28 August 2021: Clinical Research
Characteristics of the Computed Tomography Imaging Findings in 72 Patients with Airway-Invasive Pulmonary Aspergillosis
Jing Wu1ABCDEF, Tao Zhang1BCDEF, Junping Pan2ABCD, Qian Zhang3B, Xin Lin4B, Ligong Chang5B, Yu-Chen Chen1ACDF*, Xinying Xue6ADFDOI: 10.12659/MSM.931162
Med Sci Monit 2021; 27:e931162
Abstract
BACKGROUND: This multiple-center retrospective study aimed to investigate computed tomography (CT) imaging findings in 72 patients with airway-invasive pulmonary aspergillosis.
MATERIAL AND METHODS: Seventy-two patients with airway-invasive pulmonary aspergillosis confirmed by pathology results were divided into 3 types according to image characteristics. Type I involved the trachea or the main bronchus. Type II involved the lobular and segmental bronchi, which manifested early as bronchial wall thickening, and later development was divided into types IIa and IIb. Type IIa manifested as bronchiectasis, and type IIb manifested as consolidation around the bronchus. Type III involved the bronchioles and pulmonary parenchyma, with tree-in-bud sign and acinar nodules around. CT signs of the various types and their differentiation were investigated.
RESULTS: The main clinical manifestations of the 72 patients with airway-invasive pulmonary aspergillosis were shortness of breath (55/72, 76.4%), cough (40/72, 55.6%), expectoration (35/72, 48.6%), dyspnea (8/72, 11.1%), weight loss (2/72, 2.8%), and fever (30/72, 41.7%). CT typing identified 3 types: 2 patients (2.8%) had type I, presenting as thickening of trachea or main bronchial walls; 3 patients (4.2%) had early type II, manifesting as thickening of lobular or segmental bronchial walls; 27 patients (37.5%) developed type IIa, manifesting as bronchiectasis; 22 patients (30.6%) had type IIb, manifesting as consolidation around the bronchus; and 18 patients (25.0%) had type III, presenting as nodules and patchy shadows with small cavities in the periphery of the lung.
CONCLUSIONS: Airway pulmonary aspergillosis has characteristic imaging findings, which can help early clinical diagnosis through classification according to CT imaging characteristics.
Keywords: Classification, invasive pulmonary aspergillosis, multidetector computed tomography, Aged, 80 and over, Female, Follow-Up Studies, Humans, Immunocompromised Host, Tomography, X-Ray Computed, young adult
Background
There are 4 types of aspergillosis: (1) simple aspergilloma; (2) chronic pulmonary aspergillosis, including chronic cavitary pulmonary aspergillosis, chronic necrotizing pulmonary aspergillosis, and chronic fibrotic aspergillosis; (3) allergic bronchial pulmonary aspergillosis (ABPA); and (4) invasive pulmonary aspergillosis (IPA), which includes angio-invasive aspergillosis and airway-invasive aspergillosis [6].
Invasive pulmonary aspergillosis is a severe disease generally observed in immunocompromised patients, such as those with hematological malignancies and neutropenia, bone-marrow transplantation, and solid-organ transplantation, or those undergoing immune suppressive therapy, such as corticosteroid therapy [7]. Airway IPA is a subtype of IPA, and reports on it are limited. Airway-invasive aspergillosis, like the angio-invasive form, occurs in immunocompromised patients [8], but the types have different risk factors. In recent years, with the increase of bronchoscopy, the disease has been gradually recognized and is receiving more clinical attention. In addition to the aging of the population, an increasing number of people have senile diseases, such as diabetes and chronic obstructive pulmonary disease (COPD), which damage the host immune system, and the decline of immune function increases infections with pulmonary aspergillosis, especially airway-invasive aspergillosis [3]. Although airway-invasive aspergillosis is well recognized in the pathologic and clinical literature, it has received little attention in the radiologic literature. There are few articles on the typing of imaging findings of airway-invasive aspergillosis alone. The current medical environment urgently needs a new and more detailed imaging typing system of airway-invasive aspergillosis to guide clinicians and improve the early diagnosis and survival rate.
There are many differences between airway-invasive aspergillosis and angio-invasive aspergillosis, including risk factors, clinical symptoms, and diagnostic methods or imaging [9]. Therefore, it is necessary to discuss them separately. In the past, there were few reports on airway-invasive aspergillosis, which can cause misdiagnosis of the disease. Airway pulmonary aspergillosis is commonly misdiagnosed clinically as a common bacterial infection or tuberculosis, which leads to delays in treatment. In this article, we are the first to propose the classification of airway IPA through imaging. We discuss the imaging manifestations of airway-invasive aspergillosis by subtypes, which can help increase the early diagnosis rate and reduce the misdiagnosis rate. At the same time, the reexamination of CT scans helps us to evaluate the effect of treatment and guide treatment.
Material and Methods
STATISTICAL ANALYSIS:
Clinical concomitant diseases, risk factors, and clinical symptoms were calculated as percentages. The imaging features were analyzed using percentage statistics.
Results
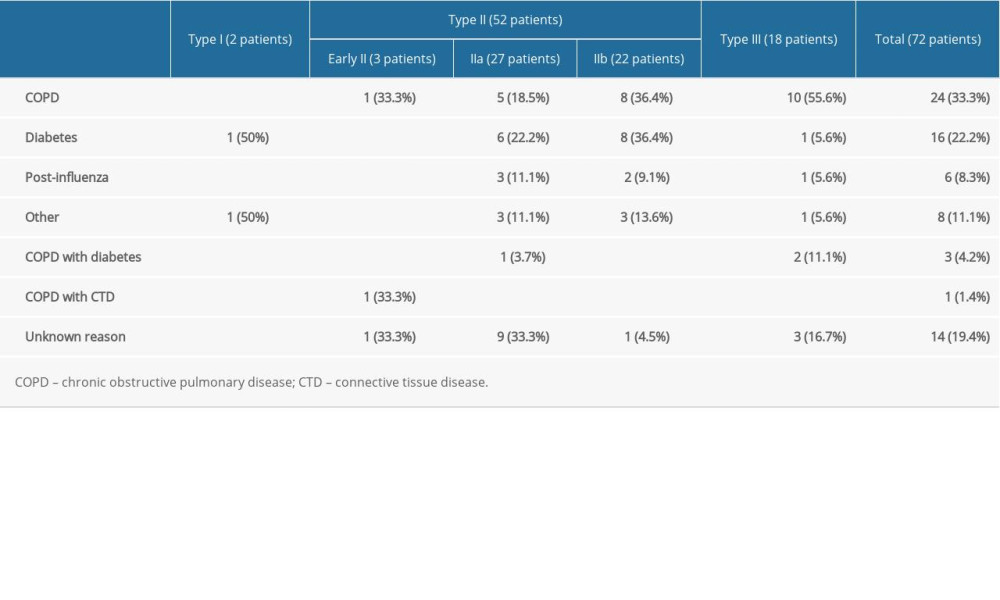
Among the 72 patients with airway IPA, there were 51 men and 21 women, aged from 22 to 88 years, with an average age of 57.97±13.73. COPD was found in 24 patients (33.3%), diabetes in 16 patients (22.2%), previous influenza infection in 6 patients (8.3%), “other” in 8 patients (including hematological diseases without chemotherapy, occupational disease history, connective tissue disease with use of hormone therapy, renal insufficiency treated by hemodialysis, HIV infection, and bronchial dysplasia), COPD complicated by diabetes in 3 patients (4.2%), COPD complicated by connective tissue disease in 1 patient (1.4%), and unknown causes in 14 patients (19.4%). Table 1 shows the history of each type of airway-invasive aspergillosis. The main clinical manifestations of the patients were shortness of breath (55/72, 76.4%), cough (40/72, 55.6%), expectoration (35/72, 48.6%), dyspnea (8/72, 11.1%), weight loss (2/72, 2.8%), and fever (30/72, 41.7%). Shortness of breath was the most typical symptom.
