09 July 2021: Clinical Research
Secondary Immunodeficiency and Hypogammaglobulinemia with IgG Levels of <5 g/L in Patients with Multiple Myeloma: A Retrospective Study Between 2012 and 2020 at a University Hospital in China
Chunmei Ye12BCEF, Weiwei Chen3EF, Qi Gao12B, Yanxia Chen4B, Xiaolu Song5B, Sujie Zheng2C, Jinlin Liu2AEG*DOI: 10.12659/MSM.930241
Med Sci Monit 2021; 27:e930241
Abstract
BACKGROUND: Infections are the main cause of mortality and morbidity in multiple myeloma (MM) patients. However, adult immunodeficiency specialists in China are lacking, and the care of secondary immunodeficiency (SID) and the prognostic role of hypogammaglobulinemia in MM is unknown.
MATERIAL AND METHODS: MM patients (295) were retrospectively analyzed between January 2012 and 2020 in Zhejiang Provincial People’s Hospital, Hangzhou Medical College. MM patients with immunoglobulin (Ig) G <5 g/L were defined as SID patients. The care of these patients and the prognostic role of IgG <5 g/L were analyzed
RESULTS: Forty-five of 295 MM patients with IgG <5 g/L were defined as SID patients. These 45 patients mainly had recurrent infections, especially pulmonary bacterial infections; 2 patients had them 5 times/year. The median survival time was significantly shorter in MM patients with SID (24 vs 66 months). More importantly, the multivariate and univariate analysis revealed that IgG <5 g/L was an independent prognostic factor for MM patients.
CONCLUSIONS: Ig replacement therapy or prophylactic antibiotics for MM patients with SID were lacking in this single retrospective study. IgG <5 g/L could be a prognostic marker for MM patients.
Keywords: Antibiotic Prophylaxis, Immunoglobulins, Intravenous, Multiple Myeloma, Agammaglobulinemia, Immunoglobulin G
Background
Multiple myeloma (MM) is a neoplastic plasma cell proliferative disorder and the second most common hematological malignancy [1], accounting for 0.9% of all cancers worldwide in 2018 and 1.1% of cancer deaths [2]. Current treatment options, such as a regimen of vincristine, Adriamycin, and dexamethasone (VAD), and a regimen of bortezomib, cyclophosphamide, and dexamethasone (PCD), have significantly improved the survival rate of patients with MM [3,4]. However, these drugs exert tremendous effects on the immune system, resulting in the impairment of immunity. These patients have a very high risk of developing secondary immunodeficiency (SID) and increased infection risk, and the drugs continue to be a main cause of mortality and morbidity for MM patients [5,6]. Therefore, care is critical for the quality of life and long survival for MM patients with SID [6].
SID patients also present a wide range of special problems and are have an increased risk of infection [6]. Thus, prophylactic antibiotics and immunoglobulin replacement therapy (IgRT) are 2 main strategies for the care of SID patients, especially secondary to hematological diseases [5]. Recent data demonstrated that compared with patients not receiving IgRT, MM patients receiving IgRT had lower infection rates, fewer days of hospitalization, and longer time free of infections [7,8]. Recently, in a European online survey of about 230 physicians responsible for diagnosing SID and administration of IgRT in hematological malignancy patients, 85% of physicians prescribed IgRT for patients with ≥2 severe infections. Additionally, in Italy, the United States, Germany, and Spain, IgRT use was above average in hypogammaglobulinemia patients, whereas considerably fewer patients received IgRT in the UK [9].
Furthermore, because of the scarce and expensive resources of IgRT, failure to respond to a 3-month course of prophylactic antibiotics is required for use of IgRT under the guidelines in the UK [9] and 86% of patients receive a trial of prophylactic antibiotics before consideration for IgRT by Irish and British immunologists [10], although prophylactic antibiotic usage can be controversial in an era of increasing antibiotic stewardship.
On the contrary, in the Chinese mainland, hematologic SID specialists are still lacking, IgRT for SID patients is not covered by health insurance, and the optimal treatment of SID caused by MM is still unknown. In the richest Hong Kong special administrative area, immunology service care for adult immunodeficiency patients was only established in 2016; prognosis and care of MM patients with SID in the Chinese mainland are still lacking. Therefore, we conducted this single-center retrospective study to describe the prognostic role of IgG <5 g/L and care of MM patients with SID in a university hospital in China.
Material and Methods
STUDY DESIGN:
We conducted this retrospective review of all MM patients in our university hospital and laboratory database from January 2012 to January 2020 in Zhejiang Provincial People’s Hospital, Hangzhou Medical College. Patients’ electronic medical records were reviewed through the hospital and laboratory information system database, including clinical characteristics, laboratory results, treatment, and survival outcomes. This study was conducted after approval by the Zhejiang Provincial People’s Hospital Ethics Committee (no. 2020QT138).
CLINICAL AND LABORATORY DATA:
The diagnosis of infection was made via clinical presentation, along with positive radiologic findings and positive microbiological cultures indicative of infection according to standard practice [11]. The complete data on infections, including frequency, location, and type, are listed in Table 1 and Supplementary Table 1. Moreover, the details of treatment, including prophylactic antibiotics, antibiotics treatment after infections, IgRT, and chemotherapy regimens, are documented in Table 2 and Supplementary Table 1.
Data on demographics and the subtype of MM are summarized in Table 3. Additionally, the results of kappa and lambda light chain determinations were measured by immunofixation electrophoresis. The serum IgG level was measured by nephelometry (Beckman Coulter, IMMAGE800) and the IgG reference values were 7.51–15.6 g/L.
END POINTS AND SURVIVAL ANALYSIS:
Survival data of all MM patients were telephoned in and documented in August 2020. IgG was first measured at diagnosis, monitored at admission, and followed up when MM patients received treatment in our hospital. The start point of survival time correlation with IgG level was based on the first IgG results in our hospital. Notably, SID patients were defined as having IgG <5 g/L at least twice. For the group with IgG <5 g/L, the overall survival (OS) was calculated from the first hypogammaglobulinemia (IgG <5 g/L) event in our hospital until the end point. In the cohort with IgG ≥5 g/L, the start point of OS was from the first occurrence of IgG ≥5 g/L in our hospital.
STATISTICAL ANALYSIS:
Statistical tests for data analysis included Cox survival analysis. Multivariate statistical analysis was performed via a Cox regression model. Statistical analysis was performed via the SPSS 19.0 statistical software package.
