06 August 2021: Lab/In Vitro Research
Human Umbilical Cord Mesenchymal Stem Cell Therapy Mitigates Interstitial Cystitis by Inhibiting Mast Cells
Yuancheng Xu1ABDEF*, Fei Yang1BC, Juncong Xie1BC, Wenbiao Li1BC, Bolong Liu1BD, Jialiang Chen1BC, Honglu Ding1BC, Jiarong Cai1EFDOI: 10.12659/MSM.930001
Med Sci Monit 2021; 27:e930001
Abstract
BACKGROUND: Interstitial cystitis (IC) is a recurrent and chronic inflammatory disease that compromises patients’ quality of life. Effective treatments for IC are limited. This study aimed to evaluate the therapeutic potency of human umbilical cord-derived mesenchymal stem cells (UC-MSCs) in an IC-induced rat model and investigate the potential molecular mechanism in a mast cell model (rat basophilic leukemia cells, RBL-2H3) in treating IC in a coculture system.
MATERIAL AND METHODS: The rat model of IC was induced by cyclophosphamide (CYP). Rats were randomly divided into 3 groups: sham, IC+PBS, and IC+MSC. In the coculture system, RBL-2H3 cells were sensitized overnight to Compound 48/80 (C48/80), cocultured with UC-MSCs for 3 days, and collected for subsequent experiments. RBL-2H3 cells were randomly divided into 3 groups: sham, C48, and UC-MSCs (C48+MSC).
RESULTS: The UC-MSCs marked by thymidine analog 5-ethynyl-2-deoxyuridine (EdU) were transplanted in the treatment group, and were densely distributed in the bladder. Accordingly, the conscious cystometry was measured and the bladder tissues were harvested. Compared with the sham group, the treated IC rats exhibited shorter bladder voiding intervals (307±35 vs 217±37 s; P<0.01), more integral epithelia, and less collagen fiber aggregation, infiltration and degranulation of mast cells, and inflammatory cytokines in the bladder tissue. In the coculture system, compared with the C48 group, the UC-MSC-treated RBL-2H3 cells had suppressed degranulation.
CONCLUSIONS: UC-MSCs treatment showed a promising therapeutic effect on treating IC in vivo and in vitro. UC-MSCs inhibit mast cell degranulation in IC and could be a potential therapeutic target to ameliorate inflammation in IC.
Keywords: adult stem cells, Cell Degranulation, Cystitis, Interstitial, Mast Cells, Coculture Techniques, Cord Blood Stem Cell Transplantation, Cytokines, Mesenchymal Stem Cells, Umbilical Cord, Urination, p-Methoxy-N-methylphenethylamine
Background
Interstitial cystitis (IC) is characterized by urinary urgency, urinary frequency, pelvic pain, and nocturia without bacterial infection or identifiable pathology [1,2]. IC is a recurrent and chronic inflammatory disease that affects patient quality of life. Berry et al reported that, on average, 2.7% of women in the United States aged over 18 years had IC-associated symptoms between 2007 and 2009 [3]. Shin et al reported in 2020 that the prevalence of IC might be more than 2% in the female population [4]. Although the etiology of IC is not yet fully understood, generally, an increased number of mast cells infiltrate the bladder walls in IC and participate in the inflammatory process by releasing a wide range of mediators, such as histamine, 5-hydroxytryptamine (5-HT), and tumor necrosis factor-α (TNF-α), which promote bladder inflammation and cause pain [5–7]. Several available treatments primarily inhibit inflammation by suppressing mast cell degranulation. However, these treatments have a limited effect on preventing recurrence [8]. In the last decade, the alternative treatment of human umbilical cord-derived mesenchymal stem cell (UC-MSC) transplantation has been increasingly investigated, and may provide a potential nonsurgical intervention to fundamentally cure the inflammation of IC.
Song et al reported that MSC treatment successfully alleviated IC in an IC-induced rat model via the Wnt pathways by stimulating the regeneration of damaged bladder epithelium [9]. However, the present study demonstrated that UC-MSCs ameliorated IC in vivo and in vitro through inhibiting mast cell infiltration and degranulation.
This study aimed to investigate the therapeutic effect of UC-MSCs in a cyclophosphamide (CYP)-induced rat model of IC and a Compound 48/80 (C48/80)-induced mast cell model.
Material and Methods
UC-MSC CULTURE:
The present study used UC-MSCs in third passages, which had been collected and cultured according to the requirements of the Ethics Committee of the Department of Biotherapy Center of the Third Affiliated Hospital of Sun Yat-Sen University (Guangzhou, China). The UC-MSCs were cultured in a humidified 37°C, 5% CO2 incubator with Dulbecco’s modified Eagle’s medium (DMEM), containing 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin (all from Gibco; Thermo Fisher Scientific, Inc.). Surface markers of the UC-MSCs were confirmed by staining with the following antibodies: PE mouse anti-human CD31, FITC mouse anti-human CD34, PE mouse anti-human CD44, FITC mouse anti-human CD45, PE mouse anti-human CD73, FITC mouse anti-human CD90, and PE mouse anti-human CD105 (BD Biosciences, New Jersey, USA). Suitable isotype controls were also used. The UC-MSCs were stained with each antibody for 30 min at 4°C and analyzed with a flow cytometer (FACS Verse TM, BD Biosciences, USA) and Flow Jo 7.6 (Treestar, Ashland, Oregon, USA). The UC-MSCs in the third passage were used in this study [10].
RBL-2H3 CELL CULTURE AND ASSESSMENT OF DEGRANULATION:
The RBL-2H3 cells were purchased from the Cell Bank of the Chinese Academy of Science (Shanghai, China) and were cultured under the same conditions as the UC-MSCs. The RBL-2H3 cell line has been widely regarded as a mast cell line [11,12]. Briefly, the RBL-2H3 cells (2×105 cells/well in 24-well plates) were sensitized via exposure to C48/80 (Sigma-Aldrich; Merck KGaA) at 10 μg/mL overnight, which caused degranulation of the RBL-2H3 cells [11–13]. The concentration of β-hexosaminidase liberated from RBL-2H3, which was tested using an enzyme-linked immunosorbent assay (ELISA) kit (Elabscience Biotechnology Co., Ltd.), was used as a marker of degranulated mast cells [14].