The images were typed into 3 types according to the different manifestations of airway-invasive aspergillosis identified on the CT scans (Figure 1).
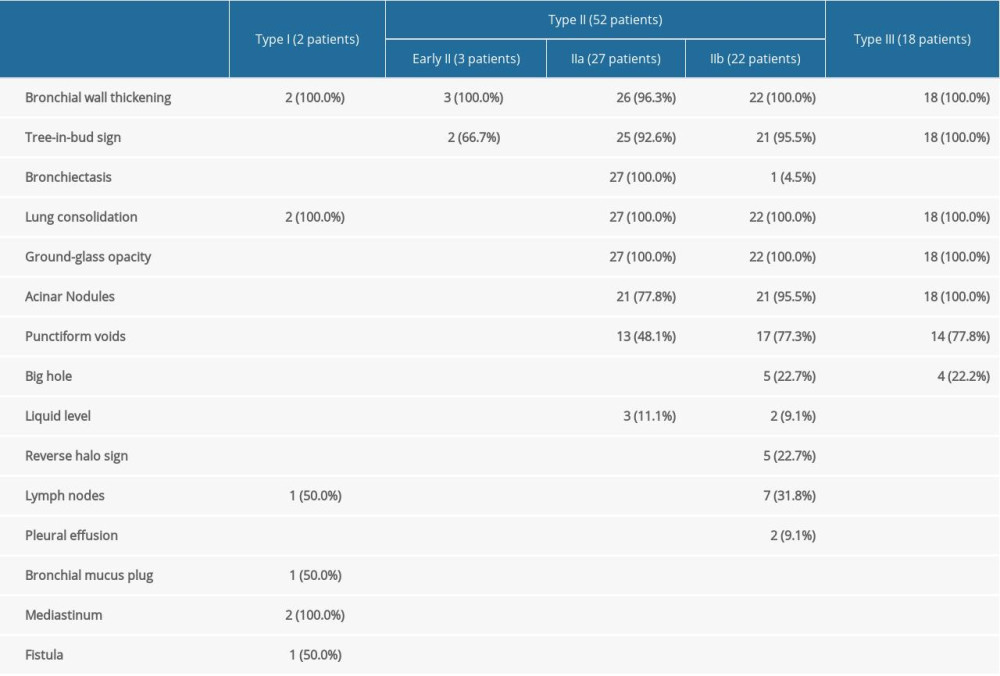
Type I (2 patients, 2.8%) (Figure 2) presented as thickening of the trachea or left/right main bronchial walls. The accompanying signs included mediastinum involvement, such as mediastinal emphysema, obstructive atelectasis, tree-in-bud sign, and mucus plug in the bronchi.
Type II involved the lobular and segmental bronchi, which manifested as bronchial wall thickening in the early stage (3 patients, 4.2%). The patients’ CT images showed diffuse thickening of the lobular and segmental bronchus accompanied by peribronchial ground-glass opacities or tree-in-bud signs and acinar nodules at the distal end of the bronchus. If not detected and treated in the early stage, the lesions continued to infiltrate around the bronchus, manifesting as types IIa or IIb. Bronchiectasis was the most typical manifestation of type IIa (27 patients, 37.5%) (Figures 3, 4). The wall of the bronchus was thickened, but peripheral consolidation was not obvious. After treatment, the dilated bronchus gradually recovered and partly returned to normal. Type IIb in 22 patients (30.6%) manifested as thickening of the bronchial wall with surrounding tissue consolidation (Figure 5). The peribronchial consolidation was predominant in the development, but bronchiectasis was not obvious. The accompanying signs included tree-in-bud sign, acinar nodules, ground-glass opacity, and reversed halo sign, a special sign showing as a central ground-glass opacity surrounded by a more or less complete ring of consolidation on CT. The reversed halo sign might be found in this type, and in the late stage, it would develop to encompass the cavity.
Type III involved the bronchioles and pulmonary parenchyma (18 patients, 25.0%) (Figure 6). The bronchial wall was slightly thickened, and there were tree-in-bud signs and acinar nodules in the periphery. With the development of the disease, consolidation and ground-glass opacities formed by the fusion of multiple nodules, which were mainly distributed subpleurally, with tree-in-bud signs and acinar nodules. Also, multiple punctate necrosis and small cavities could be seen in the consolidation. Table 2 shows the imaging features of airway IPA. Although Figures 4 and 6 seem to be similar on the surface, with both showing low-density shadows, they are completely different. Figure 4 shows bronchiectasis, which belongs to type IIA, while Figure 6 demonstrates lesions within the cavity. It is not difficult to distinguish between these 2 types through continuous layer observation or multi-planar reconstruction.