Results
FORTY-FIVE OF 266 MM PATIENTS WERE DEFINED AS SID PATIENTS:
We retrospectively analyzed data from 295 MM patients in Zhejiang Provincial People’s Hospital, Hangzhou Medical College from January 2012 to January 2020. Twenty-nine outpatients with incomplete information were excluded (Figure 1). On the basis of the criterion of IgG <5 g/L for SID patients [12,13], 266 MM patients were separated into 2 groups: 74 MM patients with SID (IgG <5 g/L) and 192 MM patients without SID (IgG ≥5 g/L). In addition, 29 MM patients with SID and 89 MM patients whose phone numbers were wrong or changed, resulting in lost contact, were also excluded. Thus, data from 45 MM patients with SID and 103 MM patients without SID were analyzed (Figure 1).
CLINICAL AND LABORATORY CHARACTERISTICS OF THESE 148 MM PATIENTS WITH AND WITHOUT SID:
According to the cutoff value IgG <5 g/L, 148 MM patients identified in this study were separated into 2 groups, the IgG <5 g/L group and the IgG ≥5 g/L group. Among these 45 MM patients with SID, 34 (75.56%) patients were men and the other 11 (24.44%) were women. Twenty-three (51.11%) patients were ≤65 years old, and 22 (48.89%) were >65 years old (Table 1). In these 45 MM patients with SID, the antibody composition was 15 λ and 12 κ IgA, 5 λ IgD and 1 λ IgG, 6 λ and 4 κ, and 2 unclear types (Table 3). Most importantly, on review of the patient history, the SID diagnosis had never been listed for these 45 MM patients. In the IgG ≥5 g/L cohort, 51 (49.51%) patients were ≤65 years and 63 MM patients (61.17%) were men. On the basis of the result of immunofixation electrophoresis, 11, 3, 4, 35, 25, 6, 8, 1, 1, 1, and 8 MM patients were IgA, λ light chain; IgA, κ light chain; IgD, λ light chain; IgG, λ light chain; IgG, κ light chain; λ light chain; κ light chain; IgA; IgG; IgG, IgA, λ light chain, and unknown, respectively (Table 3).
MM PATIENTS WITH AND WITHOUT SID HAD RECURRENT INFECTIONS, ESPECIALLY PULMONARY BACTERIAL INFECTION:
Among these 45 MM patients with SID, 41 had at least 1 infection; 11 of these patients had infection twice, 6 patients had it 3 times, 3 patients had it 4 times, and 2 patients had infection 5 times in 1 year (infection time available in Zhejiang Provincial People’s Hospital is fully listed in Table 1 and Supplementary Table 1). Of these infections, 31 were in the lung, 8 in the upper respiratory tract, 5 in the genitourinary tract, and 2 in the gingiva (Table 1). Forty-two patients had bacterial infections, 2 had proven fungal infections, 3 patients had probable fungal infections, and 5 patients had viral infections (Table 1). For the group with IgG ≥5 g/L, 70 patients had at least 1 infection 18 patients had infections twice, 4 patients had them 3 times, 2 patients had them 4 times, and 2 patients had 5 infections in 1 year. The most common location of infections was the lung (57 cases) and the upper respiratory tract (11 cases). As shown in Table 1, 82 bacterial infections, 4 proven fungal infections, 5 probable fungal infections, and 6 viral infections were documented. Thus, MM patients with IgG <5 g/L and with IgG ≥5 g/L both mainly had pulmonary bacterial infections. Notably, compared with the IgG ≥5g/L group, the proven fungal infection rate and probable fungal infection rate in the IgG <5 g/L group were significantly higher.
FOURTEEN MM PATIENTS WITH SID RECEIVED ANTIMICROBIAL PROPHYLAXIS AND ONLY 3 PATIENTS RECEIVED IGRT:
As we know, chemotherapy can be the major cause of SID in hematological diseases [6]. Thus, the treatment for these MM patients was systematically analyzed; the most common regimen was VAD and PCD therapy (Supplementary Table 1). During the hospitalization, all 41 MM patients with SID were treated with antimicrobials when they encountered infection, but only 14 patients received it to prevent these infections. As shown in Table 4, 14 MM patients with SID received antimicrobial prophylaxis, including antibiotic, antifungal, and antivirus treatment. Twelve patients with infection at admission were ruled out, and the other 19 MM patients with SID had not received antimicrobial prophylaxis. The results show that the infection rate was lower in the antimicrobial prophylaxis group within 2 weeks (21.43% vs 31.58%), but no significantly different change was seen within 1 month (42.85% vs 42.11%) (Table 2). Moreover, only 3 patients had received IgRT among these 45 MM patients with SID.
IGG <5 G/L PREDICTED POOR PROGNOSIS IN MM PATIENTS:
To further evaluate the role of hypogammaglobulinemia in the clinical progression of MM patients with SID, we compared the survival status of MM patients with or without SID. Notably, 1-year and 3-year mortality rates of 45 SID patients associated with MM were significantly higher than the 103 MM patients without SID (33.33% vs 12.62% at 1 year; 51.11% vs 23.30% at 3 years) (Table 4). To further investigate the prognostic role of SID in MM patients’ progression, we performed Cox survival analysis to assess the role of hypogammaglobulinemia in the clinical progression of these MM patients. By using the IgG <5 g/L cutoff value, results revealed that the survival time in MM patients with IgG <5 g/L was notably lower than in the MM patients with IgG ≥5 g/L (P<0.0001, Figure 2), indicating that IgG<5g/L could be a prognosis marker for MM patients. Moreover, the median survival for the IgG ≥5 g/L group was 66 months, but the median survival for the IgG <5 g/L group was only 24 months (Figure 2).
Univariate analysis revealed that IgG <5 g/L, sex, and albumin were related to MM patients’ prognostic factors. The multivariate analysis further corroborated that IgG <5 g/L was a significant independent prognostic factor for MM patients (hazard ratio: 0.335; 95% confidence interval: 0.197–0.571; P<0.001, Tables 5, 6).
Discussion
With the introduction of novel agents, including bortezomib and lenalidomide, the overall survival and progression-free time for MM patients significantly improved [14]. However, current evidence suggests that chemotherapy is significantly associated with myelosuppression or deoxyribonucleic acid synthesis inhibition, further reducing B or T cell proliferation and impairing humoral or cellular immunity. These chemotherapies significantly reduced Ig production and resulted in SID in MM patients [6]. Given these results, MM patients treated with chemotherapies are at a high infection risk. Infections are still the main cause of mortality and morbidity in MM patients [15,16]. A study in Sweden based on 9253 MM patients confirmed that infection represented MM patients’ main threat [15]. Additionally, a Danish nationwide retrospective cohort study revealed that risk factors for pneumonia were male sex, International Staging System (ISS) II and ISS III, and elevated lactate dehydrogenase in MM patients who were newly diagnosed [16]. Therefore, the infectious risk assessment should be defined before and during the active treatment of MM patients [17].