IC-INDUCED RAT MODEL:
The present study was approved by the Ethics Committee of Zhongshan Medical School, Sun Yat-Sen University. A total of 45 female Sprague-Dawley rats (weight, 200–250 g) were purchased from the Institute of Experimental Animals of Sun Yat-Sen University. The rats were equally divided into a sham group, an IC+ phosphate-buffered saline (PBS) group, and an IC+MSC group (n=15 per group). The rats in the IC+PBS and IC+MSC groups received 3 doses of 75 mg/kg cyclophosphamide (CYP) on the first, third, and fifth day of the experiment by intraperitoneal injection to induce chronic urinary cystitis. This method has been widely used to establish rat models of IC [15,16]. The sham group received an intraperitoneal injection of PBS. After the first week, a single dose of UC-MSCs (1×106 cells) was injected through the tail vein in the IC+MSC group, and PBS was injected through the tail vein in the IC+PBS and sham groups.
5-ETHYNYL-2′-DEOXYURIDINE LABELING OF UC-MSCS:
The UC-MSCs were seeded in a 6-well plate with DMEM. Thymidine analog 5-ethynyl-2-deoxyuridine (EdU; Invitrogen; Thermo Fisher Scientific, Inc.) was added to the medium at a concentration of 20 μM [17]. After 24 h, the cells were immobilized with methanol, washed twice with PBS, incubated in 3% bovine serum albumin in PBS, and incubated in 0.5% Triton X-100 in PBS for 20 min at room temperature. Next, the cells were incubated with a freshly made Click-iT reaction cocktail containing Apollo 567 for 30 min at room temperature in the dark, counterstained with Hoechst 33342, and imaged by fluorescence microscopy (Nikon TE2000-U).
TRACKING OF TRANSPLANTED UC-MSCS:
Approximately 1×106 EdU-labeled UC-MSCs were transplanted into the rats via intravenous injection in the IC+MSC group. After 1 week, the bladders were harvested for histological examination. The bladder tissues were immobilized with methanol, washed twice with PBS, incubated in 3% bovine serum albumin in PBS, and incubated in 0.5% Triton X-100 in PBS for 20 min at room temperature. The bladder tissues were then incubated with newly made Click-iT reaction cocktail containing Apollo 567 for 30 min in the dark, counterstained with Hoechst 33342, and imaged by fluorescence microscopy (Nikon TE2000-U).
CONSCIOUS CYSTOMETRY:
Conscious cystometry was analyzed after 1 week by using a urodynamic measurement system UDS-120XLT (Laborie Medical Technologies, Inc.) [18,19]. An epidural catheter (diameter, 1 mm) was used as the cystometry catheter and was inserted into the bladders of the rats with a saline infusion rate of 6 mL/h in a syringe pump. The rats were in a supine position with secured limbs. The images of the curve displaying intravesical pressure were automatically generated by the urodynamic measurement system. Conscious cystometry tests were repeated 5 times in each rat.
HISTOCHEMICAL ANALYSIS:
After 1 week, the animals were killed, and the bladders were harvested for histological examination. After 24 h of fixation in 4% paraformaldehyde, each bladder was embedded in paraffin and sliced to 3-mm-thick sections using a microtome. Epithelial denudation was assessed by hematoxylin and eosin staining so that hemorrhage, submucosal edema, and vascular structure destruction could be observed. Mast cell infiltration was assessed by counting cells with positive toluidine blue staining (Beijing Solarbio Science & Technology). Tissue fibrosis was assessed using Masson’s trichrome staining (Servicebio). All procedures were performed according to the manufacturers’ protocols. Each slide was microscopically visualized, and 10 random light microscopic areas from the images (magnification ×200) were selected for the evaluation. Quantification of histochemical staining was measured by ImageJ software [20].
MEASUREMENT OF CYTOKINE LEVELS:
The concentrations of histamine, 5-HT, and TNF-α isolated from bladder tissues, and the concentrations of histamine, 5-HT, and β-hexosaminidase (β-hex) isolated from the supernatant of RBL-2H3 cells were measured using a standard ELISA kit (Elabscience Biotechnology Co., Ltd). The bladder samples were minced into small pieces and homogenized in PBS (1 g of tissue pieces with 9 mL of PBS) with a glass of homogenizer on ice. Subsequently, the homogenates were centrifuged at 5000×g for 5 min at 4°C to obtain the supernatant. The supernatant of RBL-2H3 cells was separated via centrifugation at 1500×g for 5 min at 4°C. Quantification was performed on Bio-Plex 200 readers at 450 nm, together with Bio-Plex Pro wash stations (Bio-Rad Laboratories, Inc.), and analyzed using Bio-Plex Manager software (version 3.0). The level of each cytokine was measured 8 times for each sample.
ELECTRON MICROSCOPY:
Mast cell degranulation in bladder tissue was observed via electron microscopy, which was performed as follows [21]: First, isolated bladders were immediately immobilized with 2.5% glutaraldehyde in 0.1 mol/L PBS for 6 h at 4˚C. The buffer was then replaced with PBS including 5% sucrose. The bladders were treated with 1% OsO4 in 0.1 mol/L PBS for 2 h at 4°C. Along with dehydration by a series of alcohol washing steps, the immobilized bladders were fixed with Quetol 812 resin (Nisshin EM). Ultra-thin sections were cut at 100 nm using a diamond knife and mounted on gold grids. Next, the sections were stained with saturated uranyl acetate in 50% ethanol solution for 30 min at room temperature, and with 0.1% lead citrate solution for 15 min at room temperature. The sections were measured with an HT7700 transmission electron microscope (Hitachi) at ×1500 magnification. Ten random microscopic areas were observed in each specimen.
COCULTURE SYSTEM:
The UC-MSCs (5×105) were cocultured in the upper compartment in 6-well Transwell plates (0.4 μm pore size insert; Corning Inc.), while RBL-2H3 cells (5×105) were seeded in the lower compartment. The sensitization of RBL-2H3 cells was achieved by exposure to C48/80 at 10 ug/mL overnight. The coculture system consisted of 3 groups: (I) sham group (RBL-2H3 cells alone); (II) C48 group (RBL-2H3 cells+C48/80); and (III) C48+MSC group (MSCs+RBL-2H3 cells+C48/80).
STATISTICAL ANALYSIS:
The statistical analysis was performed using SPSS software (version 22.0; IBM Corp., Armonk, NY, USA). As obtained data were normally distributed, according to the normality test and the Shapiro-Wilk normality test, parametric tests were used for estimation of statistical significance of results. All continuous variables were expressed as the mean±standard error of the mean. Comparisons of continuous variables among groups were analyzed using the two-sample
Results
IDENTIFICATION OF UC-MSCS:
The surface markers of the UC-MSCs were analyzed by flow cytometry. The negative markers included CD31, CD34, and CD45, and positive markers included CD29, CD44, CD73, CD90, and CD105. All data are expressed as percentages from FACS measurements (Supplementary Figure 1).