Discussion
Of the 72 patients included in this study, 33.3% of patients had COPD, 22.2% had diabetes, and 8.3% had previous influenza infection. Our results were consistent with other studies reporting that COPD, diabetes, and previous influenza infection were most common risk factors in patients [13,14], but they were inconsistent with other reports [15–18] in which most of the risk factors summarized in the reports were neutrophil deficiency, blood disease, AIDS, chemotherapy, and organ transplantation; however, the reports did not distinguish between angio-invasive and airway-invasive aspergillosis. In addition, most of our patient cases were provided by the respiratory departments of the hospitals, which excluded angio-IPA in patients with particle deficiency; therefore, most patient risk factors in our research were COPD, diabetes, and previous influenza infection. In recent years, many reports have identified COPD as an underlying disease related to IPA [19]. The susceptibility of patients with COPD to invasive fungal infections can be due to ciliated dysfunction of airway epithelial cells. The long-term use of corticosteroids inhibits the function of alveolar macrophages and neutrophils, the use of broad-spectrum antibiotics exposes the body to certain fungal pathogens, and lung disease leads to changes in lung structure and malnutrition [20]. In addition, in some patients with COPD, abnormalities or defects in surfactant proteins, alveolar macrophages, and Toll-like receptors play an important role [21]. Huang et al reported that patients with COPD had a sufficient number of neutrophils compared with patients with neutrophil deficiency, and that their lung damage was mainly due to host overreaction rather than direct invasion by fungi [22]. Previously, diabetes was rarely associated with IPA, and only a few patients were reported [23,24]. These patients have more serious clinical manifestations and are prone to treatment failure, recurrence, and even death [25]. Most patients with subacute or chronic pulmonary aspergillosis have been thought to have potential diabetes; therefore, diabetes was considered to be an important risk factor for pulmonary aspergillosis [20,26]. Patientswith diabetes often have weight loss and decreased albumin levels, leading to malnutrition and decreased immunity and increasing the chance of
In the present study, the main clinical manifestations of the patients were shortness of breath, cough, expectoration, dyspnea, weight loss, and fever. Shortness of breath was the most common symptom (76.4%), which was generally consistent with the previous reports [16,18,30,31]. The symptom of shortness of breath can be associated with
The clinical classification of aspergillosis includes 4 types: simple aspergilloma, chronic pulmonary aspergillosis, ABPA, and IPA. IPA as the most serious type with high mortality includes angio-invasive aspergillosis and airway-invasive aspergillosis. The 2 have significant differences in risk factors, clinical manifestations, imaging features, and prognosis. The clinical literature is increasingly concerned with the airway invasion of
Type I referred to lesions mainly invading the trachea and the main bronchus, and might have also involved the distal end, showing that the wall of the trachea and the main bronchus were thickened. This type was rare, accounting for 2.8% of cases, suggesting that it is mainly related to the clearance function of atmospheric duct cilia and the defense function of inflammatory cells, and fungi do not easily invade there. Due to the small number of cases of this type, its image characteristics need to be summarized in further research with a larger sample size. Type I sometimes needs to be distinguished from ABPA and bronchial tuberculosis. ABPA bronchiectasis is more obvious, there are more mucus plugs, the density is higher, and the outer wall of the bronchus is clear, and the extramural invasion is milder, but the most important difference is that the clinical manifestations are different and that ABPA has specific diagnostic criteria. The outer wall of the bronchial tuberculosis is clear. The adjacent fat space is relatively clear. The bronchial wall is irregularly thickened and the inner edge and thickness of the lumen are uneven. Other lesions in the lung can be accompanied by calcification and tree-in-bud sign, and ground-glass opacity is rare. Type I aspergillosis is rare; therefore, imaging diagnosis should be prudent, and the most important examination is bronchoscopy.
Type II mainly involved the lobular and segmental bronchi. In the early stage of type II, only the lobular and segmental bronchial wall was thickened, and it was difficult to consider early diagnosis. In the late stage of follow-up, type IIa and type IIb were found. Both types mainly involved lobular and segmental bronchi; type IIa was associated with obvious bronchiectasis, and type IIb infiltrated along the periphery of the bronchus without obvious bronchiectasis. These 2 types were most common, accounting for 37.5% and 30.6% respectively. Identifying these 2 types helped us diagnose the disease early. The specificity of this type of image is high, and the differential diagnosis is relatively easy to perform. Organizing pneumonia, lymphoma, and bacterial infections, such as
Type III involved the bronchioles and pulmonary parenchyma, pathological changes in the terminal bronchus, image findings of mainly tree-in-bud signs, and image findings of respiratory bronchus as acinar nodules. Along with the surrounding lung tissue invasion, type III can include fused pieces of ground-glass opacities and consolidation and easily formed cavities because of the necrosis of the lung tissue caused by aspergillosis [14]. Type III was also very common, accounting for 25.0% of cases. In the early stage of type III airway-invasive aspergillosis, the acinar nodules need to be differentiated from those of blood-disseminated
Thickening of the trachea/main bronchial wall was the most important feature of type I, which was different from the other types. Thickening of lobular/segmental bronchial walls and bronchiectasis were the most characteristic imaging findings of type II, which could be distinguished from the other types. Tree-in-bud sign and acinar nodules were the most characteristic imaging findings of type III. In summary, the large airways have strong defense capabilities, and it is difficult for
Imaging manifestations can sometimes explain clinical symptoms. For example, imaging manifestations were mainly bronchial wall thickening, and the clinical symptoms were mainly shortness of breath, rather than sputum expectoration (involving the lung parenchyma). As the image spread to the lung parenchyma and formed consolidation in the lung, the clinical symptoms were aggravated and sputum expectoration and even fever could appear. When necrosis and voids formed, symptoms of hemoptysis could also appear. At the same time, the different types suggested that the method for obtaining pathological specimens in the clinic was also different. For example, type I and type II lesions were mainly obtained by bronchoscopy, while type III lesions were mainly located in the periphery of the lung, and pathological specimens were mainly obtained by puncture. Therefore, typing is not only helpful for early diagnosis of imaging, but also helps to indicate the method of obtaining pathological specimens. In addition, airway-invasive aspergillosis should be differentiated from angio-invasive aspergillosis, and imaging manifestations have a certain value for the identification of both. Angio-invasive aspergillosis mainly manifests as subpleural nodules with a clear boundary and central cavity. Generally, the trachea and bronchi are not invaded, so the walls of the trachea and bronchi are not thickened.
In summary, various imaging manifestations such as bronchial wall thickening, tree-in-bud signs, and acinar nodules represent the bronchial invasion after the inhalation of
There are several limitations of this paper. First, only CT findings and types of airway-invasive aspergillosis were analyzed, and there was no control group, especially important for angio-invasive aspergillosis. Second, the sample volume was insufficient, and because airway aspergillosis is rare, there were few patients with type I, which is less common than other types of aspergilloses. In a future study, we will collect more cases and use the classification method in this paper to compare angio-invasive aspergillosis with airway-invasive aspergillosis to observe whether the imaging diagnosis is consistent with the clinical diagnosis. Finally, certain errors may have occurred because airway-invasive aspergillosis is rare, the data we collected came from different hospitals, and the hospitals used different equipment.
In this study, we discussed the imaging manifestations of airway-invasive aspergillosis by subtypes. The subtypes were mainly divided according to the location of the lesions involving the bronchus. Type I referred to the lesions mainly invading the trachea and main bronchus, which was the least common type, with bronchial walls having uniform thickening. Type II referred to lesions mainly involving the lobular and segmental bronchi. In the early stage, the bronchus wall was thickened, and in the later period, it could infiltrate the surrounding lung tissue or appear as bronchiectasis. Type III mainly involved the bronchioles and pulmonary parenchyma, with tree-in-bud signs and acinar nodules in the periphery. With the development of the disease, consolidation and ground-glass opacity, with multiple punctate necrosis and small cavities could be found.