Among all sorts of treatment for infection in patients with SID, antimicrobial prophylaxis and IgRT are commonly used to prevent infections [18]. In this study, 41 patients were admitted to the hospital at least 1 time per year because of infections; 2 patients had infections 5 times in a year, including infectious fever and pneumonia. When comparing the infection rate in patients with or without antimicrobial prophylaxis, the antimicrobial prophylaxis significantly decreased the infection rate within 2 weeks, but had no change within 1 month. Notably, no MM patient developed fungal infections after administration of antifungal prophylaxis. Moreover, of these infections, the 4.44% proven invasive fungal disease (IFD) and 6.67% possible IFD in MM patients with IgG <5 g/L were relatively higher than previously reported (<2%) [19]. In addition to the conventional chemotherapy, we speculate that high frequency of immunomodulatory drug administration may be associated with this high rate and further impair the immune system in these MM patients [20].
Compared with patients not receiving IgRT, patients receiving IgRT can reduce the use of antibiotics, the number of infections, and the days of hospitalization, which is of benefit in terms of conserving hospital resources, and can dramatically improve the quality of life for MM patients [7,18], although IgRT is not routinely recommended for MM patients [17]. A recent randomized trial study showed that using the prophylactic administration of subcutaneous Ig improved both adherence to chemotherapy and health quality of life and was cost effective by reducing the need for hospitalization and the use of antibiotics among MM patients [7]. However, in this single-center Chinese university hospital retrospective study, only 3 patients had received IgRT. Without the IgRT, the survival of MM patients with SID was significantly lower than in the MM patients without SID. Moreover, compared with the MM patients without SID, the mortality rate of MM patients with SID was significantly higher in the first year and third year after diagnosis. Also, the multivariate analysis of different prognostic factors in MM patients revealed that IgG <5 g/L was an independent prognostic factor of MM.
Currently, updated criteria or guidelines on IgRT use for SID patients with hematological diseases have been issued in the UK, United States, and Australia [13,21–23]. IgRT is taken into consideration by hematologists when the indicators fulfill the criteria and guidelines on hypogammaglobulinemia associated with MM [9]. Physicians often prescribe prophylactic IgRT after any infection occurs, after lower respiratory infection, after the first severe infection, and even before any infection occurs [24]. However, no specific criteria have been issued for the treatment of MM patients with SID in China, and the IgRT or prophylactic antibiotic for the care of the MM patients with SID is still lacking [25,26]. In the present study, 41 patients were admitted to the hospital at least 1 time per year because of infections. Two patients had infections 5 times in a year, including infectious fever and pneumonia. Therefore, great attention should be focused on the care of MM patients with SID, which could significantly improve the life quality and prolong survival of MM patients.
With excellent tolerability of IgRT treatment, IgRT was feasible in SID patients to reduce infection rates and improve life quality [24]. In a noninterventional prospective French longitudinal study, data also confirmed the efficacy of IgRT in reducing infection risks in hematological malignancies associated with SID, which fulfilled physicians’ main expectations [8]. In the United States, use of IgRT should be considered in chronic lymphoid leukemia (CLL) or MM patients who had hypogammaglobulinemia with pneumococcal infection or recurrent bacterial infections [27]. In the UK, the Department of Health’s selection criteria for IgRT of hypogammaglobulinemia were recommended, particularly with MM, non-Hodgkin lymphoma, CLL, or other relevant B cell malignancy in combination with recurrent or severe bacterial infections, despite patients receiving continuous antibiotic treatment for 3 months [13]. In contrast, the present study revealed discrepancies in the use of prophylactic antibiotics and IgRT in a Chinese university hospital, which shows that more attention should be paid to MM patients with SID and then optimize the treatment regimens to allow those patients to benefit from IgRT.
Notably, MM is characterized by impaired immune surveillance mechanisms, such as altered antibody production, disruption of antigen presentation processes of dendritic cells, dysregulation of natural killer and T cell proliferation and activation, and upregulation of checkpoint and immunosuppressive mediators, thereby creating a suppressive microenvironment allowing the malignant cells to evade immune control [28,29]. New and emerging immunotherapeutic agents, such as cellular (chimeric antigen receptor T cell, vaccine, and allogeneic stem cell transplantation) therapies and antibody-based therapies (anti-CD38 daratumumab, anti-SLAMF7 elotuzumab, anti-CD28 antibody, or checkpoint inhibitor) have been developed to target evasion tactics of MM [29–31]. However, the present study revealed that evaluation of the immunodeficiency status by using the index of hypogammaglobulinemia (IgG <5 g/L) should be consistently monitored and administration of IgRT could significantly improve the survival and life quality of MM patients.
The strength of this study is that the serum IgG levels were consistently measured by the Beckman Coulter IMMAGE800 nephelometerin at this university hospital and the reference value never changed, which helped in reducing interlaboratory variation in technique and reporting methods. Also, only 3 MM patients with SID had received IgRT. Without IgRT treatment, this study further corroborated the critical and prognostic role of hypogammaglobulinemia in the survival of MM patients.
However, our study had limitations. β2-microglobulin data of patients were incomplete, and some baseline characteristics, such as ISS stage, were also missing. Moreover, 29 outpatients and 118 MM inpatients of a total of MM 295 patients were excluded and this may have resulted in selection bias of the MM cohort patients. Additionally, only 1 IgG-type MM patient was documented in the IgG <5 g/L group of this study when using the IgG <5 g/L cutoff value to define the SID patients [32], whereas the polyclonal, not monoclonal, IgG was detected in these guidelines. However, MM patients with IgG-type antibody were in a special condition; their IgG was monoclonal. Current quantitative IgG detection methods cannot differentiate the polyclonal or monoclonal IgG concentration; they can only examine total polyclonal IgG and monoclonal IgG. Therefore, when using the IgG <5 g/L for IgG-type MM patients, the inclusion of some MM patients with high monoclonal IgG concentrations and low polyclonal IgG concentrations in the IgG ≥5 g/L group may have resulted in bias.
Conclusions
IgG <5 g/L appears to be a reliable prognostic marker for MM patients, and the use of IgRT or prophylactic antibiotics should be seriously considered for these SID patients. Our study revealed that IgG <5 g/L could be a prognostic marker for survival in MM patients. The use of IgRT or prophylactic antibiotic for the care of these MM patients with SID was lacking in this single-center Chinese university study. Therefore, it is important to pay more attention to the screening and management of MM patients with SID.