EDU-LABELED UC-MSCS AND TRACKING OF INTRAVENOUSLY TRANSPLANTED UC-MSCS:
The UC-MSCs in EdU-containing media exhibited intense red fluorescence in the nuclei when stained with Apollo 567 (Figure 1A). The detection of the EdU was confirmed by the blue fluorescent nuclear label, Hoechst 33342. The fluorescent probe of EdU was nuclear-specific, presenting with clear co-localization with Hoechst 33342 staining (Figure 1B). The EdU-labeled UC-MSCs were transplanted into the IC-induced rats by injection through the tail vein. After 1 week, the rat bladders were harvested for histological examination, which identified the presence of the EdU-labeled cells in the submucosal connective tissue of the rat bladders (Figure 1E–1H).
CONSCIOUS CYSTOMETRY:
The contraction intervals were significantly longer in the IC+MSC group (307±35 s) than in the IC+PBS group (217±37 s; P<0.01, with post hoc adjustment), but were similar to those of the sham group (319±18 s; P=0.27, with post hoc adjustment). The non-voiding contractions were significantly more stable in the IC+MSC group than in the IC+PBS group, but were similar to those of the sham group. There were no differences of micturition pressure among the 3 groups. This demonstrated that UC-MSC therapy restored bladder voiding function in the IC-induced rat model (Figure 2).
HISTOLOGICAL FINDINGS AND INFLAMMATORY CYTOKINE ASSAY FOLLOWING UC-MSC TREATMENTS:
Our results showed much less submucosal edema and hemorrhage, fewer mast cells, and fewer collagen fibers in the IC+MSC group than in the IC+PBS group, and the levels of the IC+MSC group were similar to those of the sham group (Figure 3A, 3C). The concentrations of inflammatory cytokines, including histamine, 5-HT, and TNF-α, in bladder tissue were significantly lower in the IC+MSC group than in the IC+PBS group, and the concentrations of the IC+MSC group were similar to those of the sham group (Figure 3B).
ELECTRON MICROSCOPY:
Although both non-degranulated and degranulated mast cells were measured in the sham, IC+MSC, and IC+PBS groups, the most degranulated mast cells were found in the IC+PBS group. When viewed under light microscopy, in the submucosal layer of the rat bladder, the IC+MSC group had fewer mast cells than did the IC+PBS group, but had a similar amount as the sham group, which was consistent with the toluidine blue staining results (Figure 3C). Furthermore, under electron microscopy, we study found that non-degranulated mast cells contained many small granules (Figure 4A), and degranulated mast cells exhibited vacant spaces, where the granules had been lost and released into the extracellular matrix (Figure 4B).
CYTOKINE PRODUCTION IN COCULTURE SYSTEM:
The β-hex ELISA demonstrated that β-hex secreted by the RBL-2H3 cells increased significantly following overnight exposure to C48/80 (Figure 5A). Histamine and 5-HT were shown to be significantly lower in the C48+MSC group than in the C48 group, when using ELISA (Figure 5A, 5C).
Discussion
The major findings of our study were as follows: In the CYP-induced rat model of IC, UC-MSCs showed promising therapeutic effects on IC. In a urodynamic aspect, UC-MSCs restored the voiding function of IC rats. In tissues, UC-MSCs markedly alleviated inflammation in the connective tissue of the IC rat bladder, including edema, hemorrhage, mast cell infiltration, and formation of fibrosis. On the cellular and cytokine level, UC-MSCs inhibited the degranulation of mast cells and the generation of inflammatory cytokines, including histamine, 5-HT, and TNF-α, which was essential to inhibit the degranulation of RBL-2H3 cells and the resulting inflammatory cascade.
In the past decade, stem cell treatment has been proposed as a viable treatment strategy [22–24]. A small number of studies assessed the therapeutic use of stem cells in IC [9,18]. A study by Xie et al focused on the coculture experimental system of UC-MSCs with SV-HUC-1 in a rat model of epithelial cells of the bladder mucosa, which aimed to determine whether the UC-MSC treatment could restore the epithelial cells in bladder mucosa, which was most related to the thesis of epithelium denudation in IC. However, in the present study, the coculture experimental system of UC-MSCs with RBL-2H3 cells (the model of mast cells) aimed to determine whether UC-MSC treatment contributes to inhibiting the inflammation of mast cells in IC; the present study was most related to the thesis of mast cell infiltration and degranulation in IC. Consequently, as the research hypothesis of these 2 studies were significantly different, the coculture systems of the 2 studies were also distinct (UC-MSC+SV-HUC-1s [the model of epithelial cell in bladder mucosa] vs UC-MSC+RBL-2H3 cells [the model of mast cells]). To date, no study has reported the relative mechanism underlying mast cells in IC. In the present study, we were the first to demonstrate that UC-MSCs mitigate inflammation of mast cells in the disease setting of IC, which has been investigated in some inflammatory diseases but not yet in IC [25–27]. As our hypothesis was proven true, UC-MSCs may potentially become a novel therapeutic target of IC. Notably, the therapeutic effects of UC-MSC in the study by Xie et at and the present study focused on epithelium denudation and mast cell inflammation, respectively, which were remarkably different, yet potentially complementary.
We tried to explore the relationship between UC-MSC treatment and mast cells in IC in 2 steps.
First, we demonstrated that UC-MSC treatment inhibited mast cell infiltration and degranulation in IC. Toluidine blue staining demonstrated that UC-MSC treatment mitigated the infiltration of mast cells in the IC-induced rat bladder. Transmission electron microscopy examination demonstrated that UC-MSC treatment suppressed the degranulation of mast cells. In degranulated mast cells, the granules were present and large vacuoles existed in the cytoplasm, which means the degranulated mast cells had generated and secreted cytokines. This was in accordance with the results that UC-MSC treatment decreased the inflammatory cytokine concentration of the IC rat bladder, particularly histamine and 5-HT, which were specifically associated with mast cell degranulation.
Second, we showed that the treatment effect of UC-MSCs on the mast cells in IC results from the paracrine effect. We showed that UC-MSCs inhibited the degranulation of RBL-2H3 cells (mast cell line) and decreased the secretion of inflammatory cytokines from RBL-2H3 cells, particularly histamine and 5-HT, which are important triggers of mast cell degranulation. UC-MSCs play an essential role in inhibiting mast cells, which was demonstrated in other diseases [25–27], but we were the first to demonstrate this in the disease setting of IC.
