03 February 2021: Clinical Research
U-Shaped Relationship Between Cardiovascular Mortality and Serum Uric Acid May Be Attributed to Stroke- and Heart-Specific Mortality, Respectively, Among Hypertensive Patients: A Nationally Representative Cohort Study
Hao You1ABCE, Kegong Chen2BCE, Pengfei Han3C, ChaoFu Yue4B, Xia Zhao5ADEG*DOI: 10.12659/MSM.928937
Med Sci Monit 2021; 27:e928937
Abstract
BACKGROUND: Serum uric acid (UA) is involved in the development of hypertension. However, its impact on mortality in hypertension remains unclear. We aimed to assess the association of cardiovascular and all-cause mortality with UA in a hypertensive population.
MATERIAL AND METHODS: This study included 15 583 hypertensive patients from the NHANES study during 1999-2014. Weighted Cox regression analyses and cubic spline fitting were used to assess the relationship between UA and mortality risk.
RESULTS: Over a median follow-up of 7.4 years (116 351 person-years), a total of 3291 deaths occurred. Mortality was examined according to 5 predefined UA levels: £3.5, 3.5–5, 5–6, 6–7.5, and >7.5 mg/dL. In multivariable analysis with 5–6 mg/dL as a reference, the hazard ratios (95% confidence interval) of total mortality across the 5 groups were 1.40 (1.05–1.88), 1.08 (0.95–1.21), 1.00 (reference), 1.14 (1.02–1.29), and 1.74 (1.50–2.02), respectively. According to a restricted cubic spline, we noted a U-shaped relationship between UA and total mortality. The U-shaped relationship between UA and cardiovascular mortality remained in both females and males. The increased cardiovascular mortality in the lowest and highest UA groups was attributed to stroke and heart-specific mortality, respectively. However, serum UA was not significantly associated with cancer mortality.
CONCLUSIONS: Our findings showed a U-shaped relationship between serum UA levels and total and cardiovascular mortality in patients with hypertension. Furthermore, low UA was associated with stroke mortality, while higher UA was associated with heart-related mortality. Further research is needed to identify the potential mechanisms of UA in hypertension.
Keywords: Antioxidant Response Elements, Cardiovascular Diseases, Hypertension, Uric Acid, Cardiovascular System, Cause of Death, Cohort Studies, Follow-Up Studies, Proportional Hazards Models, Risk Factors, Stroke, United States
Background
Hypertension is a well-known and strong risk factor for cardiovascular diseases (CVD), which are the major cause of mortality [1,2]. Uric acid (UA), an end-product metabolite of purine nucleotide in great apes and humans, has been considered as one of the cardiometabolic risk factors in CVD, diabetes, and hypertension [3–5]. Increased serum UA is one of the strongest factors for hypertensive development [6]. Experimental results support that raising uric acid levels causes hypertension in rats [7]. Moreover, pilot studies indicate that lowering serum UA levels can decrease blood pressure in hypertensive patients [8–10].
Interestingly, numerous experimental studies demonstrated that uric acid exerts a beneficial role due to its antioxidant properties [3,11]. It has been demonstrated that uric acid explains about 50% of the total antioxidant capacity in vivo [3]. However, under the setting of pathological acidic or hydrophobic milieu, such as atherosclerotic plaque and cytoplasm, UA becomes a pro-oxidant factor that promotes redox disorder and exerts a deleterious effect in the development and progression of cardiovascular disease [3,11].
Currently, the association of UA levels with adverse outcomes is largely controversial. So far, previous studies noted positive [12–14], negative [15,16], and neutral [17,18] relationships between serum UA concentrations and risks of cardiovascular and total mortality in general adult populations, CVD, and renal disease. Insufficient sample size and variations in study populations and analysis strategies may partly account for the heterogenicity. Two recent large population-based cohorts noted a U-shaped association of total mortality with serum UA in general adult populations [19,20]. The increased mortality risk in adults with hypo- or hyper-uricemia suggests the complex biological roles of UA in humans. In particular, the benefit and harm of strict hypouricemic therapy should be considered.
However, the evidence regarding the link between UA levels and cardiovascular mortality in hypertensive individuals is unclear. To identify this association may provide new insights into the management of UA levels in hypertension, especially the intensive anti-uric acid therapy for hypertensive patients with hyperuricemia. This study aimed to assess the association of serum UA with all-cause and cause-specific mortality among 15 583 participants with hypertension.
Material and methods
STUDY POPULATION AND DESIGN:
Our analysis was based on a dataset from a nationally representative study, the National Health and Nutrition Examination Surveys (NHANES) of the United States [21], which is a stratified and multistage probability-sampling study to assess the characteristics of the nationally non-institutionalized population. The protocols of NHANES have been reported previously [21–23]. The dataset has been built since 1999 and is released in 2-year survey cycles. The protocols and procedures of this study were agreed to by the Research Ethics Review Board of the Centers of Disease Control and Prevention of the United States (Protocol Number. 98–12, 2005–06, and 2011–17). All participants provided written informed consent. The NHANES datasets are available to all researchers to reproduce the results. (https://www.cdc.gov/nchs/index.htm) [21].
We performed the primary analysis using the datasets of 8 two-year survey cycles from 1999–2000 to 2013–2014. In those cycles, there were 38 943 participants aged ≥20 years old with serum UA measurements. Hypertension was defined by blood pressure-lowering treatment and systolic/diastolic blood pressure ≥140/90 mmHg at baseline [21]. We excluded individuals without hypertension (n=22 908). Given the possibility of reverse causality, we further excluded individuals with hypouricemic therapy in the preceding month at baseline (n= 440). Further, we excluded individuals with missing information on mortality status (n=12). In total, 15 583 hypertensive individuals were included for analysis. The flow of the study is presented in Figure 1.
SERUM URIC ACID MEASUREMENT:
The exposure variable of interest was serum uric acid concentrations as tested by the Roche Hitachi Model 917/704 multichannel analyzer, Beckman Synchron LX20, or Beckman UniCel® DxC800 Synchron, as described in prior studies [19]. The protocols have been validated. In brief, UA was oxidized with specific enzyme uricase to generate allantoin and H2O2. The H2O2 further reacted with 2,4,6-tribromo-3-hydroxybenzoic acid and 4-aminophenazone to form quinone-imine dye and hydrogen bromide [19]. The intensity of red color was used to quantify results. The coefficient of variation for UA measurements in each cycle was approximately 2%, suggesting good repeatability. All laboratory variables were assessed by validated protocols and procedures. The details were available at https://www.cdc.gov/nchs/nhanes.
COVARIATES:
Demographic and lifestyle factors at baseline, including age, gender, race/ethnicity, smoke, alcohol intake, and physical activity, and family income level were recorded during personal interviews via standardized questionnaires [21,22]. Physical examinations were performed according to standardized protocols and processes at a mobile examination center. Body mass index (BMI) was calculated as weight (kilograms) divided by height (meters) squared [21]. Average systolic blood pressure and diastolic pressure were assessed as the means of 3 measurements. The detection of biosamples was conducted in special central laboratories with validated methods. Laboratory determinations of triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), glycosylated hemoglobin (HbA1c), and creatinine were detected in each survey cycle [21]. C-reactive protein (CRP) was measured in NHANES 1999–2006. The Chronic Kidney Disease Epidemiology Collaboration method was applied to calculate the estimated glomerular filtration rate (eGFR) [21]. History of diseases and prescription agents in the preceding 30 days was also solicited at a screening interview. Diabetes mellitus was defined as taking diabetic medication or HbA1c ≥6.5% [21]. Emphysema or chronic bronchitis was identified as chronic obstructive pulmonary disease (COPD). Malignant disease was identified according to self-report [21]. Antihypertensive agents were categorized as angiotensin-converting enzyme inhibitor/angiotensin II receptor antagonist (ACEI/ARBs), β-blocker, calcium-channel blockers (CCB), diuretics, and other antihypertensive drugs [4].
OUTCOMES:
The outcomes of this study were total and cause-specific mortality. All participants were linked to the National Death Index in the National Center for Health Statistics of the US [21,22]. Mortality data were available through December 31, 2015. Using the codes of International Classification of Diseases 10th Revision (ICD-10), cause-specific mortality was categorized as death caused by CVD (heart disease: I00–I09, I11, I13, and I20–I51; stroke: I60–I69), and malignant neoplasms (C00–C97) [21].