Figures
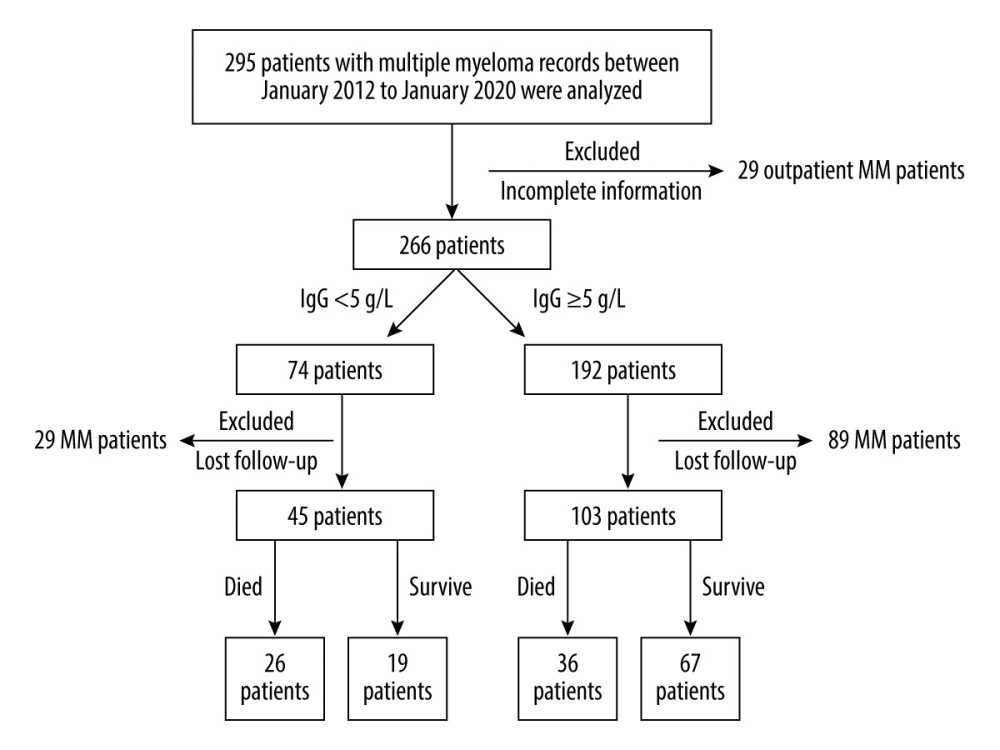 Figure 1. Flow chart of the selection of multiple myeloma (MM) patients with or without secondary immunodeficiency (SID).
Figure 1. Flow chart of the selection of multiple myeloma (MM) patients with or without secondary immunodeficiency (SID). 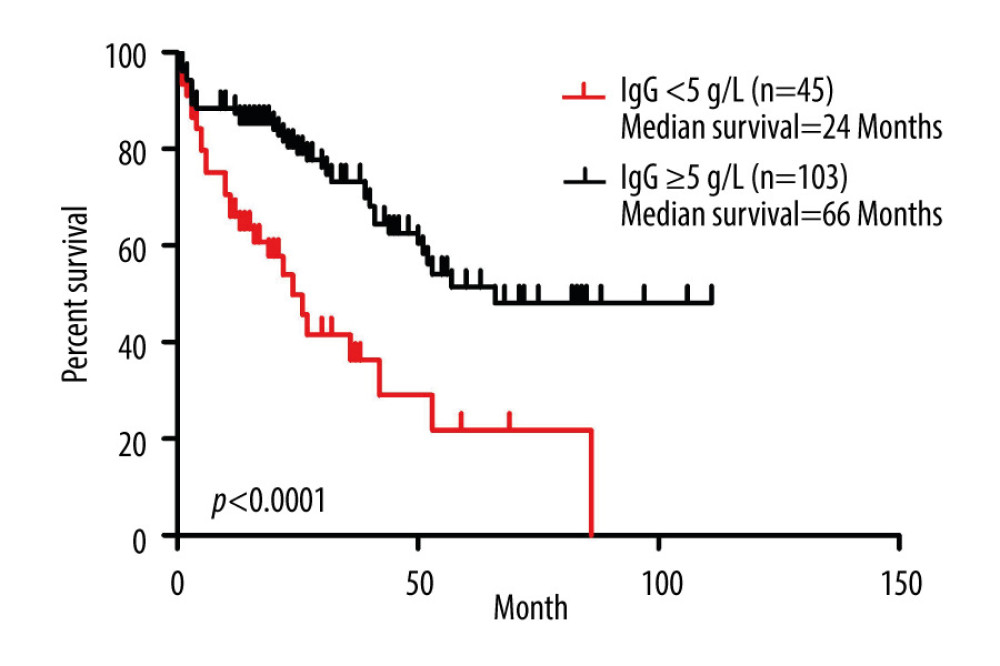 Figure 2. Prognostic role of IgG <0.5 g/L in multiple myeloma (MM) patients. Kaplan-Meier curves of MM patients with IgG <5 g/L (n=45) vs IgG ≥5 g/L (n=103) (P<0.0001, log-rank test, Cox analysis).
