24 October 2020: Animal Study
The Effects of Rhein and Honokiol on Metabolic Profiles in a Mouse Model of Acute Pancreatitis
Wei Huang12E, Hang Liu13B, Yu Li3CF, Gang Mai1AE*DOI: 10.12659/MSM.925727
Med Sci Monit 2020; 26:e925727
Abstract
BACKGROUND: Acute pancreatitis (AP) is generally a self-limiting inflammatory disease, but is associated with a high mortality rate when severe. The present study aimed to investigate the effects of rhein and honokiol on AP.
MATERIAL AND METHODS: Thirty mice were randomly divided into 5 groups (n=6 per group): blank control, AP model, AP+rhein, AP+honokiol, and AP+rhein+honokiol. The AP model was prepared by intraperitoneal injection of cerulein and lipopolysaccharide (LPS). We observed the pathological changes of the pancreas by hematoxylin and eosin (H&E) staining. A mouse amylase kit was utilized to detect the level of amylase content in serum. Gas chromatography mass spectrometer analysis was performed to detect the differences in metabolites among the blank control, AP model, and AP+rhein+honokiol groups.
RESULTS: The serum amylase level was significantly higher in the AP model, which suggested that the AP model was constructed successfully. The AP+rhein+honokiol group had significantly reduced interstitial edema, inflammatory cell infiltration, hemorrhage, and necrosis. In addition, the rhein and honokiol treatment influenced some of the metabolic pathways in AP, including riboflavin metabolism, glycerophospholipid metabolism, linoleic acid metabolism, and the pentose and glucuronate interconversions pathway.
CONCLUSIONS: This study showed that the combination of rhein and honokiol ameliorated pathological changes in the pancreas of mice with AP.
Keywords: Pathological Conditions, Signs and Symptoms, amylases, Anthraquinones, Biphenyl Compounds, Lignans, Metabolome, Pancreas
Background
Acute pancreatitis (AP), a polyetiological autodigestive inflammatory disease, is often complicated by a systemic inflammatory response and infectious process. AP is induced by abnormally activated pancreatic digestive enzymes [1] and its incidence is increasing annually [2]. Approximately 20% of patients with AP develop severe AP, which has a mortality rate of 8% to 39% [3]. Although knowledge about the potential pathophysiological mechanisms in AP is increasing, disease management is still considered to only support the organs and not address etiology [4].
Traditional Chinese medicine (TCM) has long been used in the treatment of AP. Certain Chinese medicines play an important role in maintaining intestinal barrier function. Rhein and honokiol are the most widely used TCM compounds that have protective effects on the gastrointestinal tract. Their functions include regulating gastrointestinal motility, increasing the electrical activity of intestinal smooth muscle, and releasing motilin [5].
Rhein is a quinone compound isolated from the flowers of
Honokiol is the main active constituent of
However, the exact effects of the protective mechanisms of rhein and honokiol are still unclear. Analyzing the changes in metabolite profiles after treatment with TCM can reveal their underlying efficacy, metabolism, and mechanisms of action [19]. There are few reports about the metabolic profiles involved in the pathological process of AP. Therefore, we aimed to investigate the mechanisms and metabolic profiles of rhein and honokiol in AP.
Material and Methods
ANIMALS:
Thirty healthy, specific-pathogen-free grade male C57BL/6 mice (SD, 20±15 g) were purchased. The experimental protocol was performed according to the animal ethics committee guidelines of the People\s Hospital of Deyang City (2017-040). One week after acclimation, the animals were fasted with free access to water for 12 h prior to the experiment [20].
ANIMAL MODEL CONSTRUCTION AND DRUG TREATMENT:
The mice were randomly divided into 5 groups (n=6 per group): blank control (G1 group); AP model (G2 group); AP+rhein (G3 group); AP+honokiol (G4 group); and AP+rhein+honokiol (G5 group). Except for the G1 group, the groups received 7 intraperitoneal injections of cerulein (50 μg/kg, 1 h apart) to induce pancreatitis. After the last injection, the G2 group was prepared by intraperitoneal injection of LPS (30 mg/kg) for AP model construction. In the G3 and G5 groups, 0.2% rhein (4 mg/kg) was injected into the tail vein; and in the G4 and G5 groups, honokiol (5 mg/kg) was intraperitoneally injected at 1, 5, and 9 h after the first injection of cerulein. The control G1 group was administered an equal volume of saline.
