26 June 2021: Animal Study
Value of Contrast-Enhanced Ultrasonography in Evaluating Rheumatoid Arthritis: Preliminary Research Based on an Animal Model
Yiran Gong1ABCDE, Yinan Huang1ABCE, Yiming Su2ADF, Juxin He1ABCE, Shuqiang Chen1ADFG*DOI: 10.12659/MSM.931327
Med Sci Monit 2021; 27:e931327
Abstract
BACKGROUND: The aim of this study was to evaluate the effectiveness of grayscale ultrasound (GSUS), power Doppler imaging (PDI), and contrast-enhanced ultrasonography (CEUS) in early rheumatoid arthritis (RA) diagnosis through animal experiments.
MATERIAL AND METHODS: A rabbit RA model was constructed. The animals were randomly divided into 2 groups, namely, the RA model group and the control group. GSUS, PDI, and CEUS were performed in the model group during early RA and were compared with pathology of synovial biopsies. The consistency of 3 types of ultrasonography was evaluated in tandem with pathological grading.
RESULTS: 23 rabbits in the RA model group completed the experiment. GSUS showed that the synovial thickening of grades 1, 2 and 3 occurred in 12, 19, and 15 joints, respectively. The sensitivity, specificity, and accuracy of PDI in the diagnosis of knee joint synovitis in RA grades 1, 2, and 3 were 80.56% (29/36), 60.00% (6/10), and 76.09% (35/46), respectively, while those with CEUS were 94.44% (34/36), 90.00% (9/10), and 93.47% (43/46), respectively. The differences in diagnostic sensitivity, specificity, and accuracy of the 2 methods were statistically significant. Additionally, the thickness of the synovium measured with GSUS precontrast was greater than that of postcontrast.
CONCLUSIONS: RA evaluated with GSUS is often more hypertrophied than when evaluated with CEUS, while evaluation by PDI is less hypertrophied than that by CEUS. However, from a practical view point, GSUS and PDI are of sufficient practical value, except for in a few special cases.
Keywords: Arthritis, Experimental, Microscopy, Acoustic, Models, Animal, Ultrasonography, Doppler, Arthritis, Rheumatoid, Biopsy, Contrast Media, Image Enhancement, Reproducibility of Results, Synovial Membrane, Ultrasonography
Background
Rheumatoid arthritis (RA) is an autoimmune-related chronic disease, with nonsuppurative joint synovitis as the major pathological feature. The pathological changes mainly involve the small joints of the hands and feet, but RA can involve joints in other regions, ultimately leading to impaired joint function and structural damage, with increased risks of disability and mortality.
Early diagnosis and active treatment of RA are recommended according to various guidelines. In fact, delayed treatment of RA can lead to a poor prognosis in patients [1,2]. Therefore, diagnosis and treatment evaluation of RA are very important for optimal patient outcomes. Recent studies have shown that no bone damage occurs in the absence of synovitis, and that the presence of synovitis is a prognostic indicator of bone damage; thus, early detection of vascularized synovia should be one of the primary goals in the assessment of RA [3–5]. Contrast-enhanced ultrasonography increases the intensity of Doppler signals from blood through the administration of microbubble contrast agents. It enhances the signal-to-noise ratio and can improve a non-diagnostic Doppler examination by raising the intensity of weak signals to a detectable level [6]; thus, it facilitates the early diagnosis of rheumatoid arthritis. CEUS also plays an important role in assessing disease activity. CEUS allows not only for significantly better differentiation of active articular synovitis, but also allows a better characterization of the synovitis around tendons. CEUS also provides better spatial resolution; therefore, this technique also allows better characterization of the pannus in terms of differentiating between hypervascularity, hypovascularity, and avascularity to evaluate synovial vascularity to determine prognosis and assess treatment response. The current evaluation for remission mainly includes clinical remission and imaging remission, in which the role of ultrasound is increasingly valued [7]. The use of US to monitor patients considered to be in remission can help predict those likely to suffer subsequent joint damage and flare-up of disease [8]. To estimate the degree of RA remission, ultrasound can be considered a semiquantitative assessment, mainly through the grayscale ultrasound (GSUS) presentation of the soft tissue around the joints and the detected blood flow signal. Based on the harmonic effect of the ultrasound contrast (contrast-enhanced ultrasonography, CEUS) agent, microbubbles, and their resolution on peripheral microcirculation, some scholars have applied CEUS in the diagnosis and evaluation of the remission of RA [9,10]. As with MRI and other techniques, these studies have often been based on serological tests [7,11], in which the clinical pathology of the diseased RA joints is often overlooked. The results of serological testing can be disturbed by many factors, both those relevant to RA activity and some uncorrelated interference factors, such as CRP and ESR. Currently, some studies have shown that clinical symptoms and serological markers in RA patients do not always match the pathological changes in involved joints [12,13]. Another study showed that ultrasound imaging changes can match pathological changes [14], but due to ethical issues, it is difficult to obtain human RA pathological specimens. Therefore, we used an animal model in this study. Studies have shown that the early pathological levels of RA detectable by ultrasound surpass serological findings [14]. Therefore, it is necessary to determine whether contrast-enhanced ultrasound can effectively reveal RA remission at early pathological levels. The purpose of our study was to assess the value of CEUS in the evaluation of RA pathology status based on an animal model.
Material and Methods
ANIMAL MODEL:
The animal use protocol listed below was reviewed and approved by the Animal Ethics and Welfare Committee of Fujian Medical University with the reference number SYXK (min) 2016-0008. The study animals included 30 male New Zealand (Fujian Medical University, Fuzhou, Fujian, China) white rabbits, ~6 months old, with an average body weight of 2.5 to 3.0 kg. The animals were randomly divided into either the RA model group (n=25) or the healthy control group (n=5), according to a randomization table. The RA model for rabbits was completed following the method of “Egg protein induction” introduced by Glynn [15]. Experimental reagents included albumin from chicken egg whites and Freund’s complete adjuvant (F-5881) supplied by Sigma (Merck KGaA, Darmstadt, Germany). The RA model group underwent ovalbumin-induced arthritis, as previously described by Atkinson et al [16], which mainly included basic sensitization and joint sensitization. For basic sensitization, 1 mL of 20 g/L Freund’s complete adjuvant solution was used for back subcutaneous sensitization, injected once every other week for 3 continuous weeks of sensitization. Joint sensitization was initiated 1 week after the last injection of basic sensitization (ie, the 4th week), at which time 1.6 mL (5 g/L) of dissolved ovalbumin solution was injected into the bilateral knee joints of each rabbit. For the control group, on each weekend for the first 3 weeks, 1 mL of saline was injected subcutaneously into the scapular area of the rabbits. During the 4th week, 1 mL of saline was injected into the bilateral knee joints of each rabbit. The feeding methods between the 2 groups were identical, and the time points for ultrasound observation and pathological specimen collection were selected in the 7th and 8th weeks.
