11 April 2021: Clinical Research
Analysis of Risk Factors for Thromboembolic Events in 88 Patients with COVID-19 Pneumonia in Wuhan, China: A Retrospective Descriptive Report
Wenyu Wang1CDE, Qingfeng Sun2CDF, Yongxia Bao1BC, Ming Liang3BC, Qingwei Meng4BC, Hong Chen1BC, Jianing Li1BC, Hongliang Wang5BC, Huiqing Li6BC, Ying Shi7BC, Zhaoguo Li1BF, Xinyan Wang1F, Shuai Zhao1F, Hongwei Wang1F, Jinling Xiao1F, Liyan Chen3F, Yan Zheng8F, Di Wang8F, Kaiyu Han1AEG*DOI: 10.12659/MSM.929708
Med Sci Monit 2021; 27:e929708
Abstract
BACKGROUND: Since the outbreak of COVID-19 in December 2019, there have been 96 623 laboratory-confirmed cases and 4784 deaths by December 29 in China. We aimed to analyze the risk factors and the incidence of thrombosis from patients with confirmed COVID-19 pneumonia.
MATERIAL AND METHODS: Eighty-eight inpatients with confirmed COVID-19 pneumonia were reported (31 critical cases, 33 severe cases, and 24 common cases). The thrombosis risk factor assessment, laboratory results, ultrasonographic findings, and prognoses of these patients were analyzed, and compared among groups with different severity.
RESULTS: Nineteen of the 88 cases developed DVT (12 critical cases, 7 severe cases, and no common cases). In addition, among the 18 patients who died, 5 were diagnosed with DVT. Positive correlations were observed between the increase in D-dimer level (≥5 µg/mL) and the severity of COVID-19 pneumonia (r=0.679, P<0.01), and between the high Padua score (≥4) and the severity (r=0.799, P<0.01). In addition, the CRP and LDH levels on admission had positive correlations with the severity of illness (CRP: r=0.522, P<0.01; LDH: r=0.600, P<0.01). A negative correlation was observed between the lymphocyte count on admission and the severity of illness (r=-0.523, P<0.01). There was also a negative correlation between the lymphocyte count on admission and mortality in critical patients (r=-0.499, P<0.01). Univariable logistic regression analysis showed that the occurrence of DVT was positively correlated with disease severity (crude odds ratio: 3.643, 95% CI: 1.218-10.896, P<0.05).
CONCLUSIONS: Our report illustrates that critically or severely ill patients have an associated high D-dimer value and high Padua score, and illustrates that a low threshold to screen for DVT may help improve detection of thromboembolism in these groups of patients, especially in asymptomatic patients. Our results suggest that early administration of prophylactic anticoagulant would benefit the prognosis of critical patients with COVID-19 pneumonia and would likely reduce thromboembolic rates.
Keywords: COVID-19, Embolism and Thrombosis, Asymptomatic Diseases, COVID-19, COVID-19 Testing, Fibrin Fibrinogen Degradation Products, Hospital Mortality, Incidence, Lower Extremity, Patient Admission, Risk Assessment, Risk Factors, SARS-CoV-2, Severity of Illness Index, Ultrasonography, Venous Thrombosis
Background
Since the outbreak of COVID-19 in December 2019, there have been 96 623 laboratory-confirmed cases and 4784 deaths by December 29 in China [1]. Over 70 million people were diagnosed with COVID-19 globally. Over 1.6 million people died directly because of the COVID-19 [2]. It was identified that the initial symptoms of COVID-19 pneumonia are usually fever, dry cough, and fatigue, dyspnea, and/or hypoxemia and these often occur in severe patients 1 week after onset. Serious cases can rapidly progress to acute respiratory distress syndrome, coagulation dysfunction, and disseminated intravascular coagulation (DIC). Rapid deterioration and sudden death occurring in critical patients highlight the high risk of thrombosis [3]. Clinical observations have observed a hypercoagulable state in severely and critically ill patients with COVID-19 pneumonia [4–6], and the diagnostic rate of ultrasound was 22.7% for deep vein thrombosis (DVT) [7].