Figure 2. Prognostic role of IgG <0.5 g/L in multiple myeloma (MM) patients. Kaplan-Meier curves of MM patients with IgG <5 g/L (n=45) vs IgG ≥5 g/L (n=103) (P<0.0001, log-rank test, Cox analysis). Tables
Table 1. Past history of infection of multiple myeloma patients with and without secondary immunodeficiency.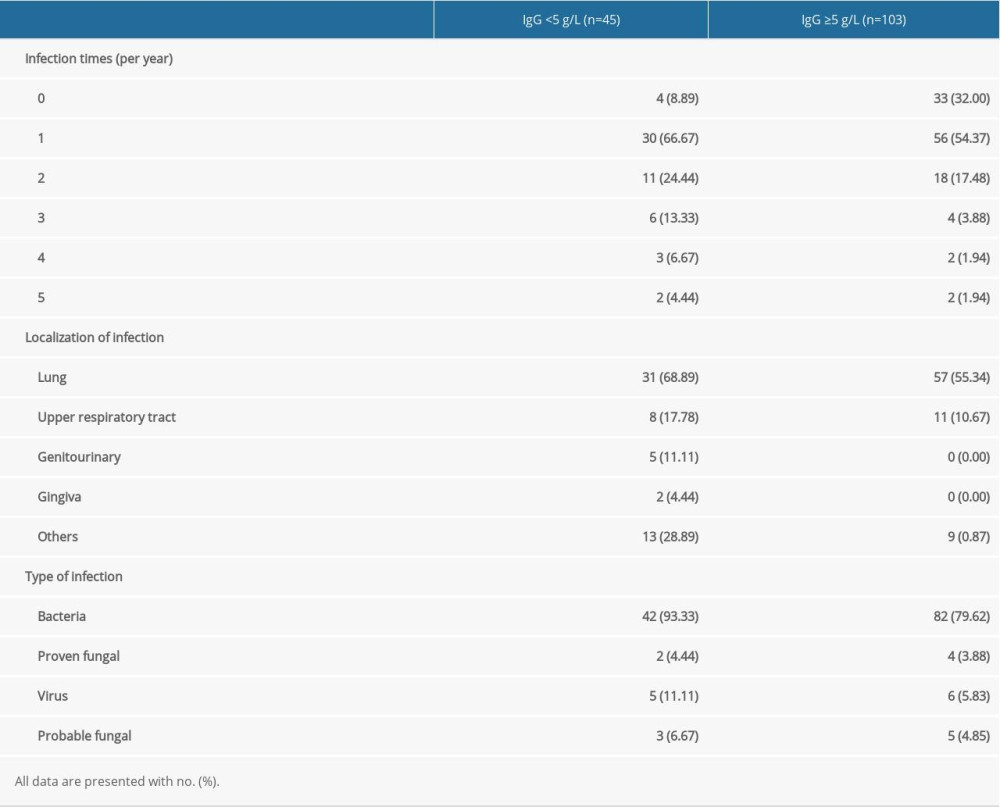 Table 2. Effectiveness of antimicrobial prophylaxis in the following 2 weeks or 1 month.
Table 2. Effectiveness of antimicrobial prophylaxis in the following 2 weeks or 1 month.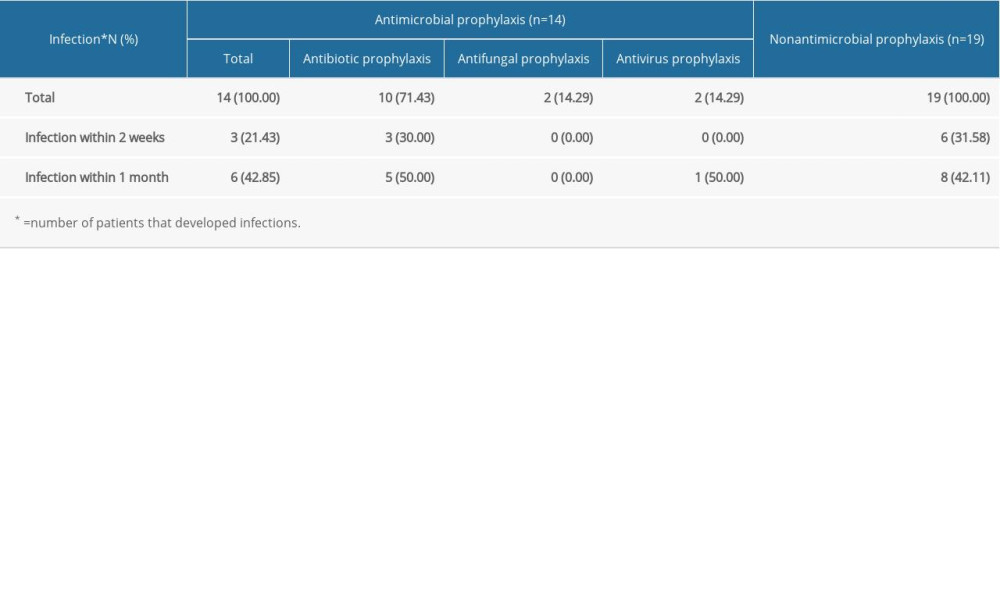 Table 3. Clinical characteristics of multiple myeloma patients with and without secondary immunodeficiency.
Table 3. Clinical characteristics of multiple myeloma patients with and without secondary immunodeficiency.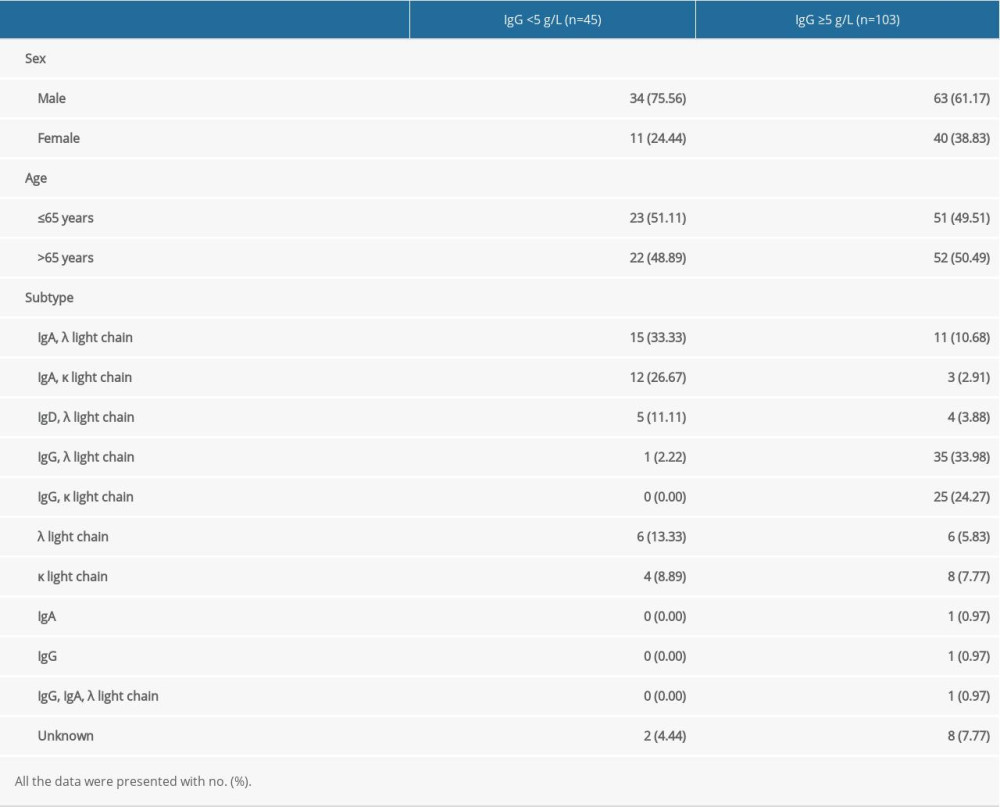 Table 4. Death rate of multiple myeloma patients with or without secondary immunodeficiency at 1 year and 3 years.