COLLECTION OF SERUM AND PANCREAS:
The mice were anesthetized with 3% sodium pentobarbital and euthanized by neck dislocation. We conducted orbital blood sample collection from the mice under aseptic conditions. The whole blood was placed at room temperature for 2 h, then centrifuged at 6000 r/min for 5 min. The serum was collected and stored at −80°C. The pancreas was also collected.
DETECTION OF SERUM AMYLASE WITH AN ELISA ASSAY AND HEMATOXYLIN AND EOSIN STAINING:
To observe the effects of the TCMs rhein and honokiol on the pancreas, we performed hematoxylin and eosin (H&E) staining. A mouse amylase kit (Shanghai Enzyme Biotechnology Co, Ltd, China) was used to detect the amylase content in serum. Pancreatic tissues were fixed in 4% paraformaldehyde and embedded in paraffin. The tissues were sliced and stained with H&E. Next, the tissue sections were dewaxed in xylene and rehydrated in gradient alcohol. After washing with running water and distilled water, slices were stained with hematoxylin for 3 to 5 min. The slices were washed a second time with tap water and then differentiated with a solution of 1% hydrochloric acid in 70% ethanol. After a third wash with running water, the sections were stained with eosin for 1 to 4 min. Once dehydrated and differentiated, the sections were mounted on a cover glass slide and observed under an optical microscope [1].
METABOLITE EXTRACTION:
An amount of 100 mg of pancreatic tissue was taken from the G1, G2 and G5 groups, and 900 uL of lysate (MeOH: H2O=1: 1) was added. In a crushing instrument, the tissue was smashed at 6500 MHz for 1 min and oscillated 3 times. After vortex mixing, the samples were placed overnight in −20°C, and then centrifuged at 13 000 r/min at 4°C for 20 min. The same volume of supernatant was freeze-dried, and 100 uL lysate was added for thawing and ultrasound treatment for 5 min. After centrifugation at 13 000 r/min at 4°C for 20 min, the supernatant was analyzed.
GAS CHROMATOGRAPHY MASS SPECTROMETER ANALYSIS:
In order to identify the differences in the metabolic profiles among the 3 groups, hydrogen nuclear magnetic resonance spectra was segmented and subjected to orthogonal projections to latent structures discriminant analysis (OPLS-DA). A DB-5 MS capillary column (30×0.25×0.25 mm; Agilent, USA) was used for the non-targeted metabolite study. The temperature program of the gas chromatography was optimized as follows: an initial temperature of 70°C was held for 4 min, then increased to 110°C at a rate of 20°C per min, and finally increased to 270°C at a rate of 8°C per min and held for 7 min. The total program time was 33 min with a 5-min solvent cut time. Helium (99.99%) was used as the carrier gas with a flow rate of 1.3 mL per min. The injection volume was 1 mL with a split ratio of 30: 1. The temperatures of the injector, ion source, and interface were 280°C, 200°C, and 275°C, respectively. The mass spectrometer was operated under an electron impact mode set at 70 eV, and a detector voltage of 0.94 kV in full-scan mode at 0.2 s per scan (m/z 35e750) [21]. To explore the metabolic pathways influenced by rhein and honokiol, pathway analysis was performed by MetaboAnalyst 3.0, which combined the results from the pathway enrichment analysis with the topology analysis.
STATISTICAL ANALYSIS:
The statistical analyses were performed using GraphPad Prism (GraphPad Software, La Jolla, CA, USA). One-way ANOVA followed by Fisher’s least significant difference test was used to assess statistical significance among different groups. A
Results
TCM DECREASED THE SERUM AMYLASE LEVEL:
Serum amylase activity is used for the diagnosis of AP. The serum amylase level in the G2 AP model group was significantly higher after treatment for 7 h (P<0.01) and 12 h (P<0.05) than that in the G1 control group, indicating that the AP model was successfully constructed. In Figure 1, the results show that the levels of serum amylase in the G3 (P<0.05) and G5 (P<0.01) groups were significantly decreased compared with those of the G2 group after treatment for 7 h.