In an effort to determine the etiology of IC, a number of studies have reported that epithelium denudation, collagen fiber aggregation, and mast cell infiltration and degranulation are essential in the pathophysiology of IC [28]. Song et al reported that treatment with MSCs successfully alleviated IC in a rat model induced by hydrochloric acid via the Wnt pathway, by stimulating the regeneration of damaged bladder epithelium [9]. The work of Song et al also partially involved mast cell infiltration, but did not include mast cell-related mechanisms. Mast cell infiltration is just a single aspect of the therapeutic effect of MSC treatment. However, the focus of our work was remarkably different from that of Song et al. In the present study, not only mast cell infiltration, but also mast cell degranulation, as the major pathogenesis of IC, was comprehensively investigated. Fundamentally, we tried to explore the role of mast cell infiltration and degranulation in IC and the mechanism of the treatment effect of UC-MSCs on these mast cell activities.
There were some limitations in our study. First, we did not measure β-hexosaminidase in the UC-MSC-treated groups. However, histamine and 5-HT, which are hallmarks of degranulation, were measured to identify the inhibiting effect of UC-MSC on mast cell degranulation. Second, there may be some signaling pathways playing an important role in the inhibiting effect of UC-MSC on mast cell activities. We attempted to identify the underlying role molecular activities play in the inhibition of UC-MSC on mast cells.
Conclusions
The present study showed that UC-MSCs therapy successfully alleviated IC in vivo and in vitro by inhibiting mast cell degranulation. This observation provides a novel insight into UC-MSC therapy for treating IC.
Figures
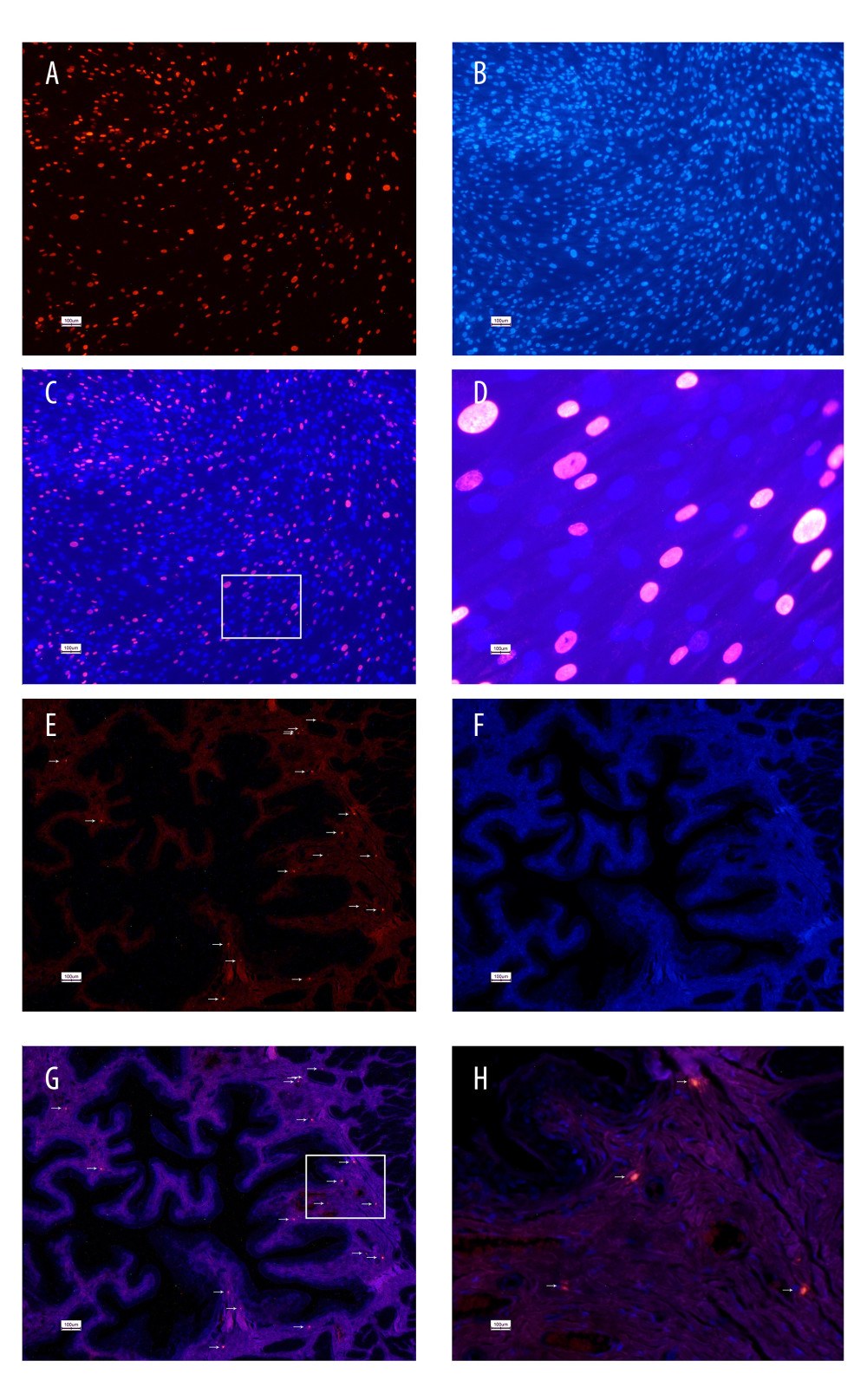 Figure 1. 5-Ethynyl-2-deoxyuridine (EdU) labeling of UC-MSCs in vitro and tracking EdU-labeled human umbilical cord-derived mesenchymal stem cells (UC-MSCs) in vivo. UC-MSCs labeled by EdU and stained with Apollo 567 (A, red fluorescence) and Hoechst 33342 (B, blue fluorescence). EdU-labeled UC-MSCs stained with Apollo 567 in harvested rat bladders (E, red fluorescence), Hoechst 33342 (F, blue fluorescence). The boxed areas in the ×40 magnified images in (C) and (G) were amplified ×200 in the corresponding (D) and (H), respectively. White arrows point to nuclei that are presented in the graphs.
