30 March 2021: Database Analysis
Prognostic Nomograms to Predict Survival of Patients with Resectable Gallbladder Cancer: A Surveillance, Epidemiology, and End Results (SEER)-Based Analysis
Yan LinCDEF, Hua ChenCDEF, Fan PanABDOI: 10.12659/MSM.929106
Med Sci Monit 2021; 27:e929106
Abstract
BACKGROUND: Gallbladder adenocarcinoma (GBAC) is globally acknowledged as one of the most common malignancies among all gastrointestinal cancers. Despite prognosis of GBAC patients remains poor, patients with early-stage disease can be observed with long-term survival.
MATERIAL AND METHODS: In this study, 2556 patients with pathological GBAC between 2010 and 2015 were derived from the Surveillance, Epidemiology, and End Results (SEER) database. The prognostic nomograms containing all independent prognostic factors for predicting overall survival (OS) and cancer-specific survival (CSS) were constructed to achieve superior prognostic discriminatory ability.
RESULTS: Based on the AJCC 7th TNM staging system, we found the TNM substaging was not accurate enough to predict the survival and stratify the risk. Based on the results of univariate and multivariate analyses, a more precise prognostic nomogram was constructed containing all significant independent prognostic factors (age, grade, TNM stage, bone metastasis, and chemotherapy) for OS, while age, grade, TNM stage, bone metastasis and radiotherapy significant independent prognostic factors for CSS. The C-index of the constructed nomogram for predicting OS and CSS was 0.740 and 0.737 higher than that of TNM staging alone (0.667 for OS and 0.689 for CSS), respectively. In addition, the calibration curves and decision curve analysis further showed its robust power in survival prediction.
CONCLUSIONS: The constructed nomograms showed better discrimination abilities to predict OS and CSS rates at 1, 3, and 5 years. In the future, these constructed models for this disease will assist in risk stratification to guide GBAC treatment.
Keywords: Decision Support Techniques, nomograms, SEER Program, Survival, Aged, 80 and over, Risk
Background
Gallbladder cancer (GBC) globally accounts for 80–95% of all biliary tract cancers and is the sixth most common malignancy among all gastrointestinal cancers [1]. Among all GBC, gallbladder adenocarcinoma (GBAC) is well-known as the most common subtype. The annual incidence of GBAC is estimated at 3 per 100 000 population. However, its 5-year overall survival (OS) rate of GBAC remains poor, which is the lowest among gastrointestinal cancers [2,3]. However, the 5-year OS of GBAC patients is variable depending on American Joint Committee on Cancer (AJCC) staging (range: 10–85%) [4,5]. Although the prognosis of advanced-stage GBAC patients remains poor, patients diagnosed and treated at an early stage of disease can have good long-term survival [6]. Surgery remains the first-line therapy for patients with resectable GBAC [7]. Thus, the prediction of survival for patients with resectable GBAC seems to be quite important.
Although there have been many reports on the prognosis of GBAC, most studies have focused on OS based on a single indicator, such as the AJCC Tumor, Node, Metastasis (TNM) staging system [8], lymph node metastasis [9], tumor location [10], and biological biomarkers [11,12]. However, for resectable GBAC patients, there are few appropriate risk factors for OS and cancer-specific survival (CSS) owing to the lack of a large-scale population-based study or inappropriate dataset construction. Although Xiao et al [13] developed a nomogram to predict the survival of resected GBC patients, adjuvant therapies and CSS were ignored in their model. Therefore, it was urgent to find a method to evaluate the prognosis of resected GBAC patients through research in a large cohort.
Nomograms are widely regarded as useful and reliable tools to stratify risk by containing vital indicators for oncologic prognosis [14]. However, the clinical application of nomograms in GBAC patients with surgery based on basic clinicopathological characteristics remains unclear. In this study, data derived from the Surveillance, Epidemiology, and End Results (SEER) database were used to identify significant prognostic indicators influencing OS and CSS of resected GBAC patients and we formulated separate nomograms for predicting the prognosis.
Material and Methods
DATA COLLECTION AND INCLUSION:
The data used in this study were retrieved and are freely available from SEER*Stat Database (
In this study, the inclusion criteria included that patients were pathologically diagnosed with GBAC from 2010 to 2015 as other published studies, and all patients need to receive surgical resection. The exclusion criteria excluded patients with unknown seventh edition of the AJCC TNM staging or more than one primary malignancy. The following data from each patient were collected for analysis: race, gender, age, differentiation grade, T stage, N stage, M stage, chemotherapy, radiotherapy, bone, brain, liver and lung metastasis, AJCC 7th TNM staging, survival data, and cause of death. OS was calculated by the time interval between diagnosis and death or censoring, and CSS was defined as the time interval from the date of diagnosis to the time of cancer-related death or censoring.
STATISTICAL ANALYSIS:
Statistical analyses were applied with R software version 4.0.2 and SPSS software version 21.1. Continuous variables ae presented as medians with inter quartile ranges (IQRs), and categorical variables are shown as frequencies with percentages. With univariate and multivariate Cox proportional hazards regression analyses, independent prognostic factors were identified. In addition, survival curves were depicted by the Kaplan-Meier method and compared by the log-rank test.
According to the results of multivariate survival analyses, separate prognostic nomograms for OS and CSS prediction were established with R software. Then, the predictive performance of the formulated nomograms was further assessed by concordance index (C-index), calibration curves and decision curve analyses (DCA) as in other studies [14,15]. In the internal validation, bootstraps with 500 resamples were used for these activities. P value <0.05 was defined as statistical significance.
Results
CLINICOPATHOLOGICAL CHARACTERISTICS:
Based on the inclusion and exclusion criteria, 2556 patients with resected GBAC were finally enrolled. There were 6548 patients from 2010 to 2015 diagnosed as having gallbladder cancer, and among which, 5340 patients had GBAC. Then, 1992 patients were excluded as no surgery was performed and 94 patients with unknown TNM staging were excluded. Finally, 698 patients with more than one primary malignancy during their lifetime were excluded.