Several hypotheses have been proposed for the incidence of thrombosis among patients infected by COVID-19. First, inflammatory mediators, respiratory failure, and local hypoxia by venous stasis may lead to vascular endothelial activation and consequent endothelial injury [8]. Subsequently, a dysfunctional endothelium can initiate thrombosis and promote atherosclerosis [9]. Second, the massive release of inflammatory mediators in patients with severe or critical COVID-19 pneumonia, and the application of glucocorticoids in patients with excessively activated inflammatory responses, can contribute to hypercoagulability [10]. Water loss caused by sweating during fever, body fluid loss caused by vomiting, and/or diarrhea can also lead to hemoconcentration and exacerbate the hypercoagulable state. The immune mechanism also can contribute to pulmonary intravascular coagulopathy [11]. Third, most patients presented with fatigue and dyspnea [12]. Critical patients who depend on a ventilator and patients in the intensive care unit are bedridden during hospitalization, which may cause venous blood flow stasis. Additionally, metabolic abnormalities, obesity, and other underlying diseases are more common in older people. Moreover, thrombosis developed not only in the lower-extremity deep veins but also in small pulmonary vessels [13]. In summary, the endothelial injury, hypercoagulable state, and venous stasis contribute to thrombosis in COVID-19 pneumonia patients. Nevertheless, the risk factors for COVID-19 pneumonia-induced DVT remain to be further explored.
Previously, due to a lack of specific antiviral medicines, treatment strategies have focused on anti-inflammation and anti-respiratory failure treatments. After experience with our previous DVT case in the clinical work in Wuhan, we began to pay attention to the relationship between DVT and prognosis in COVID pneumonia patients. In our study, all critical patients had a risk of developing thrombosis. Among the critical patients, the rate of DVT complications was 38.7% (12/31). The present retrospective study describes the occurrence of DVT in 88 patients with COVID-19 pneumonia and the relationship between thrombosis and prognosis. With the number of cases increasing globally, it will be important to prevent and diagnose DVT in patients with severe and critical COVID-19 pneumonia.
Material and Methods
DATA SOURCES:
We conducted a retrospective study focusing on the thrombotic risks of confirmed cases of COVID-19 pneumonia at Wuhan Union Hospital (Wuhan, China). This study was approved by the Institutional Research Ethics Boards of the Union Hospital, Tongji Medical College, Huazhong University of Science & Technology, Wuhan. Data were collected from 88 patients with COVID-19 pneumonia (confirmed by positive polymerase chain reaction test results on nasopharyngeal swab specimens) admitted to the hospital between 29 January 2020 and 29 February 2020. Information was collected on dates of illness onset and hospital admission. Only patients (both sexes, and age range 19–79 years) who tested positive in a nucleic acid test for SARS-CoV-2, with typical radiological findings of pneumonia, and negative results for other pathogen tests (influenza A and B, parainfluenza, respiratory syncytial virus, rhinovirus, adenovirus, and mycoplasma), and onset in Wuhan were included in this study. Patients with a history of underlying respiratory diseases, heart failure, hepatic insufficiency, or renal insufficiency before COVID-19 pneumonia onset, and patients with active malignancy, were excluded from the present study. The objective of the exclusion was to reduce the clinical effect of other severe disease on the prognosis of patients with COVID-19 pneumonia.
CLINICAL CLASSIFICATION:
Classification as mild, common, severe, and critical was carried out according to the diagnosis and treatment program of novel coronavirus pneumonia (Trial Seventh Edition) issued by the National Health Commission. The diagnosis of critical COVID-19 pneumonia was based on the fulfillment of 1 out of 3 of the following criteria: 1) developed respiratory failure requiring mechanical ventilation, 2) developed shock, and 3) combined with other organ failure requiring intensive care treatment. Severe patients fulfilled 1 out of 3 of the following criteria: 1) respiratory frequency ≥30 breaths/min; 2) blood oxygen saturation ≤93% at rest; and 3) blood oxygen saturation (PaO2)/inspired oxygen fraction (FiO2) ≤300 mmHg.