Figure 1. 5-Ethynyl-2-deoxyuridine (EdU) labeling of UC-MSCs in vitro and tracking EdU-labeled human umbilical cord-derived mesenchymal stem cells (UC-MSCs) in vivo. UC-MSCs labeled by EdU and stained with Apollo 567 (A, red fluorescence) and Hoechst 33342 (B, blue fluorescence). EdU-labeled UC-MSCs stained with Apollo 567 in harvested rat bladders (E, red fluorescence), Hoechst 33342 (F, blue fluorescence). The boxed areas in the ×40 magnified images in (C) and (G) were amplified ×200 in the corresponding (D) and (H), respectively. White arrows point to nuclei that are presented in the graphs. 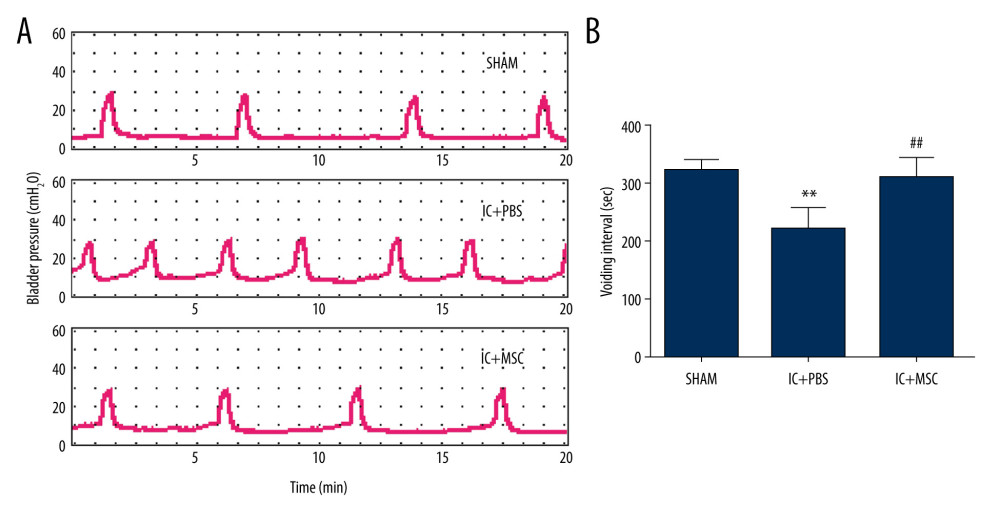 Figure 2. Human umbilical cord-derived mesenchymal stem cells (UC-MSCs) restored bladder function in the interstitial cystitis (IC)-induced rat model. (A) Conscious cystometry results. (B) Micturition intervals of the IC+MSC group (307±35 s) were significantly longer than that of the IC+PBS group (217±37 s; P<0.01, with post hoc adjustment), but similar to that of the sham group (319±18 s; P=0.27, with post hoc adjustment). ** P<0.01 vs sham group; ## P<0.01 vs IC+PBS group.
Figure 2. Human umbilical cord-derived mesenchymal stem cells (UC-MSCs) restored bladder function in the interstitial cystitis (IC)-induced rat model. (A) Conscious cystometry results. (B) Micturition intervals of the IC+MSC group (307±35 s) were significantly longer than that of the IC+PBS group (217±37 s; P<0.01, with post hoc adjustment), but similar to that of the sham group (319±18 s; P=0.27, with post hoc adjustment). ** P<0.01 vs sham group; ## P<0.01 vs IC+PBS group. 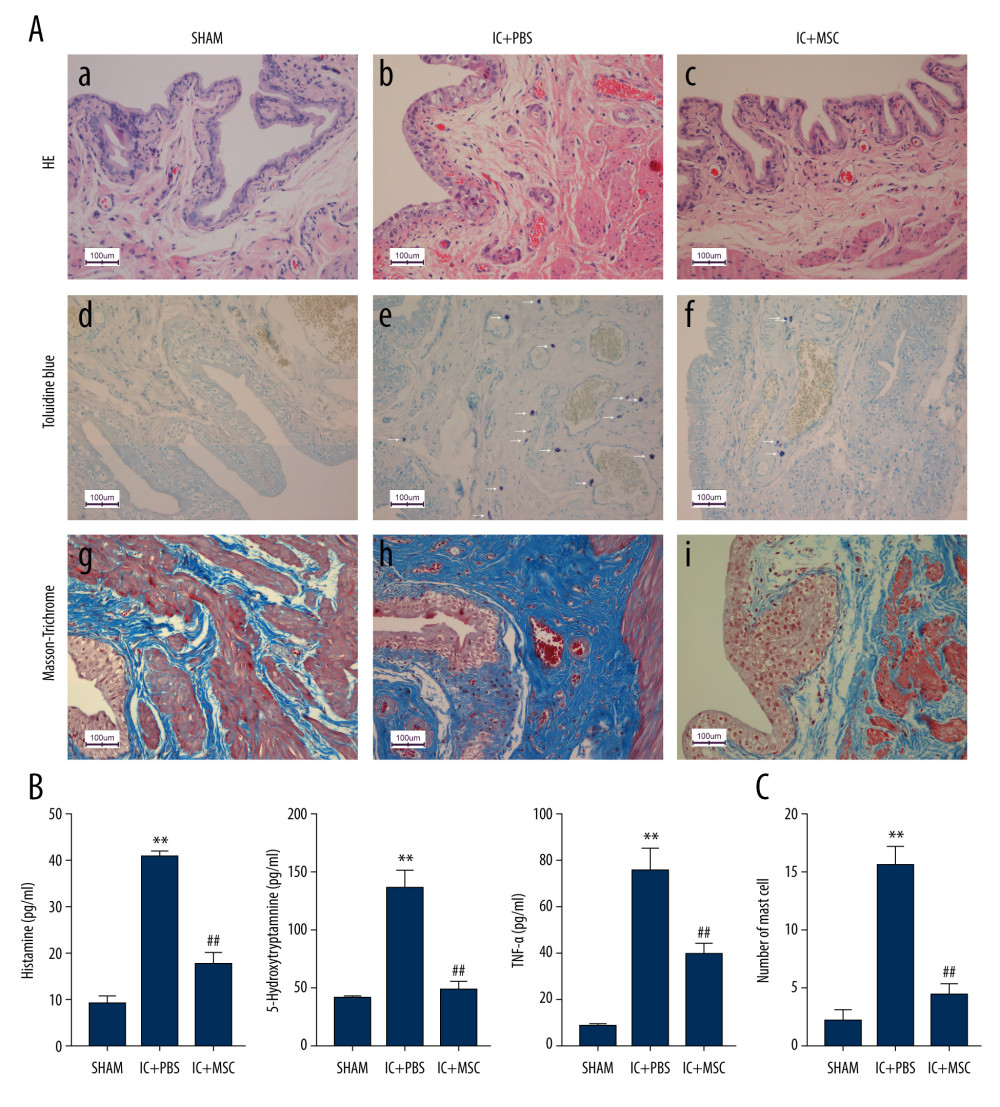 Figure 3. Human umbilical cord-derived mesenchymal stem cell (UC-MSC) treatment ameliorated inflammation in the interstitial cystitis (IC)-induced rat model. (A) (a–c): Hematoxylin and eosin staining; (d–f): Toluidine blue staining; (g–i): Masson’s trichrome staining. Hemorrhage, submucosal edema, vascular structure destruction (a–c) were more severe in the IC+PBS group, and recovered better in the IC+MSC group. The mast cell infiltration (white arrows in e and f) could be more easily observed in the IC+PBS group, compared with in the IC+MSC and sham groups. Tissue fibrosis (blue straining in g–i) was significantly decreased below the urothelium in the IC+MSC group (collagenous fiber, 24.52%) compared with in the IC+PBS group (collagenous fiber, 50.19%), but were similar to the sham group (collagenous fiber, 20.33%). Magnification ×200. (B) Comparison of histamine, 5-hydroxytryptamine (5-HT), and TNF-α among the 3 groups in bar charts. (C) Comparison of the number of infiltrating mast cells in the bladder of rats among the 3 groups in bar charts. ** P<0.01 vs sham group; ## P<0.01 vs IC+PBS group.