Among the enrolled cohort, the median age was 69 (IQR 60–79) years old and 1796 (70.3%) patients were female. Most were White (76.0%), 11.8% were Black, and 11.6% were of other races (American Indian/AK Native, Asian/Pacific Islander). Following the AJCC 7th TNM staging system, the number of patients with stage I, II, III, and IV was 356 (14.3%), 716 (28.0%), 855 (33.5%), and 620 (24.3%), respectively. Some of the patients had distal metastasis: 23 had bone metastasis, 3 had brain metastasis, 337 had liver metastasis, and 38 had lung metastasis. Regarding treatment, 417 (16.3%) patients received radiotherapy and 941 (36.8%) received chemotherapy.
INDEPENDENT PROGNOSTIC INDICATORS FOR RESECTED GBAC:
In this cohort, the median OS and CSS were 17 and 20 months (IQR, 4–24 months), respectively. Concomitantly, the 1-, 3-, and 5-year OS rates were 57.8%, 32.7%, and 23.9%, respectively, while the 1-, 3-, and 5-year CSS rates were 61.4%, 37.3%, and 29.0%, respectively.
According to the univariate and multivariate analysis for OS, older age, advanced grade, higher 7th edition of AJCC TNM staging, bone metastasis, and no chemotherapy were independent prognostic risk factors (Table 1). However, for CSS, radiotherapy (P<0.001; HR, 0.670; 95% CI, 0.573–0.784) was confirmed as an independent prognostic indicator instead of chemotherapy, and the other independent risk factors were the same as those for OS (Table 2).
DEFECTS IN SEVENTH EDITION OF AJCC TNM STAGING SYSTEM (IIIA AND IIIB, IVA, AND IVB):
According to the AJCC 7th TNM staging system, the detailed substage data were recorded. Although both the AJCC TNM staging and substaging were independent prognostic indicators, some defects were observed in the TNM substage. It was obvious that the HR of stage IIIB by multivariate analyses was 3.545, which was less than stage IIIA with HR as 3.695, and the HR of stage IVB was 4.838, which was much less than IVA with HR 6.945 when compared with stage I tumor for OS (Table 1). Similar results were found in multivariate analysis for CSS, showing that the HR of stage IIIB was 2.934 less than IIIA 3.614, and stage IVB was 4.536 less than IVA 6.313 (Table 2). Thus, the TNM substaging was not accurate enough to predict the survival and stratify the risk for OS and CSS (Figure 1A, 1B). However, survival curves of all stages (I, II, III and IV) were well-separated with reasonable HRs and statistical significance for OS and CSS (Figure 1C, 1D).
ESTABLISHING A PROGNOSTIC NOMOGRAM FOR OS AND CSS:
Based on the above findings, a more precise prognostic nomogram was established containing all significant independent prognostic factors (age, grade, TNM stage, bone metastasis, and chemotherapy) for OS (Figure 2A) and age, grade, TNM stage, bone metastasis, and radiotherapy for CSS (Figure 2B). The C-index for OS and CSS prediction with the constructed nomogram was separately 0.740 (95% CI: 0.7397–0.7403) and 0.737 (95% CI: 0.7367–0.7373), which was higher than that of the TNM staging alone (0.667 for OS and 0.689 for CSS). The higher C-index was significantly associated with better predictive accuracy for OS and CSS. In addition, the calibration curves depicted a high consistency between the actual survival and nomogram-predicted survival for the probability of 1-, 3-, and 5-year OS or CSS (Figure 3A–3F).
DCA-BASED COMPARISON OF THE CONSTRUCTED NOMOGRAMS TO AJCC TNM STAGING AS PREDICTORS FOR OS AND CSS:
As a powerful and novel evaluation method, DCA highlights the predictive power with perceivable clinical net benefit. Better net benefit was observed in the constructed nomograms with a wider range of threshold probability when compared to the AJCC TNM staging system alone, which revealed increased predictive power in predicting OS and CSS at 1, 3, and 5 years with our constructed nomograms (Figure 4A–4F). Meanwhile, higher threshold probability levels are always associated with the best decision outcomes.
Discussion
We analyzed more than 2500 cases selected from the SEER database. Through the univariate and multivariable analysis, we found that the age, tumor grade, AJCC TNM staging, bone metastasis, and chemotherapy were independent prognostic factors for OS, while for CSS, the age, tumor grade, AJCC TNM staging, bone metastasis, and radiotherapy were confirmed as independent prognostic indicators. Novel nomograms for predicting the 1-, 3-, and 5-year OS or CSS rates of GBAC patients after surgery were constructed based on the sperate significant independent prognostic factors. The C-index of the constructed nomograms was 0.740 for OS and 0.737 for CSS, higher than the C-index of TNM staging alone (0.667 for OS and 0.689 for CSS).
In previous research, age was confirmed as a meaningful risk indicator for GBAC diagnosis [16]. Age is also widely considered as a prognostic predictor in several malignancies, such as gastric cancer [17], pancreatic cancer [18], and colorectal cancer [19], as well as GBAC [20]. Chen et al reported that node-negative GBAC patients older than 70 years (multivariate HR=2.906) had significantly higher HR than those age 60–70 years (multivariate HR=1.935) or less than 60 years (HR=1) [21]. In addition, in a Japanese nationwide database, 4424 patients with GBAC in Japan were analyzed, showing that the survival rate for patients aged less than 60 years was significantly better, while the survival rate for patients aged more than 69 years was significantly worse [22]. Thus, age as a personal feature is always regarded as a diagnostic and prognostic factor for GBAC patients.
Besides personal features, tumor biology may play another vital role in GBAC patients’ prognosis. Tumor grade and the TNM staging system reveals tumor malignant behavior, tumor activity, and tumor metastatic ability. Many studies have validated that poorer tumor differentiation grade and higher TNM staging may have poorer survival for GBAC patients [6,23,24]. From our study, we further validated that TNM stage and tumor grade were the independent risk factors for resected GBAC patients, which represented the tumor intrinsic characteristics influencing prognosis. However, single AJCC TNM staging system or tumor grade was not enough to predict individual survival with high power, suggesting the need for a modified or integrated model [21,24]. Thus, prognostic prediction models seem to be rather valuable, especially for resected GBAC patients, owing to the group’s potential heterogeneity. Additionally, distal metastasis always predicts poor survival for cancer patients. Here, we found that only bone metastasis was a significant prognostic indicator for resected GBAC patients. The mechanism underlying its metastasis has been studied at different levels, such as the TASP1-PI3K/AKT-FAM49B pathway [25], miR-101-MAPK/Erk-Smad-ZFX pathway [26], and PLGF/c-MYC/miR-19a [27]. GBAC preferentially metastasizes to regional lymph nodes (LNs) and liver parenchyma. Bone metastases from GBAC are a rare presentation [28]. GBAC commonly spreads by direct extension to the liver and adjacent organs of the gastrointestinal tract, followed by regional LNs. Only a few case reports of GBAC with limited bone metastasis have been published. Presentation with GBAC metastases to bone without noticeable symptoms is extremely rare, so bone scanning is not routinely done for GBAC [29,30]. However, bone metastasis, but not liver, lung, and brain metastasis, was significantly associated with survival in resected GBAC patients owing to its malignant character.