TCM TREATMENT ALLEVIATED THE PATHOLOGICAL FEATURES OF THE PANCREAS:
The pancreatic tissue H&E staining results are shown in Figure 2. Normal morphology of the pancreas was observed in the G1 group. Morphological changes such as interstitial edema, interstitial hemorrhage, hemorrhage, necrosis, and fat necrosis occurred in the G2 group. Compared with that of the G1 group, the G2 group had larger intercellular spaces and more nuclei, indicating that the AP caused macrophages to accumulate around the inflammation. In the G3 and G4 groups, the intercellular spaces and the number of nuclei were reduced. The G5 group had significantly reduced intercellular spaces and numbers of nuclei. These results indicated that the TCM treatments could relieve inflammation in pancreatitis.
TOTAL ION CHROMATOGRAM AND ANALYSIS OF PRINCIPAL COMPONENT ANALYSIS AND OPLS-DA MODEL:
A total of 15 013 and 10 431 features were detected in the positive ions and negative ions, respectively. The base peak intensity (BPI) and total ion chromatogram (TIC) of the samples are shown in Figure 3. The principal component analysis (PCA) showed that different samples in the same group were clustered in a relatively concentrated range and could be distinguished from the data aggregation areas of other groups (Figure 4A, 4B). Construction of the OPLS-DA model contained 2 main predictive ingredients (R2Y=99%, Q2=98%; Figure 4C). The samples of the G1, G2, and G5 groups exhibited clearly distinguishable clustering, as well as good fitting and predictive ability.
RELATED METABOLIC PATHWAY ANALYSIS FOR DIFFERENT TREATMENTS OF AP:
From the identified significant metabolites, heat map analysis was used to classify the upregulated and downregulated metabolites (Figure 5). Metabolic pathway analysis revealed that more than 11 pathways were influenced in small molecule negative ions, including riboflavin metabolism, glycerophospholipid metabolism, and linoleic acid metabolism. In addition, glycerophospholipid metabolism and the pentose and glucuronate interconversions pathway were obviously enriched in the small molecule positive ions (Figures 6, 7).
Discussion
In recent years, systematic research on the efficacy of different TCM treatments has been carried out, with significant results. The effect of TCM on AP is achieved by inhibiting the function of inflammatory mediators such as cytokines, improving pancreatic microcirculation, protecting pancreatic cells, and inhibiting pancreatic enzyme function [22]. Herein, we evaluated the effect of rhein and honokiol for the treatment of AP in a mouse model.
Interestingly, serum amylase levels have long been used for AP diagnosis. Early animal studies showed that serum amylase levels were correlated with the histological changes of AP [23]. In the present study, we detected the level of serum amylase after rhein, honokiol, and rhein+honokiol treatment. The results showed that the level of serum amylase in the AP+rhein (
Metabolomics is a powerful method for studying pathophysiological processes. Metabolomics analysis of human samples can help clarify the mechanisms of AP and identify potential therapeutic targets [24]. The results of the OPLS-DA score plots revealed clear group separations among the blank control group, AP model group, and AP+rhein+honokiol group in our study. Metabolic pathway analysis revealed that more than 11 pathways were influenced, including riboflavin metabolism, glycerophospholipid metabolism, and linoleic acid metabolism in small molecule negative ions. Accordingly, the pentose and glucuronate interconversion pathway was obviously enriched in small molecule positive ions. The anti-inflammatory activity of riboflavin has been found in peritonitis and severe endotoxemia [25]. In addition, a riboflavin deficiency is found in patients with AP [26]. Glycerophospholipids are key structural components of all biological membranes and bioactive molecules involved in cellular functions [27]. It was found that the glycerophospholipid metabolic pathway is related to pancreatic cancer [28]. High concentration of linoleic acid has been found in human pancreatic necrosis collections, which can lead to necrotic cell death by inhibiting mitochondrial complexes I and V [29]. In patients with mild AP of nonalcoholic etiology, the percentage of total gamma linoleic acid is increased [30]. Stevens et al. found that oxidized metabolites of linoleic acid were elevated in chronic pancreatitis [31]. In addition, the association between linoleic acid metabolism and the severity of AP has been demonstrated [32]. Pentose and glucuronate interconversions are an insulin-relevant tissue-specific signaling pathway [33], which is the pathway associated with metabolites in fecal samples of patients with pancreatic cancer [34]. The pathological changes observed in the pancreatic tissues of the AP group were in line with the injuries induced by cerulein. Following treatment with rhein and honokiol, the pathological features of the pancreas were alleviated, indicating that rhein and honokiol were able to improve the pathological changes induced by AP.
