28 October 2020: Clinical Research
Need for Greater Attention to Joint Damage in Rhupus Patients: Results from an Ultrasound Study
Zhi-Xin Chen123BCE, Pei-Dan Yang12CDE, Min-Ying Liu12BCE, Ping-Fang Song4EF, Qiang Xu12AEFG*DOI: 10.12659/MSM.927104
Med Sci Monit 2020; 26:e927104
Abstract
BACKGROUND: The aim of this study was to evaluate the prevalence of inflammation and bone destruction of hand joints in rhupus patients through ultrasound examination.
MATERIAL AND METHODS: Ten rhupus patients and 33 systemic lupus erythematosus (SLE) patients with hand arthropathy were recruited in this single-center study, and the clinical features and ultrasound manifestations of these patients were analyzed.
RESULTS: We discovered that rhupus patients were older (47.31±4.35 years vs. 38.58±2.50 years, P=0.040), had longer duration of disease (median 72 months vs. median 12 months, P=0.040), had a higher positive rate (70% vs. 10.71%, P<0.001), and had higher titers of anti-CCP antibody (42.633±14.520 vs. 2.121±0.970, P<0.001) than SLE patients with arthropathy. More importantly, the prevalence rates of synovial hyperplasia (90% vs. 42.42%, P=0.008), synovitis (90% vs. 18.18%, P<0.001), synovial hyperplasia (70% vs. 10.71%, P<0.001), and bone destruction (70% vs. 6.06%, P<0.001) were higher in rhupus patients than in SLE patients with arthropathy.
CONCLUSIONS: Rhupus patients are more prone to develop synovitis, synovial hyperplasia, and bone destruction. Therefore, more attention should be paid to protection of the joints in rhupus patients.
Keywords: Joint Diseases, Lupus Erythematosus, Systemic, Ultrasonography, Doppler, Arthritis, Rheumatoid, Hand Joints, Prevalence, Wrist Joint
Background
Rhupus syndrome is characterized by both rheumatoid arthritis (RA)-specific arthritis and systemic lupus erythematosus (SLE)-specific antibodies such as anti-dsDNA antibodies and anti-cyclic citrullinated peptide (anti-CCP) antibodies [1]. The concept of “rhupus” was proposed by Schur in 1971 [2]. Patients who fulfill both RA and SLE clinical classification criteria are diagnosed with rhupus in clinical practice. There is no widely accepted classification standard or diagnosis criteria for rhupus [3,4]. Due to differences in classification criteria, the prevalence of rhupus in SLE patients ranges from 0.09% to 9.7% [4,5]. Two reports recently observed that the prevalence of rhupus was about 1.5% [3,6]. Unfortunately, there is currently no effective strategy to treat rhupus syndrome, most of which are based on clinical experience or literature reviews.
Most clinical reports focused on describing the disease characteristics of rhupus [3,4] and its pathogenesis [7]. There were also several studies on joint symptoms and bone destruction in SLE patients [8–10]. However, the inflammation and bone destruction of the joints of rhupus patients were rarely described.
Therefore, we aimed to assess the prevalence rates of inflammation and bone destruction of the wrist and hand joints in rhupus patients as determined by ultrasonography, compared with SLE patients with wrist or hand joint arthropathy.
Material and Methods
Data collection
BASELINE CHARACTERISTICS AND MEDICATION USE:
We collected baseline data on: age (years), onset age (years), sex (male or female), duration of disease (months), and extra-articular manifestations of rhupus (cutaneous involvement, Raynaud syndrome, renal involvement, serositis, neuropsychiatric involvement, cytopenia, lung involvement, interstitial lung disease, and pulmonary artery pressure). Medications used were prednisone, methotrexate (MTX), hydroxychloroquine (HCQ), leflunomide (LEF), biologics such as Adalimumab, Etanercept, and
LABORATORY INDICATORS AND TESTING METHODS:
We performed the following tests: blood cell counting and classification, anti-nuclear antibody (ANA), double-stranded DNA (dsDNA), anti-extractable nuclear antibody (anti-ENA), lupus anticoagulant (LAC), anticardiolipin antibody (ACA), C reactive protein (CRP), erythrocyte sedimentation rate (ESR), complementary 3 (C3), complementary 4 (C4), RF (rheumatoid factor), and anti-citrullinated protein antibody (anti-CCP).
ANA was measured by indirect immunofluorescence method, with a normal reference value of negative. dsDNA was measured by enzyme-linked immunosorbent assay (ELISA) method, with a normal reference value of negative. Anti-ENA antibodies were measured by immunoblotting method, with a normal reference value of each antibody of negative. LAC was measured by silica clotting time method, with a normal reference value of 31–44 s. ACA was measured by ELISA method, with a normal reference value of negative. CRP was detected by the nephelometry method with a normal reference value of 0–8 mg/L. ESR was measured by the Westergren method, with a normal reference value of 0–15 mm/h. C3 was measured by scatter turbidimetry method, with a normal reference value of 0.79–1.52 g/L. C4 was measured by scatter turbidimetry method, with a normal reference value of 0.16–0.38 g/L. Anti-CCP was measured by microparticle enzyme-linked immunoassay (MEIA) method, with a normal reference value of 0–5 IU/ml. RF was measured by immunoturbidimetry and latex agglutination, with a normal reference value of negative.