LABORATORY RESULTS, EXAMINATION RESULTS, AND TREATMENT:
The D-dimer value was measured by STAGO-R (Succeeder, Beijing, China). The data were reported in FEU. The absolute measurement unit was ug/mL and the cut-off value was 0.5 ug/mL. Lymphocyte count, hemoglobin, C reactive protein (CRP), lactate dehydrogenase (LDH), and serum calcium assays were performed using conventional methods. The blood samples for these laboratory assays were collected on the day of admission and were reviewed every 3–5 days, as needed. DVT was evaluated using Doppler ultrasound. The Doppler ultrasound examination was performed both on patients with symptomatic DVT and on asymptomatic patients with a D-dimer value increase. Computed tomographic pulmonary angiography was the method of choice for imaging the pulmonary vasculature in patients with suspected pulmonary thromboembolism (PTE) [14]. Patients received anticoagulants and prophylaxis therapy according to the guidelines of the Padua risk assessment scale (medical patients’ venous thromboembolism risk assessment recommended by guidelines from the American College of Chest Physicians) [15]. In the Padua scale, the high risk of thrombosis was defined by a cumulative score ≥4 points. Each of the following 6 risk factors: elderly (≥70 years), heart and/or respiratory failure, acute myocardial infarction or ischemic stroke, acute infection and/or rheumatologic disorder, obesity (BMI ≥30) and ongoing hormonal treatment, was scored with 1 point. The risk factor recent (≤1 month) trauma and/or surgery increased the score by 2 points. Each of 4 risk factors – active cancer, previous venous thromboembolism (VTE), reduced mobility (bed rest ≥3 days), and already known thrombophilia – added 3 points [16].
STATISTICAL ANALYSIS:
All analyses were performed using SPSS software (version 19.0; IBM Corp.). Continuous and categorical variables are presented as median (interquartile range) and n (%), respectively. We used the Mann-Whitney U test, χ2 test, or Fisher’s exact test to compare differences between critical and severe groups, as appropriate. Spearman’s correlation analysis was conducted to evaluate the correlation between variables. The multivariable adjustment was performed using linear regression for continuous data and logistic regression for dichotomous data.
Results
CLINICAL CHARACTERISTICS OF PATIENTS WITH COVID-19 PNEUMONIA:
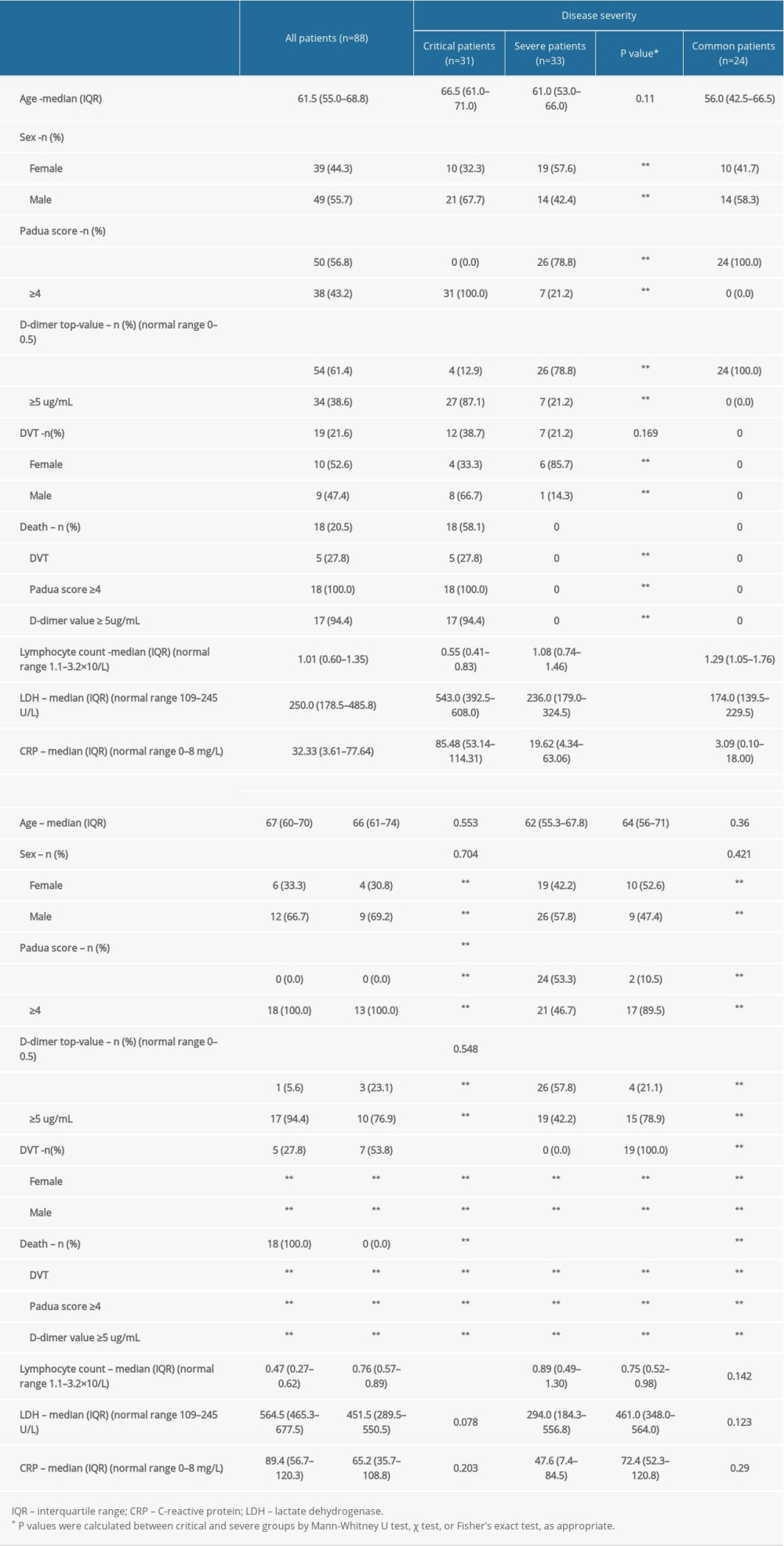
A total of 88 patients with confirmed COVID-19 pneumonia treated between 29 January 2020 and 17 March 2020 (Table 1) were reviewed in this report. Up to the time of submission of this article for publication, 19 patients (12 critical cases and 7 severe cases) developed DVT during hospitalization. Among the 18 patients who died, 5 were diagnosed with DVT. All 31 patients with critical COVID-19 pneumonia exhibited a Padua score increase (≥4), and 27 of them also exhibited a D-dimer value increase (≥5 ≤g/mL). Because the common group generally had a low score of Padua without any thrombotic events, they were not included in the comparison. The results indicated a positive correlation between the increase in D-dimer value (≥5 ≤g/mL) and the severity of COVID-19 pneumonia (r=0.679, P<0.01) and between the high Padua score (≥4) and the severity (r=0.799, P<0.01). In addition, the CRP and LDH levels on admission had positive correlations with the severity of illness (CRP: r=0.522, P<0.01; LDH: r=0.600, P<0.01). A negative correlation was observed between the lymphocyte count on admission and the severity of illness (r=−0.523, P<0.01). There was also a negative correlation between the lymphocyte count on admission and mortality in critical patients (r=−0.499, P<0.01). A weak correlation was also noticed between sex and illness severity (r=0.254, P<0.05). Moreover, univariable logistic regression analysis showed that the occurrence of DVT was positively correlated with disease severity (crude odds ratio: 3.643, 95% CI: 1.218–10.896, P<0.05). Based on the Padua score (≥4), a total of 38 patients received anticoagulants or prophylaxis therapy; 17 of these patients had a diagnosis of DVT during hospital, but there were also 2 other patients with confirmed DVT who had not received prophylaxis therapy.
CLINICAL CHARACTERISTICS OF THROMBOTIC COMPLICATIONS IN 19 PATIENTS WITH COVID-19 PNEUMONIA AND DVT:
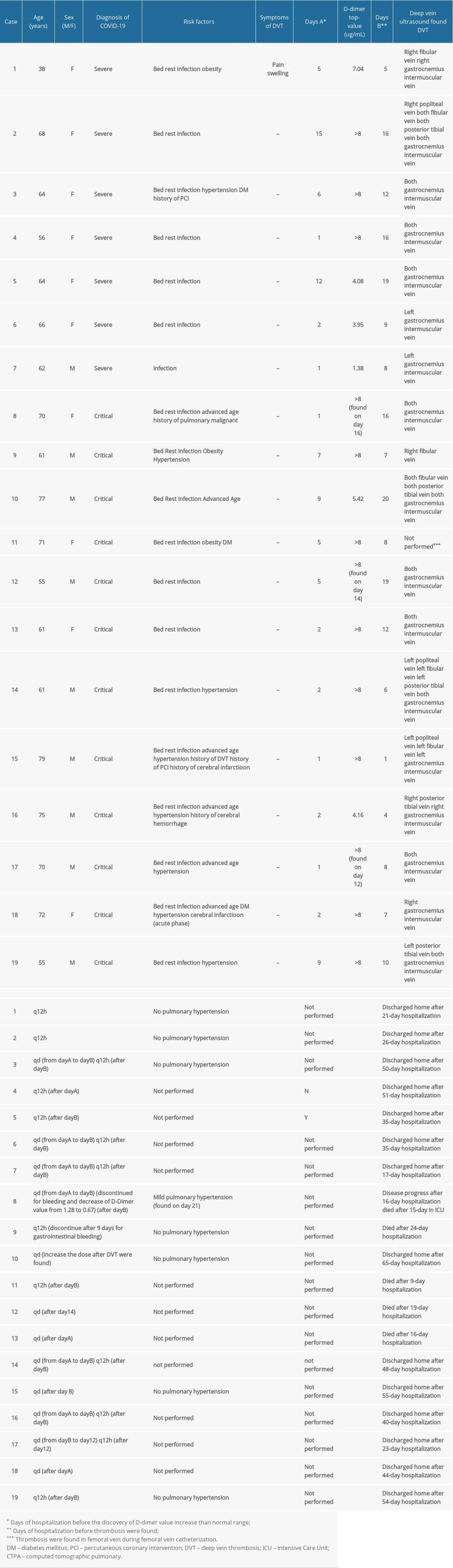
The clinical characteristics of the 19 patients exhibiting complications in the form of DVT are shown in Table 2. Except for one 38-year-old female patient who reported pain and swelling in her right lower limb, DVT was identified in all cases by ultrasound screening following an increase in the D-dimer value. All these patients had no previous history of DVT. Most of these patients