Table 4. Death rate of multiple myeloma patients with or without secondary immunodeficiency at 1 year and 3 years.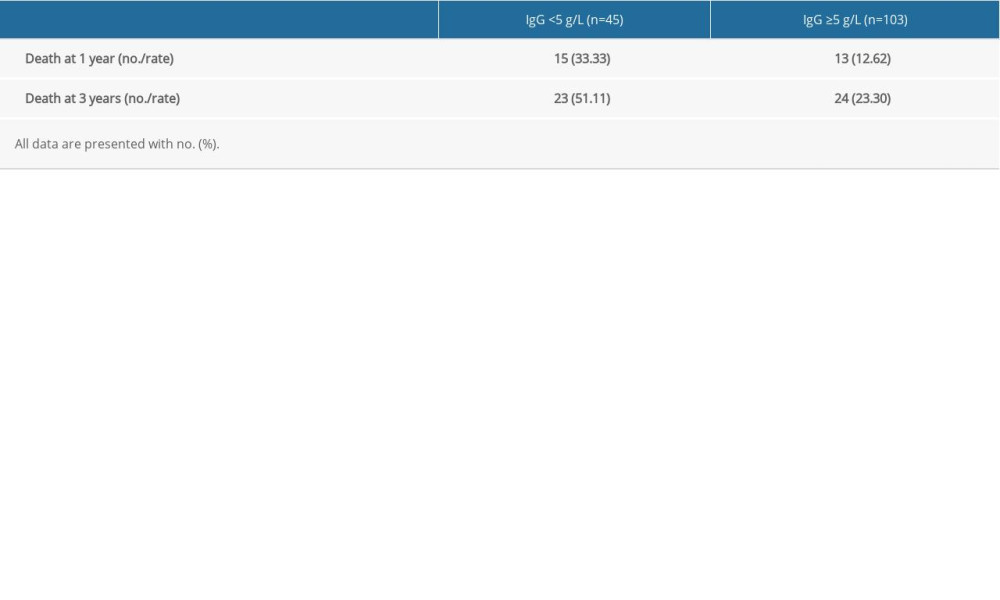 Table 5. Univariate analysis of secondary immunodeficiency in patients with multiple myeloma (MM) and matched MM patients.
Table 5. Univariate analysis of secondary immunodeficiency in patients with multiple myeloma (MM) and matched MM patients.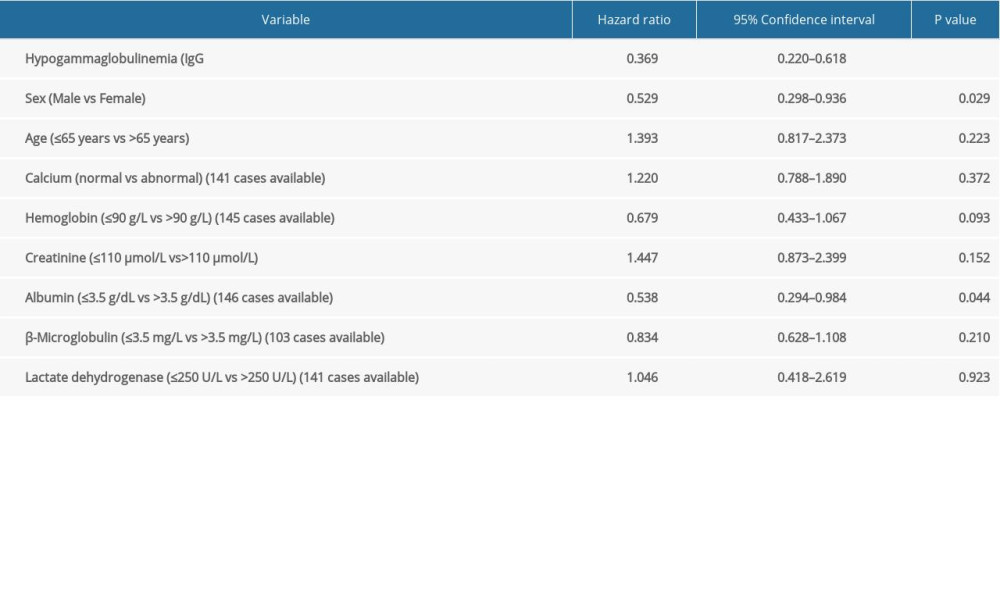 Table 6. Multivariate analysis of secondary immunodeficiency in patients with multiple myeloma (MM) and matched MM patients.
Table 6. Multivariate analysis of secondary immunodeficiency in patients with multiple myeloma (MM) and matched MM patients.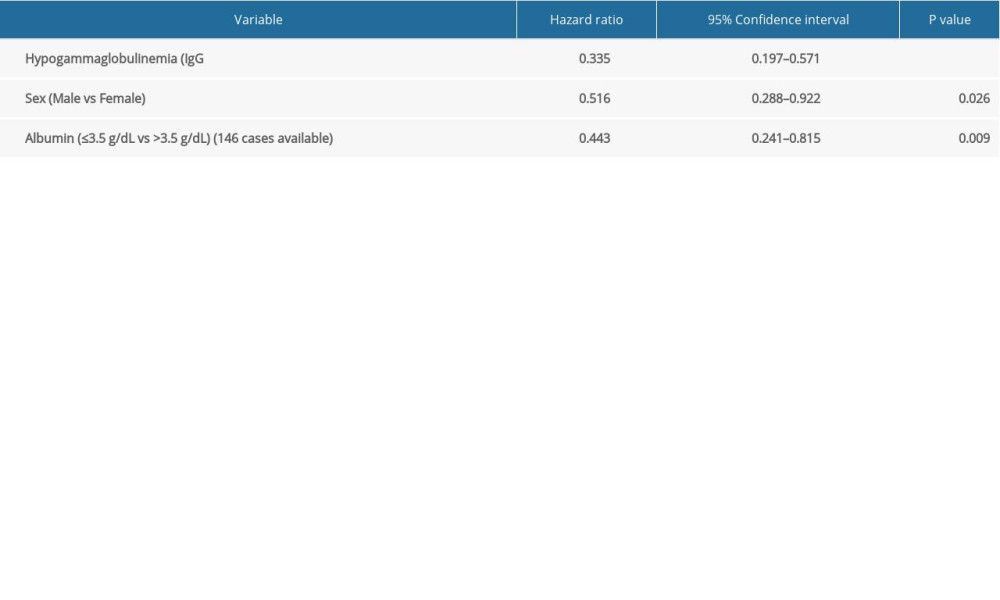 Supplementary Table 1. Details of the clinical characteristics of 45 multiple myeloma patients.