ULTRASOUND TEST:
All the recruited patients were tested by high-frequency ultrasound method on both hands, including proximal interphalangeal (PIP), metacarpophalangeal (MCP), and wrist joints using the HITACHI EZU-MT29-S1 device (Hitachi Medical Corporation, Tokyo, Japan). A rheumatologist blinded to the diagnosis of rhupus performed the ultrasonography examinations. Communication between doctors and patients was allowed during the ultrasound test.
The consensus US definition of OMERACT [13] was used for the pathologic changes of ultrasound test in hand joints of patients. Synovial hypertrophy is an abnormal hypoechoic intra-articular tissue that is non-displaceable and poorly compressible. Synovitis is hypoechoic or anechoic thickened tissue with or without fluid. Bone erosion is a visible intra-articular discontinuity of bone surfaces that is visible in 2 perpendicular planes.
STATISTICAL ANALYSIS:
Our analysis was performed using Graphpad Prism 7.0 statistical software (La Jolla, CA, USA). Categorical variables are expressed as numbers (percentage), and continuous variables are expressed as mean±standard deviation (SD) or median [interquartile range, IQR]. Chi-square and Fisher’s exact test were used to compare qualitative differences between joint groups, while Wilcoxon’s test or Mann-Whitney U test was performed to compare parametric variables. All statistical analyses were 2-sided and
Results
CLINICAL CHARACTERISTICS OF RHUPUS AND SLE WITH JOINT ARTHROPATHY:
Overall, 10 rhupus and 33 SLE patients with hand arthropathy were included in this study. The mean age of the rhupus patients was slightly older than in the SLE patients with joint arthropathy (47.31±4.35 years old vs. 38.58±2.50 years old, P=0.040). The median disease duration of rhupus patients was also slightly longer than in the SLE patients with joint arthropathy (median 72 months vs. median 12 months, P=0.040). The 2 groups showed no significant differences in sex (male: female, 2: 8 vs. 7: 26, p=0.934) or onset age (41.53±4.68 vs. 35.58±2.48, P=0.083). The extra-articular manifestations of rhupus were cytopenia (80%), lung involvement (60%), pulmonary artery pressure (44.44%), interstitial lung disease (40%), cutaneous involvement (10%), renal involvement (10%), and serositis (10%). There were no significant differences (p<0.05) between the 2 groups in prevalence of cutaneous involvement, Raynaud syndrome, renal involvement, serositis, neuropsychiatric involvement, cytopenia, lung involvement, interstitial lung disease (ILD), or pulmonary artery pressure (PAH) (Table 1).
LABORATORY CHARACTERISTICS OF RHUPUS:
All the rhupus patients had positive ANA and dsDNA antibody. The rhupus patients had lower positive prevalence of total anti-ENA antibodies (70%
In the rhupus patient group, the levels of CRP (36.471±10.232 mg/L
The titer of RF in rhupus patients was higher than in the SLE patients (798.212±653.235 IU/ml vs. 60.624±18.873IU/ml, P=0.045), but there was no significant difference in the positive prevalence of RF (80% vs. 48.48%, P=0.079) between the 2 groups (Table 2).
TREATMENT OF RHUPUS WITH MEDICATION:
A lower proportion of patients were treated with prednisone in the rhupus patient group (50% vs. 93.9%, p=0.001). Prednisone and HCQ were the most frequently used medications in SLE patients with arthropathy (93.97% and 90.1%, respectively). The most frequently used medications in treating rhupus patients were MTX (60%) and THH (60%), then prednisone (50%) and HCQ (40%). A few rhupus patients were treated with biologics (20%) (Figure 3, Table 3).
ULTRASOUND FINDINGS IN RHUPUS PATIENTS:
The prevalence rates of synovial hyperplasia, synovitis, and bone erosion in rhupus patients were 90%, 90% and 70%, respectively, which were higher than in SLE patients (42.42%, 18.18%, and 6.06%, respectively) (all
After further analysis, the number of affected joints (regardless of PIP, MCP, or wrist joints) with synovial hyperplasia, synovitis, and bone erosion was much higher in rhupus patients than in SLE patients with arthropathy.
In further analysis, we assessed the ultrasound results in various joints. We found there were more PIP, MCP, and wrist joints affected by synovial hyperplasia in rhupus patients than in SLE patients, and the same results were found in PIP, MCP, and wrist joints affected by synovitis and bone erosion (Table 4, Figure 4).
Discussion
Compared to SLE patients with arthropathy, the rhupus patients were slightly older and had a longer disease duration. Similar to our study, Mu et al. [14] found that rhupus patients were significantly older than patients with SLE, and the average age of rhupus patients was about 45 years old.
RA-like arthritis is an obvious feature of rhupus patients [15]. Our results agree with those of Tani et al. [4], showing that the prevalence rates of synovial hyperplasia, synovitis, and bone erosion in hand joints (including PIP, MCP, and wrist joints) in rhupus patients were much higher, and there were many more joints affected with synovial hyperplasia, synovitis, and bone erosion in rhupus patients than in SLE patients. According to the ultrasound results, the affected joints in rhupus patients had characteristics of RA, which can cause disability and lower the quality of life of rhupus patients. Unlike previous research, we found that extra-articular manifestations of rhupus patient were not significantly different from those of SLE patients with arthropathy.