Moreover, adjuvant chemoradiotherapy is always recommended for resected GBAC patients. In addition to the traditional first-line chemotherapy regimen, Ostwal et al recommended use of adjuvant chemotherapy with gemcitabine-cisplatin (GC) in stage II–III GBC patients undergoing R0 resections by group. They found patients receiving adjuvant GC had good tolerance, high completion rates, and encouraging outcomes [31]. Ren et al [32] performed a meta-analysis of 21 clinical trials, showing the 5-year OS rate was higher in the adjuvant radiotherapy group than in the group with no radiotherapy (OR=0.63; 95% CI=0.50–0.81, p=0.0002). Similarly, Kim et al [33] also reported that adjuvant chemotherapy/radiotherapy was utilized in 36% of patients with curative-intent resection for GBC, and after adjusted analyses, chemotherapy/radiotherapy was independently associated with improved long-term survival, but the benefit was isolated to only patients with high-risk characteristics. In addition, Wang et al [34] found that the immune index including cytotoxic T cells, regulatory T cells, mast cells, neutrophils, and macrophages could effectively predict prognosis and the benefits of gemcitabine-based chemotherapy, which might improve the TNM staging system. In another subgroup analysis for adjuvant radiotherapy for stage IIIB GBAC, no survival benefit was received from adjuvant radiotherapy [35]. The endpoints of OS may include many events, and GBAC may have poor response to chemotherapy, so chemotherapy was not a predictor of CSS in our analysis. Radiotherapy targets local tissues and can harm the cardiovascular system and organ function, so radiotherapy may be a predictor of CSS. Therefore, nomograms for OS including chemotherapy or for CSS including radiotherapy may be influenced by the tumor biology, immune pattern, or individual status.
Although there are many models for prognosis analysis of GBC based on the SEER database [36–40], the studies either analyzed the data of all patients with GBC cancer or did not include the data on neoadjuvant therapy. The present study only focused on patients with postoperative GBAC and included the data on neoadjuvant therapy, and we also analyzed the types of postoperative organ metastasis. In the current study, the calibration curves revealed promising consistencies between predictive survival and actual survival, and DCA revealed the constructed nomograms showed better net benefits with a wider range of threshold probability when compared to the AJCC TNM staging system alone. In our constructed models, the nomograms with common and easily-accessible factors were easy to use. However, our study has several limitations. First, it is just a retrospective study, and lacked sufficient validations and many important indicators such as chemotherapy regimens, CA 19-9, and preoperative hyperbilirubinemia, so a large multicenter prospective study is required. Second, the C-index of the nomograms was good but not perfect, and further research is needed to improve the quality of the nomograms.
Conclusions
Our present study concluded that age, tumor grade, AJCC TNM staging, bone metastasis, and chemotherapy were independent prognostic factors for OS, while age, tumor grade, AJCC TNM staging, bone metastasis, and radiotherapy were independent prognostic factors for CSS of resected GBAC patients. Furthermore, nomograms constructed in this study showed better discrimination abilities to predict 1-, 3-, and 5-year OS and CSS rates. These constructed models for this disease may help in risk stratification to guide GBAC treatment.
Figures
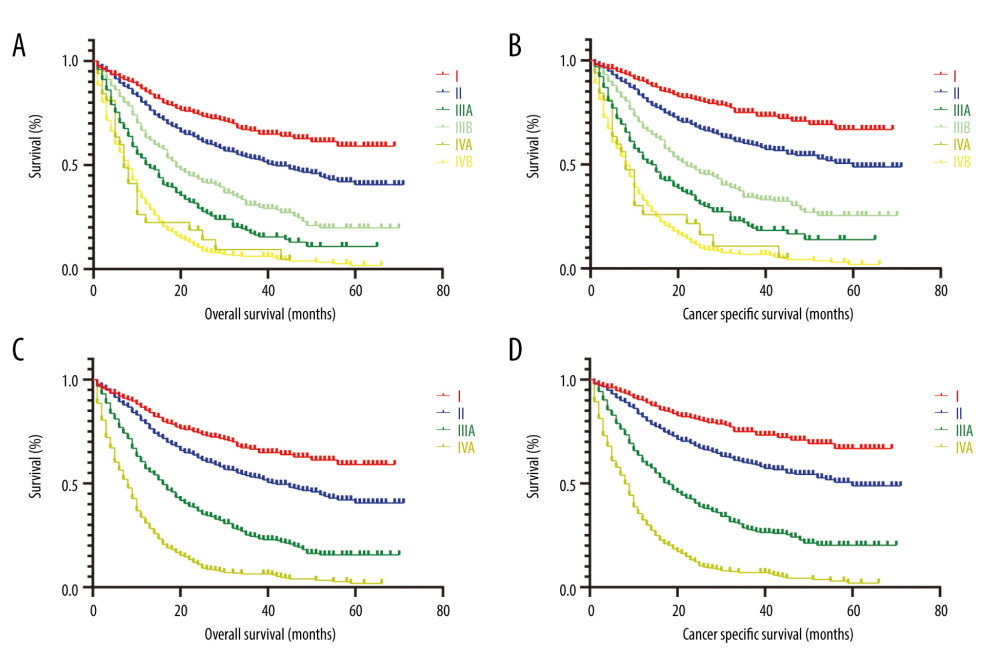 Figure 1. Kaplan-Meier survival curves for OS and CSS of patients with resected GBAC according to AJCC TNM staging system. (A) OS and (B) CSS with detailed substaging system; (C) OS and (D) CSS with overall staging system.