Figure 3. Human umbilical cord-derived mesenchymal stem cell (UC-MSC) treatment ameliorated inflammation in the interstitial cystitis (IC)-induced rat model. (A) (a–c): Hematoxylin and eosin staining; (d–f): Toluidine blue staining; (g–i): Masson’s trichrome staining. Hemorrhage, submucosal edema, vascular structure destruction (a–c) were more severe in the IC+PBS group, and recovered better in the IC+MSC group. The mast cell infiltration (white arrows in e and f) could be more easily observed in the IC+PBS group, compared with in the IC+MSC and sham groups. Tissue fibrosis (blue straining in g–i) was significantly decreased below the urothelium in the IC+MSC group (collagenous fiber, 24.52%) compared with in the IC+PBS group (collagenous fiber, 50.19%), but were similar to the sham group (collagenous fiber, 20.33%). Magnification ×200. (B) Comparison of histamine, 5-hydroxytryptamine (5-HT), and TNF-α among the 3 groups in bar charts. (C) Comparison of the number of infiltrating mast cells in the bladder of rats among the 3 groups in bar charts. ** P<0.01 vs sham group; ## P<0.01 vs IC+PBS group. 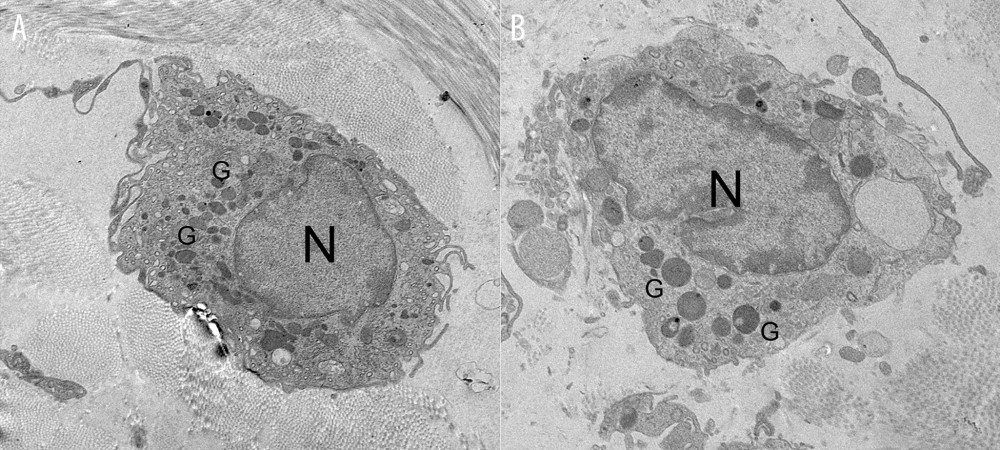 Figure 4. Representative transmission electron microscope images. (A) The non-degranulated mast cell. (B) The degranulated mast cell. The intracellular granules in degranulated mast cells were larger than those of non-degranulated mast cells. Many large granules released by degranulated mast cells appeared in the extracellular matrix around degranulated mast cells, but were absent around non-degranulated mast cells.
Figure 4. Representative transmission electron microscope images. (A) The non-degranulated mast cell. (B) The degranulated mast cell. The intracellular granules in degranulated mast cells were larger than those of non-degranulated mast cells. Many large granules released by degranulated mast cells appeared in the extracellular matrix around degranulated mast cells, but were absent around non-degranulated mast cells. 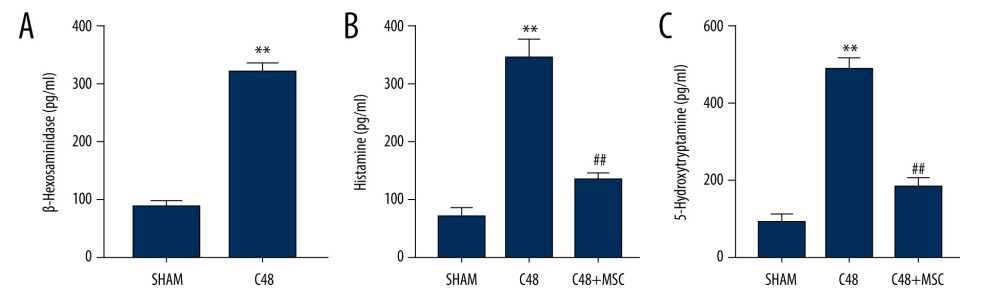 Figure 5. β-Hexosaminidase secretion was evaluated by ELISA. (A) β-Hexosaminidase assay: the concentration of β-hex released from the sensitized RBL-2H3 cells was significantly higher than that of normal RBL-2H3 cells. ** P<0.01 vs sham group. (B, C) Histamine and 5-hydroxytryptamine (5-HT) levels in the C48+MSC group were significantly lower than in the C48 group. ** P<0.01 vs sham group; ## P<0.01 vs C48 group.