Conclusions
In conclusion, the most important risk factors of airway-invasive aspergillosis are COPD, diabetes, and previous influenza infection. These diseases can lead to the decrease of immunity in varying degrees, thus increasing the infection rate and incidence of aspergillosis. Although the clinical manifestations varied, shortness of breath was most common. The CT appearance of airway-invasive aspergillosis was divided into 3 types according to the location of the bronchus involved and the imaging findings, including distribution, bronchial wall thickening, bronchiectasis, consolidation around the bronchus, peripheral alveolar nodules, consolidation, and cavities. Combining risk factors with clinical symptoms can help increase the early diagnosis rate, reduce the misdiagnosis rate, and assess the progress of the disease. Also, repeat CT scanning can help to evaluate the therapeutic effect and guide treatment.
Figures
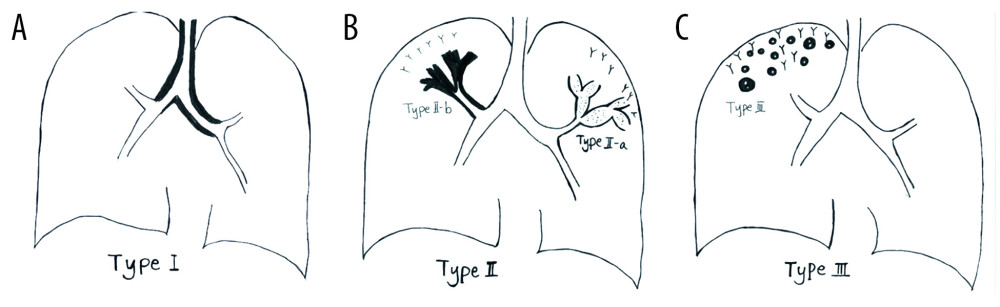 Figure 1. Schematic diagram of different types of airway-invasive pulmonary aspergillosis. (A) Type I: mainly invading the trachea and the main bronchus and the wall of the trachea, and the main bronchus is thickening. (B) Type II mainly involves the lobular and segmental bronchi. In the early stages of type II, only the bronchial wall is thickened. In the late stage of follow-up, type IIa or type IIb can be found. Type IIa is associated with obvious bronchiectasis, and type IIb mainly infiltrates along the periphery of the bronchus without obvious bronchiectasis. (C) Type III involves the bronchioles and pulmonary parenchyma, manifested as peripheral bronchioles and acinar nodules. As the course of the disease progresses, the nodules fuse to form patches of consolidation, with small cavities often appearing inside.
Figure 1. Schematic diagram of different types of airway-invasive pulmonary aspergillosis. (A) Type I: mainly invading the trachea and the main bronchus and the wall of the trachea, and the main bronchus is thickening. (B) Type II mainly involves the lobular and segmental bronchi. In the early stages of type II, only the bronchial wall is thickened. In the late stage of follow-up, type IIa or type IIb can be found. Type IIa is associated with obvious bronchiectasis, and type IIb mainly infiltrates along the periphery of the bronchus without obvious bronchiectasis. (C) Type III involves the bronchioles and pulmonary parenchyma, manifested as peripheral bronchioles and acinar nodules. As the course of the disease progresses, the nodules fuse to form patches of consolidation, with small cavities often appearing inside. 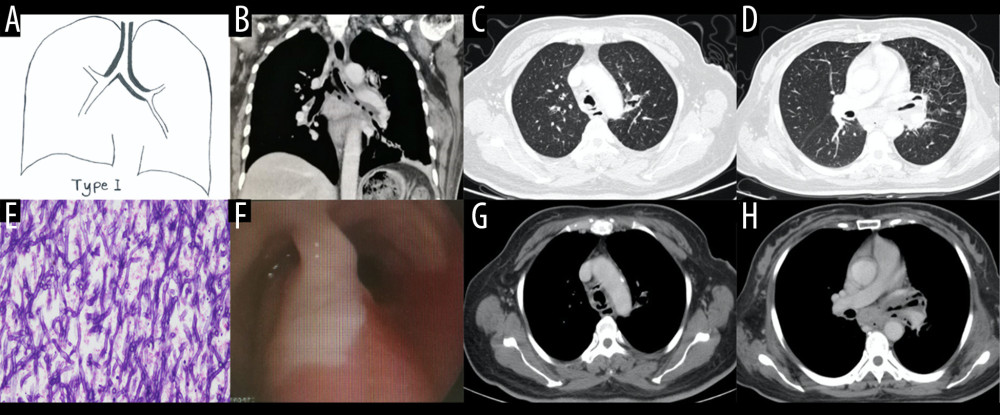 Figure 2. Case 1. A 48-year-old woman had cough and shortness of breath for 1 month, with a past history of diabetes mellitus. (A, B) The coronal view of the mediastinum, indicating major thickening along the left main bronchus, partial stenosis of the lumen, and inflammation of the distal lung tissue, which is similar to the schematic diagram. (C, D) Thickening at the walls of the trachea and left main bronchus in the lung window. (E) Pathological Aspergillus hyphae and small round spores (HE ×400). (F) The trachea and left main bronchus were covered by pus-like exudates via bronchoscopy. (G, H) Thickening of the wall of the bronchus, the soft tissue shadow in the mediastinum (inflammatory infiltration or lymph node enlargement) and extraluminal air, indicating the formation of mediastinitis and bronchial fistula.
Figure 2. Case 1. A 48-year-old woman had cough and shortness of breath for 1 month, with a past history of diabetes mellitus. (A, B) The coronal view of the mediastinum, indicating major thickening along the left main bronchus, partial stenosis of the lumen, and inflammation of the distal lung tissue, which is similar to the schematic diagram. (C, D) Thickening at the walls of the trachea and left main bronchus in the lung window. (E) Pathological Aspergillus hyphae and small round spores (HE ×400). (F) The trachea and left main bronchus were covered by pus-like exudates via bronchoscopy. (G, H) Thickening of the wall of the bronchus, the soft tissue shadow in the mediastinum (inflammatory infiltration or lymph node enlargement) and extraluminal air, indicating the formation of mediastinitis and bronchial fistula. 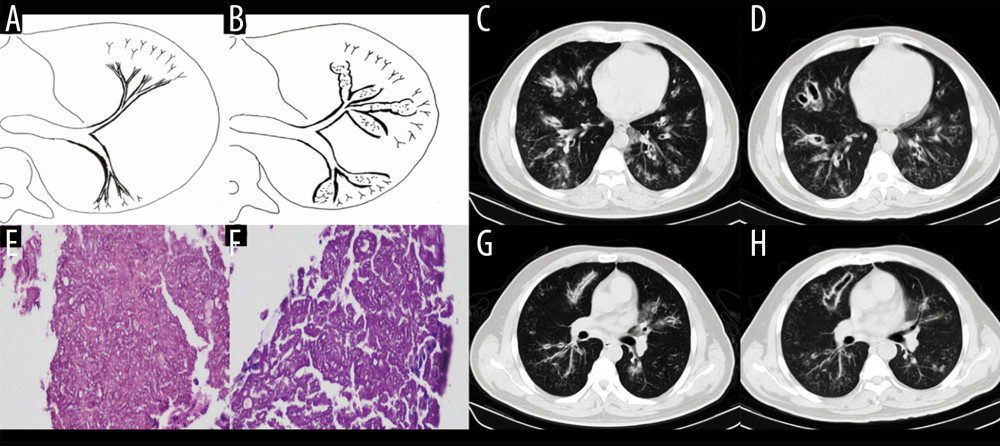 Figure 3. Case 2. A 43-year-old man had cough and shortness of breath for 10 days. (A, B) Schematic diagrams of type II airway-invasive pulmonary aspergillosis, which showed the early stage of type II to type IIa. (C, G) Computed tomography (CT) scan images taken at admission. As shown in the images, there was thickening of the lobar and segmental bronchus, diffuse tree-in-bud sign at the distal end, which was consistent with type II airway-invasion Aspergillus. Images (D) and (H) are CT scan images on the same slice with (C) and (G) after 7 days, showing bronchiectasis, which was consistent with type IIa airway-invasive aspergillosis. Image (E) shows pathological Aspergillus hyphae and small round spores, the hyphae were branched and segmented (HE ×200); (F) (HE ×400).