INSTRUMENTS AND DIAGNOSTIC METHODS:
The instruments used included a Philips IU 22 Doppler ultrasound diagnostic apparatus (Philips, Best, the Netherlands), with the probe frequency setting of 7–12 MHz. Low-speed blood flow conditions were utilized for bone and muscle. Abdominal anesthetic was administered using 1% sodium pentobarbital at a dose of 6 mg/kg body weight. After the rabbits were fully anesthetized, they were placed in an appropriate knee flexion and extension position, and a scan of the bilateral knee joints was obtained according to the standard procedure. The main observations were: 1) joint cavity fluid thickness; 2) joint synovial thickness (the joints were scanned and checked, the thickness of each thickest synovium was measured 3 times to obtain the mean, and the means were graded as levels 0–3 according to the semiquantitative criteria for synovial hyperplasia proposed by Szkudlarek [17]; and 3) the color signal of synovial blood flow, measured by PDI, and the synovial vessels semiquantitatively divided into 4 grades (0–3) according to the method described by Szkudlarek [17].
After the conventional ultrasound examination, a deep vein catheter (obtained from the contrast agent package) was inserted into the rabbits’ ear veins for the administration of contrast agent. The contrast agent used was SonoVue (Bracco, Milan, Italy), prepared according to the manufacturer’s instructions. For each joint, a single dose of 1–1.5 mL and mechanical index (MI) of 0.08 were administered. PM/PI Philips contrast-enhanced ultrasound technology was used for the measurement.
In this study, synovial CEUS was semiquantitatively assessed according to 3 levels, as referenced by the grading criteria established by IACUS (International Arthritis Contrast Ultrasound) [18]:
The ultrasound results, including CEUS, were scored by 2 doctors independently, followed by a third evaluation performed in cases of inconsistent evaluations. Overall, there was high interrater reliability.
PATHOLOGICAL SPECIMENS:
An ultrasound-guided synovial biopsy was performed around the 8th week after CEUS. A Bard-Magnum 18-G biopsy needle (Bard-Magnum Biopsy Instrument, Covington, GA, USA) was used as the puncture needle. Under ultrasound guidance, the biopsy needle was inserted approximately 0.5 cm above the attachment point of the patellar ligament. When ultrasound confirmed that the biopsy needle had entered the diseased synovial membrane of the joint cavity, the synovial specimen was quickly extracted based on the synovial condition, with careful avoidance of large blood vessels and joint effusion sites. The animals used in this experiment were euthanized in a humane way (killing by venous thromboembolism during narcotism), following the Ethics Guide of Fujian Medical University.
PATHOLOGICAL GRADING:
Synovial pathological specimens were prepared by conventional methods and were stained with HE. Synovial hyperplasia and inflammatory cell infiltration of surrounding tissue was observed under a microscope. The specimens were scored according to the synovitis pathology scoring criteria proposed by Krenn et al [19]. Each specimen was scored by 2 pathologists 3 times, and the median score was recorded. Zero points indicated no inflammatory infiltration,1 point indicated mild synovial inflammation and mild inflammatory cell infiltration, 2 points indicated moderate synovial inflammation and moderate inflammatory cell infiltration, and 3 points indicated severe synovial inflammation and severe inflammatory cell infiltration.
STATISTICAL ANALYSIS:
All of the data were analyzed using the SPSS statistical software package. The paired
Results
RABBIT RA MODELING:
The RA model group included 23 rabbits that successfully underwent the experimental procedures. Two rabbits died of an overdose of anesthesia; thus, no ultrasound or pathology evaluation was performed on them. During the stage of basic sensitization, redness and ulceration appeared gradually at the back subcutaneous injection site of the posterior parts of the body, which healed gradually in approximately 2 months. After the 4th week, all of the rabbits assigned to the RA model group showed varying degrees of redness of the bilateral knee joints, increased limb temperature, and limited activity. The rabbits in the control group (a total of 5) exhibited a symmetrical body, strong muscles, smooth and dense bright hair, quick action and reactions, and normal eating, drinking, and living habits. Two rabbits were unable to complete a biopsy because of incomplete anesthesia.
RA SYNOVITIS CONVENTIONAL ULTRASOUND PERFORMANCE:
Under GSUS, the distinction among the synovial membrane, joint capsule, and synovial fluid was not obvious among the healthy controls. In contrast, varying degrees of thickening synovium in the knee joint patellar capsule and joint cavity were observed in the RA model group. These observations were mainly low- or high-echo-based and could not be deformed and displaced when pressure was applied to the probe. Some joints showed small branching or villiform changes (Figure 2). Synovial thickening was observed in grade 1 (12 joints), grade 2 (19 joints), and grade 3 (15 joints). In this study, grade 1 PDI revealed that the thickened synovium demonstrated different degrees of blood flow signal, which could be divided into levels 0–3 according to the blood flow grading standards proposed by Chakr et al [20]. Different degrees of the blood flow signal in the thickened synovial membrane were visible by PDI (Figure 3). The 46 knee joints examined were graded as follows: grade 0: 1 (1/46, 2.17%); grade 1: 13 (13/46, 28.26%); grade 2: 14 (14/46, 30.43%); and grade 3: 18 (18/46, 39.13%). Interrater reliability was measured by the kappa index, which reached 0.79 (synovial thickening, P<0.05) and 0.80 (blood flow grading, P<0.05), showing good consistency between different observers. In animal experiments, because the animals did not coordinate like humans, it was easy to produce flash artifacts displaying as grade 1 PDI. In this evaluation, blood flow signals of 0 and 1 indicated non-disease states, while grade 2–3 blood flow was classified as disease states.
RA PERFORMANCE ASSESSED BY CEUS:
Among the 46 knee joints examined, 1 was determined to be CEUS grade 1 (1/46, 2.17%), 8 were grade 2 (8/46, 17.39%), and 37 were grade 3 (37/46, 80.43%). According the results of IACUS [18], active synovitis was defined as thickening of intra-articular tissue that is not displaceable and not at all or minimally compressible and exhibits PDUS signals or contrast enhancement at CEUS. Therefore, grade 1 or greater is defined as active synovitis. In total, the CEUS method diagnosed 45 knee joints as exhibiting active synovitis. The kappa index for evaluation of interrater reliability was 0.77 (P<0.05).
RA SYNOVIAL BIOPSY AND SYNOVIAL PATHOLOGICAL GRADING:
Twenty-three (23) of 25 animals (ie, 46 joints from the RA model group) completed the ultrasound-guided synovial biopsy (Figure 4), with a success rate of 92% (46/50). The length of the specimens was greater than 0.4 cm, and the specimen sampling was satisfactory. There was no instance of local or systemic infection after surgery. Synovial membrane semiquantitative scoring occurred as follows: 0 points for 1 joint; 1 point for 9 joints; 2 points for 20 joints; and 3 points for 16 joints. The average score of synovial inflammation was 2.14±0.78 points. The pathological manifestation of synovial hyperplasia is shown in Figure 5.
CORRECTION OF CEUS TO SYNOVIAL THICKNESS MEASURED BY GSUS:
The thickness of the synovial tissue was measured by GSUS. By CEUS, the enhanced thickness of the synovial tissue could be measured when the maximum enhancement signal appeared. For 42 joints, the synovial thickness measured before contrast was greater than the thickness measured after contrast. For 4 joints, the synovial thickness before and after contrast was equivalent (within 0.2 mm). No joints exhibited a greater synovial thickness after contrast. The paired t test was performed on synovial thickness before and after contrast. The results are shown in Table 1.