Some studies showed that major SLE characteristics of rhupus patients were skin involvement, blood involvement, and serositis involvement [16]. However, the prevalence of skin involvement, serositis involvement, and kidney involvement was lower in our study, which may be due to ethnic differences in the various study cohorts. We also found that kidney involvement and nerve involvement were less common, and our findings agree with previous studies [4,5].
Patients with rhupus had higher concentrations of CRP than in SLE patients with arthropathy, and other studies have reached the same conclusion [3,4]. Patients with rhupus syndrome have been reported to have ANA, dsDNA, RF, and anti-CCP antibodies [16]. In the present study, the positive rates of anti-CCP and titer of anti-CCP were significantly higher in rhupus patients than in the SLE with arthropathy group, which agrees with some previous studies [17,18]. Anti-CCP antibodies have been shown to play an essential diagnostic role in patients with rhupus symptom and may increase the risk of erosive arthritis in RS patients [19].
In our study, the most frequently used medications in treating rhupus patients were MTX, THH, prednisone, and HCQ. Corticosteroids and DMARDs are often used to prevent erosive arthritis in rhupus patients [16]. A few studies observed the effect of biologics on rhupus. A recent study demonstrated that TNFi was effective and safe in treating rhupus, with a follow-up period of 112 months [20]. A pilot study found that Rituximab was a potential option in treating refractory rhupus [21]. Abatacept was also effective in treating MTX-failed rhupus patients [22]. In that study, 2 in 10 patients were treated with Etanercept-Yisaipu, which is an Etanercept biomimic. Unfortunately, they were not followed-up. Clinical researchers recommend caution in use of biologics [23], although a few studies showed that biologics were effective in treating rhupus patients with joint arthropathy. Unfortunately, only 10 rhupus patients were included in the present study. In addition, this was a retrospective, cross-sectional, single-center study. Further longitudinal follow-up and multi-center studies are warranted.
Conclusions
In conclusion, rhupus is a systemic syndrome that combines the characteristics of RA and SLE. Rheumatologists should pay much more attention to protecting the joints of these patients.
Figures
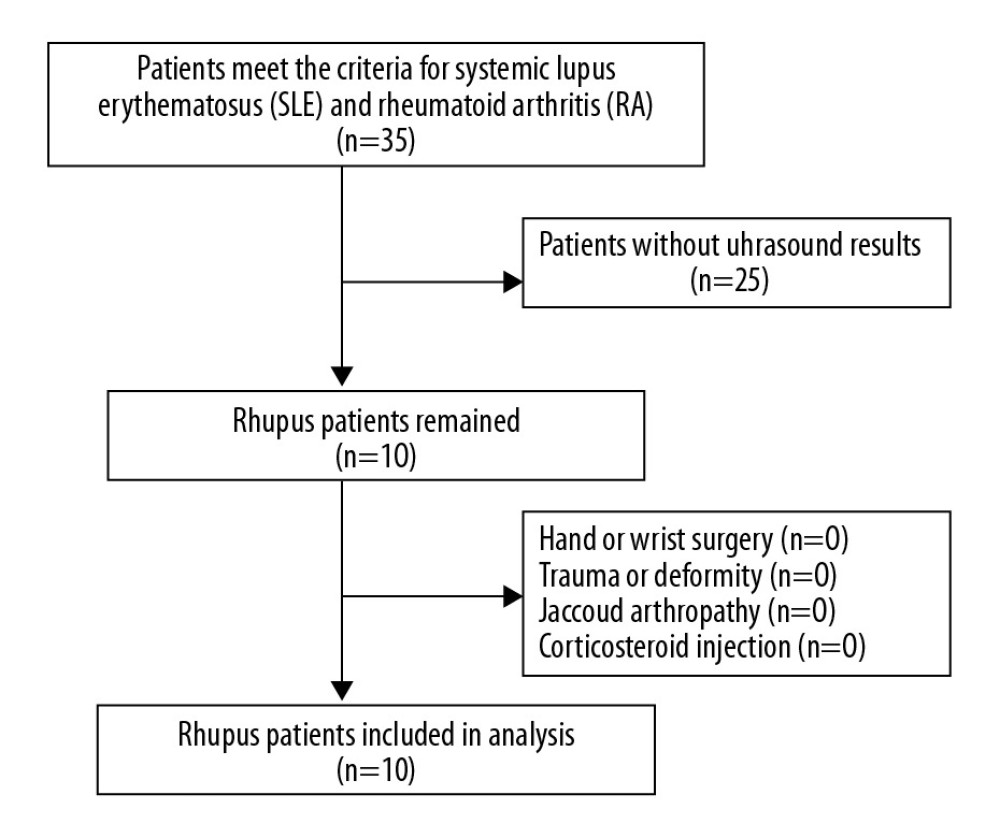 Figure 1. Enrollment of rhupus patients.