Supplementary Table 1. Details of the clinical characteristics of 45 multiple myeloma patients.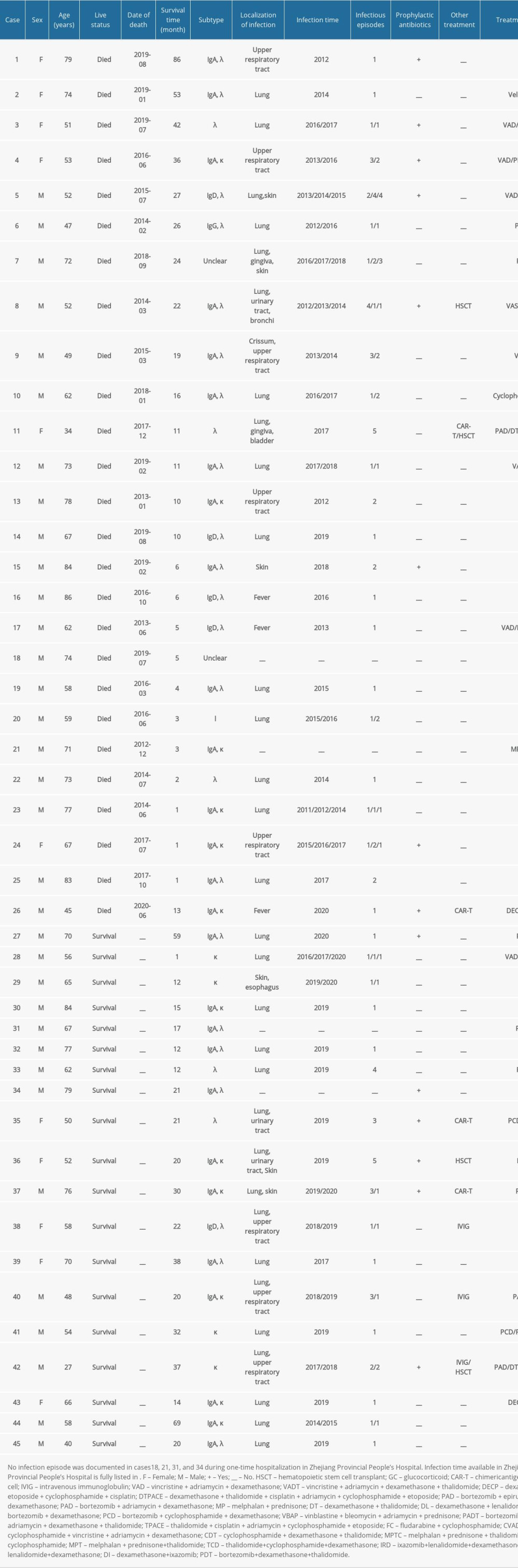
References
1. Bergstrom DJ, Kotb R, Louzada ML, Consensus guidelines on the diagnosis of multiple myeloma and related disorders: Recommendations of the Myeloma Canada Research Network Consensus Guideline Consortium: Clin Lymphoma Myeloma Leuk, 2020; 20(7); e352-67
2. Bray F, Ferlay J, Soerjomataram I, Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries: Cancer J Clin, 2018; 68(6); 394-424
3. Bergin K, McQuilten Z, Moore E, Myeloma in the real world: What is really happening?: Clin Lymphoma Myeloma Leuk, 2017; 17(3); 133-44.e131
4. Kazandjian D, Multiple myeloma epidemiology and survival: A unique malignancy: Sem Oncol, 2016; 43(6); 676-81
5. Srivastava S, Wood P, Secondary antibody deficiency – causes and approach to diagnosis: Clin Med (Lond), 2016; 16(6); 571-76
6. Patel SY, Carbone J, Jolles S, The expanding field of secondary antibody deficiency: Causes, diagnosis, and management: Front Immunol, 2019; 10; 33
7. Vacca A, Melaccio A, Sportelli A, Subcutaneous immunoglobulins in patients with multiple myeloma and secondary hypogammaglobulinemia: A randomized trial: Clin Immunol, 2018; 191; 110-15
8. Benbrahim O, Viallard JF, Choquet S, The use of octagam and gammanorm in immunodeficiency associated with hematological malignancies: A prospective study from 21 French hematology departments: Hematology, 2019; 24(1); 173-82
9. Na IK, Buckland M, Agostini C, Current clinical practice and challenges in the management of secondary immunodeficiency in hematological malignancies: Eur J Haematol, 2019; 102(6); 447-56
10. Edgar JDM, Richter AG, Huissoon AP, Prescribing immunoglobulin replacement therapy for patients with non-classical and secondary antibody deficiency: An analysis of the practice of clinical immunologists in the UK and Republic of Ireland: J Clin Immunol, 2018; 38(2); 204-13
11. Miller JM, Binnicker MJ, Campbell S, A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2018 update by the Infectious Diseases Society of America and the American Society for Microbiology: Clin Infect Dis, 2018; 67(6); e1-e94
12. Blot M, Boyer P, Samson M, Should mild hypogammaglobulinemia be managed as severe hypogammaglobulinemia? A study of 389 patients with secondary hypogammaglobulinemia: Eur J Int Med, 2014; 25(9); 837-42
13. Department of Health: Clinical guidelines for immunoglobulin use: Update to second edition, 2011; 32 https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/216671/dh_131107.pdf
14. Durie BGM, Hoering A, Abidi MH, Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem cell transplant (SWOG S0777): A randomised, open-label, phase 3 trial: Lancet, 2017; 389(10068); 519-27
15. Blimark C, Holmberg E, Mellqvist UH, Multiple myeloma and infections: A population-based study on 9253 multiple myeloma patients: Haematologica, 2015; 100(1); 107-13
16. Sørrig R, Klausen TW, Salomo M, Risk factors for infections in newly diagnosed multiple myeloma patients: A Danish retrospective nationwide cohort study: Eur J Haematol, 2019; 102(2); 182-90
17. Girmenia C, Cavo M, Offidani M, Management of infectious complications in multiple myeloma patients: Expert panel consensus-based recommendations: Blood Rev, 2019; 34; 84-94
18. Benbrahim O, Viallard JF, Choquet S, A French observational study describing the use of human polyvalent immunoglobulins in hematological malignancy-associated secondary immunodeficiency: Eur J Haematol, 2018; 101(1); 48-56
19. Maertens JA, Girmenia C, Brüggemann RJ, European guidelines for primary antifungal prophylaxis in adult haematology patients: Summary of the updated recommendations from the European Conference on Infections in Leukaemia: J Antimicrob Chemother, 2018; 73(12); 3221-30
20. Teh BW, Teng JC, Urbancic K, Invasive fungal infections in patients with multiple myeloma: A multi-center study in the era of novel myeloma therapies: Haematologica, 2015; 100(1); e28-31
21. National Blood Authority: Criteria for the clinical use of intravenous immunoglobulin in Australia, 2012 https://www.blood.gov.au/system/files/documents/NBA_IVIgCriteria_Second
22. Authority NB: National Policy Access to Government-Funded Immunoglobulin Products in Australia, 2019 https://www.blood.gov.au/ig-program
23. US FDA: Guidance for industry safety, efficacy, and pharmacokinetic studies to support marketing of immune globulin intravenous (human) as replacement therapy for primary humoral immunodeficiency, 2008 http://www.fda.gov/cber/gdlns/igivimmuno.htm
24. Reiser M, Borte M, Huscher D, Management of patients with malignancies and secondary immunodeficiencies treated with immunoglobulins in clinical practice: Long-term data of the SIGNS study: Eur J Haematol, 2017; 99(2); 169-77
25. Li L, Wang L, Multiple myeloma: What do we do about immunodeficiency?: J Cancer, 2019; 10(7); 1675-84
