07 January 2021: Clinical Research
Efficacy of 5-Aminolevulinic Acid (ALA)-Photodynamic Therapy (PDT) in Refractory Vulvar Lichen Sclerosus: Preliminary Results
Fenghua Zhang1ABCEF, Daoyun Li2BCD, Lijuan Shi2BC, Yijia Gu2C, Yun Xu2AEG, Changping Wu345ACEG*DOI: 10.12659/MSM.927406
Med Sci Monit 2021; 27:e927406
Abstract
BACKGROUND: As a chronic inflammatory skin disease of unknown etiology, vulvar lichen sclerosus (VLS) mainly affects postmenopausal and perimenopausal women. The main clinical manifestations of VLS include itching, burning pain, and sexual dysfunction, which can lead to a decline in quality of life. The existing treatment options include topical corticosteroid ointment, estrogen, and traditional Chinese medicine; however, their therapeutic effects on VLS remain unsatisfactory.
MATERIAL AND METHODS: Thirty patients with VLS and routine treatment failure were treated with 5-aminolevulinic acid (ALA)-photodynamic therapy (PDT). A 20% ALA water-in-oil emulsion was applied to the vulvar lesions and sealed with plastic film for 3 h. Patients were irradiated at a power density of 60 to 90 mW/cm² with a red light at a wavelength of 635±15 nm for 20 min, delivering a total dose of 100 to 150 J/cm² per session. The treatment was repeated 3 times every 2 weeks. The objective parameters, female sexual function index (FSFI) and quality of life (QoL) scores, were used before and after treatment to evaluate the clinical curative effect.
RESULTS: All patients completed 3 treatment cycles of ALA-PDT and follow-up visits. The clinical symptoms of pruritus completely disappeared in 27 cases, and itching improved from severe to mild in 3 cases. The pathological changes of all patients were objectively improved. FSFI score decreased significantly after treatment (P<0.001). The main adverse effects of ALA-PDT were pain, erythema, and swelling. These adverse effects were temporary and tolerable. The QoL score was significantly improved after treatment (P<0.001).
CONCLUSIONS: ALA-PDT is an effective and safe approach for the treatment of VLS.
Keywords: Lichen Sclerosus et Atrophicus, Photochemotherapy, vulvar lichen sclerosus, Aminolevulinic Acid, Photosensitizing Agents
Background
Vulvar leukoplakia, also known as white lesions of the vulva, is a term used to describe vulvar intraepithelial non-tumor-like lesions, which are caused by vulvar squamous epithelial hyperplasia, vulvar lichen sclerosus (VLS), and vulvar squamous epithelial hyperplasia with VLS. Lichen sclerosus (LS) is a chronic inflammatory mucocutaneous disease characterized by remissions and exacerbations [1]. It affects mostly genital and perianal areas, although every skin area and oral mucosa can be involved [1]. Postmenopausal women are more commonly affected by LS than are men, with a prevalence in women estimated at approximately 3%, while the prevalence in children and men is approximately 0.1% and 0.07%, respectively [2]. These percentages are possibly underestimated, as many cases are asymptomatic and undiagnosed. However, the most commonly reported symptoms include burning sensation, pain, itching, dyspareunia, dysuria, and painful defecation. Itching of the vulva is the main symptom of VLS in women and the histopathological finding of a biopsy is the main form of diagnosis. Vulvar leukoplakia histiocytosis is active disease and its pathogenesis may be related to human papillomavirus (HPV) infection. A genetic theory states that about 22% of lichens can be inherited. Autoimmune factors and oxidative stress are also understood to have a role in LS because of the strong association with autoimmune diseases and the existence of highly specific antibodies to extracellular matrix protein 1 (ECM-1). A previous study reported an autoimmune phenotype of LS that is characterized by increased expressions of Th1-specific cytokines, dense infiltration of T cells, and increased expression of BIC/miR-155 [3]. This phenotype affects genital skin and causes severe itching, skin hypopigmentation, and poor skin elasticity or atrophy, which can lead to stricture, pain in sexual intercourse, and pain during urination and bowel movements. It is the second leading cause of non-neoplastic vulvar disease and a risk factor for vulvar squamous cell carcinoma (SCC), including invasive SCC and vulvar intraepithelial neoplasia; the lifetime risk of SCC in untreated or improperly treated disease is 2% to 6% [4,5]. The diagnosis of LS depends primarily on the clinical diagnosis, and biopsy should be performed when malignant transformation is suspected. LS should be distinguished from atopic dermatitis, extramammary Paget disease, genital warts, vitiligo, non-pigmented seborrheic keratosis, and vulvar intraepithelial neoplasia. In hospitals, the cited prevalence rate of LS is roughly 0.1% to 0.3%. Therefore, LS is recognized as a rare disease by the Genetic and Rare Diseases Information Center of the US National Institutes of Health and the National Organization for Rare Diseases. Among patients in gynecology clinics, the incidence of VLS is 1.7% [6]. A recent study in the Netherlands estimated that the incidence of VLS is 14.6 per 100 000 women [4].
The difficulty in treating VLS is a problem worldwide and treatment failure is common. It is important to treat the disease as soon as possible using a method that can reverse its progression. Unfortunately, at present, there is no definite treatment for VLS. Therefore, the main purpose of currently available treatments is to relieve or eliminate symptoms (especially vulvar pruritus) rather than cure the disease. Traditional treatments include topical corticosteroids, hormones (testosterone, estrogen, and progesterone), 5-fluorouracil, and retinoic acid. Other physiotherapeutic methods have been attempted, including surgery, cryosurgery, carbon dioxide laser therapy, focused ultrasound therapy, and traditional Chinese medicine therapy. Among the above-mentioned options, effective topical corticosteroids are the first-line treatment. However, long-term use of topical corticosteroids may increase the risk of skin atrophy and pigmentation. Surgical intervention is generally used in patients with atypical hyperplasia and malignant tendency. Nevertheless, surgery is often accompanied by infection, a high recurrence rate of disease, and severe pain [7]. Therefore, there is a need for a more effective treatment of VLS, which has fewer adverse effects and can lead to a lower rate of recurrence.
Photodynamic therapy (PDT) has been recommended for the treatment of VLS [8]. As the second generation of porphyrin photosensitive drugs, 5-aminolevulinic acid (5-ALA) is an endogenous substance, the intermediate product of porphyrin metabolism, which participates in the biosynthesis of heme
Material and Methods
PATIENTS AND ENROLLMENT CRITERIA:
From January 2019 to December 2019, fifty-six patients volunteered to participate in this study, and 30 patients with vulvar leukoplakia who failed conventional treatment were enrolled (the workflow of patients recruited in the study is shown in Figure 1). Inclusion criteria were the clinical manifestations of vulvar lesions: hypopigmentation, waxy atrophy, lichenoid keratosis, or sclerosing lesions. All patients underwent vulvar biopsy, and the pathological diagnosis was consistent with VLS (Figure 2). All 30 patients received a variety of routine treatments before selection, of which 22 patients were treated with topical corticosteroids, and 1 patient was treated with vitamin E cream (Table 1). However, these treatments resulted in only temporary improvement for some patients and no improvement for others. Informed consent was obtained from all patients.