STATISTICAL ANALYSIS:
The statistical analyses were conducted following the analytical guidelines. Sampling weights, the masked variance of the primary sampling unit, and strata were used to explain the complex study design and acquire nationally representative estimates [21]. Variables are presented as weighted means (standard error, SE) and proportions unless otherwise noted. We used weighted linear regression or logistics regression to assess the difference across serum UA groups, when necessary. Restricted cubic spline based on Cox regression was applied to show the link between serum UA and total mortality, after adjustment for age, sex, race, smoking, alcohol intake, exercise, poverty-to-income ratio (PIR), BMI, cancer, COPD, diabetes, cardiovascular disease, TG, TC, HDL-C, eGFR, lipid-lowering agents, antiplatelet treatment, ACEI/ARBs, β-blocker, CCB, diuretics, and other antihypertensive drugs [21,24]. All hypertensive patients were stratified into 5 prespecified groups according to uric acid levels: ≤3.5, 3.5–5, 5–6, 6–7.5, and >7.5 mg/dL. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated by crude and multivariable Cox regression models for the relationship between UA and total or cause-specific mortality, with UA 5–6 mg/dL as a reference. Three multivariable models were applied. Model 1 was adjusted for demographic variables, including age, sex, and race/ethnicity (non-Hispanic white, black, Hispanic-Mexican, or other). Model 2 was further adjusted for PIR, BMI, smoking status, alcohol intakes, physical activity, TG, TC, HDL-C, eGFR, CVD, diabetes, COPD, and cancer. Model 3 was additionally adjusted for lipid-lowering agents, antiplatelet drugs, ACEI/ARBs, β-blocker, CCB, diuretics, and other antihypertensive drugs [21]. In secondary analyses, the association between baseline UA and all-cause, cardiovascular, heart-specific, and stroke mortality was ascertained in subgroups by age (<65 and ≥65 years), sex (female and male), race (non-Hispanic white and non-white), BMI (<30 and ≥30 kg/m2), eGFR (<60 and ≥60 mL/min/1.73 m2), and anti-hypertension medications (yes/no), with the fully adjusted model except for stratification factors [21]. We also assessed whether the main results were altered after additional adjustment for inflammation marker CRP in NHANES 1999–2006. All tests with a 2-sided P value <0.05 were considered significant using Stata (version 15) software.
Results
BASELINE CHARACTERISTICS:
Overall, 15 583 participants with hypertension (7524 men and 8059 women) were identified in the dataset from NHANES 1999–2000 to 2013–2014 cycles (Figure 1). The mean age was 55.8 years, and 47.6% were males in the study population. The median of serum UA level was 5.7 mg/dL (interquartile range [IQR], 4.8–6.7 mg/dL). Baseline characteristics of hypertensive patients across the strata of uric acid (≤3.5, 3.5–5, 5–6, 6–7.5, and >7.5 mg/dL) are shown in Table 1. Compared to those with lower UA, the participants with higher levels of UA were older and mostly males, and had higher alcohol consumption, TG, and CRP levels. The higher-UA groups were more likely to have adiposity, prior CVD, and to be taking antihypertensive agents. They also had less smoking and vigorous exercise, as well as lower eGFR.
ASSOCIATIONS OF UA WITH TOTAL AND CAUSE-SPECIFIC MORTALITY:
The median duration of follow-up was 7.4 (IQR, 4.1–11.3) years. Over 116 351 person-years of follow-up, 3291 deaths occurred among the 15 583 patients with hypertension, including 639 (19.4%) heart-related deaths and 630 (19.1%) cancer deaths. Among hypertensive participants in NHANES 1999–2006, 152 died due to stroke. The weighted mortality rates per 1000 person-years of follow-up are shown in Table 2, with 21.2 (95% CI, 20.3–22.1) for all-cause mortality, 3.9 (95% CI, 3.5–4.3) for heart-related and 4.1 (95% CI, 3.7–4.5) for cancer-related mortality, and 1.3 (95% CI 1.1–1.6) for stroke mortality. In multiple restricted cubic spline fitting, there was a U-shaped trend between UA and risks of total and cardiovascular mortality (Figure 2).
The relationships between serum UA levels and risks of all-cause, heart disease, stroke, and cancer-related mortality are shown in Table 2, assessed by several weighted multivariable Cox regression analyses with UA 5.0–6.0 mg/dL as reference. The age- and sex-adjusted HRs (95% CIs) of total mortality from ≤3.5 mg/dL to 3.5–5 mg/dL, 5–6 mg/dL, 6–7.5 mg/dL, and >7.5 mg/dL were 1.40 (1.05–1.88), 1.08 (0.95–1.21), 1.00 (reference), 1.14 (1.02–1.29), and 1.74 (1.50–2.02), respectively. The risks remained significant after adjusting for demographics, income, smoking, amateur sports activity, alcohol intakes, BMI, cancer, COPD, diabetes, cardiovascular disease, TG, TC, HDL-C, eGFR, lipid-lowering agents, antiplatelet treatment, ACEI/ARBs, β-blocker, CCB, diuretics, and other antihypertensive drugs. Compared with patients with UA 5–6 mg/dl, those with UA ≤3.5 and >7.5 mg/dL had an elevated risk of all-cause mortality, by 47% and 35%, respectively.
As expected, a similar trend of association of UA with cardiovascular mortality was noted (Table 2). In multivariable-adjusted model, the HRs (95% CI) of cardiovascular mortality from the lowest to the highest group were 1.92 (1.08–3.42), 1.27 (0.88–1.81), 1.00 (ref.), 1.19 (0.92–1.55), and 1.37 (1.00–1.90), respectively. Further, compared with patients with UA 5–6 mg/dl, the increased cardiovascular mortality in patients with UA ≤3.5 mg/dl was mainly due to stroke (HR 4.34, 95% CI 1.66–11.35), while the increased mortality in patients with UA >7.5 mg/dl was mainly due to ischemic heart disease (HR 1.46, 95% CI 1.10–1.95). However, neither lower serum UA nor higher UA was significantly related to cancer mortality.
In prespecified stratification analyses (Supplemenatry Tables 1–6), the associations of serum concentrations of UA with total and cardiovascular mortality were similar in the subgroups of hypertensive patients regarding sex (female versus male), eGFR (≥60 versus <60), and other subgroups. In the further sensitivity analysis, we assessed the relationship between UA and mortality in participants with CRP measurement. After additionally adjusting for CRP, the results did not alter significantly (Supplemenatry Table 7).
Discussion
STRENGTHS AND LIMITATIONS:
Our study has several limitations. First, the residual confounders unmeasured may not be completely ruled out. Second, the causality of this link between serum UA and mortality risk could not be determined because of the observational nature of the study, although prior experimental studies observed that UA-related metabolism activated inflammation and oxidative stress in the process of cardiovascular disease. Third, the enrolled participants in this study were US civilians, so the results extrapolated to other populations need further verification. Fourth, information on distinguishing primary and secondary hypertension was lacking in this community-based cohort, and further investigation in special hospital-based cohorts is warranted. This study may have some strengths. The NHANES study was a national sampling dataset that favored repeatability and generalization. The sample size and long-term follow-up provide favorable statistical effectiveness. We have adjusted for a variety of potential confounders, including lifestyle, laboratory data, chronic disease, and detailed information of antihypertensive agents, and the relationship between UA and all-cause and cardiovascular mortality remained significant.
Conclusions
Our findings suggest a U-shaped relationship between serum UA and risks of total and cardiovascular mortality in hypertensive patients. Both low and high levels of uric acid were independently associated with increased risks of cardiovascular mortality. Nonetheless, low UA may mainly increase stroke-related mortality, and high UA may mainly increase heart-related mortality. Further investigations are needed to elucidate the potential mechanisms and validate the role of uric acid in the progression of hypertension.