Figure 1. Kaplan-Meier survival curves for OS and CSS of patients with resected GBAC according to AJCC TNM staging system. (A) OS and (B) CSS with detailed substaging system; (C) OS and (D) CSS with overall staging system. 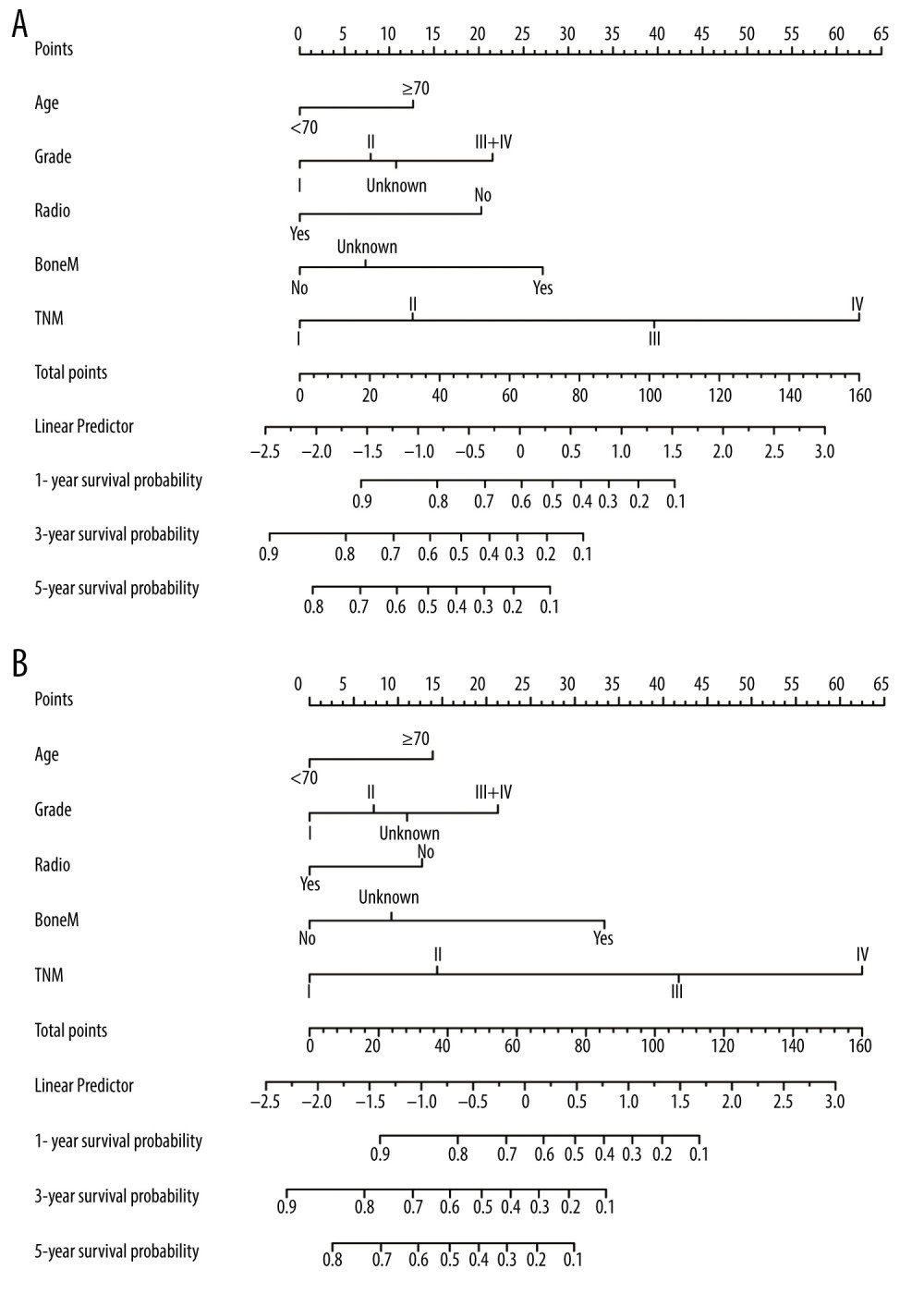 Figure 2. Prognostic nomograms constructed for resected GBAC. (A) Formulated nomogram for OS of resected GBAC and (B) formulated nomogram for CSS of resected GBAC.