Figure 5. β-Hexosaminidase secretion was evaluated by ELISA. (A) β-Hexosaminidase assay: the concentration of β-hex released from the sensitized RBL-2H3 cells was significantly higher than that of normal RBL-2H3 cells. ** P<0.01 vs sham group. (B, C) Histamine and 5-hydroxytryptamine (5-HT) levels in the C48+MSC group were significantly lower than in the C48 group. ** P<0.01 vs sham group; ## P<0.01 vs C48 group. References
1. Patnaik SS, Lagana AS, Vitale SG, Etiology, pathophysiology and biomarkers of interstitial cystitis/painful bladder syndrome: Arch Gynecol Obstet, 2017; 295(6); 1341-59
2. Kim A, Han JY, Ryu CM, Histopathological characteristics of interstitial cystitis/bladder pain syndrome without Hunner lesion: Histopathology, 2017; 71(3); 415-24
3. Berry SH, Elliott MN, Suttorp M, Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States: J Urol, 2011; 186(2); 540-44
4. Shin JH, Ryu CM, Yu HY, Shin DM, Current and future directions of stem cell therapy for bladder dysfunction: Stem Cell Rev, 2020; 16(1); 82-93
5. Gamper M, Regauer S, Welter J, Are mast cells still good biomarkers for bladder pain syndrome/interstitial cystitis?: J Urol, 2015; 193(6); 1994-2000
6. Akiyama Y, Maeda D, Morikawa T, Digital quantitative analysis of mast cell infiltration in interstitial cystitis: Neurourol Urodyn, 2018; 37(2); 650-57
7. Coates MD, Tekin I, Vrana KE, Review article: the many potential roles of intestinal serotonin (5-hydroxytryptamine, 5-HT) signalling in inflammatory bowel disease: Aliment Pharmacol Ther, 2017; 46(6); 569-80
8. Ostardo E, Impellizzeri D, Cervigni M, Adelmidrol + sodium hyaluronate in IC/BPS or conditions associated to chronic urothelial inflammation. A translational study: Pharmacol Res, 2018; 134; 16-30
9. Song M, Lim J, Yu HY, Mesenchymal stem cell therapy alleviates interstitial cystitis by activating Wnt signaling pathway: Stem Cells Dev, 2015; 24(14); 1648-57
10. Zhou X, Gu J, Gu Y, Human umbilical cord-derived mesenchymal stem cells improve learning and memory function in hypoxic-ischemic brain-damaged rats via an IL-8-mediated secretion mechanism rather than differentiation pattern induction: Cell Physiol Biochem, 2015; 35(6); 2382-401
11. Passante E, Frankish N, The RBL-2H3 cell line: Its provenance and suitability as a model for the mast cell: Inflamm Res, 2009; 58(11); 737-45
12. Palmer RK, Hutchinson LM, Burpee BT, Antibacterial agent triclosan suppresses RBL-2H3 mast cell function: Toxicol Appl Pharmacol, 2012; 258(1); 99-108
13. Gao Y, Hou R, Fei Q, The three-herb formula Shuang-Huang-Lian stabilizes mast cells through activation of mitochondrial calcium uniporter: Sci Rep, 2017; 7; 38736
14. Kandhare AD, Aswar UM, Mohan V, Ameliorative effects of type-A procyanidins polyphenols from cinnamon bark in compound 48/80-induced mast cell degranulation: Anat Cell Biol, 2017; 50(4); 275-83
15. Chen YT, Chiang HJ, Chen CH, Melatonin treatment further improves adipose-derived mesenchymal stem cell therapy for acute interstitial cystitis in rat: J Pineal Res, 2014; 57(3); 248-61
16. Auge C, Chene G, Dubourdeau M, Relevance of the cyclophosphamide-induced cystitis model for pharmacological studies targeting inflammation and pain of the bladder: Eur J Pharmacol, 2013; 707(1–3); 32-40
17. Lin G, Huang YC, Shindel AW, Labeling and tracking of mesenchymal stromal cells with EdU: Cytotherapy, 2009; 11(7); 864-73
18. Xie J, Liu B, Chen J, Umbilical cord-derived mesenchymal stem cells alleviated inflammation and inhibited apoptosis in interstitial cystitis via AKT/mTOR signaling pathway: Biochem Biophys Res Commun, 2018; 495(1); 546-52
19. Cheng WM, Fan YH, Lin ATL, Urodynamic characteristics might be variable in bladder pain syndrome/interstitial cystitis patients with different non-bladder co-morbid conditions: J Chin Med Assoc, 2018; 81(3); 248-54
20. Ruifrok AC, Johnston DA, Quantification of histochemical staining by color deconvolution: Anal Quant Cytol Histol, 2001; 23(4); 291-99
21. Michishita M, Tomita K, Yano K, Mast cell accumulation and degranulation in rat bladder with partial outlet obstruction: Adv Ther, 2015; 32(Suppl 1); 16-28
22. Harris VK, Stark J, Vyshkina T, Phase I trial of intrathecal mesenchymal stem cell-derived neural progenitors in progressive multiple sclerosis: EBioMedicine, 2018; 29; 23-30
23. Dave M, Jaiswal P, Cominelli F, Mesenchymal stem/stromal cell therapy for inflammatory bowel disease: an updated review with maintenance of remission: Curr Opin Gastroenterol, 2017; 33(1); 59-68
24. Zheng G, Huang R, Qiu G, Mesenchymal stromal cell-derived extracellular vesicles: Regenerative and immunomodulatory effects and potential applications in sepsis: Cell Tissue Res, 2018; 374(1); 1-15
25. Fernando J, Faber TW, Pullen NA, Genotype-dependent effects of TGF-β1 on mast cell function: Targeting the Stat5 pathway: J Immunol, 2013; 191(9); 4505
26. Han KH, Kim AK, Kim MH, Enhancement of angiogenic effects by hypoxia-preconditioned human umbilical cord-derived mesenchymal stem cells in a mouse model of hindlimb ischemia: Cell Biol Int, 2016; 40(1); 27-35
27. Kim HS, Yun JW, Shin TH, Human umbilical cord blood mesenchymal stem cell-derived PGE2 and TGF-beta1 alleviate atopic dermatitis by reducing mast cell degranulation: Stem Cells, 2015; 33(4); 1254-66
28. Shie JH, Kuo HC, Higher levels of cell apoptosis and abnormal E-cadherin expression in the urothelium are associated with inflammation in patients with interstitial cystitis/painful bladder syndrome: BJU Int, 2011; 108(2 Pt 2); E136-41
Figures
 Figure 1. 5-Ethynyl-2-deoxyuridine (EdU) labeling of UC-MSCs in vitro and tracking EdU-labeled human umbilical cord-derived mesenchymal stem cells (UC-MSCs) in vivo. UC-MSCs labeled by EdU and stained with Apollo 567 (A, red fluorescence) and Hoechst 33342 (B, blue fluorescence). EdU-labeled UC-MSCs stained with Apollo 567 in harvested rat bladders (E, red fluorescence), Hoechst 33342 (F, blue fluorescence). The boxed areas in the ×40 magnified images in (C) and (G) were amplified ×200 in the corresponding (D) and (H), respectively. White arrows point to nuclei that are presented in the graphs.