The above statistical results demonstrated that contrast enhancement with GSUS significantly impacted measured synovial thickness.
COMPARISON OF SYNOVITIS EVALUATED BY PDI BEFORE AND AFTER CEUS CONTRAST:
A total of 32 knee joints were diagnosed as having hypertrophied synovitis by PDI, while a total of 37 were classified as hypertrophied synovitis cases by CEUS. Among the total of 46 knee joints evaluated, only 1 joint did not show obvious blood flow as detected by PDI without CEUS enhancement. Twenty-seven joints demonstrated a weaker blood flow signal by PDI with significant enhancement by CEUS, and 18 joints showed a rich blood flow signal by PDI (grade 2–3 blood flow) and obvious grade 2 enhancement by CEUS (Figure 1).
COMPARISON OF PDI AND CEUS WITH PATHOLOGY:
Using the pathological results as the criterion standard, the sensitivity, specificity, accuracy, negative predictive value, and positive predictive value of PDI in the diagnosis of knee joint synovitis in this RA model were 80.56% (29/36,SE), 60.00% (6/10, SP), 76.09% (35/46, AC), 46.15% (6/13, NPV), and 87.88% (29/33, PPV), respectively, while those parameters according to the CEUS methods were 94.44% (34/36, SE), 90.00% (9/10, SP), 93.47% (43/46, AC), 81.82% (9/11, NPV), and 97.14 (34/35, PPV), respectively (Table 2). The diagnostic sensitivity, specificity, and accuracy of the 2 methods showed statistically significant differences (P<0.05) (Tables 3, 4).
Discussion
In this study, the synovial thickness of 46 knees joints in the RA model group was measured before and after contrast-enhanced ultrasound. It was found that there was significant difference in synovial thickness measured by CEUS and GSUS. Contrast enhancement with GSUS had a significant effect on the measured synovial thickness. In addition, the results of PDI and CEUS semiquantitative measurement of synovium in 46 knee joints were compared with the results of synovial pathological grading. It was found that CEUS was more sensitive than PDI in measuring synovial blood perfusion. This study found that RA evaluated with GSUS is often more hypertrophied than when evaluated with CEUS, while evaluation by PDI is less hypertrophied than that by CEUS.
Rabbit antigen-induced arthritis model (AIA) is a large-animal model that has been developed over the past 30 years [21], especially in the research on imaging. Qiu [22] reported a nearly 100% success rate when 8 mg of Ova was injected into the joints. In fact, the size of the rabbit knee is comparable with that of human peripheral joints, which are the most common lesions in RA. The following observed results also indicated pathogenesis similar to human RA. Thus, the choice of a rabbit model is suitable for research [23]. The lower price, suitable feeding, and molding were other factors influencing our experimental methods.
As stated in the RA chronic disease management guide, the true state of RA improvement includes not only the disappearance of clinical symptoms and the dissipation of inflammation but also the improvement of joint outcomes and functions, resulting in the improvement of quality of life [24]. Therefore, imaging is playing an increasingly important role in the diagnosis of RA and its follow-up assessment [25–28]. Ultrasound has the inherent advantage of being convenient and cost-effective. It can also be used for the assessment of multiple joints of the body simultaneously [29]. Compared to magnetic resonance imaging (MRI), ultrasound greatly reduces the time and cost of the examination. Hence, ultrasound has obvious advantages in the assessment of RA and should be recognized as a conventional means for diagnosis and follow-up [30].
Although both GSUS and PDI can show changes in the diseased region, they cannot reveal the most distant synovial tissues with microfluidic perfusion due to the limited resolution of GSUS and the limited sensitivity of blood flow illustration. With its harmonic effects and technology, CEUS has been widely used in various clinical fields, including microcirculation perfusion, tumor chemotherapy and ablation monitoring. Currently, evaluation of the application of CEUS in RA diagnosis and its efficacy has been very limited. Comparative studies have been mainly restricted to serological changes [31–34] and no comparative studies in a pathological context have been reported. Based on the results of our study, the role of CEUS in the early diagnosis and classification of RA is of demonstrable value. The results suggested that the synovial thickness measured by CEUS and GSUS was significantly different (
Further, CEUS could determine the synovial blood flow perfusion more sensitively (
Our experimental study also found that GSUS assessment of RA often overestimated the condition compared to CEUS, while PDI demonstrated an underestimated evaluation. As such, CEUS across the 2 methods could provide more accurate assessments. Therefore, after completion of GSUS and PDI examinations, the RA status assessed by CEUS could be roughly estimated, and the pathological analysis of RA could then be determined. As a semiquantitative means of evaluation, CEUS had high effectiveness. However, in terms of the time and cost, GSUS and PDI have advantages over CEUS. Therefore, from a practical point of view, except for in a few special cases, GSUS and PDI have more practical value for extensive application.
Conversely, because the CEUS agent in the body subsides faster than MRI agent and cannot assess multiple joints simultaneously, CEUS might not be as logistically advantageous as MRI in cases of multi-joint-diseased RA. The main obstacles to using US contrast media are high costs, technical limitations (for instance in near fields), a relatively short time window of examination, and the need for optimally designed bubbles for near field investigation at higher frequencies. The main specific limitations of CEUS in RA assessment are the small number of joints (usually 1) that can be examined at one time, the blooming artifact after injection, and the lack of definition of normal joint flows, so slightly increased flows are also undefined. Thus, it is obvious that CEUS, although more accurate, still has important practical limitations. Meanwhile, ultrasound contrast agents also have potential negative effects and adverse effects. When using ultrasound contrast agent, it is necessary to understand its contraindications to avoid allergy to the corresponding components in patients with a history of allergy, but the possibility of allergic reaction is very small. The adverse effects of ultrasound contrast agents are less and the incidence is also very low. The main adverse effects are headache, nausea, pain at the injection site, and abnormal sensation. These adverse reactions are temporary and mild. In addition, CEUS in RA loses the ultrasound cost-effectiveness advantage in terms of cost and prolongation of the examination and interpretation time.
In recent years, studies have shown that texture analysis and high-frequency ultrasound are widely used [35–40]. In addition, they have lower cost and are less invasive. Texture analysis applies analysis analytic metrics to both the normals and abnormals by extracting ultrasonic image features, and allows the computer to learn from the schema. Textural analysis can help identify abnormally thickened synovium and locate the edge of the synovium clearly in RA so that synovium thickness can be measured more accurately. Meanwhile, textural analysis can reduce the influence of acoustic shadowing on contour measurement since we cannot assume that we will have a reliable contour in every case [41]. High-frequency ultrasound (HRUS) is widely used in the early diagnosis of RA, particularly in the introduction and application of high-frequency color Doppler ultrasound (HCDU), which can monitor blood flow change in the synovium and synovial thickening [42].