Figure 3. Case 2. A 43-year-old man had cough and shortness of breath for 10 days. (A, B) Schematic diagrams of type II airway-invasive pulmonary aspergillosis, which showed the early stage of type II to type IIa. (C, G) Computed tomography (CT) scan images taken at admission. As shown in the images, there was thickening of the lobar and segmental bronchus, diffuse tree-in-bud sign at the distal end, which was consistent with type II airway-invasion Aspergillus. Images (D) and (H) are CT scan images on the same slice with (C) and (G) after 7 days, showing bronchiectasis, which was consistent with type IIa airway-invasive aspergillosis. Image (E) shows pathological Aspergillus hyphae and small round spores, the hyphae were branched and segmented (HE ×200); (F) (HE ×400). 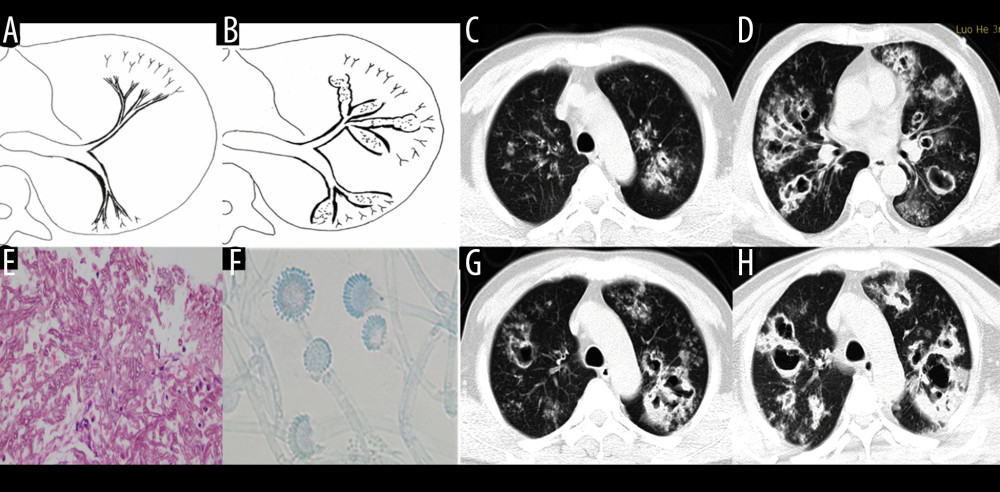 Figure 4. Case 3. A 46-year-old man was hospitalized with fever and shortness of breath for 1 week without a previous significant medical history. (A, B) Schematic diagrams of type II airway-invasive pulmonary aspergillosis, which show early stage of type II to type IIa. (C) Computed tomography (CT) scan image taken at admission, showing only diffuse thickening of the bronchial wall and tree-in-bud signs and acinar nodules at the distal end of the bronchus. (D, G) CT scan images from reexamination after 10 days of antibacterial treatment, which show obvious bronchiectasis. Image (H) was taken on the same slice 5 days later, which showed further bronchiectasis, with internal separation. (E) Shows pathological Aspergillus hyphae and small round spores (HE ×400). Image (F) shows sputum specimens, conidia of Aspergillus fumigatus; Lactophenol cotton blue dyeing, ×1000.
Figure 4. Case 3. A 46-year-old man was hospitalized with fever and shortness of breath for 1 week without a previous significant medical history. (A, B) Schematic diagrams of type II airway-invasive pulmonary aspergillosis, which show early stage of type II to type IIa. (C) Computed tomography (CT) scan image taken at admission, showing only diffuse thickening of the bronchial wall and tree-in-bud signs and acinar nodules at the distal end of the bronchus. (D, G) CT scan images from reexamination after 10 days of antibacterial treatment, which show obvious bronchiectasis. Image (H) was taken on the same slice 5 days later, which showed further bronchiectasis, with internal separation. (E) Shows pathological Aspergillus hyphae and small round spores (HE ×400). Image (F) shows sputum specimens, conidia of Aspergillus fumigatus; Lactophenol cotton blue dyeing, ×1000. 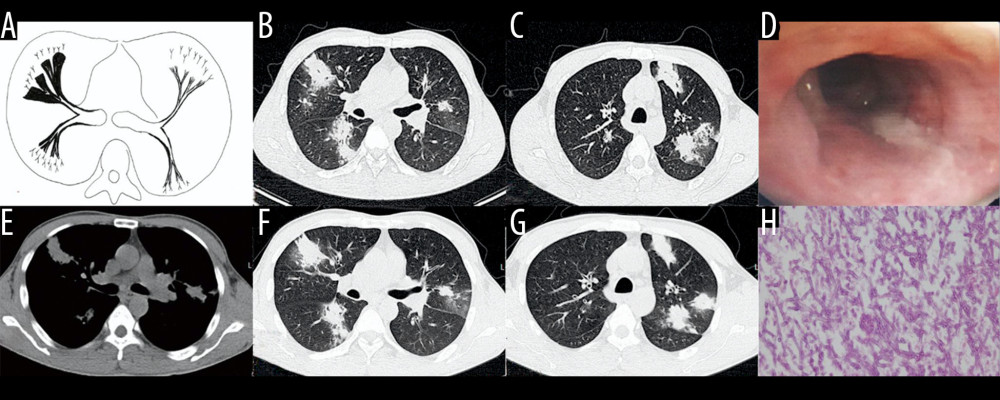 Figure 5. Case 4. A 45-year-old man had cough, sputum, and fever for 2 weeks, accompanied by shortness of breath, and he had been previously in good health. (A) Schematic diagram of type IIb airway-invasive pulmonary aspergillosis. (B, C) Computed tomography (CT) scan images taken at admission, showing both lungs with diffuse lesions distributed along the long axis of the bronchial alignment, with consolidation opacity and stenosis of the bronchus. (B) Arrow shows the location of the pathology. (D) The bronchial wall in the segmental bronchus was covered by pus-like exudates via bronchoscopy. (E) Mediastinal window shows the lesion distributed along the bronchus, and the edge was straight, consistent with the inflammatory changes. (F, G) CT scan images of the lung window on the same slice after 5 days of anti-infective treatment, showing no obvious improvement, diffuse lesions that are distributed along the periphery of the bronchus, and no bronchiectasis. (H) pathological Aspergillus hyphae and small round spores (HE ×400).
