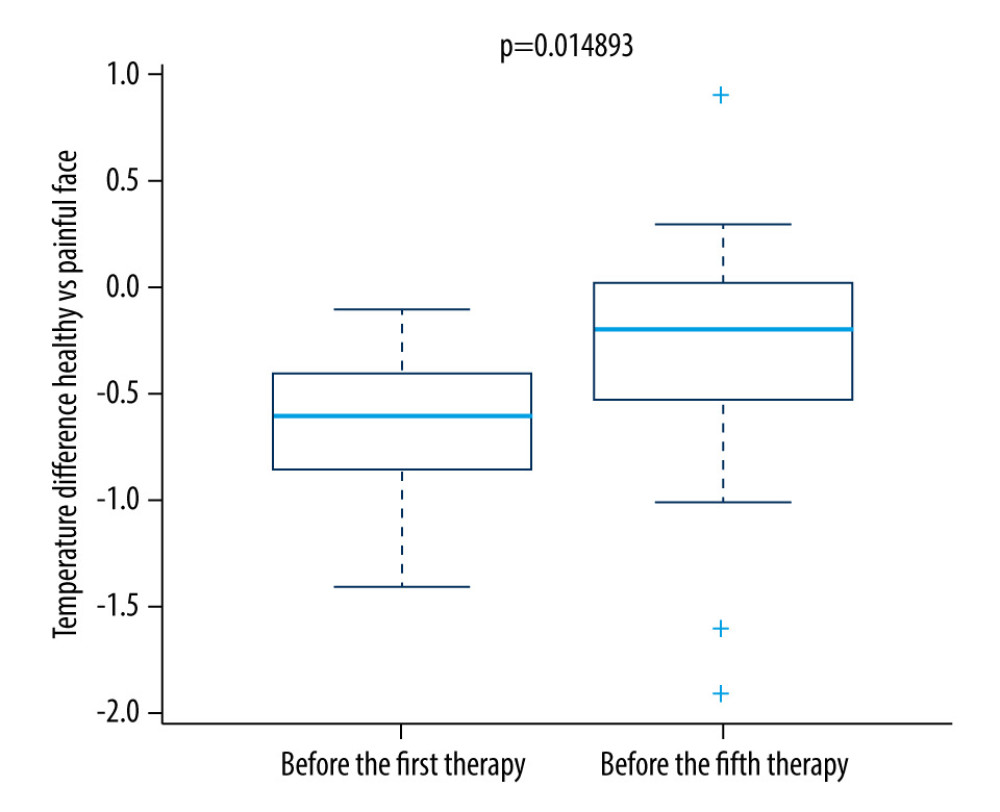 Figure 5. Difference in temperature between the first and fifth treatment day in the research group.
Figure 5. Difference in temperature between the first and fifth treatment day in the research group. 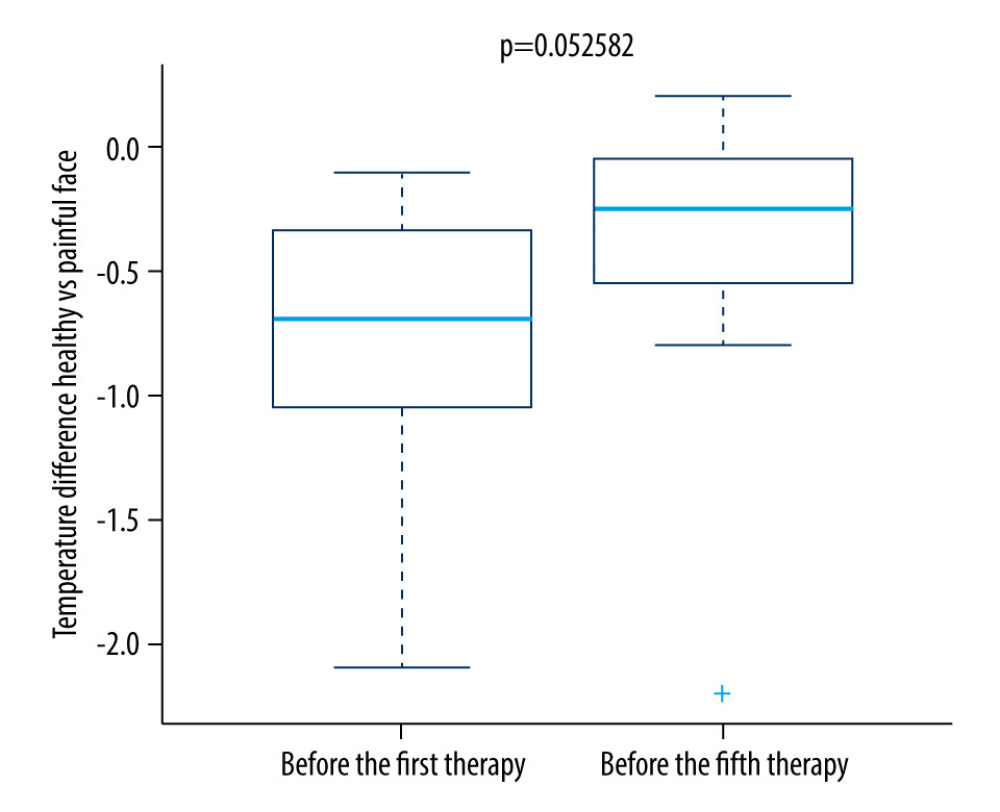 Figure 6. Difference in