This study in and of itself also had some limitations. Due to the lack of CEUS in the rabbit RA model, the 1–1.5 mL dose of ultrasound contrast agent of SonoVue was used by us in repeated experiments. It is important to note that great differences exist between human and rabbit physiology, including a much faster heart rate and the comparative dose required by a human patient. Because the synovial membrane is close to the cartilage and bone surface, there were many limitations when implanting the needle to avoid injury to the cartilage and bone. At the same time, because of the different depths of the needle, the synovial membrane tissue obtained by puncture could not completely represent the full condition of the synovial lesions, especially in cases of synovial fibrosis. Even if the approximate range of the hypertrophied synovium was determined beforehand by CEUS, some deviation in the operation was still inherently inevitable, and the situation of the synovium only represents one part of the whole joint pathology and not all. It has been reported that immunohistochemical analysis of the synovial membrane could be another means to evaluate the activity of RA, and it is a complimentary method to be considered in future studies [43–45]. This method could largely avoid the errors caused by sampling and more accurately reflects RA activity.
Conclusions
In summary, CEUS can accurately reflect the microcirculation status of RA synovium. It is good for the determination of synovial thickness and even better for the selection of ultrasound-guided synovial puncture biopsy sites. Although CEUS can more accurately determine the microcirculation and activity of RA synovial pathology, it will require refinement and optimization for large-scale application in routine and daily clinical applications. Nonetheless, this study elucidates the advantages of CEUS in a pathological context, encouraging future endeavors to expand our current understanding. The limitation of this study is that there was no ultrasonic observation of the knee joint in the model group and the control group at the 1st week and the 2nd week (ie, the 5th and the 6th week) after joint sensitization (ie, the 4th week). Therefore, we could not get the ultrasonic observation results of the model group and the control group during the period of reactive arthritis in which synovial pannus had not been formed. In the follow-up study, we will further improve the experiment, and rabbits with induced reactive arthritis will be included in the inflammatory control group. Ultrasound observation and pathological specimen collection will be selected in the inflammatory control group at the 1st week (ie, the 5th week) after joint sensitization (ie, the 4th week), while the time points for ultrasound observation and pathological specimen collection of the model group will be selected in the 7th and 8th weeks, respectively. The ultrasonographic and pathological results of the RA model group and the inflammatory control group will be compared to detect the difference between rheumatoid arthritis and reactive arthritis in the early stage by use of the above ultrasonic methods. In this study, we only drew conclusions based on the animal model. Regarding its application for RA in humans, further clinical studies are needed.
Figures
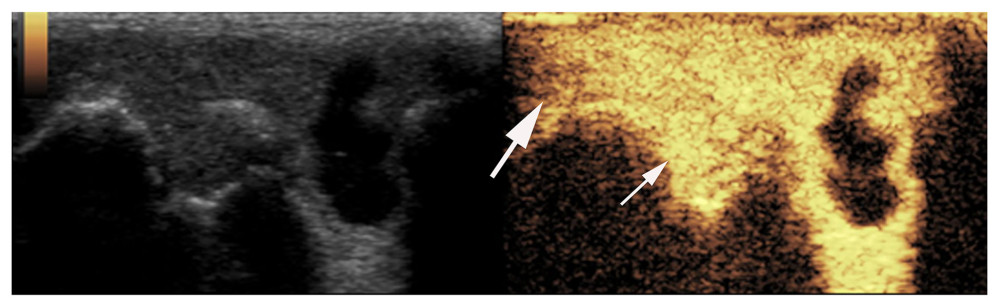 Figure 1. Synovial ultrasound contrast. Synovial enhancement (small arrow) was significantly greater than the surrounding tissue (large arrow).
Figure 1. Synovial ultrasound contrast. Synovial enhancement (small arrow) was significantly greater than the surrounding tissue (large arrow). 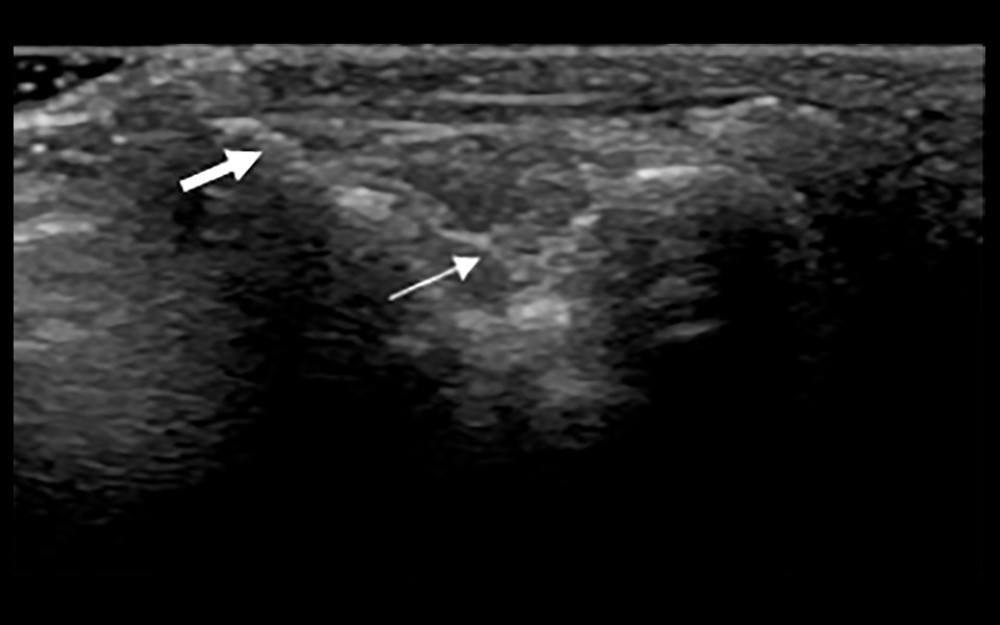 Figure 2. Synovial membrane grade 2 thickening. Thickening of the synovial tissue reached the joint head (small arrow), but did not reach the diaphysis (big arrow).
Figure 2. Synovial membrane grade 2 thickening. Thickening of the synovial tissue reached the joint head (small arrow), but did not reach the diaphysis (big arrow). 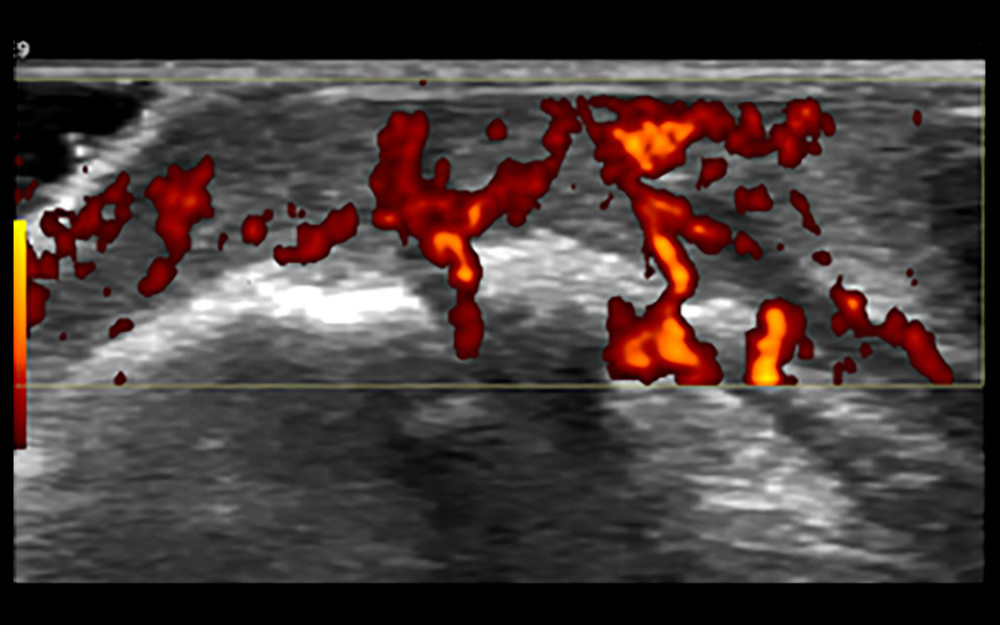 Figure 3. Synovial grade 3 blood flow signal. Blood flow was detected through the synovium.