had no complaint of lower limb discomfort. One patient was finally diagnosed with PTE (case 5). The most common risk factors observed in the present study were bed rest and infection. The average hospitalization period was 6.7 days prior to the discovery of a marked D-dimer value increase (≥5 ≤g/mL) in the patients who were eventually diagnosed with DVT. Univariable logistic regression analysis showed an increased odd of DVT with D-dimer levels greater than or equal to 5 ≤g/mL (crude odds ratio: 4.539, 95% CI: 1.289–15.991, P<0.05). Twelve of the 19 patients with DVT received prophylaxis after the discovery of D-dimer value increasing to above the normal range; 6 of them received antithrombotic therapy after the diagnosis of DVT, and 1 79-year-old patient received antithrombotic therapy after the diagnosis of DVT. For patients with confirmed DVT, anticoagulant therapy was provided according to antithrombotic therapy for VTE disease guidelines (Tenth Edition) issued by the American College of Chest Physicians [12]. The adjuvant treatment included physical therapy and reduced activity. Six patients were discharged successfully with further oral rivaroxaban medication (cases 1, 2, 5, 6, 7, and 17).
THROMBOSIS RISK IN 18 PATIENTS WITH COVID-19 PNEUMONIA WHO DIED:
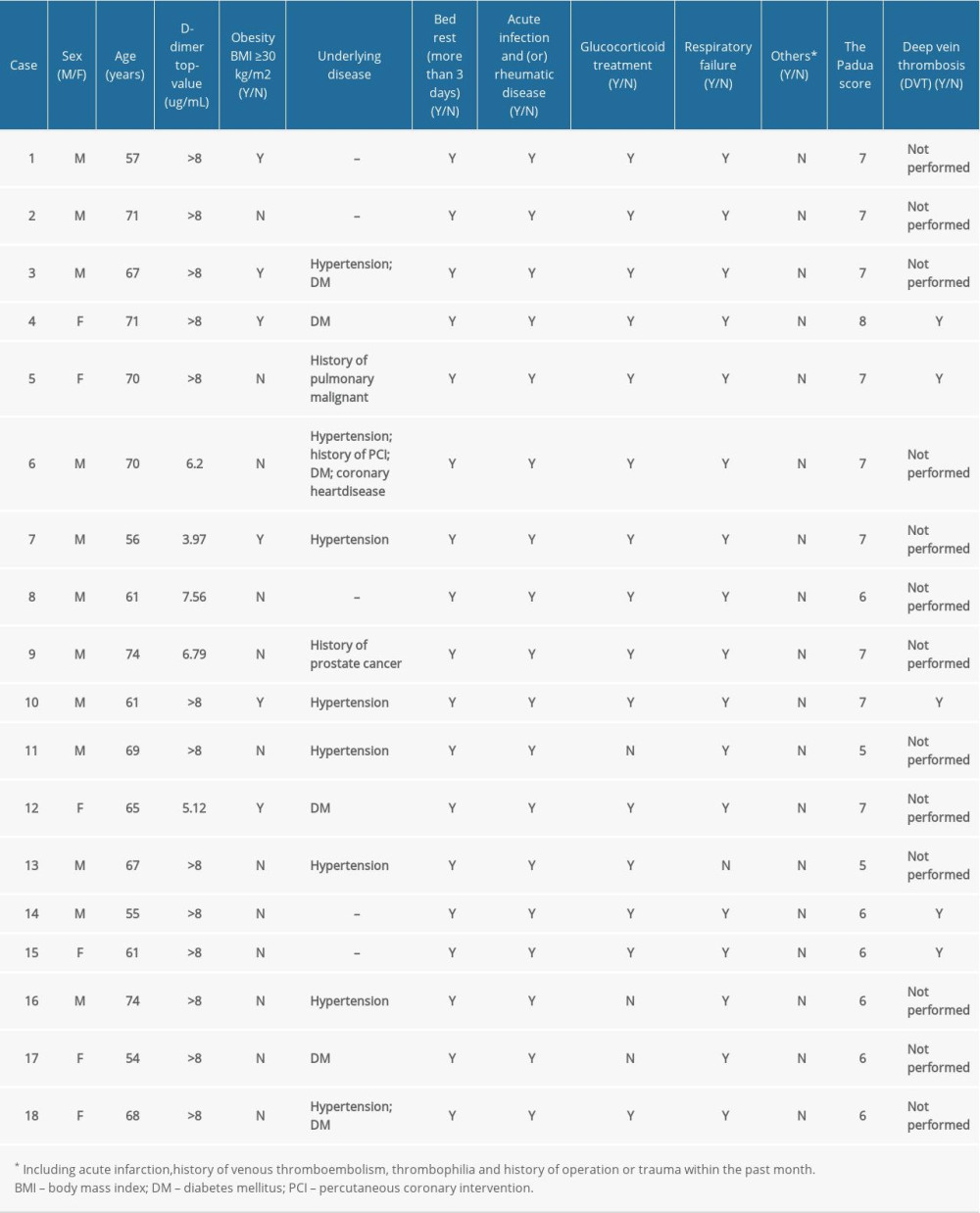
Among the 31 patients with confirmed critical COVID-19 pneumonia, 18 eventually died (Table 3). We observed that they may all have suffered from coagulation dysfunction with a marked increase in D-dimer levels before death. We also identified 6 deaths suspected to be associated with thrombosis (cases 4, 5, 6, 10, 13, and 14). One patient suspected of having PTE developed acute respiratory failure before death and exhibited continuously rising D-dimer and troponin levels without any myocardial infarction change (case 6). The other 13 critical patients’ Padua scores were generally high, and 7 were diagnosed with DVT. The decrease in the D-dimer level and no signs of thrombosis indicated that they might benefit from anticoagulation therapy. Non-critical patients without DVT were also scored to assess whether anticoagulation should be administered for prevention.
Discussion