Figure 2. Prognostic nomograms constructed for resected GBAC. (A) Formulated nomogram for OS of resected GBAC and (B) formulated nomogram for CSS of resected GBAC. 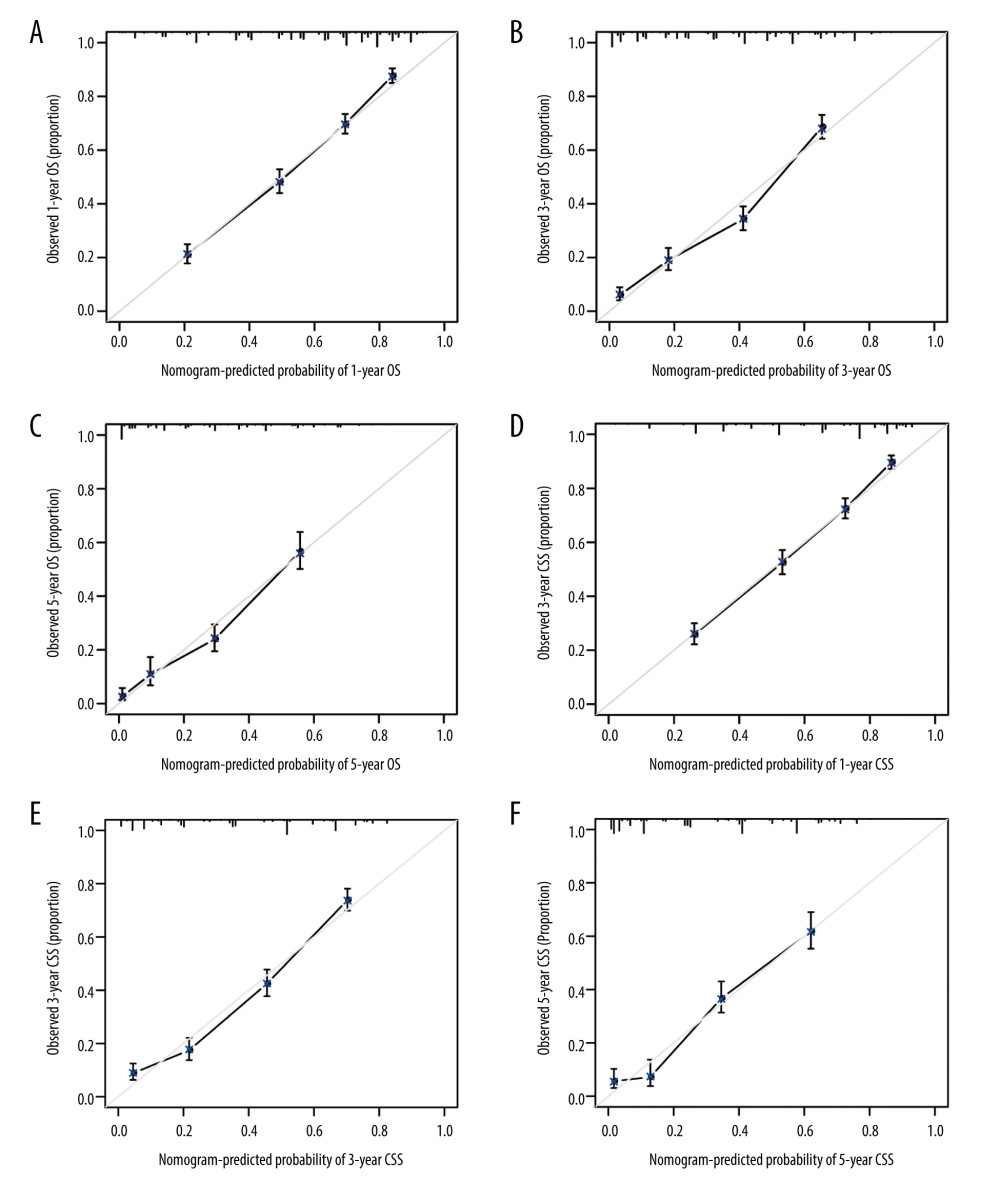 Figure 3. Calibration curves validation for resected GBAC. The calibration curves predict OS at (A) 1 years, (B) 3 years and (C) 5 years and CSS at (D) 1 years, (E) 3 years, (F) 5 years.
Figure 3. Calibration curves validation for resected GBAC. The calibration curves predict OS at (A) 1 years, (B) 3 years and (C) 5 years and CSS at (D) 1 years, (E) 3 years, (F) 5 years. 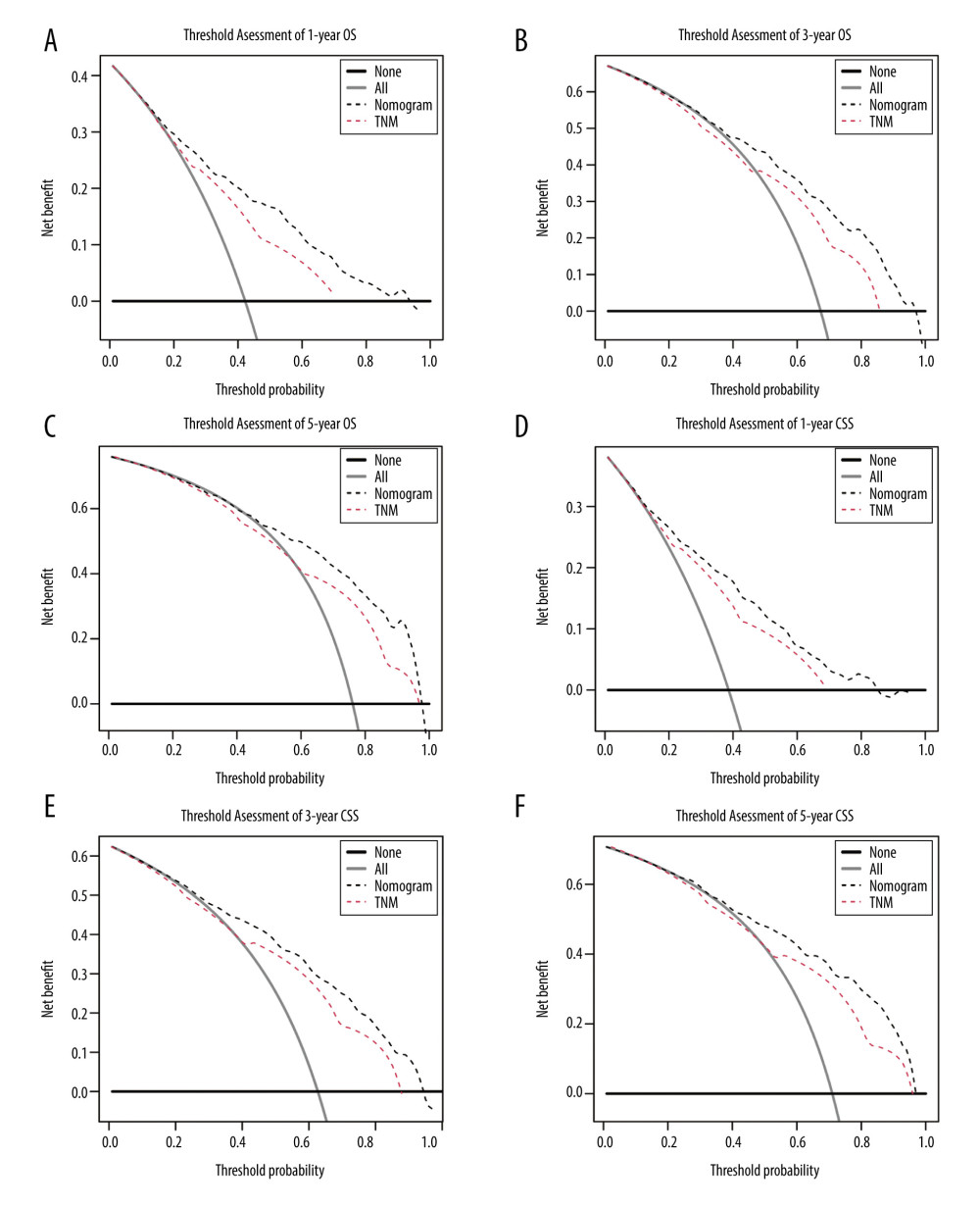 Figure 4. Decision curve analyses for resected GBAC. The decision curve analyses depict the clinical net benefit with threshold probability to compare the constructed nomograms and AJCC TNM staging system by assessing (A) 1-year, (B) 3-year, (C) 5-year OS and (D) 1-year, (E) 3-year, (F) 5-year CSS.