Figures
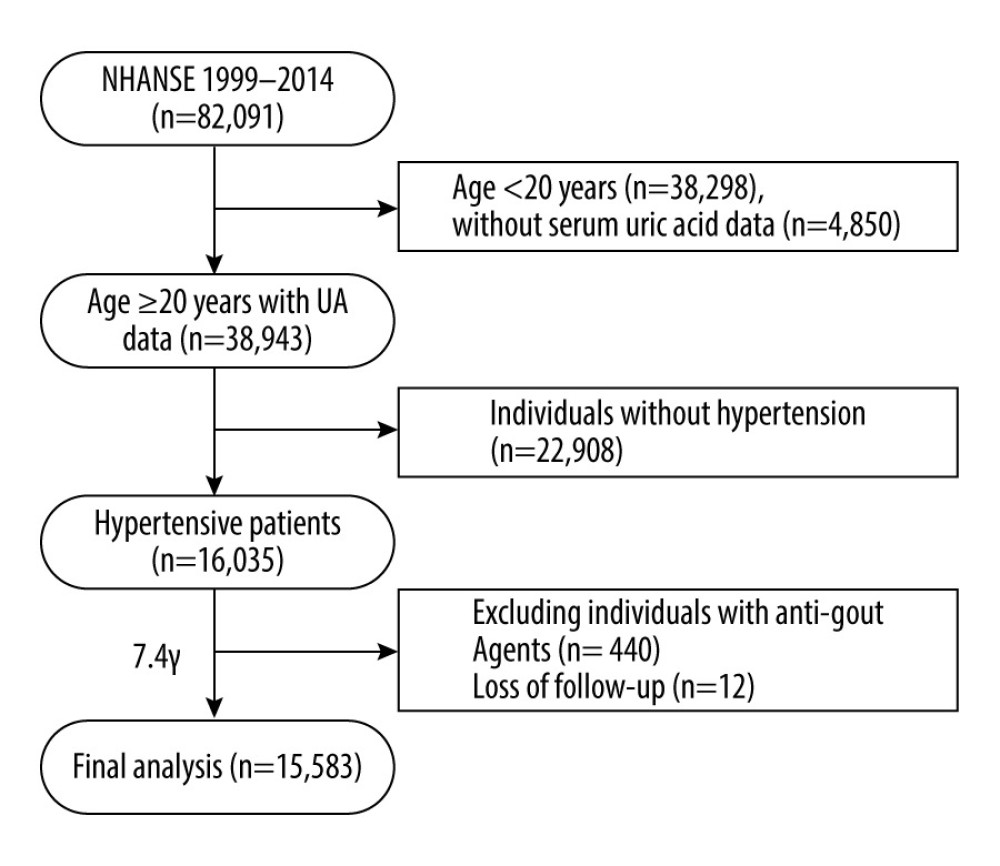 Figure 1. Flow diagram of this study.
Figure 1. Flow diagram of this study. 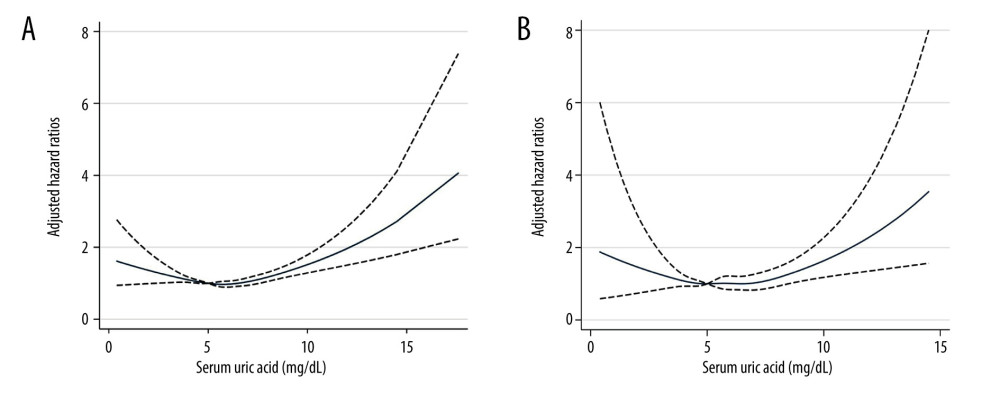 Figure 2. The non-linear associations between uric acid levels and all-cause and cardiovascular mortality. The restricted cubic spline shows the relationship between UA and all-cause (A) and cardiovascular (B) mortality risk. The fitting includes 5 knots: the 5th, 27.5th, 50th, 72.5th, and 95th percentiles. HR (95% CI) was estimated with multivariable Cox regression analysis after adjustment for Model 3. The solid and dashed lines represent point estimates and 95% CIs, respectively. The non-linear trend was significant for the relationship between UA and all-cause and cardiovascular mortality (P for non-linearity ≤0.035).
Figure 2. The non-linear associations between uric acid levels and all-cause and cardiovascular mortality. The restricted cubic spline shows the relationship between UA and all-cause (A) and cardiovascular (B) mortality risk. The fitting includes 5 knots: the 5th, 27.5th, 50th, 72.5th, and 95th percentiles. HR (95% CI) was estimated with multivariable Cox regression analysis after adjustment for Model 3. The solid and dashed lines represent point estimates and 95% CIs, respectively. The non-linear trend was significant for the relationship between UA and all-cause and cardiovascular mortality (P for non-linearity ≤0.035). Tables
Table 1. Characteristics of patients with hypertension in NHANES 1999–2014 at baseline.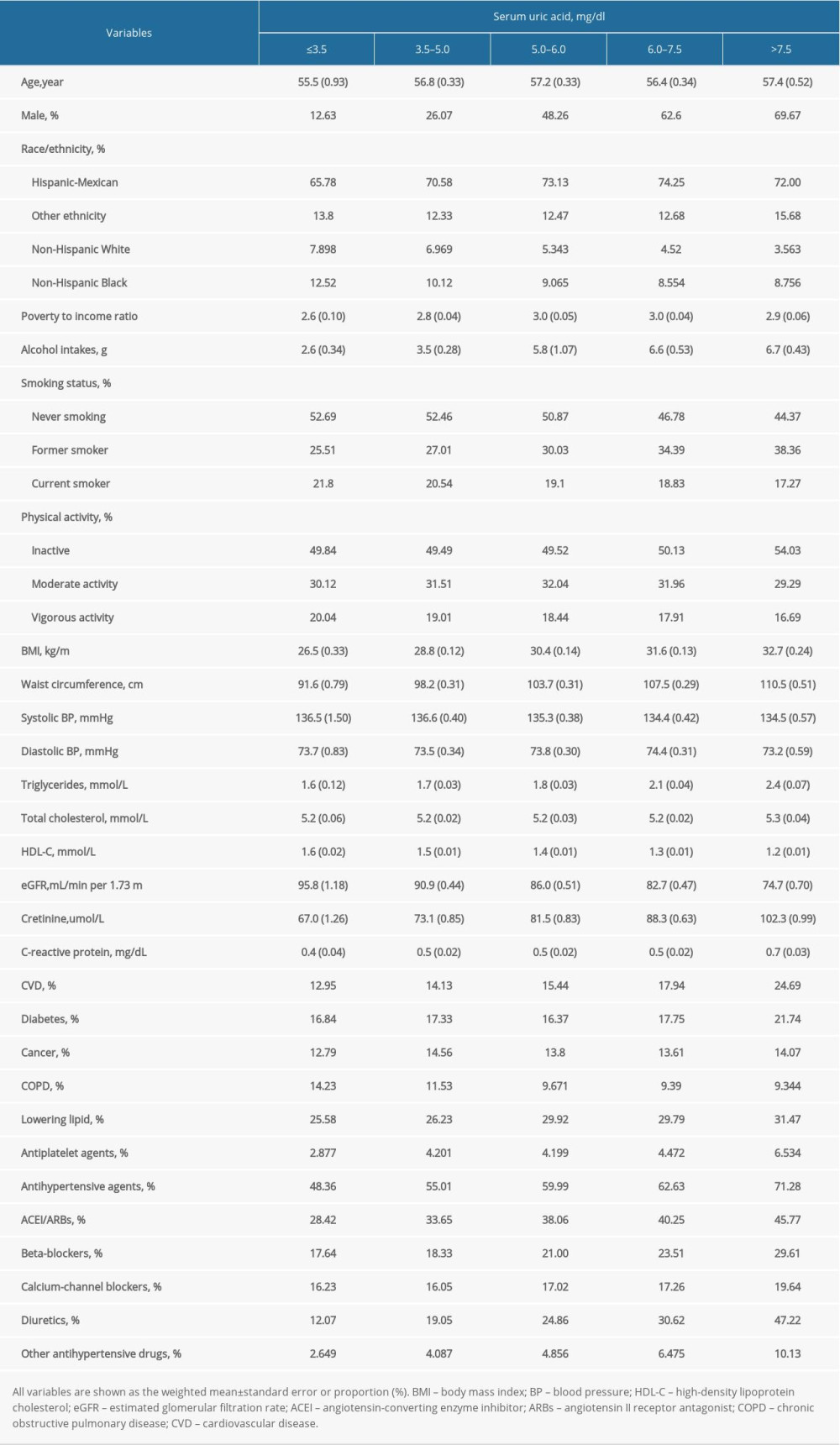 Table 2. The relationship between serum uric acid and all-cause and cause-specific mortality in hypertensive patients.