Figure 1. Enrollment of rhupus patients. 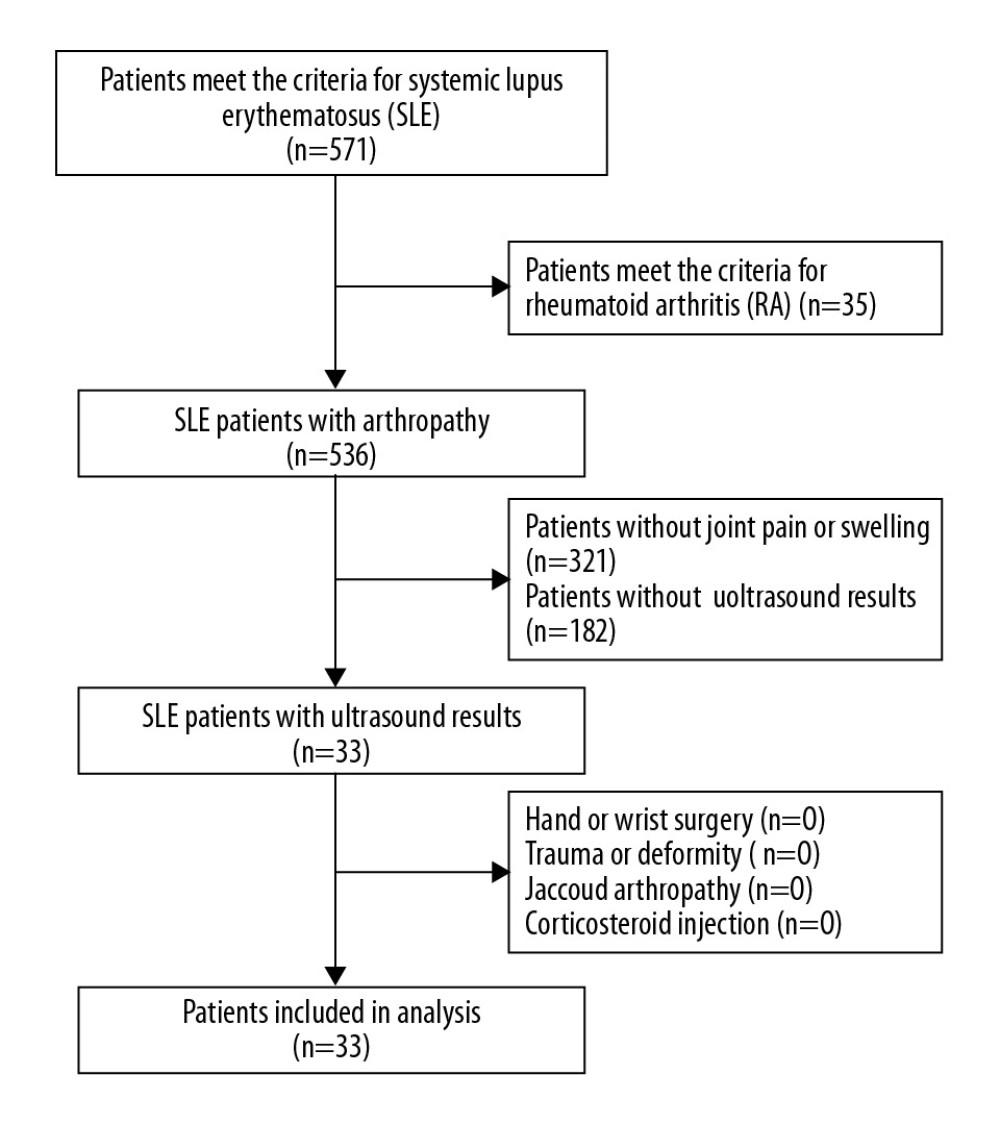 Figure 2. Enrollment of SLE patients with arthropathy.
Figure 2. Enrollment of SLE patients with arthropathy. 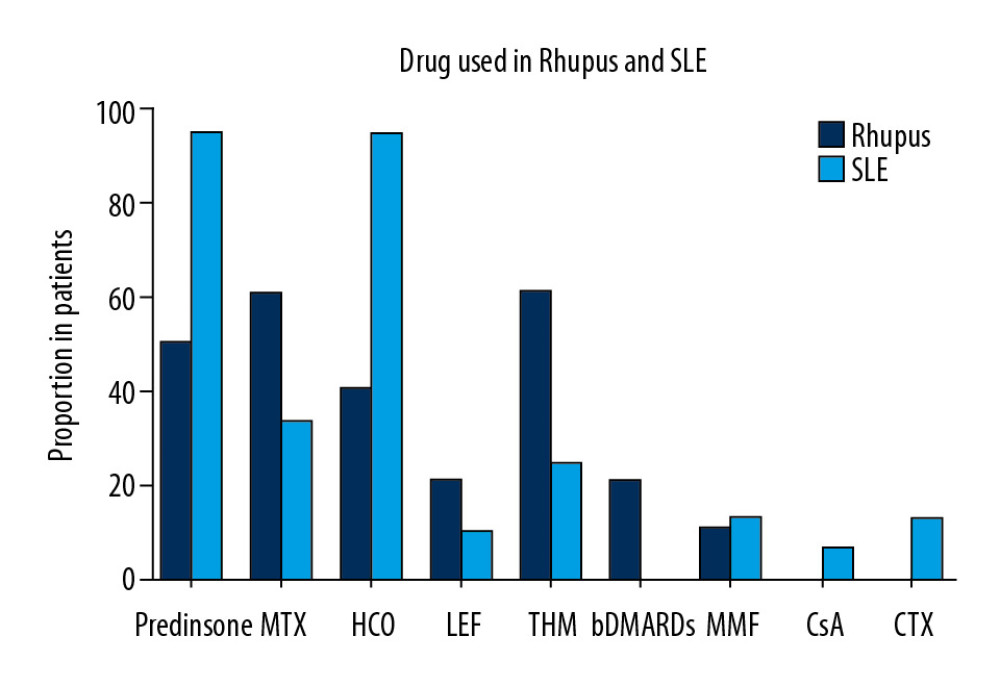 Figure 3. Proportion of patients in the 2 groups who received steroids, MTX, HCQ, LEF, THH, bDMARDS, MMF, CsA, and CTX.