Figure 5. Case 4. A 45-year-old man had cough, sputum, and fever for 2 weeks, accompanied by shortness of breath, and he had been previously in good health. (A) Schematic diagram of type IIb airway-invasive pulmonary aspergillosis. (B, C) Computed tomography (CT) scan images taken at admission, showing both lungs with diffuse lesions distributed along the long axis of the bronchial alignment, with consolidation opacity and stenosis of the bronchus. (B) Arrow shows the location of the pathology. (D) The bronchial wall in the segmental bronchus was covered by pus-like exudates via bronchoscopy. (E) Mediastinal window shows the lesion distributed along the bronchus, and the edge was straight, consistent with the inflammatory changes. (F, G) CT scan images of the lung window on the same slice after 5 days of anti-infective treatment, showing no obvious improvement, diffuse lesions that are distributed along the periphery of the bronchus, and no bronchiectasis. (H) pathological Aspergillus hyphae and small round spores (HE ×400). 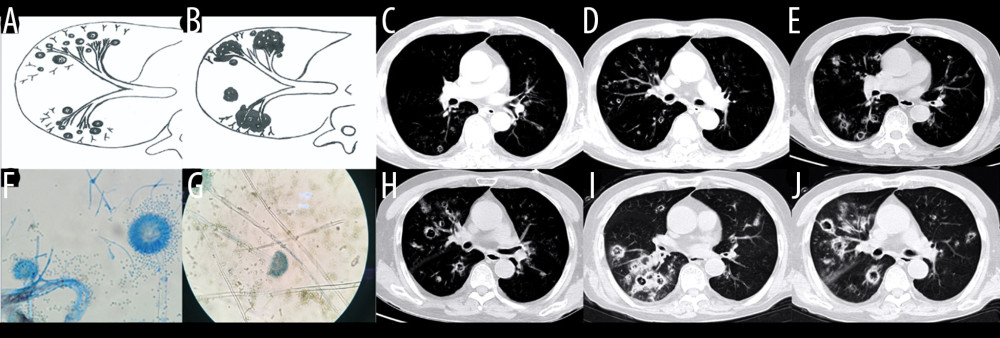 Figure 6. Case 5. An 83-year-old man had chronic obstructive pulmonary disease for more than 20 years had been aggravated for 10 days, with fever of 38°C and shortness of breath. (A, B) Schematic diagram of type III airway-invasive pulmonary aspergillosis, which showed early stage of type III and its progress. (C, D) Computed tomography (CT) scan images on the second day of admission, showing diffuse acinar nodules with punctate cavities in peripheral lungs, with internal separation. (E, H) CT scan images taken on day 5 after admission and reexamination after anti-infective treatment, showing the lung window of the same slice, specifically the lesion and the cavity were further enlarged with compartmentalized changes in the cavity. (I, J) CT scan images taken on the day 8 after admission showing the lung window on the same slice; the lesion was further enlarged, with fusion of small lesions, and the cavity was enlarged and increased, with obvious internal separation. (F, G) Sputum specimens, conidia of Aspergillus flavus; Lactophenol cotton blue dyeing, ×1000.
Figure 6. Case 5. An 83-year-old man had chronic obstructive pulmonary disease for more than 20 years had been aggravated for 10 days, with fever of 38°C and shortness of breath. (A, B) Schematic diagram of type III airway-invasive pulmonary aspergillosis, which showed early stage of type III and its progress. (C, D) Computed tomography (CT) scan images on the second day of admission, showing diffuse acinar nodules with punctate cavities in peripheral lungs, with internal separation. (E, H) CT scan images taken on day 5 after admission and reexamination after anti-infective treatment, showing the lung window of the same slice, specifically the lesion and the cavity were further enlarged with compartmentalized changes in the cavity. (I, J) CT scan images taken on the day 8 after admission showing the lung window on the same slice; the lesion was further enlarged, with fusion of small lesions, and the cavity was enlarged and increased, with obvious internal separation. (F, G) Sputum specimens, conidia of Aspergillus flavus; Lactophenol cotton blue dyeing, ×1000. References
1. Panse P, Smith M, Cummings K, The many faces of pulmonary aspergillosis: Imaging findings with pathologic correlation: Radiol Infect Dis, 2016; 3(4); 192-200
2. Krenke R, Grabczak EM: Sci World J, 2011; 11; 2310-29
3. Xu XY, Sun HM, Zhao BL, Shi Y, Diagnosis of airway-invasive pulmonary aspergillosis by tree-in-bud sign in an immunocompetent patient: Case report and literature review: J Mycol Med, 2013; 23(1); 64-69
4. Chotirmall SH, Martin-Gomez MT: Mycopathologia, 2018; 183(1); 45-59
5. Buckingham SJ, Hansell DM: Eur Radiol, 2003; 13(8); 1786-800
6. Davda S, Kowa XY, Aziz Z, The development of pulmonary aspergillosis and its histologic, clinical, and radiologic manifestations: Clin Radiol, 2018; 73(11); 913-21
7. Ohta H, Yamazaki S, Miura Y, Invasive tracheobronchial aspergillosis progressing from bronchial to diffuse lung parenchymal lesions: Respirol Case Rep, 2016; 4(1); 32-34
8. Logan PM, Primack SL, Miller RR, Muller NL, Invasive aspergillosis of the airways: Radiographic, CT, and pathologic findings: Radiology, 1994; 193(2); 383-88
9. Henzler C, Henzler T, Buchheidt D, Diagnostic performance of contrast enhanced pulmonary computed tomography angiography for the detection of angioinvasive pulmonary Aspergillosis in immunocompromised patients: Sci Rep, 2017; 7(1); 4483
10. De Pauw B, Walsh TJ, Donnelly JP, Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group: Clin Infect Dis, 2008; 46(12); 1813-21
11. Maturu VN, Agarwal R, Acute invasive pulmonary aspergillosis complicating allergic bronchopulmonary aspergillosis: Case report and systematic review: Mycopathologia, 2015; 180(3–4); 209-15
12. Roden AC, Schuetz AN, Histopathology of fungal diseases of the lung: Semin Diagn Pathol, 2017; 34(6); 530-49
13. Ader F, Nseir S, Le Berre R, Invasive pulmonary aspergillosis in chronic obstructive pulmonary disease: An emerging fungal pathogen: Clin Microbiol Infect, 2005; 11(6); 427-29
14. Wessolossky M, Welch VL, Sen A: Infect Drug Resist, 2013; 6; 33-39
15. Kosmidis C, Denning DW, Republished: The clinical spectrum of pulmonary aspergillosis: Postgrad Med J, 2015; 91(1077); 403-10
16. Desoubeaux G, Bailly É, Chandenier J, Diagnosis of invasive pulmonary aspergillosis: Updates and recommendations: Med Mal Infect, 2014; 44(3); 89-101
17. Park SY, Lim C, Lee S-O, Computed tomography findings in invasive pulmonary aspergillosis in non-neutropenic transplant recipients and neutropenic patients, and their prognostic value: J Infect, 2011; 63(6); 447-56
18. Tunnicliffe G, Schomberg L, Walsh S, Airway and parenchymal manifestations of pulmonary aspergillosis: Respir Med, 2013; 107(8); 1113-23
19. Guinea J, Torres-Narbona M, Gijon P, Pulmonary aspergillosis in patients with chronic obstructive pulmonary disease: Incidence, risk factors, and outcome: Clin Microbiol Infect, 2010; 16(7); 870-77