Table 2. The relationship between serum uric acid and all-cause and cause-specific mortality in hypertensive patients.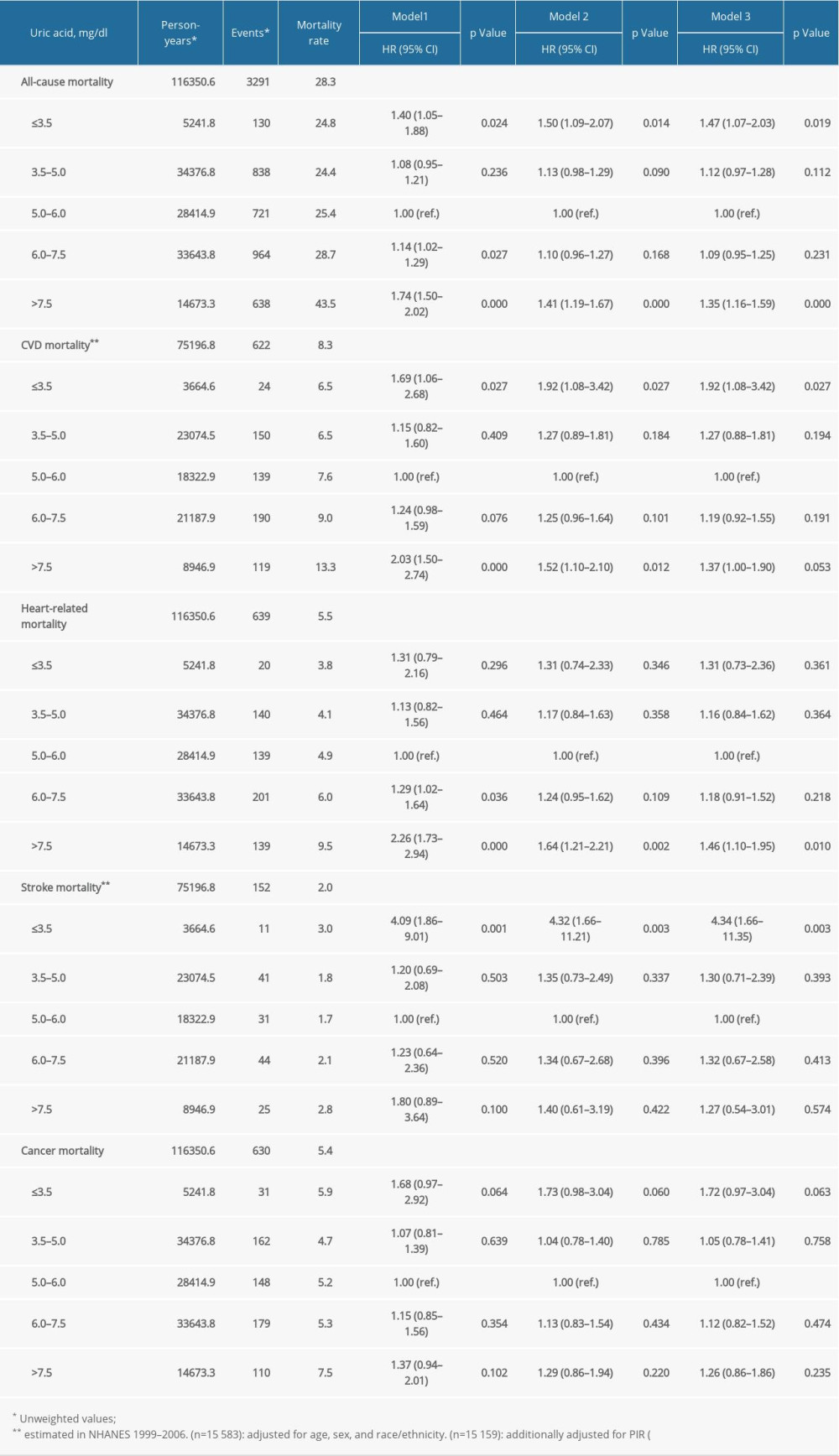 Supplementary Table 1. Stratification analysis of the association between uric acid and mortality by sex.
Supplementary Table 1. Stratification analysis of the association between uric acid and mortality by sex.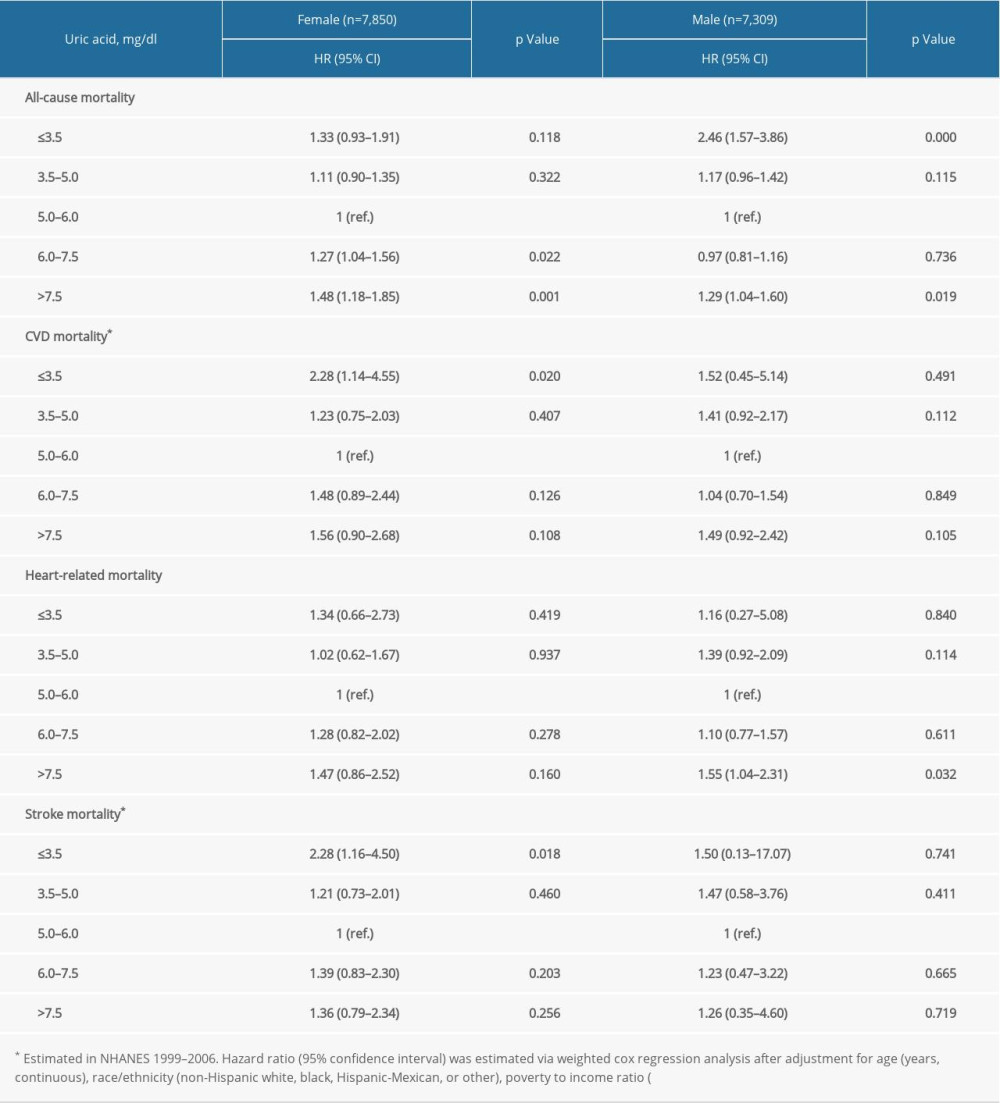 Supplementary Table 2. Stratification analysis by estimated glomerular filtration rate.