Since the outbreak of COVID-19 in December 2019, this pandemic continues to evolve worldwide. To date, there are still no sufficient and totally effective vaccines or effective antiviral agents available anywhere in the world. Moreover, considering the potential virus variation and the vaccine acceptance rates in normal healthy people, the effects of current vaccines still need to be observed. The virus continues to cause a large number of deaths. We performed a single-center study of patients with COVID-19 pneumonia. This study found high mortality in critical COVID-19 pneumonia patients. We also reported the factors that affect disease severity and the association between the possible methods (like early ultrasound screening and/or prophylactic anticoagulant) and the improvement of the prognosis.
In the present study, we observed that the mortality rate of critically ill patients was 58.1%. According to previous reports, the mortality rate of hospitalized COVID-19 patients ranged from 1.4 to 25.7% [17–19], and the rate was significantly higher (39.3–61.5%) in patients with severe hypoxemia and who required mechanical ventilation [20–22].
Several reasons probably explain the high mortality rate. First, older people with complications are more susceptible to COVID-19 infection [23]. The average age of the critically ill and common groups was 65.9 and 48.5 years, respectively. Second, an objective reason was that the medical institutions lacked sufficient medical resources at that time. The critical group had an average of 11.7 days of symptoms at home before admission, which caused the continuous progression of the disease without systematic intervention. Furthermore, not every inpatient could receive invasive mechanical ventilation therapy at the early stage of the COVID-19 pandemic. Third, one main reason was that the critical patients were commonly had multiple complications and organ failure, which predicts a higher risk of poor outcomes. Recent evidence [24,25] indicates that coagulation dysfunction and thrombosis-related diseases, including DVT, PTE, and DIC, were present in the majority of deceased patients. The clinically determined causes of death seemed to correspond to the autopsy results. An autopsy study [26] of 12 deceased patients reported that 33.3% were diagnosed with pulmonary embolism as a direct cause of death, and 58.3% of the patients were confirmed to have DVT. In another autopsy study [27], involving 80 deceased patients, the incidence rates of PTE and DVT complications were 10% and 40%, respectively.