Figure 3. Synovial grade 3 blood flow signal. Blood flow was detected through the synovium. 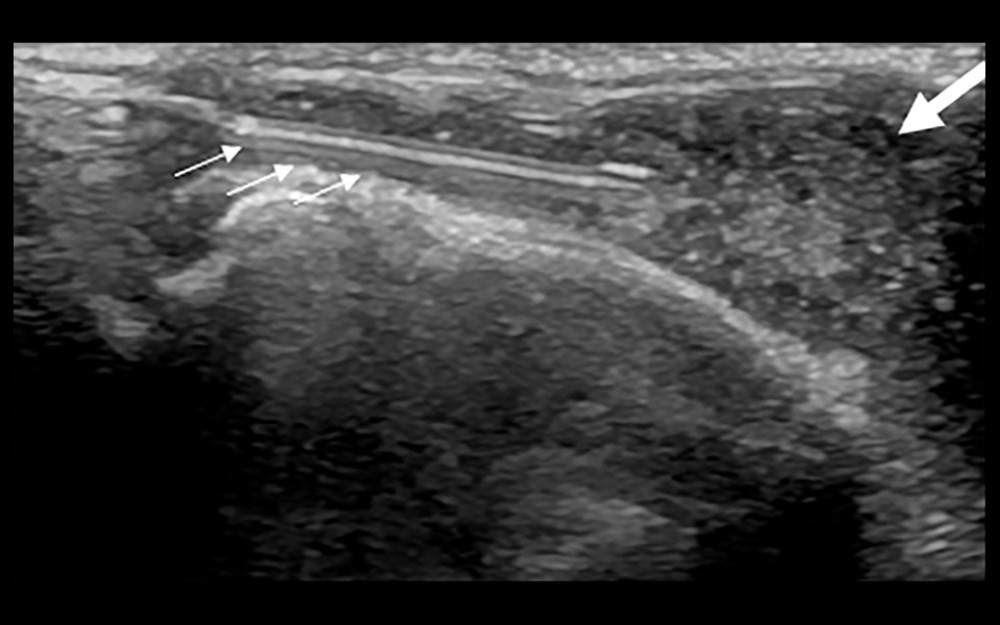 Figure 4. Ultrasound-guided synovial puncture. 18-G puncture needle (small arrows) accurately entered the synovial tissue (large arrow).
Figure 4. Ultrasound-guided synovial puncture. 18-G puncture needle (small arrows) accurately entered the synovial tissue (large arrow). 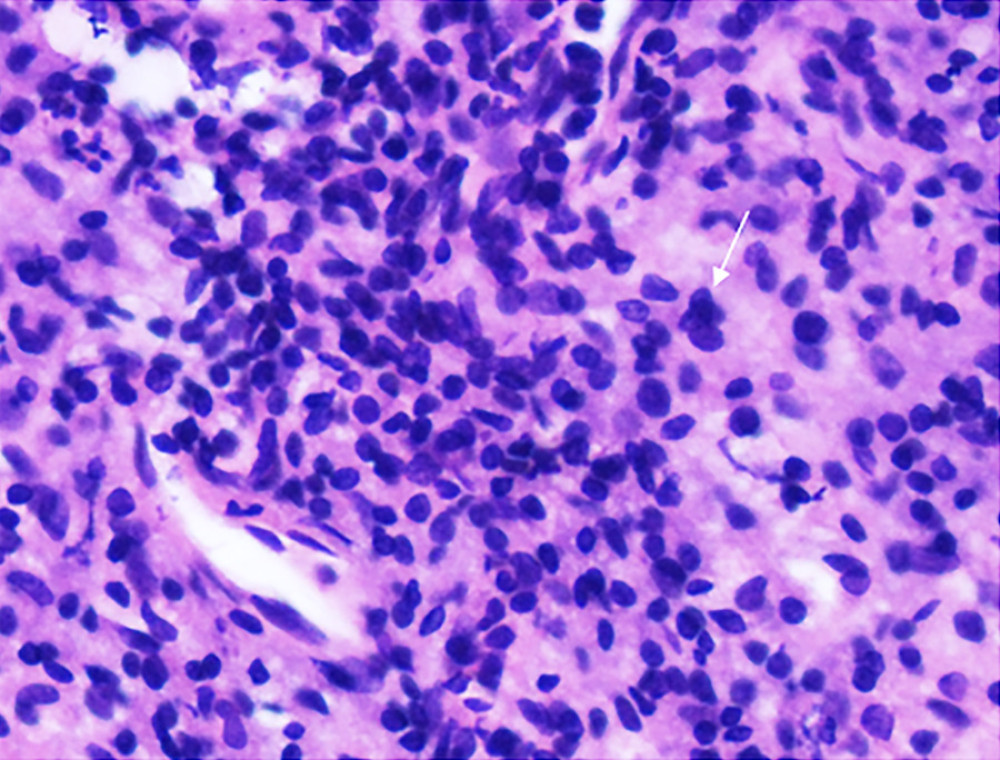 Figure 5. Pathology of synovial biopsy. Representative image showing neutrophil (small arrow) infiltration in grade 3 synovitis.