Supplementary Table 2. Stratification analysis by estimated glomerular filtration rate.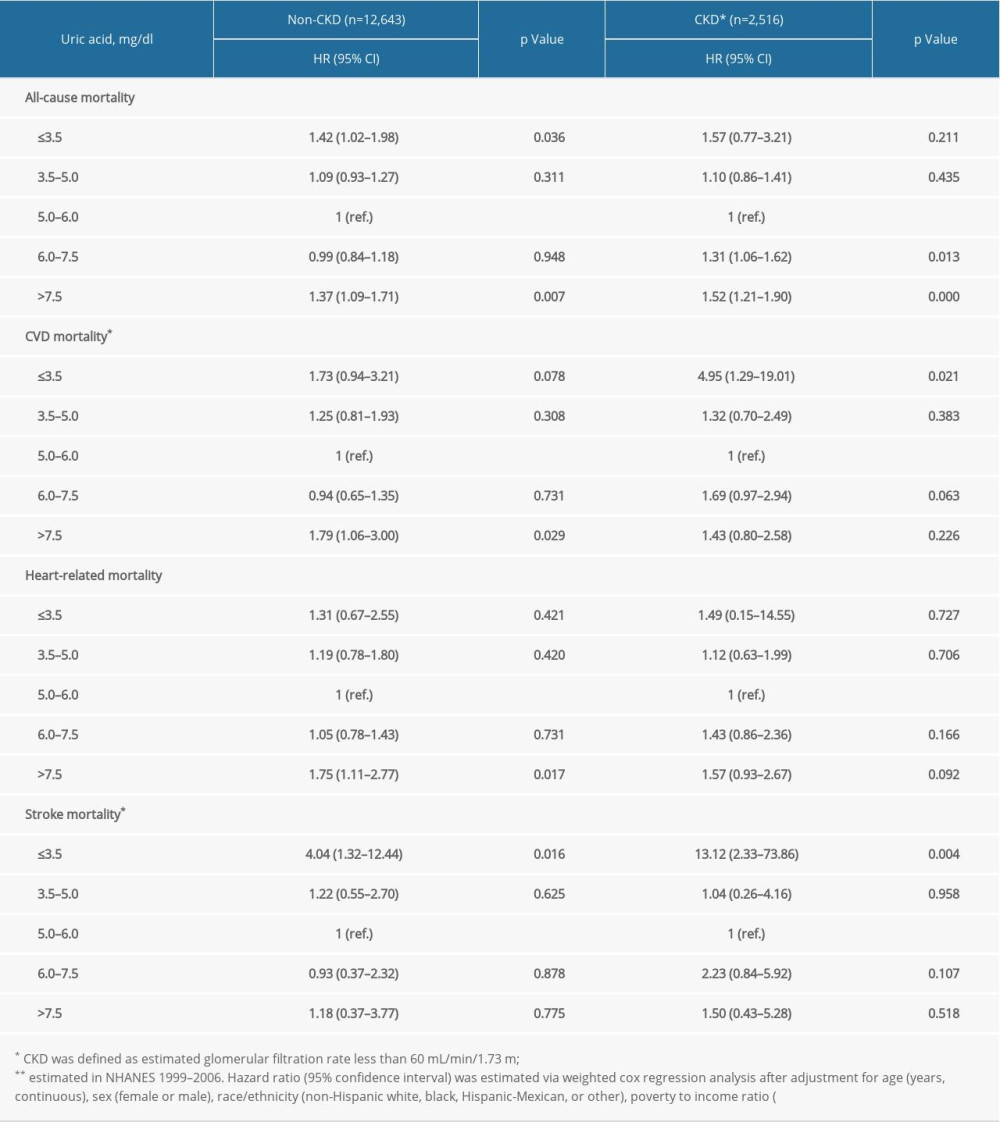 Supplementary Table 3. Stratification analysis by race/ethnicity.
Supplementary Table 3. Stratification analysis by race/ethnicity.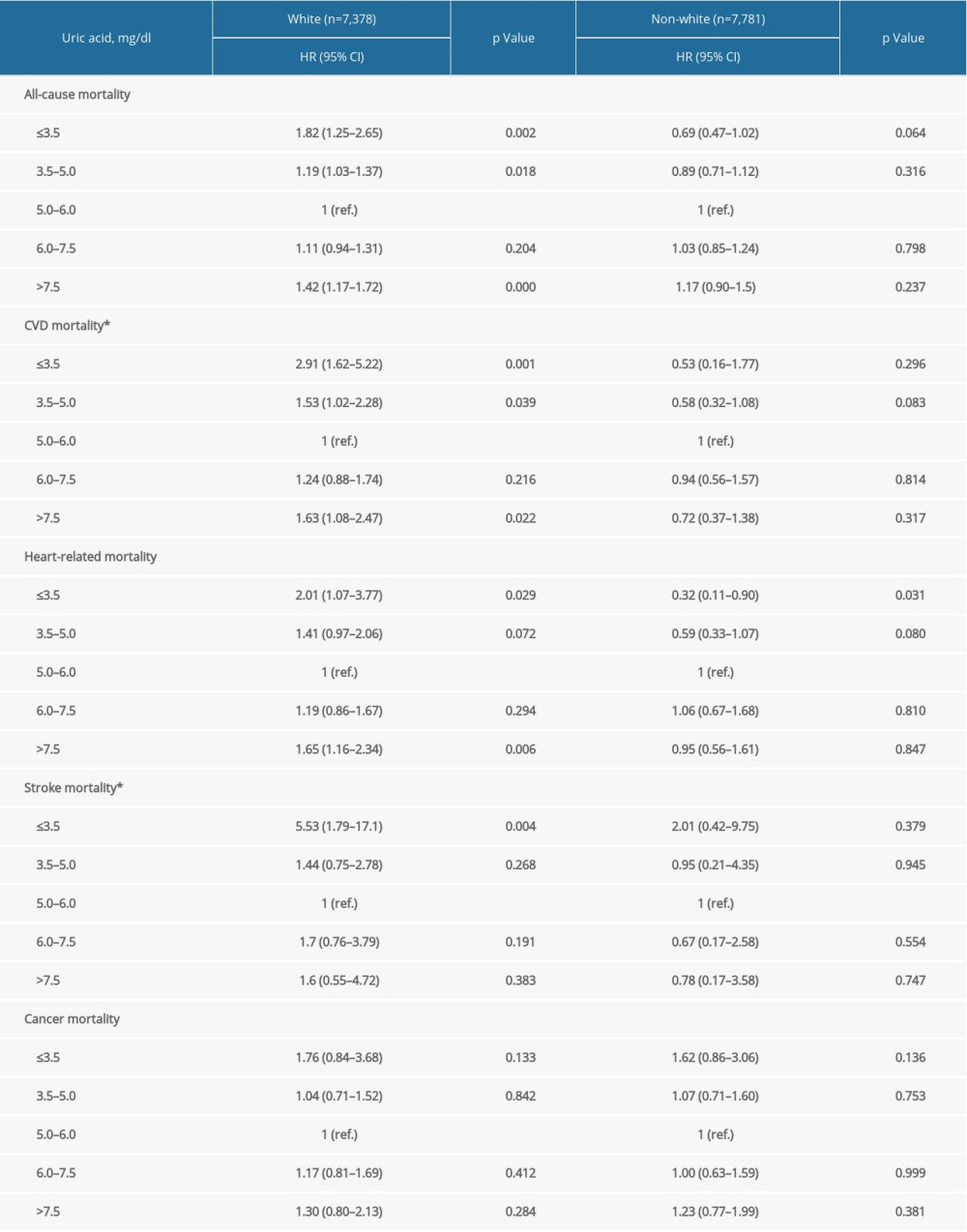 Supplementary Table 4. Stratification analysis by BMI.