Figure 3. Proportion of patients in the 2 groups who received steroids, MTX, HCQ, LEF, THH, bDMARDS, MMF, CsA, and CTX. 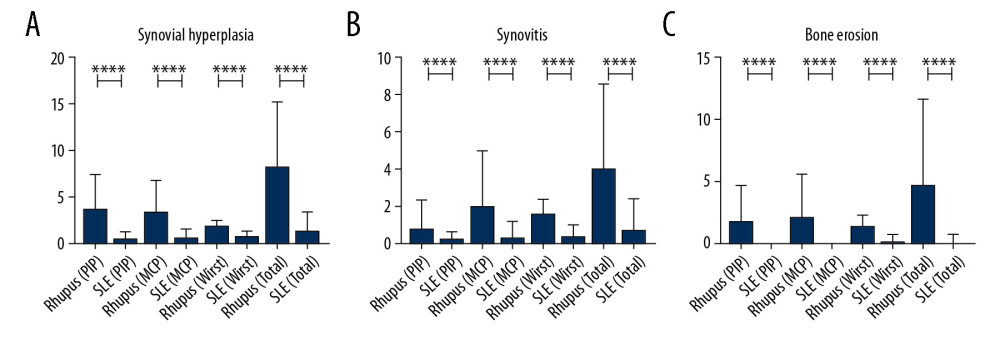 Figure 4. The number of affected hand joints in the 2 groups assessed by ultrasound testing, showing that rhupus patients had more affected joints (including PIP, MCP, and wrist) than SLE patients. (A) Synovial hyperplasia. (B) Synovitis. (C) Bone erosion.
Figure 4. The number of affected hand joints in the 2 groups assessed by ultrasound testing, showing that rhupus patients had more affected joints (including PIP, MCP, and wrist) than SLE patients. (A) Synovial hyperplasia. (B) Synovitis. (C) Bone erosion. Tables
Table 1. Clinical characteristics of rhupus patients and SLE with arthropathy patients.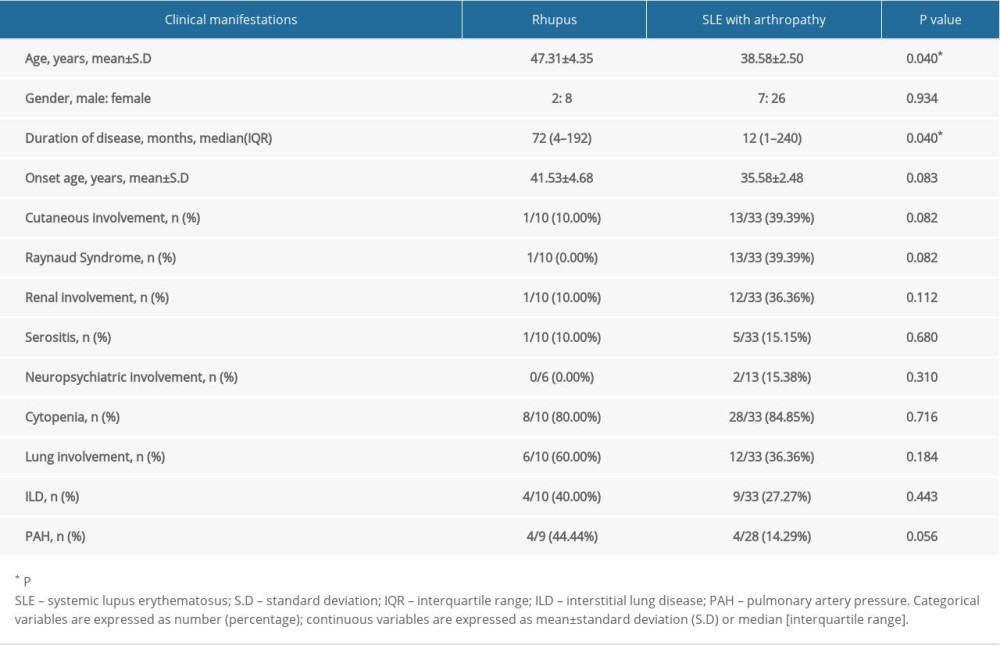 Table 2. Laboratory manifestations of rhupus patients and SLE with arthropathy patients.