Figure 5. Pathology of synovial biopsy. Representative image showing neutrophil (small arrow) infiltration in grade 3 synovitis. Tables
Table 1. Comparison of synovial thickness pre- and postcontrast (paired t test).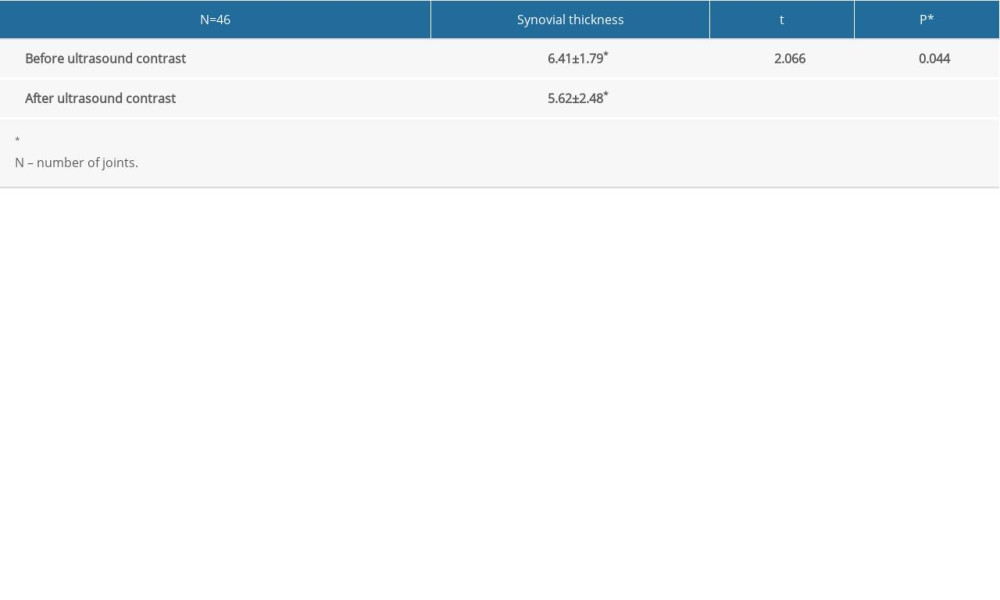 Table 2. Comparative diagnosis of sensitivity, specificity, accuracy, negative predictive value, and positive predictive value between Doppler ultrasound and contrast-enhanced ultrasound.
Table 2. Comparative diagnosis of sensitivity, specificity, accuracy, negative predictive value, and positive predictive value between Doppler ultrasound and contrast-enhanced ultrasound.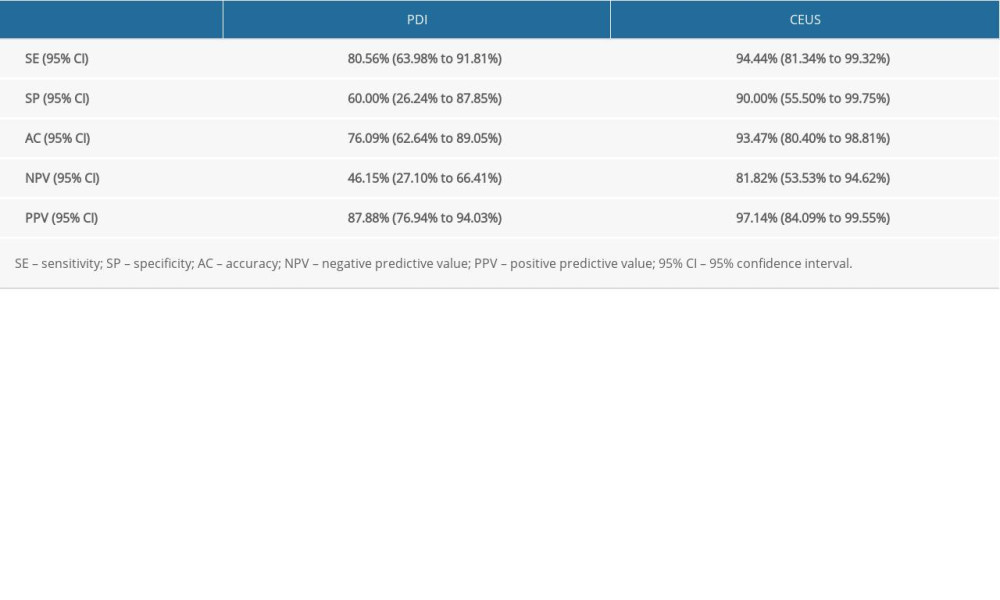 Table 3. Comparative diagnosis of knee joint synovitis by CEUS and standard clinical pathology (N: number of joints).
Table 3. Comparative diagnosis of knee joint synovitis by CEUS and standard clinical pathology (N: number of joints).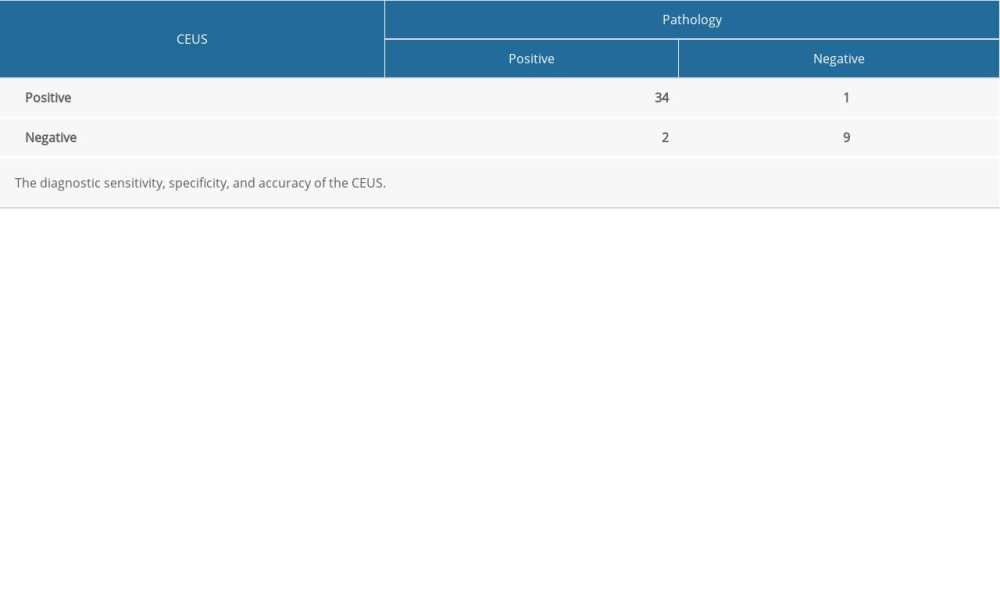 Table 4. Diagnosis of knee joint synovitis by Doppler ultrasonography (number of knee joints), using pathology as the criterion standard.