Figure 4. Decision curve analyses for resected GBAC. The decision curve analyses depict the clinical net benefit with threshold probability to compare the constructed nomograms and AJCC TNM staging system by assessing (A) 1-year, (B) 3-year, (C) 5-year OS and (D) 1-year, (E) 3-year, (F) 5-year CSS. References
1. Hundal R, Shaffer EA, Gallbladder cancer: Epidemiology and outcome: Clin Epidemiol, 2014; 6; 99-109
2. Lazcano-Ponce EC, Miquel JF, Munoz N, Epidemiology and molecular pathology of gallbladder cancer: Cancer J Clin, 2001; 51; 349-64
3. Mittal B, Desmin dysregulation in gall bladder cancer: Indian J Med Res, 2020; 151; 273-74
4. Levy AD, Murakata LA, Rohrmann CA, Gallbladder carcinoma: Radiologic-pathologic correlation: Radiographics, 2001; 21; 295-314 questionnaire, 549–55
5. Mayo SC, Shore AD, Nathan H, National trends in the management and survival of surgically managed gallbladder adenocarcinoma over 15 years: A population-based analysis: J Gastrointest Surg, 2010; 14; 1578-91
6. Chen C, Geng Z, Shen H, Long-term outcomes and prognostic factors in advanced gallbladder cancer: Focus on the advanced T stage: PLoS One, 2016; 11; e0166361
7. Kondo S, Takada T, Miyazaki M, Guidelines for the management of biliary tract and ampullary carcinomas: surgical treatment: J Hepatobiliary Pancreat Surg, 2008; 15; 41-54
8. Yadav S, Tella SH, Kommalapati A, A novel clinically based staging system for gallbladder cancer: J Natl Compr Canc Netw, 2020; 18; 151-59
9. Shi Z, Xiao Z, Li L, Application of nomogram containing log odds of metastatic lymph node in gallbladder cancer patients: Ann Transl Med, 2020; 8; 655
10. Lafaro K, Blakely AM, Melstrom LG, Prognostic impact of tumor location in resected gallbladder cancer: A national cohort analysis: J Surg Oncol, 2020 [Online ahead of print]
11. Wu Z, Miao X, Zhang Y, XRCC1 is a promising predictive biomarker and facilitates chemo-resistance in gallbladder cancer: Front Mol Biosci, 2020; 7; 70
12. Zheng X, Gao L, Wang BT, Overexpression of EIF5A2 is associated with poor survival and aggressive tumor biology in gallbladder cancer: Histol Histopathol, 2020; 35; 579-87
13. Xiao Z, Shi Z, Hu L, A new nomogram from the SEER database for predicting the prognosis of gallbladder cancer patients after surgery: Ann Transl Med, 2019; 7; 738
14. Pu N, Lv Y, Zhao G, Survival prediction in pancreatic cancer patients with no distant metastasis: A large-scale population-based estimate: Future Oncol, 2018; 14; 165-75
15. Gao S, Pu N, Liu L, The latest exploration of staging and prognostic classification for pancreatic neuroendocrine tumors: A large population-based study: J Cancer, 2018; 9; 1698-706
16. Alvi AR, Siddiqui NA, Zafar H, Risk factors of gallbladder cancer in Karachi – a case-control study: World J Surg Oncol, 2011; 9; 164
17. Alshehri A, Alanezi H, Kim BS, Prognosis factors of advanced gastric cancer according to sex and age: World J Clin Cases, 2020; 8; 1608-19
18. Pu N, Yin L, Habib JR, Optimized modification of the eighth edition of AJCC TNM staging system for resected pancreatic ductal adenocarcinoma: Future Oncol, 2019; 15; 3457-65
19. Li J, Wang Z, Yuan X, The prognostic significance of age in operated and non-operated colorectal cancer: BMC Cancer, 2015; 15; 83