Exclusion criteria were as follows: skin thickening, ulcer, and chapping, in which the possibility of malignancy could not be ruled out; severe pelvic inflammation, cervical inflammation, or other serious gynecological inflammation; undiagnosed vaginal bleeding; current allergic diseases; suspected or known porphyria or known history of allergy to experimental drugs and drugs with a similar chemical structure; severe diseases of the cardio-cerebral, vascular, nervous, mental, endocrine, and hematopoietic systems; known severe low immune function or long-term use of glucocorticoids and immunosuppressants; presence of malignant tumor; abnormal liver function (ALT, AST, total bilirubin 1.5 times higher than the normal upper limit) or renal dysfunction (creatinine and blood urea nitrogen 1.5 times higher than the normal upper limit); current pregnancy or lactation; previous physiotherapeutic measures for vulvar leukoplakia after diagnosis; participation in any drug clinical trial within 30 days before this trial; and judgment of researchers that patient was otherwise unsuitable to participate.
TREATMENT:
Pictures of the VLS lesions before each treatment and during follow-up were acquired using the same colposcope. The patients in the treatment group were treated with bladder lithotomy, the vulva was cleaned with normal saline and gently wiped using medical gauze, and the vagina was blocked with condoms to avoid contamination by secretion. During the treatment, the ALA dose was determined according to the area of the lesions, whereby an area of 1 cm2 was covered by 0.5 mL of gel containing 118 mg ALA (Shanghai Fudan Zhangjiang Biopharmaceutical Co., Ltd., China). Briefly, 118 mg 20% ALA was applied to the hypopigmented area of vulvar skin (1 cm boundary) and covered with gauze and fresh-keeping film for 3 h. The patients were treated within 7 days after menstruation using a 635-nm LED gynecological therapeutic instrument (Wuhan Accord Optoelectronic Technology Co, Ltd, China) with a power density of 60 to 90 mW/cm2 and exposure time of 20 min (Figure 3). The treatment was given every 14 days for a total of 3 times. Sexual activity was prohibited during the treatment period.
EVALUATION AND FOLLOW-UP:
A clinical evaluation was conducted before each treatment and follow-up, and patients were also asked to assess their degree of subjective symptoms, such as itching, burning pain, and difficulty in sexual intercourse caused by VLS, according to the female sexual function index (FSFI) [9]. The patients were followed up at 1, 3, and 6 months after treatment (Figure 4). The histological biopsy examinations after treatment (Figure 5) were performed and analyzed by 2 senior pathologists.
The objective clinical and morphological manifestations and the size of the lesions before and during treatment and at 1, 3, and 6 months after treatment were independently assessed by 2 gynecologists. The 4 objective parameters (atrophy, hyperkeratosis, depigmentation, and sclerosis) were graded according to the following scores: 3=severe; 2=moderate; 1=mild; 0=absence [10]. In addition, the anatomical scope of the disease was evaluated according to the following grading criteria: 0.5=vulvar surface involvement of less than 30%; 1=vulvar surface involvement of 30% to 60%; 1.5=vulvar surface involvement of more than 60% [9]. The total score was obtained by summing the scores of each parameter [10].
In addition, during each treatment and follow-up, the patients’ quality of life (QoL) score was evaluated, and a higher score indicated worse QoL [11].
In previous studies [12,13], the recurrence of VLS was defined as a total score of the objective parameters exceeding 3 or a sclerosing lesion showing increased symptoms.
STATISTICAL ANALYSIS:
Statistical analysis was carried out with SPSS 20.0 (IBM Corporation, Armonk, NY, USA). The distribution of data was determined by the Shapiro-Wilk test. Continuous variables were presented as mean±standard deviation. The comparison before and after treatment was performed by a paired
Results
PATIENT CHARACTERISTICS:
All 30 patients completed the entire treatment and were followed for 6 months. The average age of the patients was 48 years and half of the women were before menopause and half were after menopause. The patients’ medical history of VLS ranged from 1 to 12 years, with an average course of 80 months. Among these 30 patients, all had hyperkeratosis and depigmentation. The other clinical features of the patients are listed in Table 1.
EVALUATION OF OBJECTIVE PARAMETERS:
At the 6-month follow-up, the clinical signs of all patients were significantly improved. The average scores of clinical signs at baseline were significantly higher than those at the 6-month follow-up. Before the first administration of PDT, all patients showed hypopigmentation and atrophy in the vulvar lesion area. Hyperkeratosis was found in 7 patients and vulvar sclerosis was found in 5 patients. The vulvar lesions were significantly reduced in 25 patients after treatment and had completely disappeared in 5 patients. The average score at 6 months after the last treatment was lower than the score before treatment. Before the treatment, the vulvar lesions of 21 patients were more than 60% of the vulvar surface, the lesions of 7 patients were 30% of the vulvar surface, and the lesions of 2 patients were less than 30% of the vulvar surface. At 6 months after the last treatment, the vulvar lesions of all patients were less than 30%. Further, the vulvar lesions of 17 patients had nearly or completely disappeared (Table 2). The total scores after treatment were significantly lower than those before treatment (P<0.001). After the PDT, the thickness of the epidermis was reduced (Figure 4) and the inflammatory cell infiltration of the dermis was ameliorated. The pathology reports showed that the chronic inflammation, acellular collagen band in the dermis, and superficial epithelial vacuolar degeneration improved (Figure 5), suggesting that ALA was effective in the treatment of VLS and had a therapeutic effect on vulvar inflammation.
FSFI AND QOL EVALUATION:
Before treatment, the median FSFI score was 1 and after treatment it was 0. Patients sexual intercourse difficulty was significantly decreased (P<0.001, Table 3). The score of the female QoL was significantly improved after treatment (P<0.001, Figure 6).
RECURRENCE:
Three patients manifested signs of recurrence. After 6 months of treatment, evaluation of the patients’ objective parameters showed that 2 patients had a total score of 3.5, and 1 patient had a total score of 5.5 (Table 2).
ADVERSE REACTIONS:
No serious adverse reactions were observed during or after the treatment. The main adverse reactions of ALA-PDT were pain and burning sensations during irradiation, which gradually disappeared within 3 to 48 h after treatment. Moreover, 28 patients were able to tolerate the pain, and 2 patients were given painkillers and lidocaine to relieve the pain. The local vulvar pain during ALA-PDT treatment is its main disadvantage. The analgesic methods in our study mainly included the cold wind system and local cold compresses on the vulva. Among 30 patients, 14 patients were treated with analgesics during treatment, such as oral ibuprofen and 2% lidocaine topical application or injection (Figure 6). Slight erythema and swelling were seen in all patients after each irradiation. These symptoms lasted for 4 days and were almost gone after 3 days. No infection, blistering, or erosion was observed during or after the treatment (Table 4).