Table 4. Diagnosis of knee joint synovitis by Doppler ultrasonography (number of knee joints), using pathology as the criterion standard.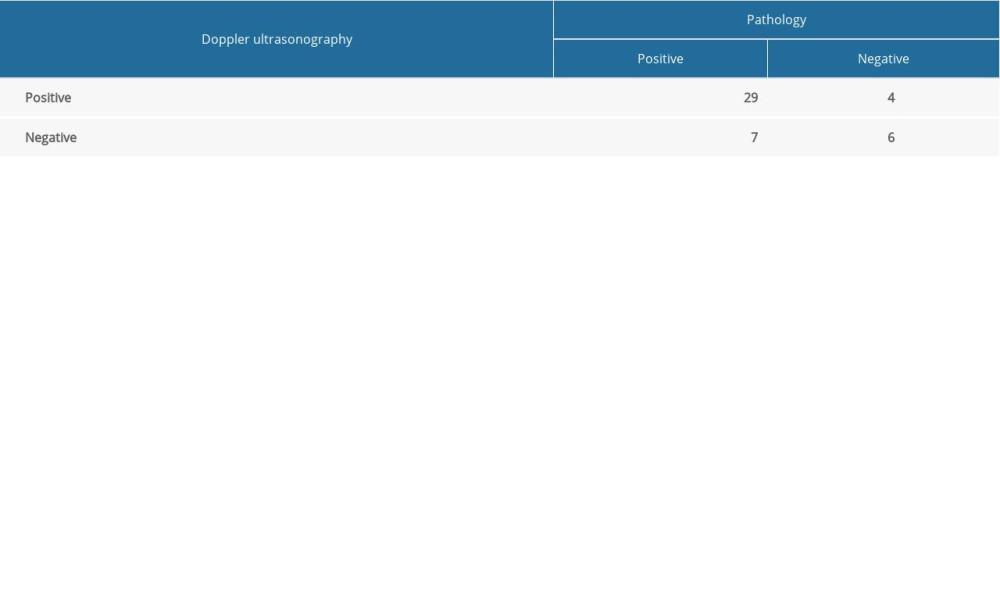
References
1. Singh JA, Furst DE, Bharat A, 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis: Arthritis Care Res, 2012; 64(5); 625-39
2. Felson DT, Smolen JS, Wells G, American College of Rheumatology/European League against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials: Ann Rheum Dis, 2011; 70(3); 404-13
3. Nell V, Machold K, Eberl G, Benefit of very early referral and very early therapy with disease-modifying anti-rheumatic drugs in patients with early rheumatoid arthritis: Rheumatology (Oxford), 2004; 43(7); 906-14
4. Ostergaard M, Stoltenberg M, Løvgreen-Nielsen P, Magnetic resonance imaging-determined synovial membrane and joint effusion volumes in rheumatoid arthritis and osteoarthritis: Comparison with the macroscopic and microscopic appearance of the synovium: Arthritis Rheum, 1997; 40(10); 1856-67
5. Ostergaard M, Hansen M, Stoltenberg M, Magnetic resonance imaging-determined synovial membrane volume as a marker of disease activity and a predictor of progressive joint destruction in the wrists of patients with rheumatoid arthritis: Arthritis Rheum, 1999; 42(5); 918-29
6. Blomley M, Cooke J, Unger E, Microbubble contrast agents: A new era in ultrasound: BMJ, 2001; 322(7296); 1222-25
7. D’Agostino MA, Terslev L, Wakefield R, Novel algorithms for the pragmatic use of ultrasound in the management of patients with rheumatoid arthritis: From diagnosis to remission: Ann Rheum Dis, 2016; 75(11); 1902-8
8. Saleem B, Brown A, Quinn M, Can flare be predicted in DMARD treated RA patients in remission, and is it important? A cohort study: Ann Rheum Dis, 2012; 71(8); 1316-21
9. Cai XH, Yang SP, Shen HL, Application of contrast-enhanced ultrasonography and ultrasonography scores in rheumatoid arthritis: Int J Clin Exp Med, 2015; 8(11); 20056-64
10. Zhao C, Jiang Y, Li J, Role of contrast-enhanced ultrasound in the evaluation of inflammatory arthritis: Chin Med J, 2017; 130(14); 1722-30
11. Haavardsholm EA, Aga AB, Olsen IC, Ultrasound in management of rheumatoid arthritis: ARCTIC randomised controlled strategy trial: BMJ, 2016; 354; i4205
12. de Hair MJ, van de Sande MG, Ramwadhdoebe TH, Features of the synovium of individuals at risk of developing rheumatoid arthritis: Implications for understanding preclinical rheumatoid arthritis: Arthritis Rheumatol, 2014; 66(3); 513-22
13. Pincus T, Yazici Y, Sokka T, Complexities in assessment of rheumatoid arthritis: Absence of a single gold standard measure: Rheum Dis Clin North Am, 2009; 35(4); 687-97
14. Chen S, Zheng Q, Liu H, Sonography is superior to serum-based biomarkers for measuring disease status in experimental rheumatoid arthritis: J Ultrasound Med, 2016; 35(10); 2223-30
15. Glynn LE, Experimental model of rheumatoid arthritis: J Belge Rhumatol Med Phys, 1967; 22(4); 201-3
16. Atkinson EG, Dinning WJ, Kasp E, Precipitation of experimental autoallergic uveoretinitis by cyclosporin A withdrawal: An experimental model of uveitis relapse: Clin Exp Immunol, 1989; 78(1); 108-14
17. Szkudlarek M, Court-Payen M, Jacobsen S, Interobserver agreement in ultrasonography of the finger and toe joints in rheumatoid arthritis: Arthritis Rheum, 2003; 48(4); 955-62
18. Klauser A, Demharter J, De Marchi A, Contrast enhanced gray-scale sonography in assessment of joint vascularity in rheumatoid arthritis: Results from the IACUS study group: Eur Radiol, 2005; 15(12); 2404-10
19. Krenn V, Morawietz L, Burmester GR, Synovitis score: Discrimination between chronic low-grade and high-grade synovitis: Histopathology, 2006; 49(4); 358-64
20. Chakr RM, Mendonca JA, Brenol CV, Assessing rheumatoid arthritis disease activity with ultrasound: Clin Rheumatol, 2013; 32(9); 1249-54
21. Lei Z, Feng G, Xu N, Early extremity MRI findings and pathological synovial changes in antigen-induced arthritis rabbit model: J Magn Reson Imaging, 2014; 39(6); 1366-73
22. Qiu L, Jiang Y, Luo Y, Antigen-induced arthritis in rabbits: A comparative study between high-resolution ultrasound and contrast-enhanced ultrasound and pathologic findings: Rheumatol Int, 2012; 32(6); 1569-80
23. Wang K, Amirabadi A, Moineddin R, Longitudinal assessment of bone loss using quantitative ultrasound in a blood-induced arthritis rabbit model: Haemophilia, 2015; 21(5); e402-10
24. Balsa ADefining remission in Rheumatoid Arthritis. New ACR/EULAR criteria: Reumatol Clin, 2011; 6S3; S12-15 [in Spanish]
25. Schleich C, Buchbender C, Sewerin P, Evaluation of a simplified version of the Rheumatoid Arthritis Magnetic Resonance Imaging Score (RAMRIS) comprising 5 joints (RAMRIS5): Clin Exp Rheumatol, 2015; 33(2); 209-15
26. Ramirez J, Celis R, Usategui A, Immunopathologic characterization of ultrasound-defined synovitis in rheumatoid arthritis patients in clinical remission: Arthritis Res Ther, 2016; 18; 74
27. Aga A, Hammer H, Olsen I, First step in the development of an ultrasound joint inflammation score for rheumatoid arthritis using a data-driven approach: Ann Rheum Dis, 2016; 75(8); 1444-51
28. Aga A, Berner Hammer H, Christoffer Olsen I, Development of a feasible and responsive ultrasound inflammation score for rheumatoid arthritis through a data-driven approach: RMD Open, 2016; 2(2); e000325
29. Dale J, Stirling A, Zhang R, Targeting ultrasound remission in early rheumatoid arthritis: The results of the TaSER study, a randomised clinical trial: Ann Rheum Dis, 2016; 75(6); 1043-50
30. Baillet A, Gaujoux-Viala C, Mouterde G, Comparison of the efficacy of sonography, magnetic resonance imaging and conventional radiography for the detection of bone erosions in rheumatoid arthritis patients: A systematic review and meta-analysis: Rheumatology (Oxford), 2011; 50(6); 1137-47
31. Stramare R, Raffeiner B, Ciprian L, Evaluation of finger joint synovial vascularity in patients with rheumatoid arthritis using contrast-enhanced ultrasound with water immersion and a stabilized probe: J Clin Ultrasound, 2012; 40(3); 147-54