We found that 12 of the 31 (38.7%) critical patients and 7 of the 33 (21.2%) severe patients developed isolated distal deep vein thrombosis (IDDVT). The univariate analysis showed an association between IDDVT and disease severity (odds ratio=3.643
Therefore, it is crucial to use appropriate methods for early judgment of DVT occurrence. The Padua prediction score contains 11 common VTE risk factors, including age over 70 years, overweight, bed rest over 3 days, acute infection and/or rheumatic diseases, respiratory and/or heart failure, acute myocardial infarction and/or ischemic stroke, receiving hormone therapy, history of venous thromboembolism, history of operation or trauma within the past month, active tumors, and has disease with thrombotic tendency. This score can be easily evaluated and applied by clinicians. In addition, the increase in D-dimer level generally reminds us of thrombotic events. Our results showed that 78.9% (15/19) of the patients with DVT had a high Padua score (>4) and a high D-dimer level (≥5 ≤g/mL). Meanwhile, the 18 dead critical cases exhibited a Padua score increase (>4), and 94.4% (17/18) of them also showed a D-dimer value increase (≥5 ≤g/mL) before death. Eventually, the univariate analysis confirmed that the 2 factors were positively associated with disease severity. This result indirectly confirms the viewpoint of McFadyen et al. [30], who wrote that the increase in plasma D-dimer levels has emerged as a prognostic marker in COVID-19 pneumonia. D-dimer value is easy to access, and it can be helpful to perform a lower limb venous ultrasound screening subsequently both on asymptomatic and symptomatic patients.
Low-molecular-weight heparin (LMWH) was administered to the patients according to their risk of thrombosis, as evaluated by the Padua score and D-dimer level. We noticed that no further deterioration had occurred and no DVT had developed in patients who had a moderate increase in D-dimer level (1–5 μg/mL) and a high Padua score (>4), receiving prophylactic anticoagulant. Additionally, the disease state was stable in the severe patients treated for DVT with LMWH (Table 2, cases 1–7). However, anticoagulation did not result in disease reversal in patients who subsequently died. We think that the disease progression was not only promoted by the coagulation disorder, but also by the inflammatory storm, acute organ failure, and so on. An early appropriate anticoagulation treatment probably assists in improving the outcome of COVID-19 pneumonia patients with a high Padua score and an elevated D-dimer value. Considering the limited sample size of the present study, the effect of the prophylactic anticoagulant in the critical patients warrants further investigation.
In summary, to improve the outcome of the critical patients with COVID-19 pneumonia, it is helpful to evaluate risk factors for DVT as early as possible. This report highlights the advantage of performing D-dimer and lower-extremity vascular ultrasound screening. It is possible that a D-dimer level-guided positive thrombosis prophylaxis in COVID-19 pneumonia patients, especially in asymptomatic patients, will help decrease the potential for VTE and mortality. Moreover, rehabilitation treatment, graded compression stockings, and intermittent pneumatic compression should be used as soon as possible, especially for the critical and bedridden patients whose Padua scores are high [31]. The present research was a retrospective descriptive study in a single center, and the small sample size might have affected the accuracy of risk factors assessment and the prognosis analysis. A multi-center, prospective study is needed to further clarify the value of early diagnosis and treatment of DVT in critically ill COVID-19 pneumonia patients.
Conclusions
Our report illustrates that critically or severely ill patients have an associated high D-dimer value and high Padua scores, and suggests that a low threshold to screen for DVT may help improve detection of thromboembolism in these groups of patients, especially in asymptomatic patients. Our results suggest that early administration of prophylactic anticoagulant would benefit the prognosis of critical patients with COVID-19 pneumonia and would likely reduce thromboembolic rates.