20. Bao Z, Chen H, Zhou M, Invasive pulmonary aspergillosis in patients with chronic obstructive pulmonary disease: A case report and review of the literature: Oncotarget, 2017; 8(23); 38069-74
21. Kousha M, Tadi R, Soubani AO, Pulmonary aspergillosis: A clinical review: Eur Respir Rev, 2011; 20(121); 156-74
22. Huang L, He H, Ding Y, Values of radiological examinations for the diagnosis and prognosis of invasive bronchial-pulmonary aspergillosis in critically ill patients with chronic obstructive pulmonary diseases: Clin Respir J, 2018; 12(2); 499-509
23. Janes SM, Barker KF, Mak V, Bell D, Invasive pulmonary aspergillosis in an insulin-dependent diabetic: Respir Med, 1998; 92(7); 972-75
24. Watanabe N, Saito K, Kiritani A, A case of invasive pulmonary aspergillosis diagnosed by transbronchial lung biopsy during treatment for diabetic ketoacidosis in a type 1 diabetic patient: J Infect Chemother, 2020; 26(2); 274-78
25. Sanghani RN, Udwadia ZF, The association of diabetes and tuberculosis: Impact on treatment and post-treatment outcomes: Thorax, 2013; 68(3); 202-3
26. Ekwueme C, Otu AA, Chinenye S: Clin Case Rep, 2016; 4(4); 432-36
27. Ghanaat F, Tayek JA, Weight loss and diabetes are new risk factors for the development of invasive aspergillosis infection in non-immunocompromized humans: Clin Pract, 2017; 14(5 Spec Iss); 296-301
28. Crum-Cianflone NF: Open Forum Infect Dis, 2016; 3(3); ofw171
29. Vanderbeke L, Spriet I, Breynaert C, Invasive pulmonary aspergillosis complicating severe influenza: Epidemiology, diagnosis and treatment: Curr Opin Infect Dis, 2018; 31(6); 471-80
30. Chabi ML, Goracci A, Roche N, Pulmonary aspergillosis: Diagn Interv Imaging, 2015; 96(5); 435-42
31. Kim JH, Lee HL, Kim L, Airway centered invasive pulmonary aspergillosis in an immunocompetent patient: Case report and literature review: J Thorac Dis, 2016; 8(3); E250-54
32. De Soyza A, Aliberti S: Med Mycol, 2017; 55(1); 69-81
33. Gadkowski LB, Stout JE, Cavitary pulmonary disease: Clin Microbiol Med, 2008; 21(2); 305-33 table of contents
Figures
 Figure 1. Schematic diagram of different types of airway-invasive pulmonary aspergillosis. (A) Type I: mainly invading the trachea and the main bronchus and the wall of the trachea, and the main bronchus is thickening. (B) Type II mainly involves the lobular and segmental bronchi. In the early stages of type II, only the bronchial wall is thickened. In the late stage of follow-up, type IIa or type IIb can be found. Type IIa is associated with obvious bronchiectasis, and type IIb mainly infiltrates along the periphery of the bronchus without obvious bronchiectasis. (C) Type III involves the bronchioles and pulmonary parenchyma, manifested as peripheral bronchioles and acinar nodules. As the course of the disease progresses, the nodules fuse to form patches of consolidation, with small cavities often appearing inside.
Figure 1. Schematic diagram of different types of airway-invasive pulmonary aspergillosis. (A) Type I: mainly invading the trachea and the main bronchus and the wall of the trachea, and the main bronchus is thickening. (B) Type II mainly involves the lobular and segmental bronchi. In the early stages of type II, only the bronchial wall is thickened. In the late stage of follow-up, type IIa or type IIb can be found. Type IIa is associated with obvious bronchiectasis, and type IIb mainly infiltrates along the periphery of the bronchus without obvious bronchiectasis. (C) Type III involves the bronchioles and pulmonary parenchyma, manifested as peripheral bronchioles and acinar nodules. As the course of the disease progresses, the nodules fuse to form patches of consolidation, with small cavities often appearing inside. Figure 2. Case 1. A 48-year-old woman had cough and shortness of breath for 1 month, with a past history of diabetes mellitus. (A, B) The coronal view of the mediastinum, indicating major thickening along the left main bronchus, partial stenosis of the lumen, and inflammation of the distal lung tissue, which is similar to the schematic diagram. (C, D) Thickening at the walls of the trachea and left main bronchus in the lung window. (E) Pathological Aspergillus hyphae and small round spores (HE ×400). (F) The trachea and left main bronchus were covered by pus-like exudates via bronchoscopy. (G, H) Thickening of the wall of the bronchus, the soft tissue shadow in the mediastinum (inflammatory infiltration or lymph node enlargement) and extraluminal air, indicating the formation of mediastinitis and bronchial fistula.
Figure 2. Case 1. A 48-year-old woman had cough and shortness of breath for 1 month, with a past history of diabetes mellitus. (A, B) The coronal view of the mediastinum, indicating major thickening along the left main bronchus, partial stenosis of the lumen, and inflammation of the distal lung tissue, which is similar to the schematic diagram. (C, D) Thickening at the walls of the trachea and left main bronchus in the lung window. (E) Pathological Aspergillus hyphae and small round spores (HE ×400). (F) The trachea and left main bronchus were covered by pus-like exudates via bronchoscopy. (G, H) Thickening of the wall of the bronchus, the soft tissue shadow in the mediastinum (inflammatory infiltration or lymph node enlargement) and extraluminal air, indicating the formation of mediastinitis and bronchial fistula. Figure 3. Case 2. A 43-year-old man had cough and shortness of breath for 10 days. (A, B) Schematic diagrams of type II airway-invasive pulmonary aspergillosis, which showed the early stage of type II to type IIa. (C, G) Computed tomography (CT) scan images taken at admission. As shown in the images, there was thickening of the lobar and segmental bronchus, diffuse tree-in-bud sign at the distal end, which was consistent with type II airway-invasion Aspergillus. Images (D) and (H) are CT scan images on the same slice with (C) and (G) after 7 days, showing bronchiectasis, which was consistent with type IIa airway-invasive aspergillosis. Image (E) shows pathological Aspergillus hyphae and small round spores, the hyphae were branched and segmented (HE ×200); (F) (HE ×400).