20. Mady M, Prasai K, Tella SH, Neutrophil to lymphocyte ratio as a prognostic marker in metastatic gallbladder cancer: HPB (Oxford), 2020; 22(10); 1490-95
21. Chen M, Cao J, Zhang B, A Nomogram for prediction of overall survival in patients with node-negative gallbladder cancer: J Cancer, 2019; 10; 3246-52
22. Kayahara M, Nagakawa T, Nakagawara H, Prognostic factors for gallbladder cancer in Japan: Ann Surg, 2008; 248; 807-14
23. Lee AJ, Chiang YJ, Lee JE, Validation of American Joint Committee on Cancer eighth staging system for gallbladder cancer and its lymphadenectomy guidelines: J Surg Res, 2018; 230; 148-54
24. Wang J, Bo X, Shi X: Eur J Surg Oncol, 2020; 46; 527-33
25. Zhang Y, Du P, Li Y, TASP1 promotes gallbladder cancer cell proliferation and metastasis by up-regulating FAM49B via PI3K/AKT pathway: Int J Biol Sci, 2020; 16; 739-51
26. Bao RF, Shu YJ, Hu YP, miR-101 targeting ZFX suppresses tumor proliferation and metastasis by regulating the MAPK/Erk and Smad pathways in gallbladder carcinoma: Oncotarget, 2016; 7; 22339-54
27. Li H, Jin Y, Hu Y, The PLGF/c-MYC/miR-19a axis promotes metastasis and stemness in gallbladder cancer: Cancer Sci, 2018; 109; 1532-44
28. Chaudhari S, Hatwal D, Bhat P, A rare presentation of gallbladder carcinoma metastasis: J Clin Diagn Res, 2014; 8; FD19-20
29. Prakash M, Aiyappan SK, Kumar A, Solitary skeletal metastasis in carcinoma gallbladder: Two case reports: Cancer Imaging, 2010; 10; 121-23
30. Sameer G, Naseem A, Vijay K, Skeletal metastasis in gallbladder cancer from a high-volume tertiary care center of north India: A series of rare occurrence: J Gastrointest Cancer, 2015; 46; 36-41
31. Ostwal V, Swami R, Patkar S, Gemcitabine-cisplatin (GC) as adjuvant chemotherapy in resected stage II and stage III gallbladder cancers (GBC): A potential way forward: Med Oncol, 2018; 35; 57
32. Ren B, Guo Q, Yang Y, A meta-analysis of the efficacy of postoperative adjuvant radiotherapy versus no radiotherapy for extrahepatic cholangiocarcinoma and gallbladder carcinoma: Radiat Oncol, 2020; 15; 15
33. Kim Y, Amini N, Wilson A, Impact of chemotherapy and external-beam radiation therapy on outcomes among patients with resected gallbladder cancer: A multi-institutional analysis: Ann Surg Oncol, 2016; 23; 2998-3008
34. Wang J, Bo X, Wang C, Low immune index correlates with favorable prognosis but with reduced benefit from chemotherapy in gallbladder cancer: Cancer Sci, 2020; 111; 219-28
35. Cai YL, Lin YX, Xiong XZ, Postsurgical radiotherapy in stage IIIB gallbladder cancer patients with one to three lymph nodes metastases: A propensity score matching analysis: Am J Surg, 2020 [Online ahead of print]
36. Xiao Z, Shi Z, Hu L, A new nomogram from the SEER database for predicting the prognosis of gallbladder cancer patients after surgery: Ann Transl Med, 2019; 7; 738
37. Zhu X, Zhang X, Hu X, Survival analysis of patients with primary gallbladder cancer from 2010 to 2015: A retrospective study based on SEER data: Medicine (Baltimore), 2020; 99(40); e22292
38. Han D, Yang J, Xu F, Prognostic factors in patients with gallbladder adenocarcinoma identified using competing-risks analysis: A study of cases in the SEER database: Medicine (Baltimore), 2020; 99(31); e21322