Supplementary Table 4. Stratification analysis by BMI.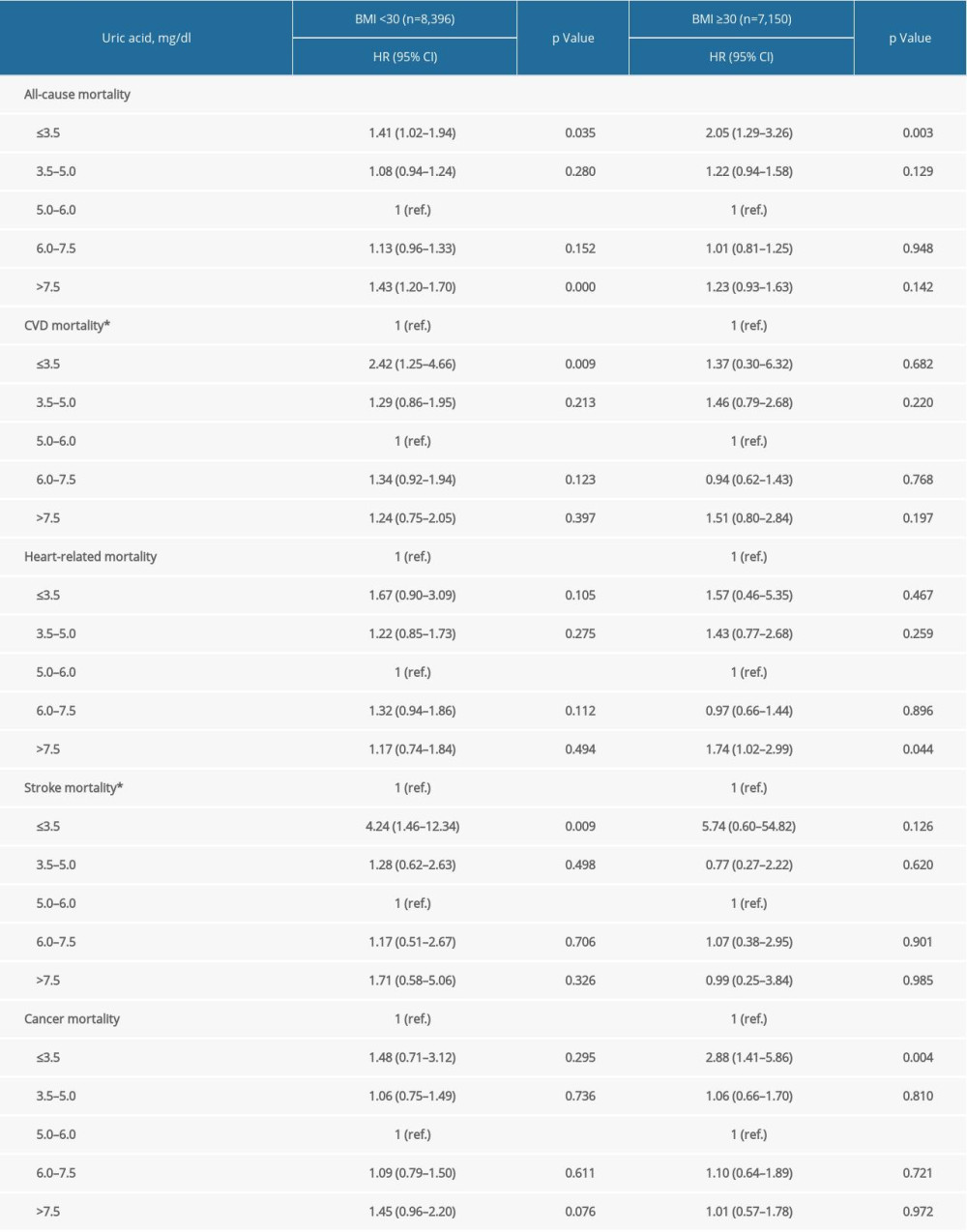 Supplementary Table 5. Stratification analysis by age groups.
Supplementary Table 5. Stratification analysis by age groups.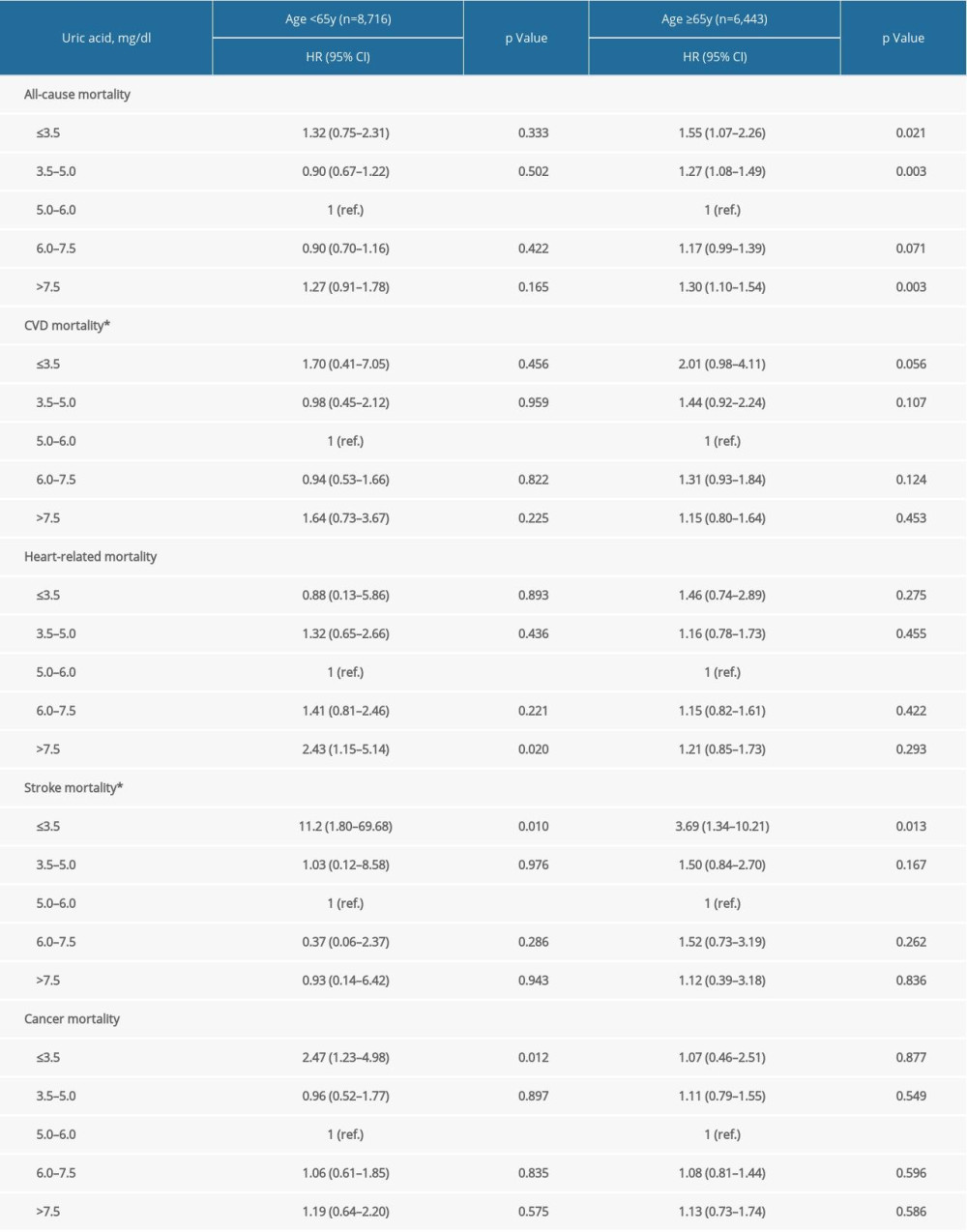 Supplementary Table 6. Stratification analysis by anti-hypertension medications.