Discussion
METHODS TO REDUCE THE ADVERSE EFFECTS OF PDT:
During the process of receiving treatment with ALA-PDT for vulvar lesions, patients will experience various degrees of vulvar pain. The pain caused by lower intensity light, which mainly causes cell apoptosis, is less than that of the higher intensity light, which causes severe cell necrosis [27]. Apoptosis does not cause inflammation, while necrosis can lead to the release of inflammatory mediators, such as adenosine and bradykinin triphosphate, resulting in activation of sensory and motor nerves and the induction of pain [28]. Cottrell et al. proposed that the initial stage of treatment should use irradiation at a light intensity of 40 mW/cm2. Photobleaching occurs in most of PPIX, followed by irradiation with high-light intensity at 150 mW/cm2. The rapid administration of a residual light dose not only significantly reduces the pain, but also does not affect the photodynamic effect [29].
In the course of treatment in the present study, we used lidocaine local external spray, wet compresses, and infiltration anesthesia, and the sensitive patients were treated with biphasic irradiation to relieve vulvar pain. Meanwhile, the subsequent treatment was changed to a 2-step radiation therapy with irradiation at 50 mW/cm2 for 5 min before the treatment. After the patients adapted to the mild pain during the initial treatment, it was then changed to irradiation at 90 mW/cm2 for 17 min of consistent radiation energy, resulting in a reduction of vulvar skin damage and exudation after treatment. This treatment was tolerable for all patients and was not interrupted.
PATHOGENESIS OF VLS:
ECM-1, which is involved in the autoimmune mechanism, is the target of humoral autoantigens. Many studies have speculated that the pathogenesis of LS may develop along the immune genetic pathway to humoral autoimmunity since the development of autoantibodies against unknown autoantigens may explain the histological changes of extracellular remodeling. The dysfunction of ECM-1 is found at the dermal-epidermal junction and has long been considered to be related to the pathogenesis of LS. It supports the loss of functional mutation of ECM-1 gene in lipoproteinosis, an autosomal recessive dermatosis with clinical symptoms similar to LS, suggesting that ECM-1 is a powerful presumptive autoantigen in LS autoimmunity [30]. Further analysis showed that ECM-1 was significantly downregulated only in childhood male LS lesions, when compared with adult men and healthy controls. Therefore, autoimmunity to ECM-1 alone cannot explain the pathogenesis of LS. The mutation of the ECM-1 gene or the loss of autoantibodies in this particular ECM-1 region effectively destroys the usual regulatory binding of ECM-1 to MMP-9, resulting in the overreaction of MMP-9 collagenase activity to destroy collagen homeostasis, which in turn may explain the focal basement membrane destruction observed in LS [31].
Overexpression of miR-155 promotes fibroblast proliferation by downregulating FOXO3 and CDKN1B. In addition to its possible role in inducing autoimmunity, miR-155 is related to the formation of sclerotic tissue. Specifically, Ren et al. [32] found that the expression level of miR-155 in LS tissue is increased, while the expression levels of FOXO3 and CDKN1B are decreased. FOXO3 and CDKN1B are related to fibroblast proliferation and cell cycle inhibition, respectively. The decreased expressions of FOXO3 and CDKN1B may promote the proliferation and persistence of fibroblasts, explaining the high level of collagen synthesis in the transparent and hardened dermis in LS [32].
Some researchers believe that the white lesions of the vulva may be related to the decreased expression of 5-α reductase in the vulva and the failed transformation of testosterone to dihydrotestosterone [33]. Federica et al. [34] found that the white lesions are mainly related to immune inflammation of lymphocytes in the dermis and patients with VLS had lower circulating CD3 and CD4 counts than did controls. Gian et al. [35] found that the expression of CDK inhibitor p27Kip1 in vulvar squamous epithelial proliferative lesions is significantly decreased and the oxidative damage of DNA is significantly increased [34]. The abnormal expression of p27Kip1 may be a therapeutic factor. ALA-PDT is effective in the treatment of female vulvar white lesions with p27Kip1 as the target.
Conclusions
Collectively, our current findings support ALA-PDT as an effective approach with fewer adverse effects for the treatment of VLS, especially for patients with recurrent episodes after drug therapy and other physiotherapies. During the treatment, vulva pain and burning sensation were effectively controlled by cold compresses and traditional painkillers, with all patients tolerating treatment. Nevertheless, there are limitations in the study, such as the small sample size and short follow-up time. Therefore, we should evaluate the efficacy and safety of ALA-PDT for the treatment of VLS with larger sample sizes. Moreover, studies with longer follow-up periods should also be carried out to assess factors including adverse reactions and recurrence. The exact mechanism underlying the improvement in VLS lesions with ALA-PDT treatment remains largely unexplored. Abnormal expression of p27Kip1 could contribute to such improvement, whereby ALA-PDT could effectively treat female vulvar white lesions by targeting p27Kip1. Therefore, P27Kip1 could be a target to investigate the underlying mechanism of ALA-PDT in the treatment of VLS.
Figures
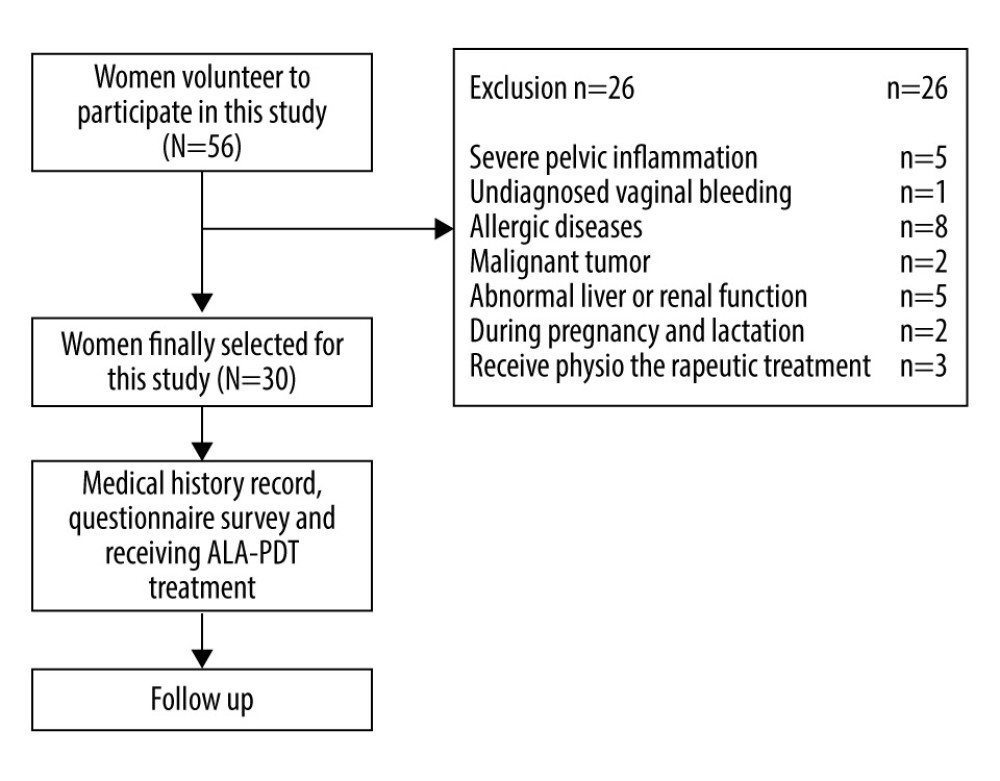 Figure 1. The workflow of this study.