Figure 1. 5-Ethynyl-2-deoxyuridine (EdU) labeling of UC-MSCs in vitro and tracking EdU-labeled human umbilical cord-derived mesenchymal stem cells (UC-MSCs) in vivo. UC-MSCs labeled by EdU and stained with Apollo 567 (A, red fluorescence) and Hoechst 33342 (B, blue fluorescence). EdU-labeled UC-MSCs stained with Apollo 567 in harvested rat bladders (E, red fluorescence), Hoechst 33342 (F, blue fluorescence). The boxed areas in the ×40 magnified images in (C) and (G) were amplified ×200 in the corresponding (D) and (H), respectively. White arrows point to nuclei that are presented in the graphs. Figure 2. Human umbilical cord-derived mesenchymal stem cells (UC-MSCs) restored bladder function in the interstitial cystitis (IC)-induced rat model. (A) Conscious cystometry results. (B) Micturition intervals of the IC+MSC group (307±35 s) were significantly longer than that of the IC+PBS group (217±37 s; P<0.01, with post hoc adjustment), but similar to that of the sham group (319±18 s; P=0.27, with post hoc adjustment). ** P<0.01 vs sham group; ## P<0.01 vs IC+PBS group.
Figure 2. Human umbilical cord-derived mesenchymal stem cells (UC-MSCs) restored bladder function in the interstitial cystitis (IC)-induced rat model. (A) Conscious cystometry results. (B) Micturition intervals of the IC+MSC group (307±35 s) were significantly longer than that of the IC+PBS group (217±37 s; P<0.01, with post hoc adjustment), but similar to that of the sham group (319±18 s; P=0.27, with post hoc adjustment). ** P<0.01 vs sham group; ## P<0.01 vs IC+PBS group. Figure 3. Human umbilical cord-derived mesenchymal stem cell (UC-MSC) treatment ameliorated inflammation in the interstitial cystitis (IC)-induced rat model. (A) (a–c): Hematoxylin and eosin staining; (d–f): Toluidine blue staining; (g–i): Masson’s trichrome staining. Hemorrhage, submucosal edema, vascular structure destruction (a–c) were more severe in the IC+PBS group, and recovered better in the IC+MSC group. The mast cell infiltration (white arrows in e and f) could be more easily observed in the IC+PBS group, compared with in the IC+MSC and sham groups. Tissue fibrosis (blue straining in g–i) was significantly decreased below the urothelium in the IC+MSC group (collagenous fiber, 24.52%) compared with in the IC+PBS group (collagenous fiber, 50.19%), but were similar to the sham group (collagenous fiber, 20.33%). Magnification ×200. (B) Comparison of histamine, 5-hydroxytryptamine (5-HT), and TNF-α among the 3 groups in bar charts. (C) Comparison of the number of infiltrating mast cells in the bladder of rats among the 3 groups in bar charts. ** P<0.01 vs sham group; ## P<0.01 vs IC+PBS group.
Figure 3. Human umbilical cord-derived mesenchymal stem cell (UC-MSC) treatment ameliorated inflammation in the interstitial cystitis (IC)-induced rat model. (A) (a–c): Hematoxylin and eosin staining; (d–f): Toluidine blue staining; (g–i): Masson’s trichrome staining. Hemorrhage, submucosal edema, vascular structure destruction (a–c) were more severe in the IC+PBS group, and recovered better in the IC+MSC group. The mast cell infiltration (white arrows in e and f) could be more easily observed in the IC+PBS group, compared with in the IC+MSC and sham groups. Tissue fibrosis (blue straining in g–i) was significantly decreased below the urothelium in the IC+MSC group (collagenous fiber, 24.52%) compared with in the IC+PBS group (collagenous fiber, 50.19%), but were similar to the sham group (collagenous fiber, 20.33%). Magnification ×200. (B) Comparison of histamine, 5-hydroxytryptamine (5-HT), and TNF-α among the 3 groups in bar charts. (C) Comparison of the number of infiltrating mast cells in the bladder of rats among the 3 groups in bar charts. ** P<0.01 vs sham group; ## P<0.01 vs IC+PBS group. Figure 4. Representative transmission electron microscope images. (A) The non-degranulated mast cell. (B) The degranulated mast cell. The intracellular granules in degranulated mast cells were larger than those of non-degranulated mast cells. Many large granules released by degranulated mast cells appeared in the extracellular matrix around degranulated mast cells, but were absent around non-degranulated mast cells.
Figure 4. Representative transmission electron microscope images. (A) The non-degranulated mast cell. (B) The degranulated mast cell. The intracellular granules in degranulated mast cells were larger than those of non-degranulated mast cells. Many large granules released by degranulated mast cells appeared in the extracellular matrix around degranulated mast cells, but were absent around non-degranulated mast cells. Figure 5. β-Hexosaminidase secretion was evaluated by ELISA. (A) β-Hexosaminidase assay: the concentration of β-hex released from the sensitized RBL-2H3 cells was significantly higher than that of normal RBL-2H3 cells. ** P<0.01 vs sham group. (B, C) Histamine and 5-hydroxytryptamine (5-HT) levels in the C48+MSC group were significantly lower than in the C48 group. ** P<0.01 vs sham group; ## P<0.01 vs C48 group.
Figure 5. β-Hexosaminidase secretion was evaluated by ELISA. (A) β-Hexosaminidase assay: the concentration of β-hex released from the sensitized RBL-2H3 cells was significantly higher than that of normal RBL-2H3 cells. ** P<0.01 vs sham group. (B, C) Histamine and 5-hydroxytryptamine (5-HT) levels in the C48+MSC group were significantly lower than in the C48 group. ** P<0.01 vs sham group; ## P<0.01 vs C48 group. In Press
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