temperature between the first and fifth treatment days in the control group.
Figure 6. Difference in temperature between the first and fifth treatment days in the control group. 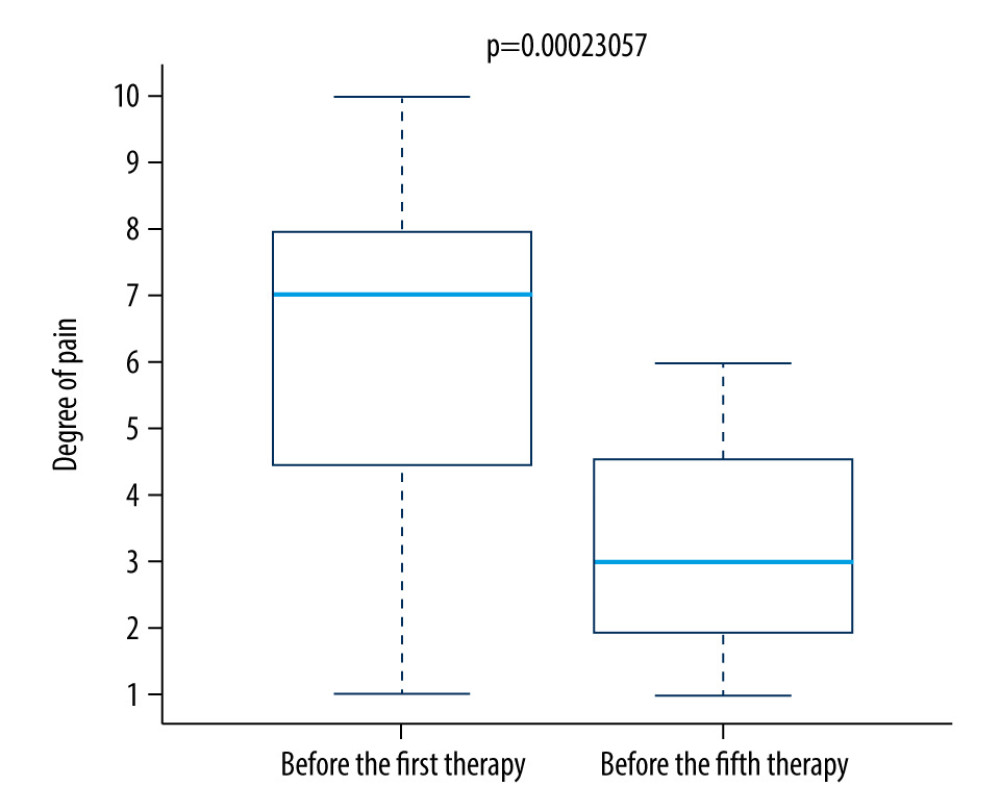 Figure 7. Difference in pain between the first and fifth treatment day in the control group.