Figure 1. The workflow of this study. 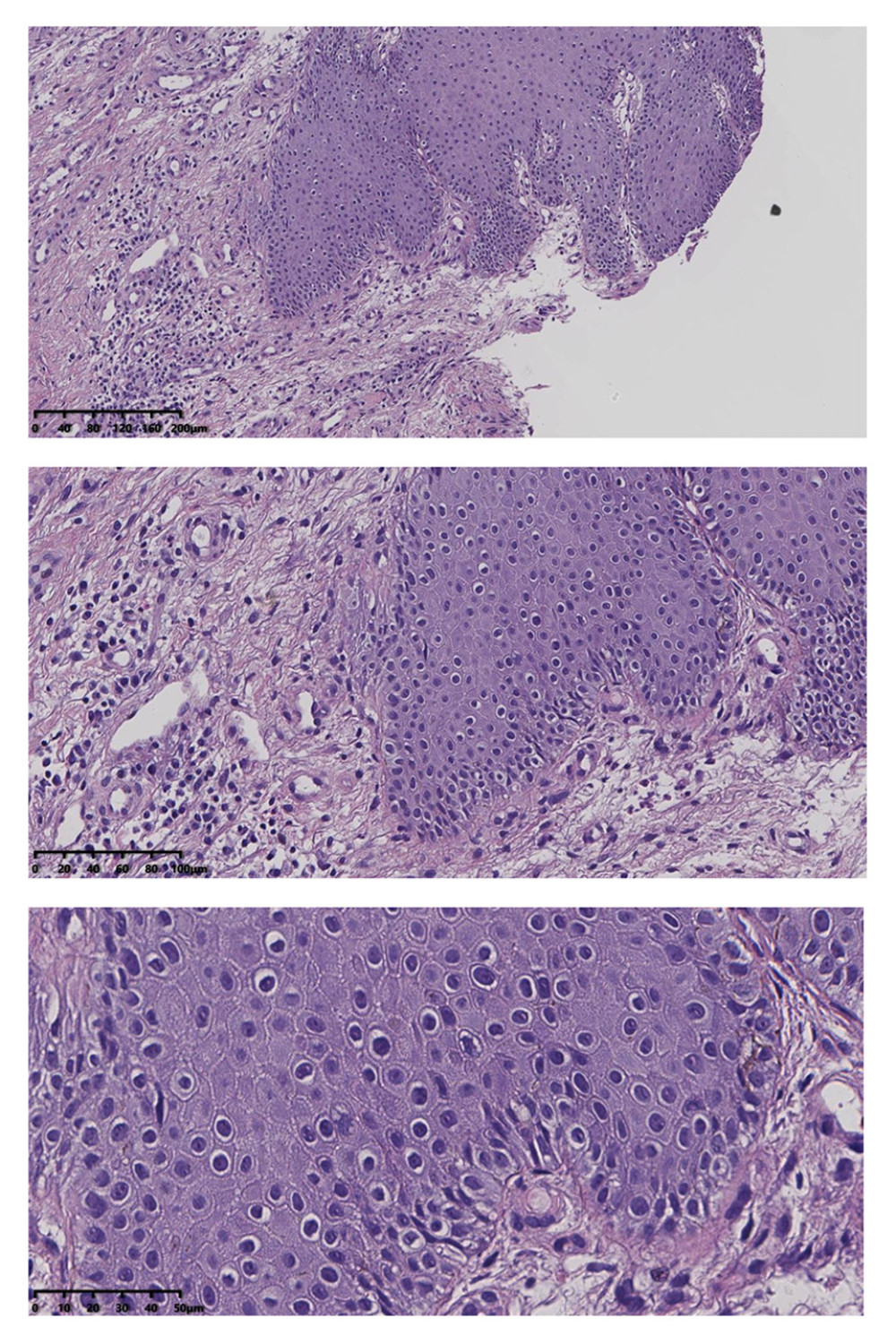 Figure 2. Squamous epithelial atrophy, acellular collagen degeneration zone in the superficial dermis, and inflammatory cell infiltration, consistent with vulvar sclerosing lichen.
Figure 2. Squamous epithelial atrophy, acellular collagen degeneration zone in the superficial dermis, and inflammatory cell infiltration, consistent with vulvar sclerosing lichen. 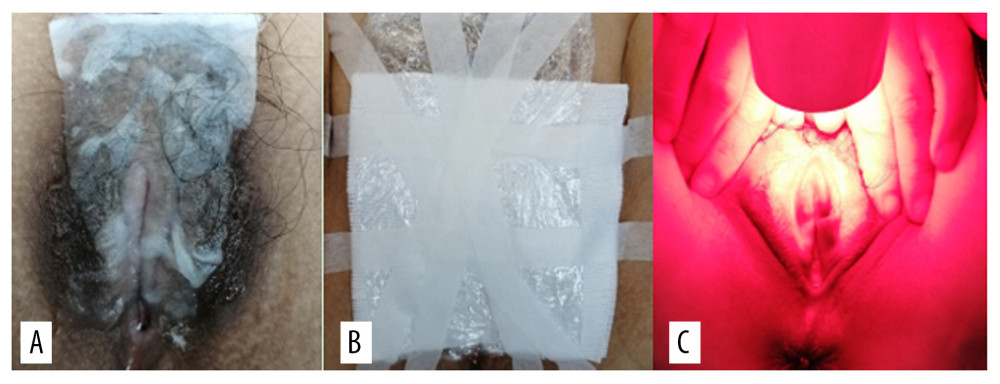 Figure 3. (A) 5-aminolevulinic acid (ALA) to the leukoplakia of the vulva. (B) Cling film to cover the ALA. (C) Illumination for 20 min exposure time at 60–90 mW/cm2 power.
Figure 3. (A) 5-aminolevulinic acid (ALA) to the leukoplakia of the vulva. (B) Cling film to cover the ALA. (C) Illumination for 20 min exposure time at 60–90 mW/cm2 power. 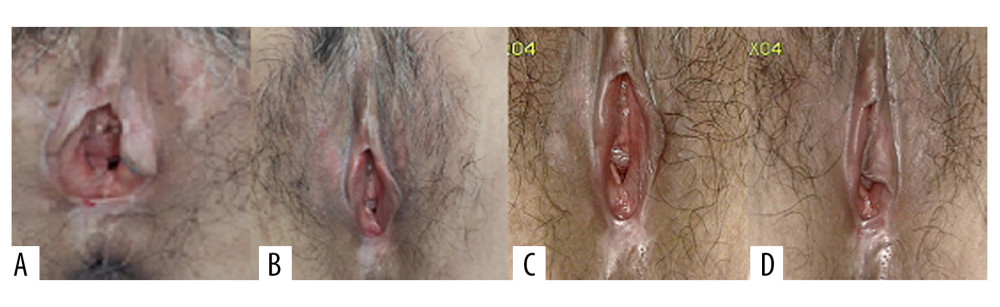 Figure 4. Representative images of vulvar lesions of 1 patient receiving 5-aminolevulinic acid-photodynamic (ALA-PDT) for 3 times during 6 months of follow-up. (A) Pre-treatment; (B) 1-month follow-up; (C) 3-month follow-up; (D) 6-month follow-up. A significant clinical improvement was achieved, showing reduced hyperkeratosis and decreased area of vulval lesions, and even the hypopigmentation was completely improved.