References
1. National Health Commission of the People’s Republic of China: Update on new coronavirus pneumonia [in Chinese]http://www.nhc.gov.cn/xcs/yqtb/202012/59afa466d1ff416f93070dd20c4f990c.shtml
2. World Health Organization: Update on new coronavirus pneumonia https://www.who.int/publications/m/item/weekly-epidemiological-update---29-december-2020
3. Mei H, Hu YCharacteristics, causes, diagnosis and treatment of coagulation dysfunction in patients with COVID-19: Zhonghua Xue Ye Xue Za Zhi, 2020; 41; E002 [in Chinese]
4. Huang CL, Wang YM, Li XW, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China: Lancet, 2020; 395; 497-506
5. Chen NS, Zhou M, Dong X, Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study: Lancet, 2020; 395; 507-13
6. Fan BE, Ng J, Chan SSW, COVID-19 associated coagulopathy in critically ill patients: A hypercoagulable state demonstrated by parameters of haemostasis and clot waveform analysis: J Thromb Thrombolysis, 2020 Online ahead of print]
7. Shi ZY, Fu GWDiagnosis and treatment recommendation for novel coronavirus pneumonia related isolated distal deep vein thrombosis: Shanghai Medical Journal, 2020; 1-7 [in Chinese]
8. Huang XJ, Dai ZY, Cai L, Endothelial p110γPI3K mediates endothelial regeneration and vascular repair after inflammatory vascular injury: Circulation, 2016; 133; 1093-103
9. Koupenova M, Kehrel BE, Corkrey HA, Thrombosis and platelets: An update: Eur Heart J, 2017; 38; 785-91
10. Gando S, Levi M, Toh CK, Disseminated intravascular coagulation: Nat Rev Dis Primers, 2016; 2; 16037
11. Zhang JJ, Dong X, Cao YY, Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China: Allergy, 2020; 75(7); 1730-41
12. National Health Commission: Diagnosis and treatment program of novel coronavirus pneumonia (Trial Seventh Edition) [in Chinees]http://www.nhc.gov.cn/xcs/zhengcwj/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml
13. McGonagle D, O’Donnell JS, Sharif K, Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia: Lancet Rheumatol, 2020; 2; e437-45
14. Konstantinides SV, Meyer G, Becattini C, 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): Eur Heart J, 2020; 41; 543-603
15. Kearon C, Akl EA, Ornelas J, Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report: Chest, 2016; 149; 315-52
16. Kahn SR, Lim W, Dunn AS, Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines: Chest, 2020; 141; e195S-226S
17. Zhou F, Yu T, Du R, Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study: Lancet, 2020; 395; 1054-62
18. Argenziano MG, Bruce SL, Slater CL, Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: Retrospective case series: BMJ, 2020; 369; m1996
19. Docherty AB, Harrison EM, Green CA, Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Prospective observational cohort study: BMJ, 2020; 369; m1985
20. Cummings MJ, Baldwin MR, Abrams D, Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: A prospective cohort study: Lancet, 2020; 395; 1763-70
21. Yang XB, Yu Y, Xu JQ, Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study: Lancet Respir Med, 2020; 8; 475-81
22. Petrilli CM, Jones SA, Yang J, Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: Prospective cohort study: BMJ, 2020; 369; m1966
23. Killerby ME, Link GR, Haight SC, Characteristics Associated with hospitalization among patients with COVID-19 – Metropolitan Atlanta, Georgia, March–April 2020: Morb Mortal Wkly Rep, 2020; 69; 790-94
24. Zhang L, Feng XK, Zhang DQ, Deep vein thrombosis in hospitalized patients with coronavirus disease 2019 (COVID-19) in Wuhan, China: Prevalence, risk factors, and outcome: Circulation, 2020; 142(2); 114-28
25. Connors JM, Levy JH, COVID-19 and its implications for thrombosis and anticoagulation: Blood, 2020; 135(23); 2033-40
26. Wichmann D, Sperhake JP, Lütgehetmann M, Autopsy findings and venous thromboembolism in patients with COVID-19: Ann Intern Med, 2020; 173(4); 268-77
27. Edler C, Schröder AS, Aepfelbacher M, Dying with SARS-CoV-2 infection-an autopsy study of the first consecutive 80 cases in Hamburg, Germany: Int J Legal Med, 2020; 134; 1275-84
28. Bilaloglu S, Aphinyanaphongs Y, Jones S, Thrombosis in hospitalized patients with COVID-19 in a New York City Health System: JAMA, 2020; 324(8); 799-801
29. Yu Y, Tu J, Lei BX, Incidence and risk factors of deep vein thrombosis in hospitalized COVID-19 patients: Clin Appl Thromb Hemost, 2020; 26 1076029620953217
30. McFadyen JD, Stevens H, Peter K, The emerging threat of (micro)thrombosis in COVID-19 and its therapeutic implications: Circ Res, 2020; 127(4); 571-87
31. Arabi YM, Al-Hameed F, Burns KEA, Adjunctive intermittent pneumatic compression for venous thromboprophylaxis: N Engl J Med, 2019; 380; 1305-13
In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952