Figure 3. Case 2. A 43-year-old man had cough and shortness of breath for 10 days. (A, B) Schematic diagrams of type II airway-invasive pulmonary aspergillosis, which showed the early stage of type II to type IIa. (C, G) Computed tomography (CT) scan images taken at admission. As shown in the images, there was thickening of the lobar and segmental bronchus, diffuse tree-in-bud sign at the distal end, which was consistent with type II airway-invasion Aspergillus. Images (D) and (H) are CT scan images on the same slice with (C) and (G) after 7 days, showing bronchiectasis, which was consistent with type IIa airway-invasive aspergillosis. Image (E) shows pathological Aspergillus hyphae and small round spores, the hyphae were branched and segmented (HE ×200); (F) (HE ×400). Figure 4. Case 3. A 46-year-old man was hospitalized with fever and shortness of breath for 1 week without a previous significant medical history. (A, B) Schematic diagrams of type II airway-invasive pulmonary aspergillosis, which show early stage of type II to type IIa. (C) Computed tomography (CT) scan image taken at admission, showing only diffuse thickening of the bronchial wall and tree-in-bud signs and acinar nodules at the distal end of the bronchus. (D, G) CT scan images from reexamination after 10 days of antibacterial treatment, which show obvious bronchiectasis. Image (H) was taken on the same slice 5 days later, which showed further bronchiectasis, with internal separation. (E) Shows pathological Aspergillus hyphae and small round spores (HE ×400). Image (F) shows sputum specimens, conidia of Aspergillus fumigatus; Lactophenol cotton blue dyeing, ×1000.
Figure 4. Case 3. A 46-year-old man was hospitalized with fever and shortness of breath for 1 week without a previous significant medical history. (A, B) Schematic diagrams of type II airway-invasive pulmonary aspergillosis, which show early stage of type II to type IIa. (C) Computed tomography (CT) scan image taken at admission, showing only diffuse thickening of the bronchial wall and tree-in-bud signs and acinar nodules at the distal end of the bronchus. (D, G) CT scan images from reexamination after 10 days of antibacterial treatment, which show obvious bronchiectasis. Image (H) was taken on the same slice 5 days later, which showed further bronchiectasis, with internal separation. (E) Shows pathological Aspergillus hyphae and small round spores (HE ×400). Image (F) shows sputum specimens, conidia of Aspergillus fumigatus; Lactophenol cotton blue dyeing, ×1000. Figure 5. Case 4. A 45-year-old man had cough, sputum, and fever for 2 weeks, accompanied by shortness of breath, and he had been previously in good health. (A) Schematic diagram of type IIb airway-invasive pulmonary aspergillosis. (B, C) Computed tomography (CT) scan images taken at admission, showing both lungs with diffuse lesions distributed along the long axis of the bronchial alignment, with consolidation opacity and stenosis of the bronchus. (B) Arrow shows the location of the pathology. (D) The bronchial wall in the segmental bronchus was covered by pus-like exudates via bronchoscopy. (E) Mediastinal window shows the lesion distributed along the bronchus, and the edge was straight, consistent with the inflammatory changes. (F, G) CT scan images of the lung window on the same slice after 5 days of anti-infective treatment, showing no obvious improvement, diffuse lesions that are distributed along the periphery of the bronchus, and no bronchiectasis. (H) pathological Aspergillus hyphae and small round spores (HE ×400).
Figure 5. Case 4. A 45-year-old man had cough, sputum, and fever for 2 weeks, accompanied by shortness of breath, and he had been previously in good health. (A) Schematic diagram of type IIb airway-invasive pulmonary aspergillosis. (B, C) Computed tomography (CT) scan images taken at admission, showing both lungs with diffuse lesions distributed along the long axis of the bronchial alignment, with consolidation opacity and stenosis of the bronchus. (B) Arrow shows the location of the pathology. (D) The bronchial wall in the segmental bronchus was covered by pus-like exudates via bronchoscopy. (E) Mediastinal window shows the lesion distributed along the bronchus, and the edge was straight, consistent with the inflammatory changes. (F, G) CT scan images of the lung window on the same slice after 5 days of anti-infective treatment, showing no obvious improvement, diffuse lesions that are distributed along the periphery of the bronchus, and no bronchiectasis. (H) pathological Aspergillus hyphae and small round spores (HE ×400). Figure 6. Case 5. An 83-year-old man had chronic obstructive pulmonary disease for more than 20 years had been aggravated for 10 days, with fever of 38°C and shortness of breath. (A, B) Schematic diagram of type III airway-invasive pulmonary aspergillosis, which showed early stage of type III and its progress. (C, D) Computed tomography (CT) scan images on the second day of admission, showing diffuse acinar nodules with punctate cavities in peripheral lungs, with internal separation. (E, H) CT scan images taken on day 5 after admission and reexamination after anti-infective treatment, showing the lung window of the same slice, specifically the lesion and the cavity were further enlarged with compartmentalized changes in the cavity. (I, J) CT scan images taken on the day 8 after admission showing the lung window on the same slice; the lesion was further enlarged, with fusion of small lesions, and the cavity was enlarged and increased, with obvious internal separation. (F, G) Sputum specimens, conidia of Aspergillus flavus; Lactophenol cotton blue dyeing, ×1000.
Figure 6. Case 5. An 83-year-old man had chronic obstructive pulmonary disease for more than 20 years had been aggravated for 10 days, with fever of 38°C and shortness of breath. (A, B) Schematic diagram of type III airway-invasive pulmonary aspergillosis, which showed early stage of type III and its progress. (C, D) Computed tomography (CT) scan images on the second day of admission, showing diffuse acinar nodules with punctate cavities in peripheral lungs, with internal separation. (E, H) CT scan images taken on day 5 after admission and reexamination after anti-infective treatment, showing the lung window of the same slice, specifically the lesion and the cavity were further enlarged with compartmentalized changes in the cavity. (I, J) CT scan images taken on the day 8 after admission showing the lung window on the same slice; the lesion was further enlarged, with fusion of small lesions, and the cavity was enlarged and increased, with obvious internal separation. (F, G) Sputum specimens, conidia of Aspergillus flavus; Lactophenol cotton blue dyeing, ×1000. In Press
05 Mar 2024 : Clinical Research
Muscular Function Recovery from General Anesthesia in 132 Patients Undergoing Surgery with Acceleromyograph...Med Sci Monit In Press; DOI: 10.12659/MSM.942780
05 Mar 2024 : Clinical Research
Effects of Thermal Insulation on Recovery and Comfort of Patients Undergoing Holmium Laser LithotripsyMed Sci Monit In Press; DOI: 10.12659/MSM.942836
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952