Figure 4. Representative images of vulvar lesions of 1 patient receiving 5-aminolevulinic acid-photodynamic (ALA-PDT) for 3 times during 6 months of follow-up. (A) Pre-treatment; (B) 1-month follow-up; (C) 3-month follow-up; (D) 6-month follow-up. A significant clinical improvement was achieved, showing reduced hyperkeratosis and decreased area of vulval lesions, and even the hypopigmentation was completely improved. 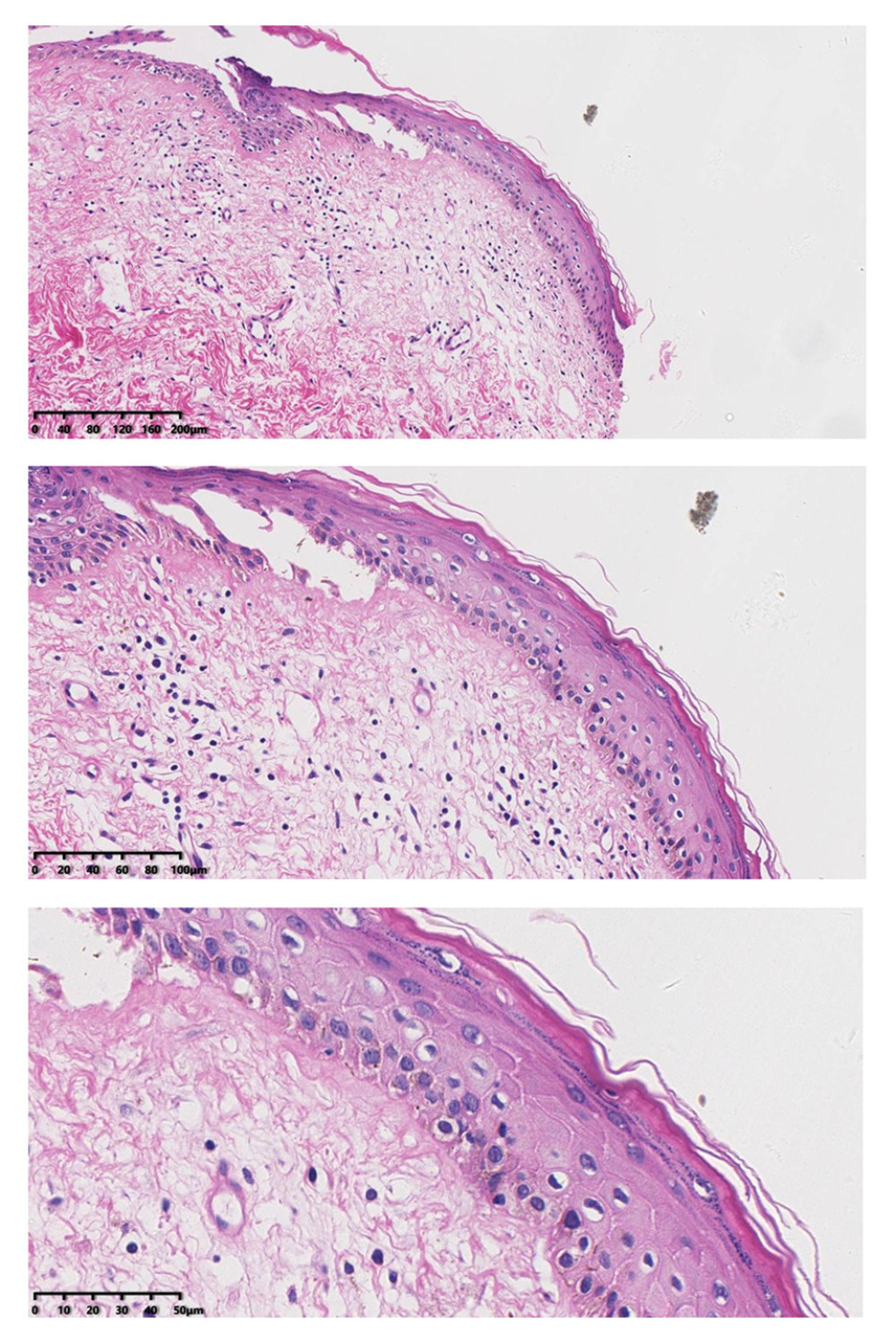 Figure 5. After treatment, histopathology results showed that chronic inflammation and acellular collagen band in dermis disappeared and superficial epithelial vacuolar degeneration improved.
Figure 5. After treatment, histopathology results showed that chronic inflammation and acellular collagen band in dermis disappeared and superficial epithelial vacuolar degeneration improved. 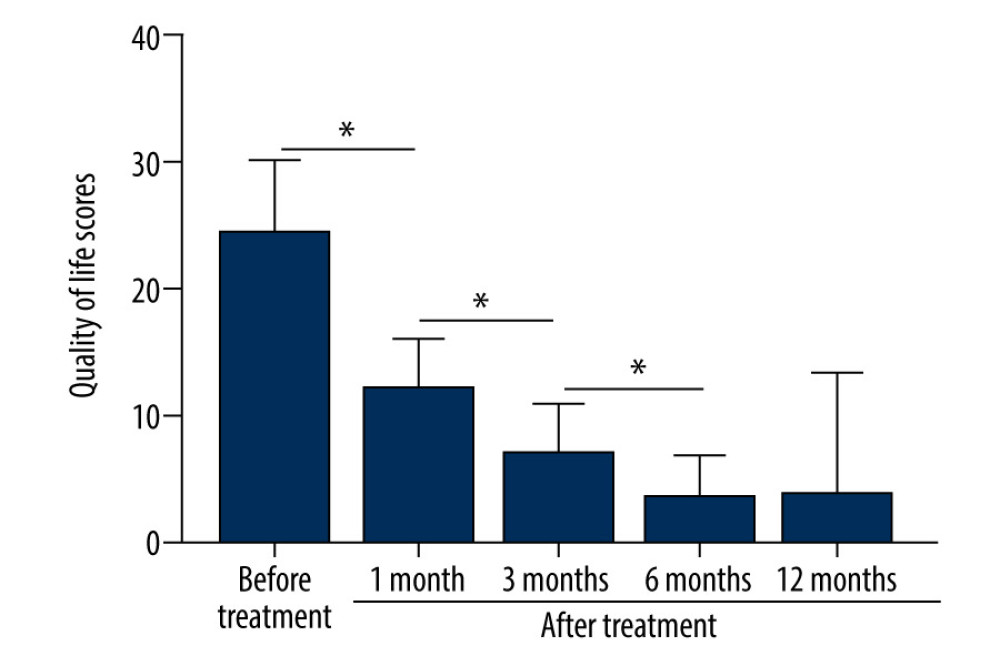 Figure 6. The patients’ QoL scores were evaluated before treatment and at 1, 3, 6, and 12 months after treatment. The comparison was performed by paired t test. * P<0.001.