Table 2. Laboratory manifestations of rhupus patients and SLE with arthropathy patients.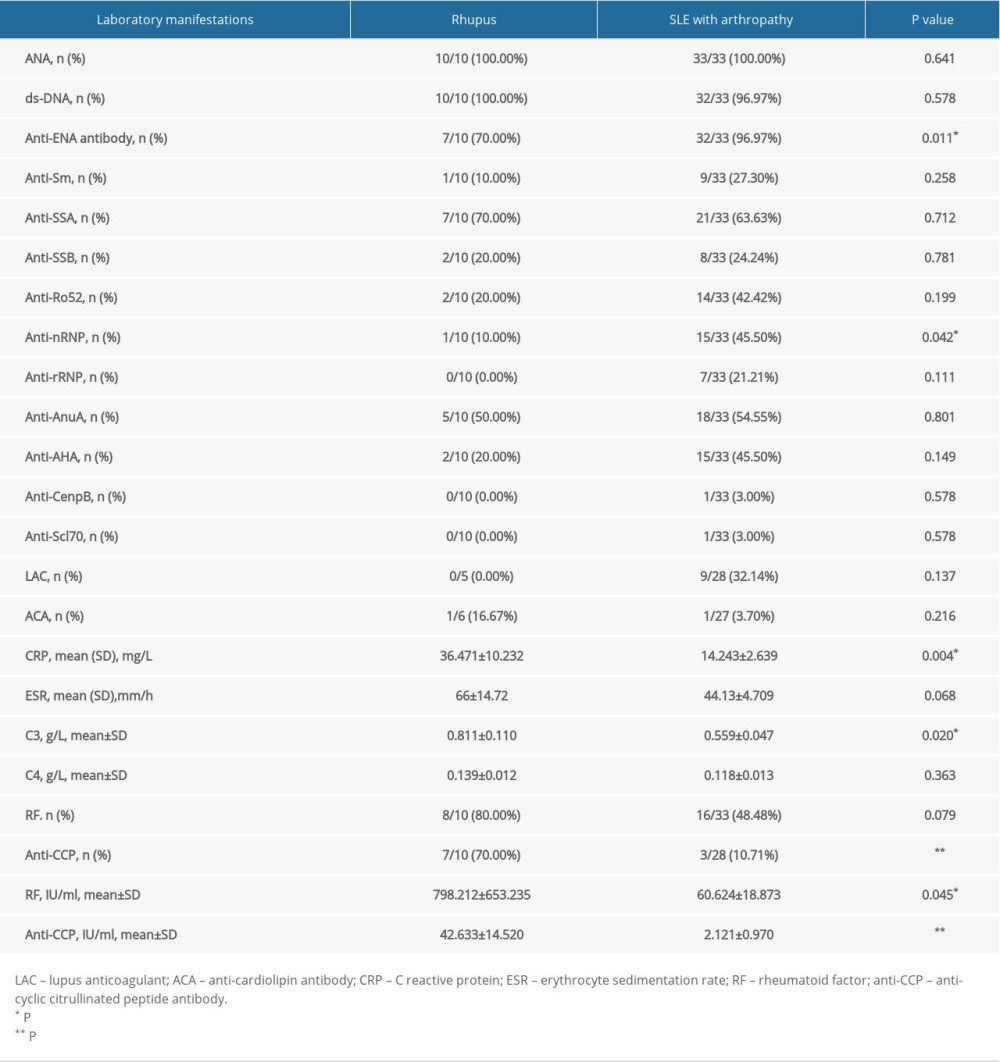 Table 3. Prednisone, DMARDs, and biologics used in rhupus patients and SLE with arthropathy patients.
Table 3. Prednisone, DMARDs, and biologics used in rhupus patients and SLE with arthropathy patients.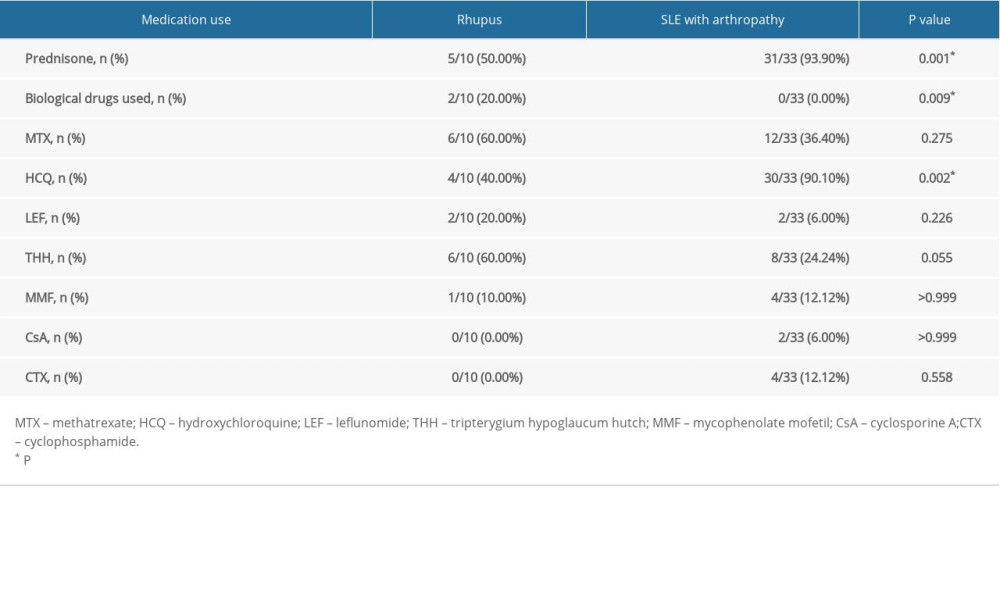 Table 4. Ultrasound results of rhupus patients and SLE with arthropathy patients.