Figure 7. Difference in pain between the first and fifth treatment day in the control group. 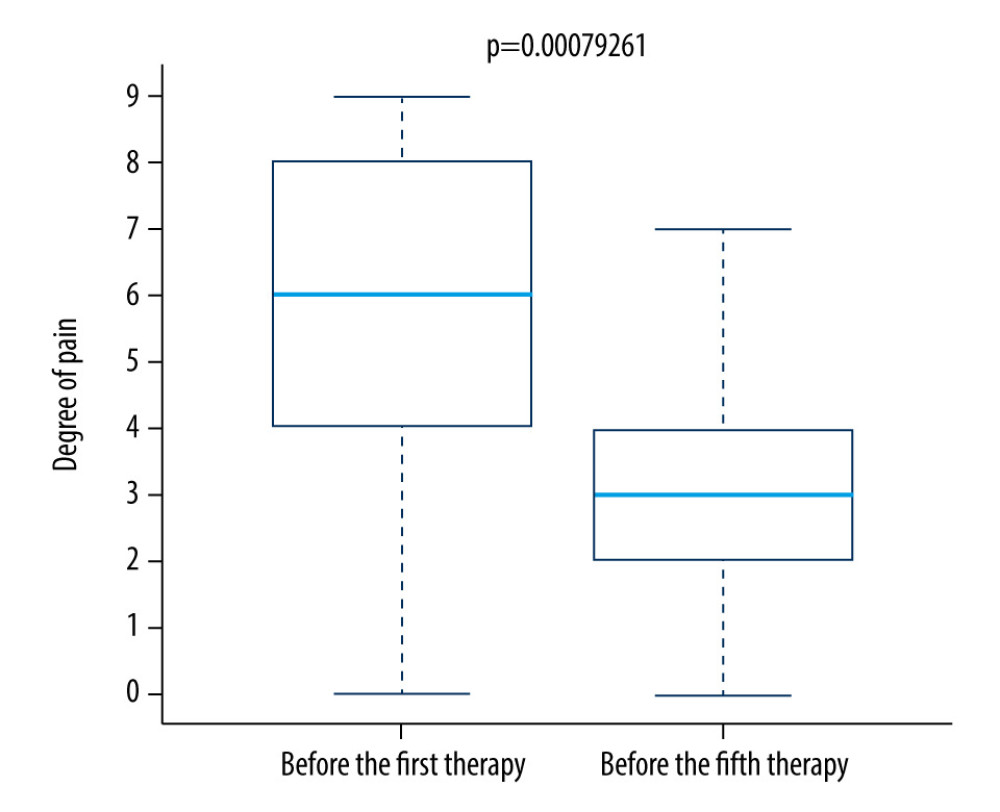 Figure 8. Difference in pain between the first and fifth treatment day in the research group.
Figure 8. Difference in pain between the first and fifth treatment day in the research group. References
1. Zakrzewska JM, Differential diagnosis of facial pain and guidelines for management: Br J Anaesth, 2013; 111(1); 95-104
2. Schiffman E, Ohrbach R, Truelove EInternational RDC/TMD Consortium Network, International association for Dental Research; Orofacial Pain Special Interest Group, International Association for the Study of Pain, Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: Recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Group: J Oral Facial Pain Headache, 2014; 28(1); 6-27
3. Benoliel R, Eliav E, Sharav Y, Classification of chronic orofacial pain: Applicability of chronic headache criteria: Oral Surg Oral Med Oral Pathol Oral Radiol Endod, 2010; 110(6); 729-37
4. Benoliel R, Birman N, Eliav E, Sharav Y, The International Classification of Headache Disorders: Accurate diagnosis of orofacial pain?: Cephalalgia, 2008; 28(7); 752-62
5. Benoliel R, Svensson P, Evers S, The IASP classification of chronic pain for ICD-11: Chronic secondary headache or orofacial pain: Pain, 2019; 160(1); 60-68
6. Smith B, Ceusters W, Goldberg L, Ohrbach R: Towards an ontology of pain, 2011, Keio University Press Inc
7. Woolf CJAmerican College of Physicians, American Physiological Society, Pain: Moving from symptom control toward mechanism-specific pharmacologic management: Ann Intern Med, 2004; 140(6); 441-51
8. https//s3.amazonaws.com/rdcmsiasp/files/production/public/Content/ContentFolders/GlobalYearAgainstPain2/20132014OrofacialPain/FactSheets/Orofacial_Pain_2016.pdf
9. Ring EFJ, Ammer K, Infrared thermal imaging in medicine: Physiol Meas, 2012; 33(3); R33-46
10. Gratt BM, Graff-Radford SB, Shetty V, A 6-year clinical assessment of electronic facial thermography: Dento Maxillo Facial Rad, 1996; 25(5); 247-55
11. Leung A, Donohue M, Xu R, rTMS for suppressing neuropathic pain: A meta-analysis: J Pain, 2009; 10(12); 1205-16
12. Selfe J, Whitaker J, Hardaker N, A narrative literature review identifying the minimum clinically important difference for skin temperature asymmetry at the knee: Thermol Int, 2008; 18(2); 41-44
13. Ammer K, Cold challenge to provoke a vasospastic reaction in fingers determined by temperature measurements: A systematic review: Thermol Int, 2009; 19(4); 109-18
14. Choi E, Lee PB, Nahm FS, Interexaminer reliability of infrared thermography for the diagnosis of complex regional pain syndrome: Skin Res Technol, 2013; 19(2); 189-93
15. Denoble AE, Hall N, Pieper CF, Kraus VB, Patellar skin surface temperature by thermography reflects knee osteoarthritis severity: Clin Med Insights Arthritis Musculoskelet Disord, 2010; 3; 69-75