Supplementary Table 6. Stratification analysis by anti-hypertension medications.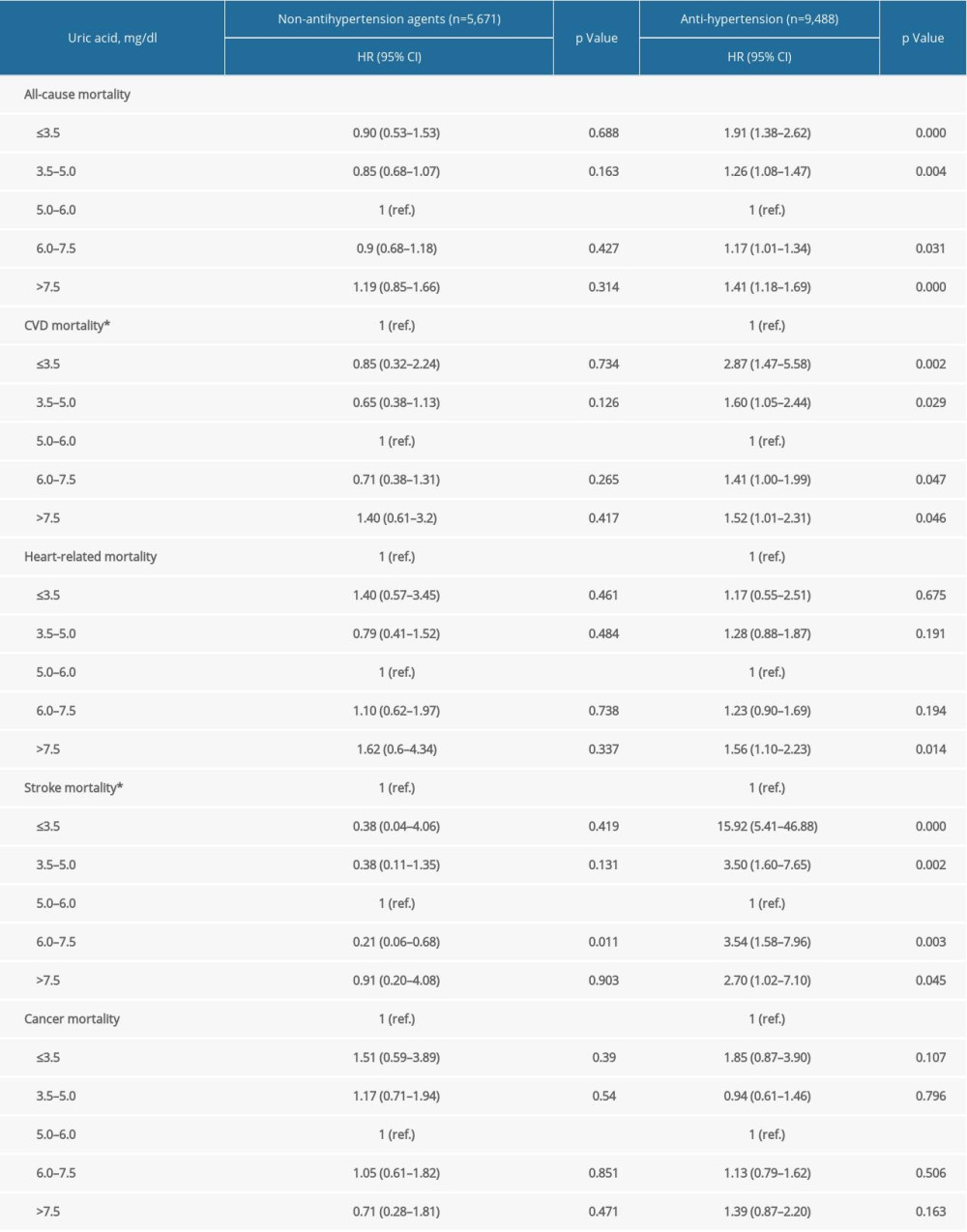 Supplementary Table 7. Repeated analysis after adjustment for C-reactive protein in NHANES 1999–2006.
Supplementary Table 7. Repeated analysis after adjustment for C-reactive protein in NHANES 1999–2006.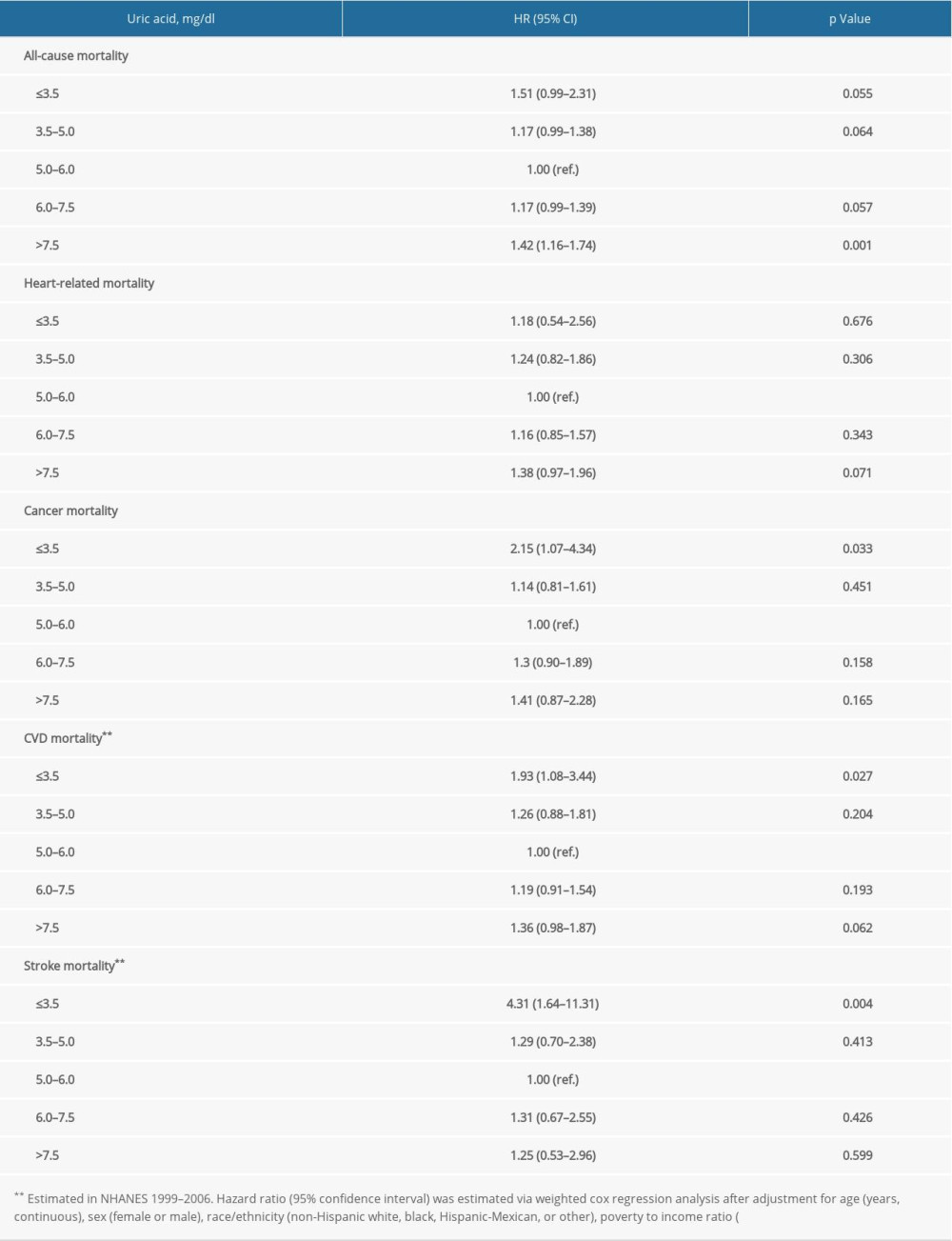
References
1. Overwyk KJ, Zhao L, Zhang Z, Trends in blood pressure and usual dietary sodium intake among children and adolescents, national health and nutrition examination survey 2003 to 2016: Hypertension, 2019; 74; 260-66
2. Benjamin EJ, Virani SS, Callaway CW, Heart disease and stroke statistics – 2018 update: A report from the American Heart Association: Circulation, 2018; 137; e67-492
3. Ndrepepa G, Uric acid and cardiovascular disease: Clin Chim Acta, 2018; 484; 150-63
4. Kuwabara M, Hisatome I, Niwa K, A 5-year Japanese cohort study: Hypertension, 2018; 71; 78-86
5. Pilemann-Lyberg S, Hansen TW, Tofte N, Uric acid is an independent risk factor for decline in kidney function, cardiovascular events, and mortality in patients with type 1 diabetes: Diabetes Care, 2019; 42; 1088-94
6. Liu L, Gu Y, Li C, A population-based prospective cohort study: J Hum Hypertens, 2017; 31; 116-20
7. Mazzali M, Hughes J, Kim YG, Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism: Hypertension, 2001; 38; 1101-6
8. Soletsky B, Feig DI, Uric acid reduction rectifies prehypertension in obese adolescents: Hypertension, 2012; 60; 1148-56
9. Higgins P, Walters MR, Murray HM, Allopurinol reduces brachial and central blood pressure, and carotid intima-media thickness progression after ischaemic stroke and transient ischaemic attack: A randomised controlled trial: Heart, 2014; 100; 1085-92
10. Madero M, Rodríguez CF, Jalal D, A pilot study on the impact of a low fructose diet and allopurinol on clinic blood pressure among overweight and prehypertensive subjects: A randomized placebo controlled trial: J Am Soc Hypertens, 2015; 9; 837-44
11. Becker BF, Towards the physiological function of uric acid: Free Radic Biol Med, 1993; 14; 615-31
12. Kleber ME, Delgado G, Grammer TB, Uric acid and cardiovascular events: A Mendelian randomization study: J Am Soc Nephrol, 2015; 26; 2831-38
13. Xia X, Zhao C, Peng FF, Serum uric acid predicts cardiovascular mortality in male peritoneal dialysis patients with diabetes: Nutr Metab Cardiovasc Dis, 2016; 26; 20-26
14. Basar N, Sen N, Ozcan F, Elevated serum uric acid predicts angiographic impaired reperfusion and 1-year mortality in ST-segment elevation myocardial infarction patients undergoing percutaneous coronary intervention: J Investig Med, 2011; 59; 931-37
15. Li M, Ye ZC, Li CM, Low serum uric acid levels increase the risk of all-cause death and cardiovascular death in hemodialysis patients: Ren Fail, 2020; 42; 315-22
16. Lai KJ, Kor CT, Hsieh YP, An inverse relationship between hyperuricemia and mortality in patients undergoing continuous ambulatory peritoneal dialysis: J Clin Med, 2018; 7(11); 416
17. Panero F, Gruden G, Perotto M, Uric acid is not an independent predictor of cardiovascular mortality in type 2 diabetes: A population-based study: Atherosclerosis, 2012; 221; 183-88
18. Zalawadiya SK, Veeranna V, Mallikethi-Reddy S, Uric acid and cardiovascular disease risk reclassification: Findings from NHANES III: Eur J Prev Cardiol, 2015; 22; 513-18
19. Hu L, Hu G, Xu BP, U-shaped association of serum uric acid with all-cause and cause-specific mortality in US adults: A cohort study: J Clin Endocrinol Metab, 2020; 105(1); dgz068
20. Cho SK, Chang Y, Kim I, Ryu S, U-shaped association between serum uric acid level and risk of mortality: A cohort study: Arthritis Rheumatol, 2018; 70; 1122-32
21. Wang S, Liu Y, Liu J, Mitochondria-derived methylmalonic acid, a surrogate biomarker of mitochondrial dysfunction and oxidative stress, predicts all-cause and cardiovascular mortality in the general population: Redox Biol, 2020; 37; 101741
22. Wang S, Tian W, Liu Y, Temporal trend of circulating trans-fatty acids and risk of long-term mortality in general population: Clin Nutr, 2020 [Online ahead of print]
23. Rossato LT, de Branco FMS, Azeredo CM, Association between omega-3 fatty acids intake and muscle strength in older adults: A study from National Health and Nutrition Examination Survey (NHANES) 1999–2002: Clin Nutr, 2020; 39(11); 3434-41
24. Zhang X, Wang S, Liu J, D-dimer and the incidence of heart failure and mortality after acute myocardial infarction: Heart, 2020 [Online ahead of print]