Table 4. Ultrasound results of rhupus patients and SLE with arthropathy patients.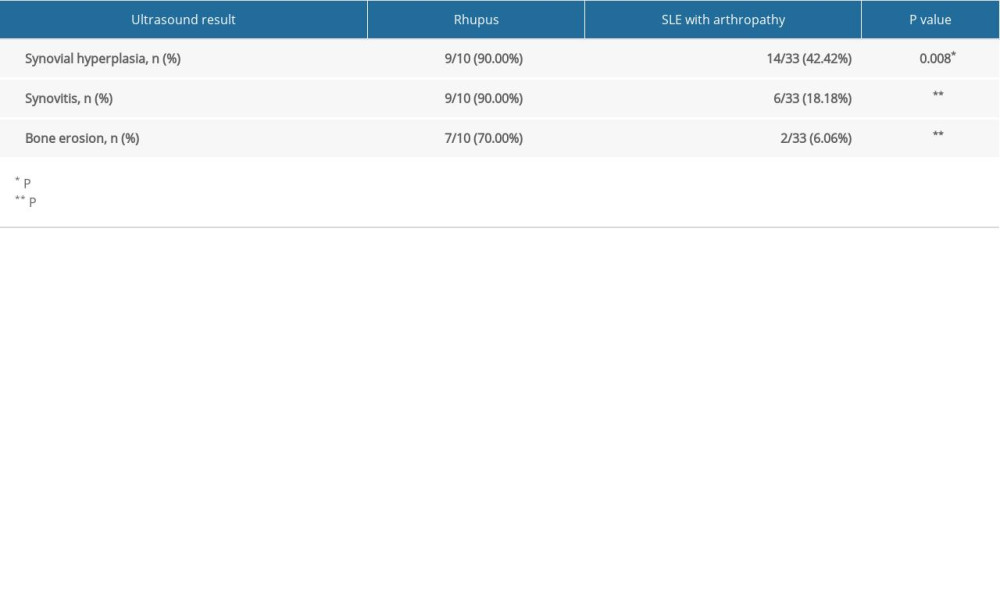
References
1. Pipili C, Sfritzeri A, Cholongitas E, Deforming arthropathy in systemic lupus erythematosus: Eur J Intern Med, 2008; 19(7); 482-87
2. Schur PH, Systemic lupus erythematosus: Cecil-Loeb Textbook of Medicine, 1971; 821, Philadelphia, Sanders
3. Li J, Wu H, Huang X, Clinical analysis of 56 patients with rhupus syndrome: Manifestations and comparisons with systemic lupus erythematosus: A retrospective case-control study: Medicine (Baltimore), 2014; 93(10); e49
4. Tani C, D’Aniello D, Delle Sedie A, Rhupus syndrome: Assessment of its prevalence and its clinical and instrumental characteristics in a prospective cohort of 103 SLE patients: Autoimmun Rev, 2013; 12(4); 537-41
5. Cohen MG, Webb J, Concurrence of rheumatoid arthritis and systemic lupus erythematosus: Report of 11 cases: Ann Rheum Dis, 1987; 46(11); 853-58
6. Liu T, Li G, Mu R, Clinical and laboratory profiles of rhupus syndrome in a Chinese population: A single-centre study of 51 patients: Lupus, 2014; 23(7); 958-63
7. Lozada-Navarro AC, Castillo-Martínez D, Moreno-Ramírez M, An imbalance in the T-helper phenotypes displayed by senescent CD4+CD28null T cells is associated with erosive arthritis (Rhupus syndrome) in systemic lupus erythematosus: Lupus, 2018; 27(13); 2155-60
8. Buosi AL, Natour J, Machado FS, Hand ultrasound: Comparative study between “no Rhupus” lupus erythematosus and rheumatoid arthritis: Mod Rheumatol, 2014; 24(4); 599-605
9. Piga M, Saba L, Gabba A, Ultrasonographic assessment of bone erosions in the different subtypes of systemic lupus erythematosus arthritis: Comparison with computed tomography: Arthritis Res Ther, 2016; 18(1); 222
10. Salliot C, Denis A, Dernis E, Ultrasonography and detection of subclinical joints and tendons involvements in Systemic Lupus erythematosus (SLE) patients: A cross-sectional multicenter study: Joint Bone Spine, 2018; 85(6); 741-45
11. Aletaha D, Neogi T, Silman AJ, 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative: Ann Rheum Dis, 2010; 69; 1580-88
12. Petri M, Orbai A-M, Alarcón GS, Derivation and validation of systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus: Arthritis Rheum, 2012; 64(8); 2677-86
13. Conaghan P, Lassere M, Østergaard M, OMERACT rheumatoid arthritis magnetic resonance imaging studies. Exercise 4: An international multicenter longitudinal study using the RA-MRI Score: J Rheumatol, 2003; 30(6); 1376-79
14. Mu R, Ye H, Chen S, Li ZGA retrospective clinical study of Rhupus syndrome: Zhonghua Nei Ke Za Zhi, 2006; 45(7); 540-43 [in Chinese]
15. Chan MT, Owen P, Dunphy J, Associations of erosive arthritis with anti-cyclic citrullinated peptide antibodies and MHC Class II alleles in systemic lupus erythematosus: J Rheumatol, 2008; 35(1); 77-83
16. Antonini L, Le Mauff B, Marcelli C, Rhupus: A systematic literature review: Autoimmun Rev, 2020; 19(9); 102612
17. Amezcua-Guerra LM, Springall R, Marquez-Velasco R, Presence of antibodies against cyclic citrullinated peptides in patients with ‘rhupus’: A cross-sectional study: Arthritis Res Ther, 2006; 8(5); R144
18. Amaya-Amaya J, Molano-González N, Franco JS, Anti-CCP antibodies as a marker of rhupus: Lupus, 2015; 24(8); 892-94
19. Ioan-Facsinay A, Willemze A, Robinson DB, Marked differences in fine specificity and isotype usage of the anti-citrullinated protein antibody in health and disease: Arthritis Rheum, 2008; 58(10); 3000-8