26. Liu J, Huang H, Li Y, Epidemiology and treatment of invasive fungal diseases in patients with multiple myeloma: Findings from a multicenter prospective study from China: Tumour Biol, 2016; 37(6); 7893-900
27. Perez EE, Orange JS, Bonilla F, Update on the use of immunoglobulin in human disease: Areview of evidence: J Allergy Clin Immunol, 2017; 139(3S); S1-46
28. Rodríguez-Otero P, Paiva B, Engelhardt M, Is immunotherapy here to stay in multiple myeloma?: Haematologica, 2017; 102(3); 423-32
29. Ghobrial I, Cruz CH, Garfall A, Immunotherapy in multiple myeloma: Accelerating on the path to the patient: Clin Lymphoma Myeloma Leuk, 2019; 19(6); 332-44
30. Liegel J, Avigan D, Rosenblatt J, Cellular immunotherapy as a therapeutic approach in multiple myeloma: Expert Rev Hematol, 2018; 11(7); 525-36
31. Baljevic M, Holstein SA, Present and future of immunotherapy in the management of multiple myeloma: J Oncol Pract, 2018; 14(7); 403-10
32. Monleón Bonet C, Waser N, Cheng K, A systematic literature review of the effects of immunoglobulin replacement therapy on the burden of secondaryimmunodeficiency diseases associated with hematological malignancies and stem cell transplants: Expert Rev Clin Immunol, 2020; 16(9); 911-21
Figures
 Figure 1. Flow chart of the selection of multiple myeloma (MM) patients with or without secondary immunodeficiency (SID).
Figure 1. Flow chart of the selection of multiple myeloma (MM) patients with or without secondary immunodeficiency (SID). Figure 2. Prognostic role of IgG <0.5 g/L in multiple myeloma (MM) patients. Kaplan-Meier curves of MM patients with IgG <5 g/L (n=45) vs IgG ≥5 g/L (n=103) (P<0.0001, log-rank test, Cox analysis).
Figure 2. Prognostic role of IgG <0.5 g/L in multiple myeloma (MM) patients. Kaplan-Meier curves of MM patients with IgG <5 g/L (n=45) vs IgG ≥5 g/L (n=103) (P<0.0001, log-rank test, Cox analysis). Tables
 Table 1. Past history of infection of multiple myeloma patients with and without secondary immunodeficiency.
Table 1. Past history of infection of multiple myeloma patients with and without secondary immunodeficiency. Table 2. Effectiveness of antimicrobial prophylaxis in the following 2 weeks or 1 month.
Table 2. Effectiveness of antimicrobial prophylaxis in the following 2 weeks or 1 month. Table 3. Clinical characteristics of multiple myeloma patients with and without secondary immunodeficiency.
Table 3. Clinical characteristics of multiple myeloma patients with and without secondary immunodeficiency. Table 4. Death rate of multiple myeloma patients with or without secondary immunodeficiency at 1 year and 3 years.
Table 4. Death rate of multiple myeloma patients with or without secondary immunodeficiency at 1 year and 3 years. Table 5. Univariate analysis of secondary immunodeficiency in patients with multiple myeloma (MM) and matched MM patients.
Table 5. Univariate analysis of secondary immunodeficiency in patients with multiple myeloma (MM) and matched MM patients. Table 6. Multivariate analysis of secondary immunodeficiency in patients with multiple myeloma (MM) and matched MM patients.
Table 6. Multivariate analysis of secondary immunodeficiency in patients with multiple myeloma (MM) and matched MM patients. Table 1. Past history of infection of multiple myeloma patients with and without secondary immunodeficiency.
Table 1. Past history of infection of multiple myeloma patients with and without secondary immunodeficiency. Table 2. Effectiveness of antimicrobial prophylaxis in the following 2 weeks or 1 month.
Table 2. Effectiveness of antimicrobial prophylaxis in the following 2 weeks or 1 month. Table 3. Clinical characteristics of multiple myeloma patients with and without secondary immunodeficiency.
Table 3. Clinical characteristics of multiple myeloma patients with and without secondary immunodeficiency. Table 4. Death rate of multiple myeloma patients with or without secondary immunodeficiency at 1 year and 3 years.
Table 4. Death rate of multiple myeloma patients with or without secondary immunodeficiency at 1 year and 3 years. Table 5. Univariate analysis of secondary immunodeficiency in patients with multiple myeloma (MM) and matched MM patients.
Table 5. Univariate analysis of secondary immunodeficiency in patients with multiple myeloma (MM) and matched MM patients. Table 6. Multivariate analysis of secondary immunodeficiency in patients with multiple myeloma (MM) and matched MM patients.
Table 6. Multivariate analysis of secondary immunodeficiency in patients with multiple myeloma (MM) and matched MM patients. Supplementary Table 1. Details of the clinical characteristics of 45 multiple myeloma patients.
Supplementary Table 1. Details of the clinical characteristics of 45 multiple myeloma patients. In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