16. Verdugo RJ, Ochoa JL, Use and misuse of conventional electrodiagnosis, quantitative sensory testing, thermography, and nerve blocks in the evaluation of painful neuropathic syndromes: Muscle Nerve, 1993; 16(10); 1056-62
17. Gratt BM, Sickles EA, Electronic facial thermography: An analysis of asymptomatic adult subjects: J Orofac Pain, 1995; 9(3); 255-65
18. Fricova J, Janatova M, Anders M, Thermovision: A new diagnostic method for orofacial pain?: J Pain Res, 2018; 11; 3195-203
19. Benoliel R, Zadik Y, Eliav E, Sharav Y, Peripheral painful traumatic trigeminal neuropathy: Clinical features in 91 cases and proposal of novel diagnostic criteria: J Orofac Pain, 2012; 26(1); 49-58
20. Fricova J, Klirova M, Masopust V, Repetitive transcranial magnetic stimulation in the treatment of chronic orofacial pain: Physiol Res Suppl, 2013; 62; 125-34
21. Rowbotham MC, Fields HL, Post-herpetic neuralgia: The relation of pain complaint, sensory disturbance, and skin temperature: Pain, 1989; 39(2); 129-44
22. Lindholm P, Lamusuo S, Taiminen T, Right secondary somatosensory cortex – a promising novel target for the treatment of drug-resistant neuropathic orofacial pain with repetitive transcranial magnetic stimulation: Pain, 2015; 156(7); 1276-83
23. Giamberardino MA, Affaitati G, Fabrizio A, Costantini R, Myofascial pain syndromes and their evaluation: Best Pract Res Clin Rheumatol, 2011; 25(2); 185-98
Figures
 Figure 1. Thermography of orofacial area before therapy (orofacial pain on right side).
Figure 1. Thermography of orofacial area before therapy (orofacial pain on right side). Figure 2. Thermography of orofacial area after therapy (orofacial pain on right side).
Figure 2. Thermography of orofacial area after therapy (orofacial pain on right side). Figure 3. Difference in temperature after a single therapeutic session in the research group.
Figure 3. Difference in temperature after a single therapeutic session in the research group. Figure 4. Difference in temperature after a single therapeutic session in the control group.
Figure 4. Difference in temperature after a single therapeutic session in the control group. Figure 5. Difference in temperature between the first and fifth treatment day in the research group.
Figure 5. Difference in temperature between the first and fifth treatment day in the research group. Figure 6. Difference in temperature between the first and fifth treatment days in the control group.
Figure 6. Difference in temperature between the first and fifth treatment days in the control group. Figure 7. Difference in pain between the first and fifth treatment day in the control group.
Figure 7. Difference in pain between the first and fifth treatment day in the control group. Figure 8. Difference in pain between the first and fifth treatment day in the research group.
Figure 8. Difference in pain between the first and fifth treatment day in the research group. In Press
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952