Figure 6. The patients’ QoL scores were evaluated before treatment and at 1, 3, 6, and 12 months after treatment. The comparison was performed by paired t test. * P<0.001. Tables
Table 1. Demographics and baseline characteristics of patients. Table 2. The clinical manifestation, lesion size, and scores before treatment and at the 6-month follow-up.
Table 2. The clinical manifestation, lesion size, and scores before treatment and at the 6-month follow-up.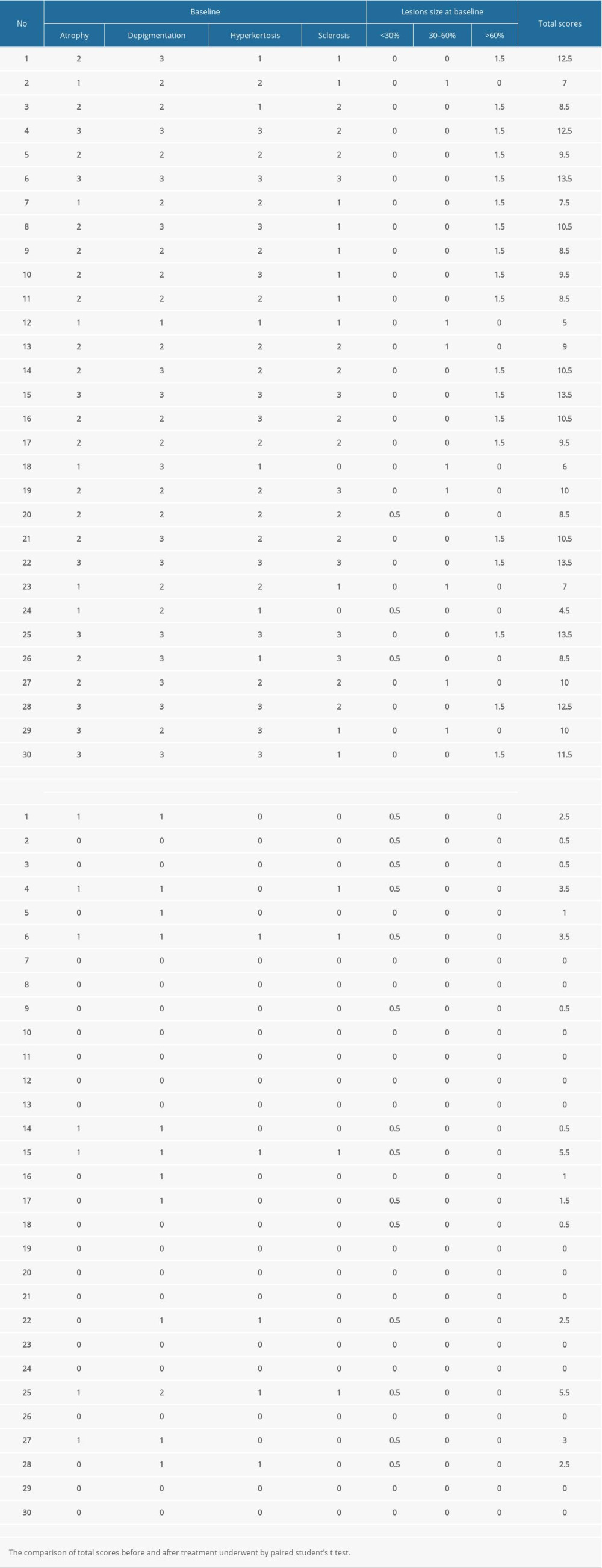 Table 3. Evaluation of the female sexual function index (FSFI) scores.
Table 3. Evaluation of the female sexual function index (FSFI) scores.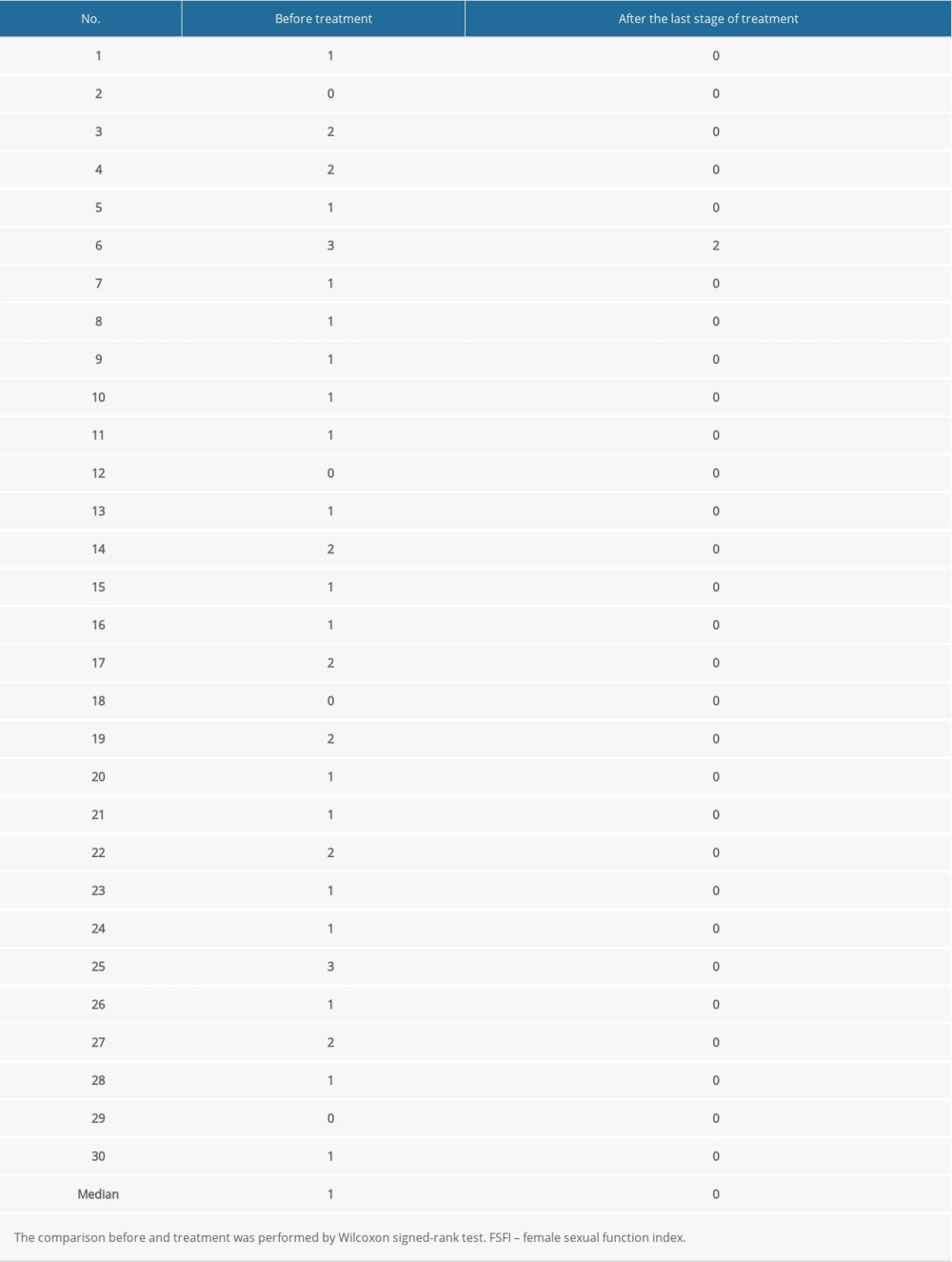 Table 4. Adverse reactions after treatment with 5-aminolevulinic acid-photodynamic (ALA-PDT) therapy in patients.