25. Wang J, Wang Y, Zhao D, Association between serum uric acid and mortality in a Chinese population of hypertensive patients: Ren Fail, 2015; 37; 73-76
26. Tseng WC, Chen YT, Ou SMTaiwan Geriatric Kidney Disease (TGKD) Research Group, U-shaped association between serum uric acid levels with cardiovascular and all-cause mortality in the elderly: The role of Malnourishment: J Am Heart Assoc, 2018; 7(4); e007523
27. Wu AH, Gladden JD, Ahmed M, Relation of serum uric acid to cardiovascular disease: Int J Cardiol, 2016; 213; 4-7
28. DeMarco VG, Aroor AR, Sowers JR, The pathophysiology of hypertension in patients with obesity: Nat Rev Endocrinol, 2014; 10; 364-76
29. Sharaf EDU, Salem MM, Abdulazim DO, Uric acid in the pathogenesis of metabolic, renal, and cardiovascular diseases: A review: J Adv Res, 2017; 8; 537-48
30. Takir M, Kostek O, Ozkok A, Lowering uric acid with allopurinol improves insulin resistance and systemic inflammation in asymptomatic hyperuricemia: J Investig Med, 2015; 63; 924-29
31. Ruggiero C, Cherubini A, Ble A, Uric acid and inflammatory markers: Eur Heart J, 2006; 27; 1174-81
32. Juraschek SP, Miller ER, Gelber AC, Effect of oral vitamin C supplementation on serum uric acid: A meta-analysis of randomized controlled trials: Arthritis Care Res (Hoboken), 2011; 63; 1295-306
Figures
 Figure 1. Flow diagram of this study.
Figure 1. Flow diagram of this study. Figure 2. The non-linear associations between uric acid levels and all-cause and cardiovascular mortality. The restricted cubic spline shows the relationship between UA and all-cause (A) and cardiovascular (B) mortality risk. The fitting includes 5 knots: the 5th, 27.5th, 50th, 72.5th, and 95th percentiles. HR (95% CI) was estimated with multivariable Cox regression analysis after adjustment for Model 3. The solid and dashed lines represent point estimates and 95% CIs, respectively. The non-linear trend was significant for the relationship between UA and all-cause and cardiovascular mortality (P for non-linearity ≤0.035).
Figure 2. The non-linear associations between uric acid levels and all-cause and cardiovascular mortality. The restricted cubic spline shows the relationship between UA and all-cause (A) and cardiovascular (B) mortality risk. The fitting includes 5 knots: the 5th, 27.5th, 50th, 72.5th, and 95th percentiles. HR (95% CI) was estimated with multivariable Cox regression analysis after adjustment for Model 3. The solid and dashed lines represent point estimates and 95% CIs, respectively. The non-linear trend was significant for the relationship between UA and all-cause and cardiovascular mortality (P for non-linearity ≤0.035). Tables
 Table 1. Characteristics of patients with hypertension in NHANES 1999–2014 at baseline.
Table 1. Characteristics of patients with hypertension in NHANES 1999–2014 at baseline. Table 2. The relationship between serum uric acid and all-cause and cause-specific mortality in hypertensive patients.
Table 2. The relationship between serum uric acid and all-cause and cause-specific mortality in hypertensive patients. Table 1. Characteristics of patients with hypertension in NHANES 1999–2014 at baseline.
Table 1. Characteristics of patients with hypertension in NHANES 1999–2014 at baseline. Table 2. The relationship between serum uric acid and all-cause and cause-specific mortality in hypertensive patients.
Table 2. The relationship between serum uric acid and all-cause and cause-specific mortality in hypertensive patients. Supplementary Table 1. Stratification analysis of the association between uric acid and mortality by sex.
Supplementary Table 1. Stratification analysis of the association between uric acid and mortality by sex. Supplementary Table 2. Stratification analysis by estimated glomerular filtration rate.
Supplementary Table 2. Stratification analysis by estimated glomerular filtration rate. Supplementary Table 3. Stratification analysis by race/ethnicity.
Supplementary Table 3. Stratification analysis by race/ethnicity. Supplementary Table 4. Stratification analysis by BMI.
Supplementary Table 4. Stratification analysis by BMI. Supplementary Table 5. Stratification analysis by age groups.
Supplementary Table 5. Stratification analysis by age groups. Supplementary Table 6. Stratification analysis by anti-hypertension medications.
Supplementary Table 6. Stratification analysis by anti-hypertension medications. Supplementary Table 7. Repeated analysis after adjustment for C-reactive protein in NHANES 1999–2006.
Supplementary Table 7. Repeated analysis after adjustment for C-reactive protein in NHANES 1999–2006. In Press
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