32. Platzgummer H, Schueller G, Grisar J, Quantification of synovitis in rheumatoid arthritis: Do we really need quantitative measurement of contrast-enhanced ultrasound? Eur J Radiol, 2009; 71(2); 237-41
33. De Zordo T, Mlekusch SP, Feuchtner GM, Value of contrast-enhanced ultrasound in rheumatoid arthritis: Eur J Radiol, 2007; 64(2); 222-30
34. Freeston JE, Brown AK, Hensor EM, Extremity magnetic resonance imaging assessment of synovitis (without contrast) in rheumatoid arthritis may be less accurate than power Doppler ultrasound: Ann Rheum Dis, 2008; 67(9); 1351
35. Vreju A, Chisalau B, Parvanescu C, High frequency ultrasonography of the hand in rheumatoid arthritis, psoriatic arthritis, gout and osteoarthritis patients, 2016; 42(1); 35-39
36. Wang M, Wang X, Sun X, Diagnostic value of high-frequency color Doppler ultrasonography examination in combination with anti-cyclic citrullinated peptide antibody testing in rheumatoid arthritis patients: Exp Ther Med, 2017; 13(3); 905-8
37. Wu CM, Chen YC, Hsieh KS, Texture features for classification of ultrasonic liver images: IEEE Trans Med Imaging, 1992; 11(2); 141-52
38. Keramida G, Siddique M, Cook G, Peters A, Texture analysis of the liver to detect steatohepatitis: J Nucl Med, 2016; 57
39. Bonet-Carne E, Palacio M, Cobo T, Quantitative ultrasound texture analysis of fetal lungs to predict neonatal respiratory morbidity: Ultrasound Obstet Gynecol, 2015; 45(4); 427-33
40. Baños N, Perez-Moreno A, Migliorelli F, Quantitative analysis of the cervical texture by ultrasound and correlation with gestational age: Fetal Diagn Ther, 2017; 41(4); 265-72
41. Nieniewski M, Chmielewski LJ, Study of classification of breast lesions using texture GLCM features obtained from the raw ultrasound signal: Image Analysis & Stereology, 2020; 39(2); 129-45
42. Patil P, Dasgupta BJT, Role of diagnostic ultrasound in the assessment of musculoskeletal diseases: Ther Adv Musculoskelet Dis, 2012; 4(5); 341-55
43. Filkova M, Vernerova Z, Hulejova H, Pro-inflammatory effects of interleukin-35 in rheumatoid arthritis: Cytokine, 2015; 73(1); 36-43
44. Imaoka A, Zhang L, Kuboyama N, Abiko Y, Reduction of IL-20 expression in rheumatoid arthritis by linear polarized infrared light irradiation: Laser Ther, 2014; 23(2); 109-14
45. Capellino S, Cosentino M, Luini A, Increased expression of dopamine receptors in synovial fibroblasts from patients with rheumatoid arthritis: Inhibitory effects of dopamine on interleukin-8 and interleukin-6: Arthritis Rheumatol, 2014; 66(10); 2685-93
Figures
 Figure 1. Synovial ultrasound contrast. Synovial enhancement (small arrow) was significantly greater than the surrounding tissue (large arrow).
Figure 1. Synovial ultrasound contrast. Synovial enhancement (small arrow) was significantly greater than the surrounding tissue (large arrow). Figure 2. Synovial membrane grade 2 thickening. Thickening of the synovial tissue reached the joint head (small arrow), but did not reach the diaphysis (big arrow).
Figure 2. Synovial membrane grade 2 thickening. Thickening of the synovial tissue reached the joint head (small arrow), but did not reach the diaphysis (big arrow). Figure 3. Synovial grade 3 blood flow signal. Blood flow was detected through the synovium.
Figure 3. Synovial grade 3 blood flow signal. Blood flow was detected through the synovium. Figure 4. Ultrasound-guided synovial puncture. 18-G puncture needle (small arrows) accurately entered the synovial tissue (large arrow).
Figure 4. Ultrasound-guided synovial puncture. 18-G puncture needle (small arrows) accurately entered the synovial tissue (large arrow). Figure 5. Pathology of synovial biopsy. Representative image showing neutrophil (small arrow) infiltration in grade 3 synovitis.
Figure 5. Pathology of synovial biopsy. Representative image showing neutrophil (small arrow) infiltration in grade 3 synovitis. Tables
 Table 1. Comparison of synovial thickness pre- and postcontrast (paired t test).
Table 1. Comparison of synovial thickness pre- and postcontrast (paired t test). Table 2. Comparative diagnosis of sensitivity, specificity, accuracy, negative predictive value, and positive predictive value between Doppler ultrasound and contrast-enhanced ultrasound.
Table 2. Comparative diagnosis of sensitivity, specificity, accuracy, negative predictive value, and positive predictive value between Doppler ultrasound and contrast-enhanced ultrasound. Table 3. Comparative diagnosis of knee joint synovitis by CEUS and standard clinical pathology (N: number of joints).
Table 3. Comparative diagnosis of knee joint synovitis by CEUS and standard clinical pathology (N: number of joints). Table 4. Diagnosis of knee joint synovitis by Doppler ultrasonography (number of knee joints), using pathology as the criterion standard.
Table 4. Diagnosis of knee joint synovitis by Doppler ultrasonography (number of knee joints), using pathology as the criterion standard. Table 1. Comparison of synovial thickness pre- and postcontrast (paired t test).
Table 1. Comparison of synovial thickness pre- and postcontrast (paired t test). Table 2. Comparative diagnosis of sensitivity, specificity, accuracy, negative predictive value, and positive predictive value between Doppler ultrasound and contrast-enhanced ultrasound.
Table 2. Comparative diagnosis of sensitivity, specificity, accuracy, negative predictive value, and positive predictive value between Doppler ultrasound and contrast-enhanced ultrasound. Table 3. Comparative diagnosis of knee joint synovitis by CEUS and standard clinical pathology (N: number of joints).
Table 3. Comparative diagnosis of knee joint synovitis by CEUS and standard clinical pathology (N: number of joints). Table 4. Diagnosis of knee joint synovitis by Doppler ultrasonography (number of knee joints), using pathology as the criterion standard.
Table 4. Diagnosis of knee joint synovitis by Doppler ultrasonography (number of knee joints), using pathology as the criterion standard. In Press
05 Mar 2024 : Clinical Research
Effects of Thermal Insulation on Recovery and Comfort of Patients Undergoing Holmium Laser LithotripsyMed Sci Monit In Press; DOI: 10.12659/MSM.942836
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