Table 4. Adverse reactions after treatment with 5-aminolevulinic acid-photodynamic (ALA-PDT) therapy in patients.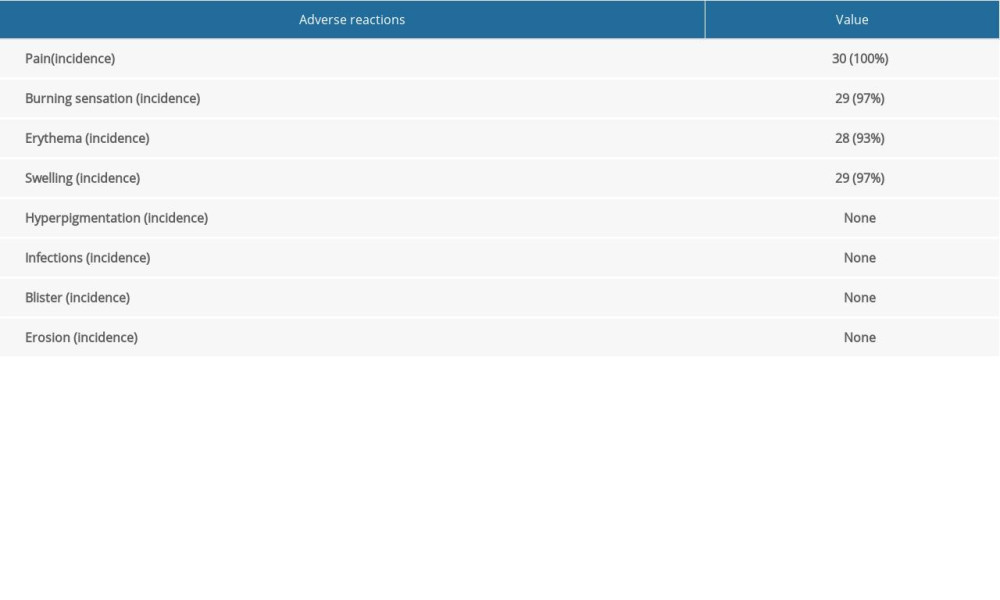
References
1. Fistarol SK, Itin PH, Diagnosis and treatment of lichen sclerosus: An update: Am J Clin Dermatol, 2013; 14(1); 27-47
2. Kirtschig G, Lichen sclerosus-presentation, diagnosis and management: Dtsch Arztebl Int, 2016; 113(19); 337-43
3. Tran DA, Tan X, Macri CJ, Lichen sclerosus: An autoimmunopathogenic and genomic enigma with emerging genetic and immune targets: Int J Biol Sci, 2019; 15(7); 1429-39
4. Bleeker MC, Visser PJ, Overbeek LI, Lichen sclerosus: Incidence and risk of vulvar squamous cell carcinoma: Cancer Epidemiol Biomarkers Prev, 2016; 25(8); 1224-30
5. Micheletti L, Preti M, Radici G, Vulvar lichen sclerosus and neoplastic transformation: A retrospective study of 976 cases: J Low Genit Tract Dis, 2016; 20(2); 180-83
6. Goldstein AT, Marinoff SC, Christopher K, Srodon M, Prevalence of vulvar lichen sclerosus in a general gynecology practice: J Reprod Med, 2005; 50(7); 477-80
7. Burger MP, Obdeijn MC, Complications after surgery for the relief of dyspareunia in women with lichen sclerosus: A case series: Acta Obstet Gynecol Scand, 2016; 95(4); 467-72
8. Liberati A, Altman DG, Tetzlaff J, The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration: BMJ, 2009; 339; b2700
9. Rosen R, Brown C, Heiman J, The Female Sexual Function Index (FSFI): A multidimensional self-report instrument for the assessment of female sexual function: J Sex Marital Ther, 2000; 26(2); 191-208
10. Wen X, Li Y, Hamblin MR, Photodynamic therapy in dermatology beyond non-melanoma cancer: An update: Photodiagnosis Photodyn Ther, 2017; 19; 140-52
11. Pinkas J, Gujski M, Humeniuk E, State of health and quality of life of women at advanced age: Med Sci Monit, 2016; 22; 3095-105
12. Li Z, Wang Y, Wang J, Evaluation of the efficacy of 5-aminolevulinic acid photodynamic therapy for the treatment of vulvar lichen sclerosus: Photodiagnosis Photodyn Ther, 2020; 29; 101596
13. Shi L, Miao F, Zhang LL, Comparison of 5-aminolevulinic acid photodynamic therapy and clobetasol propionate in treatment of vulvar lichen sclerosus: Acta Derm Venereol, 2016; 96(5); 684-48
14. Lan T, Zou Y, Hamblin MR, Yin R, 5-Aminolevulinic acid photodynamic therapy in refractory vulvar lichen sclerosus et atrophicus: Series of ten cases: Photodiagnosis Photodyn Ther, 2018; 21; 234-38
15. Lee A, Bradford J, Fischer G, Long-term management of adult vulvar lichen sclerosus: A prospective cohort study of 507 women: JAMA Dermatol, 2015; 151(10); 1061-67
16. Cooper SM, Gao XH, Powell JJ, Wojnarowska F, Does treatment of vulvar lichen sclerosus influence its prognosis?: Arch Dermatol, 2004; 140(6); 702-6
17. Renaud-Vilmer C, Cavelier-Balloy B, Porcher R, Dubertret L, Vulvar lichen sclerosus: Effect of long-term topical application of a potent steroid on the course of the disease: Arch Dermatol, 2004; 140(6); 709-12
18. Kennedy JC, Pottier RH, Pross DC, Photodynamic therapy with endogenous protoporphyrin IX: basic principles and present clinical experience: J Photochem Photobiol, 1990; 6(1–2); 143-48
19. Agostinis P, Berg K, Cengel KA, Photodynamic therapy of cancer: An update: Cancer J Clin, 2011; 61(4); 250-81
20. Wilson BC, Patterson MS, The physics, biophysics and technology of photodynamic therapy: Phys Med Biol, 2008; 53(9); R61-109
21. Mazdziarz A, Successful pregnancy and delivery following selective use of photodynamic therapy in treatment of cervix and vulvar diseases: Photodiagnosis Photodyn Ther, 2019; 28; 65-68
22. Prodromidou A, Chatziioannou E, Daskalakis G, Photodynamic therapy for vulvar lichen sclerosus – a systematic review: J Low Genit Tract Dis, 2018; 22(1); 58-65
23. Biniszkiewicz T, Olejek A, Kozak-Darmas I, Sieroń A, Therapeutic effects of 5-ALA-induced photodynamic therapy in vulvar lichen sclerosus: Photodiagnosis Photodyn Ther, 2005; 2(2); 157-60
24. Romero A, Hernández-Núñez A, Córdoba-Guijarro S, Treatment of recalcitrant erosive vulvar lichen sclerosus with photodynamic therapy: J Am Acad Dermatol, 2007; 57(2); 46-47
25. Sotiriou E, Panagiotidou D, Ioannidis D, An open trial of 5-aminolevulinic acid photodynamic therapy for vulvar lichen sclerosus: Eur J Obstet Gynecol Reprod Biol, 2008; 141(2); 187-88
26. Sotiriou E, Apalla Z, Patsatsi A, Panagiotidou D, Recalcitrant vulvar lichen sclerosis treated with aminolevulinic acid-photodynamic therapy: A report of five cases: J Eur Acad Dermatol Venereol, 2008; 22(11); 1398-99
27. François A, Salvadori A, Bressenot A, How to avoid local side effects of bladder photodynamic therapy: Impact of the fluence rate: J Urol, 2013; 190(2); 731-36
28. Wang H, Ehnert C, Brenner GJ, Woolf CJ, Bradykinin and peripheral sensitization: Biol Chem, 2006; 387(1); 11-14
29. Cottrell WJ, Paquette AD, Keymel KR, Irradiance-dependent photobleaching and pain in delta-aminolevulinic acid-photodynamic therapy of superficial basal cell carcinomas: Clin Cancer Res, 2008; 14(14); 4475-83
30. Hamada T, Lipoid proteinosis: Clin Exp Dermatol, 2002; 27(8); 624-29