39. He C, Cai Z, Zhang Y, Prognostic model to predict cancer-specific survival for patients with gallbladder carcinoma after surgery: A population-based analysis: Front Oncol, 2019; 9; 1329
40. Zhang W, Hong HJ, Chen YL, Establishment of a gallbladder cancer-specific survival model to predict prognosis in non-metastatic gallbladder cancer patients after surgical resection: Dig Dis Sci, 2018; 63(9); 2251-58
Figures
 Figure 1. Kaplan-Meier survival curves for OS and CSS of patients with resected GBAC according to AJCC TNM staging system. (A) OS and (B) CSS with detailed substaging system; (C) OS and (D) CSS with overall staging system.
Figure 1. Kaplan-Meier survival curves for OS and CSS of patients with resected GBAC according to AJCC TNM staging system. (A) OS and (B) CSS with detailed substaging system; (C) OS and (D) CSS with overall staging system. Figure 2. Prognostic nomograms constructed for resected GBAC. (A) Formulated nomogram for OS of resected GBAC and (B) formulated nomogram for CSS of resected GBAC.
Figure 2. Prognostic nomograms constructed for resected GBAC. (A) Formulated nomogram for OS of resected GBAC and (B) formulated nomogram for CSS of resected GBAC. Figure 3. Calibration curves validation for resected GBAC. The calibration curves predict OS at (A) 1 years, (B) 3 years and (C) 5 years and CSS at (D) 1 years, (E) 3 years, (F) 5 years.
Figure 3. Calibration curves validation for resected GBAC. The calibration curves predict OS at (A) 1 years, (B) 3 years and (C) 5 years and CSS at (D) 1 years, (E) 3 years, (F) 5 years. Figure 4. Decision curve analyses for resected GBAC. The decision curve analyses depict the clinical net benefit with threshold probability to compare the constructed nomograms and AJCC TNM staging system by assessing (A) 1-year, (B) 3-year, (C) 5-year OS and (D) 1-year, (E) 3-year, (F) 5-year CSS.
Figure 4. Decision curve analyses for resected GBAC. The decision curve analyses depict the clinical net benefit with threshold probability to compare the constructed nomograms and AJCC TNM staging system by assessing (A) 1-year, (B) 3-year, (C) 5-year OS and (D) 1-year, (E) 3-year, (F) 5-year CSS. Tables
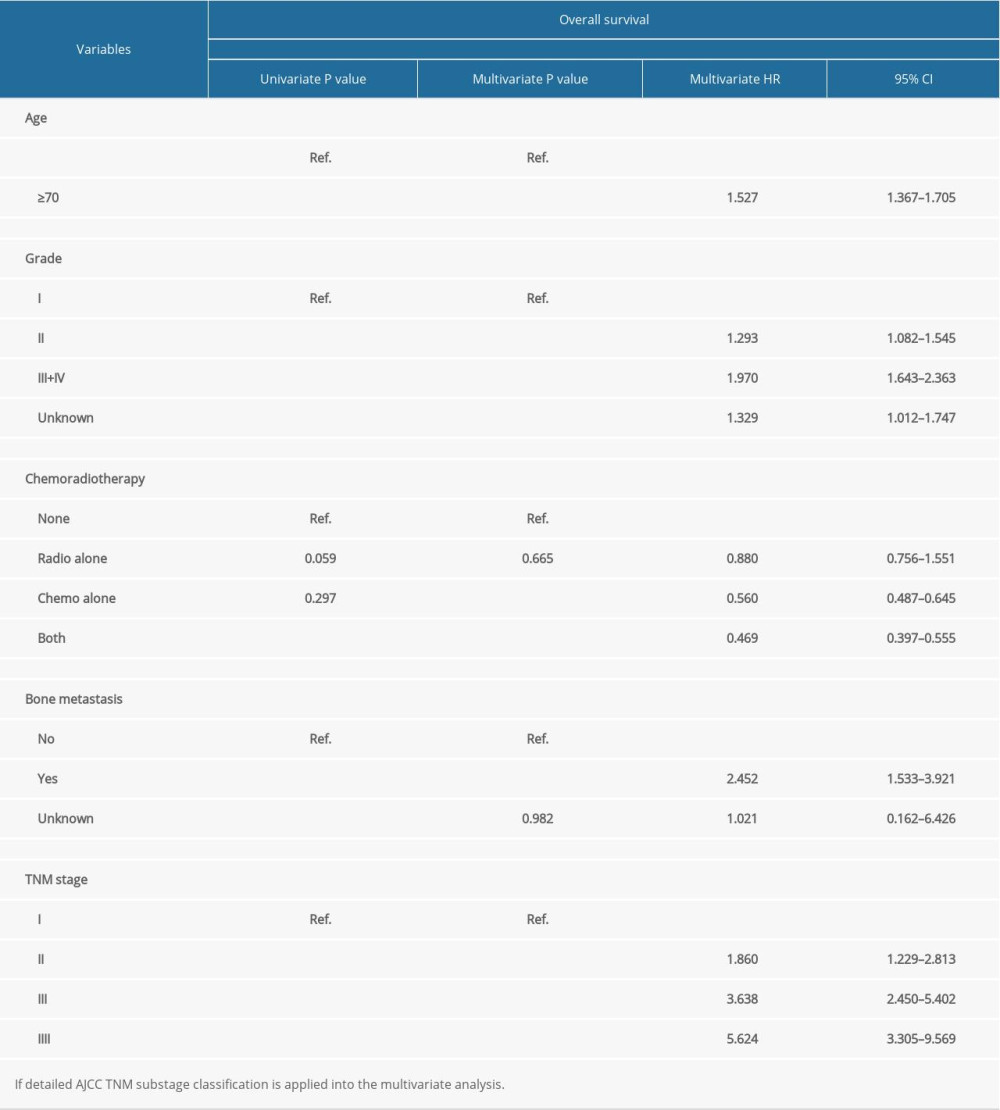 Table 1. Univariate and multivariate analysis of prognostic factors associated with overall survival.
Table 1. Univariate and multivariate analysis of prognostic factors associated with overall survival.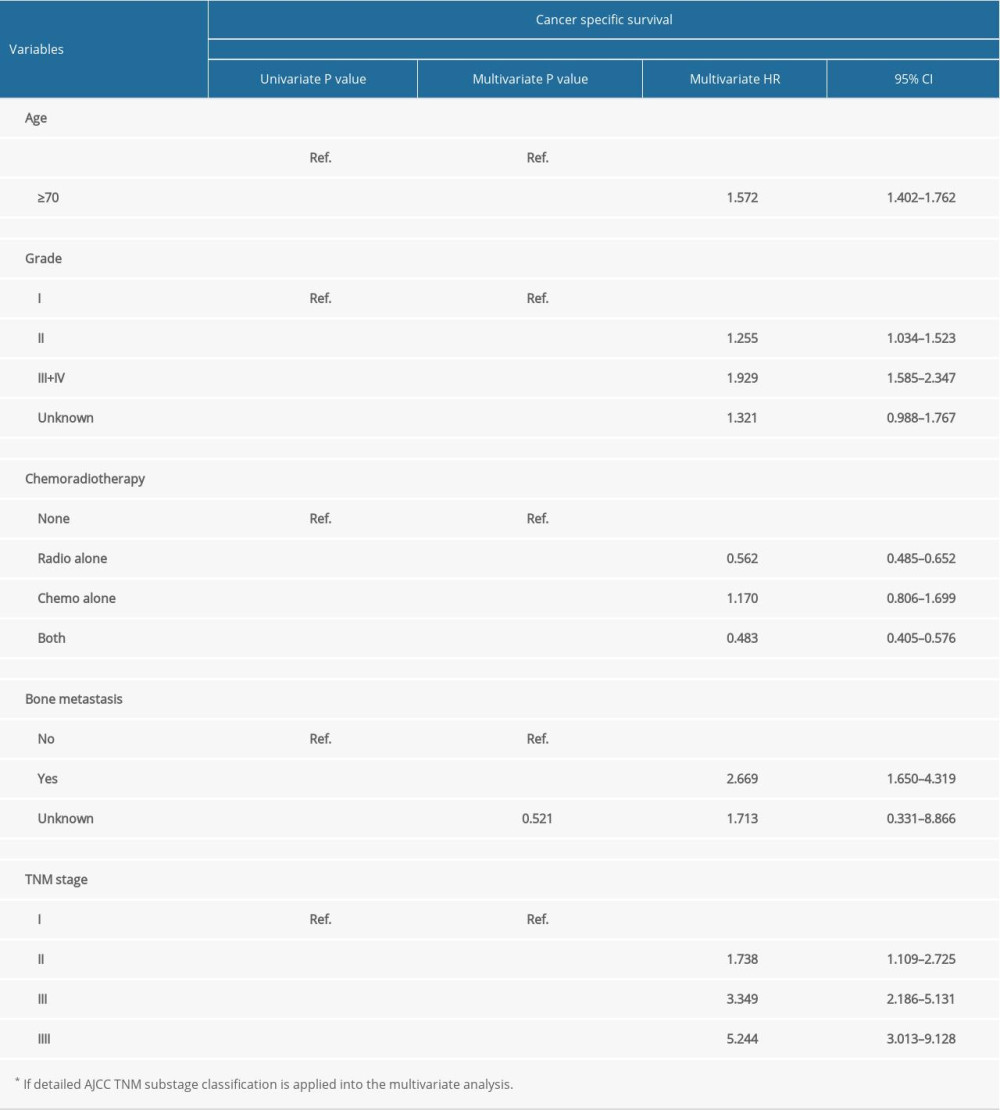 Table 2. Univariate and multivariate analysis of prognostic factors associated with cancer-specific survival.
Table 2. Univariate and multivariate analysis of prognostic factors associated with cancer-specific survival. Table 1. Univariate and multivariate analysis of prognostic factors associated with overall survival.
Table 1. Univariate and multivariate analysis of prognostic factors associated with overall survival. Table 2. Univariate and multivariate analysis of prognostic factors associated with cancer-specific survival.
Table 2. Univariate and multivariate analysis of prognostic factors associated with cancer-specific survival. In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