20. Danion F, Sparsa L, Arnaud L, Long-term efficacy and safety of antitumour necrosis factor alpha treatment in rhupus: An open-label study of 15 patients: RMD Open, 2017; 3(2); e000555
21. Piga M, Gabba A, Cauli A, Rituximab treatment for ‘rhupus syndrome’: Clinical and power-Doppler ultrasonographic monitoring of response. A longitudinal pilot study: Lupus, 2013; 22(6); 624-28
22. Ikeda K, Sanayama Y, Makita S, Efficacy of abatacept for arthritis in patients with an overlap syndrome between rheumatoid arthritis and systemic lupus erythematosus: Clin Dev Immunol, 2013; 2013 697525
23. Ramos-Casals M, Brito-Zerón P, Muñoz S, Autoimmune diseases induced by TNF-targeted therapies: Analysis of 233 cases: Medicine (Baltimore), 2007; 86(4); 242-51
Figures
 Figure 1. Enrollment of rhupus patients.
Figure 1. Enrollment of rhupus patients. Figure 2. Enrollment of SLE patients with arthropathy.
Figure 2. Enrollment of SLE patients with arthropathy. Figure 3. Proportion of patients in the 2 groups who received steroids, MTX, HCQ, LEF, THH, bDMARDS, MMF, CsA, and CTX.
Figure 3. Proportion of patients in the 2 groups who received steroids, MTX, HCQ, LEF, THH, bDMARDS, MMF, CsA, and CTX. Figure 4. The number of affected hand joints in the 2 groups assessed by ultrasound testing, showing that rhupus patients had more affected joints (including PIP, MCP, and wrist) than SLE patients. (A) Synovial hyperplasia. (B) Synovitis. (C) Bone erosion.
Figure 4. The number of affected hand joints in the 2 groups assessed by ultrasound testing, showing that rhupus patients had more affected joints (including PIP, MCP, and wrist) than SLE patients. (A) Synovial hyperplasia. (B) Synovitis. (C) Bone erosion. Tables
 Table 1. Clinical characteristics of rhupus patients and SLE with arthropathy patients.
Table 1. Clinical characteristics of rhupus patients and SLE with arthropathy patients. Table 2. Laboratory manifestations of rhupus patients and SLE with arthropathy patients.
Table 2. Laboratory manifestations of rhupus patients and SLE with arthropathy patients. Table 3. Prednisone, DMARDs, and biologics used in rhupus patients and SLE with arthropathy patients.
Table 3. Prednisone, DMARDs, and biologics used in rhupus patients and SLE with arthropathy patients. Table 4. Ultrasound results of rhupus patients and SLE with arthropathy patients.
Table 4. Ultrasound results of rhupus patients and SLE with arthropathy patients. Table 1. Clinical characteristics of rhupus patients and SLE with arthropathy patients.
Table 1. Clinical characteristics of rhupus patients and SLE with arthropathy patients. Table 2. Laboratory manifestations of rhupus patients and SLE with arthropathy patients.
Table 2. Laboratory manifestations of rhupus patients and SLE with arthropathy patients. Table 3. Prednisone, DMARDs, and biologics used in rhupus patients and SLE with arthropathy patients.
Table 3. Prednisone, DMARDs, and biologics used in rhupus patients and SLE with arthropathy patients. Table 4. Ultrasound results of rhupus patients and SLE with arthropathy patients.
Table 4. Ultrasound results of rhupus patients and SLE with arthropathy patients. In Press
06 Mar 2024 : Clinical Research
Prevalence and Variation of Medical Comorbidities in Oral Surgery Patients: A Retrospective Study at Jazan ...Med Sci Monit In Press; DOI: 10.12659/MSM.943884
08 Mar 2024 : Clinical Research
Evaluation of Foot Structure in Preschool Children Based on Body MassMed Sci Monit In Press; DOI: 10.12659/MSM.943765
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