31. Fujimoto N, Terlizzi J, Aho S, Extracellular matrix protein 1 inhibits the activity of matrix metalloproteinase 9 through high-affinity protein/protein interactions: Exp Dermatol, 2006; 15(4); 300-7
32. Ren L, Zhao Y, Huo X, Wu X, MiR-155-5p promotes fibroblast cell proliferation and inhibits FOXO signaling pathway in vulvar lichen sclerosis by targeting FOXO3 and CDKN1B: Gene, 2018; 653(1); 43-50
33. Neill S, Treatment for lichen sclerosus: BMJ, 1990; 301(6751); 555-56
34. Scrimin F, Rustja S, Radillo O, vulvar lichen sclerosus: An immunologic study: Obstet Gynecol, 2000; 95(1); 147-50
35. Zannoni GF, Faraglia B, Tarquini E, Expression of the CDK inhibitor p27kip1 and oxidative DNA damage in non-neoplastic and neoplastic vulvar epithelial lesions: Mod Pathol, 2016; 19(4); 504-13
36. Sgambato A, Cittadini A, Faraglia B, Weinstein IB, Multiple functions of p27Kip1 and its alterations in tumor cells: A review: J Cell Physiol, 2000; 183(1); 18-27
Figures
 Figure 1. The workflow of this study.
Figure 1. The workflow of this study. Figure 2. Squamous epithelial atrophy, acellular collagen degeneration zone in the superficial dermis, and inflammatory cell infiltration, consistent with vulvar sclerosing lichen.
Figure 2. Squamous epithelial atrophy, acellular collagen degeneration zone in the superficial dermis, and inflammatory cell infiltration, consistent with vulvar sclerosing lichen. Figure 3. (A) 5-aminolevulinic acid (ALA) to the leukoplakia of the vulva. (B) Cling film to cover the ALA. (C) Illumination for 20 min exposure time at 60–90 mW/cm2 power.
Figure 3. (A) 5-aminolevulinic acid (ALA) to the leukoplakia of the vulva. (B) Cling film to cover the ALA. (C) Illumination for 20 min exposure time at 60–90 mW/cm2 power. Figure 4. Representative images of vulvar lesions of 1 patient receiving 5-aminolevulinic acid-photodynamic (ALA-PDT) for 3 times during 6 months of follow-up. (A) Pre-treatment; (B) 1-month follow-up; (C) 3-month follow-up; (D) 6-month follow-up. A significant clinical improvement was achieved, showing reduced hyperkeratosis and decreased area of vulval lesions, and even the hypopigmentation was completely improved.
Figure 4. Representative images of vulvar lesions of 1 patient receiving 5-aminolevulinic acid-photodynamic (ALA-PDT) for 3 times during 6 months of follow-up. (A) Pre-treatment; (B) 1-month follow-up; (C) 3-month follow-up; (D) 6-month follow-up. A significant clinical improvement was achieved, showing reduced hyperkeratosis and decreased area of vulval lesions, and even the hypopigmentation was completely improved. Figure 5. After treatment, histopathology results showed that chronic inflammation and acellular collagen band in dermis disappeared and superficial epithelial vacuolar degeneration improved.
Figure 5. After treatment, histopathology results showed that chronic inflammation and acellular collagen band in dermis disappeared and superficial epithelial vacuolar degeneration improved. Figure 6. The patients’ QoL scores were evaluated before treatment and at 1, 3, 6, and 12 months after treatment. The comparison was performed by paired t test. * P<0.001.
Figure 6. The patients’ QoL scores were evaluated before treatment and at 1, 3, 6, and 12 months after treatment. The comparison was performed by paired t test. * P<0.001. Tables
 Table 1. Demographics and baseline characteristics of patients.
Table 1. Demographics and baseline characteristics of patients. Table 2. The clinical manifestation, lesion size, and scores before treatment and at the 6-month follow-up.
Table 2. The clinical manifestation, lesion size, and scores before treatment and at the 6-month follow-up. Table 3. Evaluation of the female sexual function index (FSFI) scores.
Table 3. Evaluation of the female sexual function index (FSFI) scores. Table 4. Adverse reactions after treatment with 5-aminolevulinic acid-photodynamic (ALA-PDT) therapy in patients.
Table 4. Adverse reactions after treatment with 5-aminolevulinic acid-photodynamic (ALA-PDT) therapy in patients. Table 1. Demographics and baseline characteristics of patients.
Table 1. Demographics and baseline characteristics of patients. Table 2. The clinical manifestation, lesion size, and scores before treatment and at the 6-month follow-up.
Table 2. The clinical manifestation, lesion size, and scores before treatment and at the 6-month follow-up. Table 3. Evaluation of the female sexual function index (FSFI) scores.
Table 3. Evaluation of the female sexual function index (FSFI) scores. Table 4. Adverse reactions after treatment with 5-aminolevulinic acid-photodynamic (ALA-PDT) therapy in patients.
Table 4. Adverse reactions after treatment with 5-aminolevulinic acid-photodynamic (ALA-PDT) therapy in patients. In Press
06 Mar 2024 : Clinical Research
Prevalence and Variation of Medical Comorbidities in Oral Surgery Patients: A Retrospective Study at Jazan ...Med Sci Monit In Press; DOI: 10.12659/MSM.943884
08 Mar 2024 : Clinical Research
Evaluation of Foot Structure in Preschool Children Based on Body MassMed Sci Monit In Press; DOI: 10.12659/MSM.943765
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