Conclusions
In summary, the present study indicates that rhein and honokiol improved the pathological changes induced by AP. In addition, rhein and honokiol may influence some metabolic pathways in AP such as riboflavin metabolism, glycerophospholipid metabolism, linoleic acid metabolism, and pentose and glucuronate interconversions pathways. However, there are limitations to our study. First, the study included a limited number of animals. Future studies with larger sample sizes and prospective trials are required. Second, intestinal injury was not investigated. Further experiments on intestinal injury, such as H&E staining and immunohistochemistry of intestine tissue, and immune response and inflammatory cytokine detection by reverse transcription polymerase chain reaction, western blot analysis, and flow cytometer should be conducted in future studies.
Figures
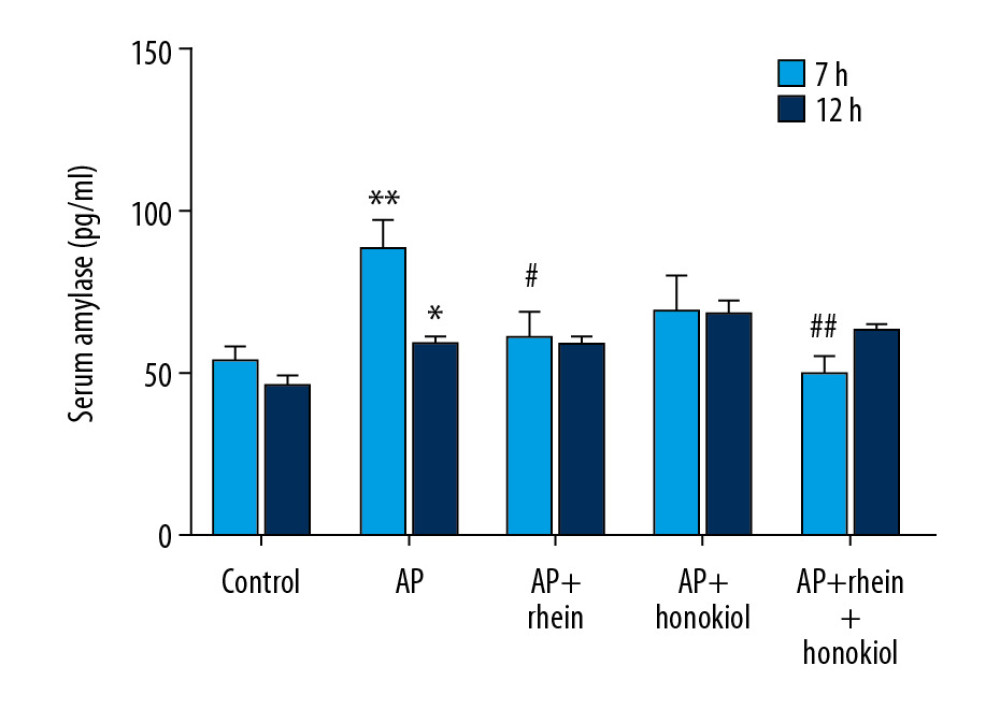 Figure 1. The effect of rhein and honokiol on serum amylase level in mice with AP. (* P<0.05 and ** P<0.01 compared with the control group, # P<0.05 and ## P<0.01 compared with the model group). Con – control group; AP – AP model group; AP+rhein – rhein treatment group; AP+honokiol – honokiol treatment group; AP+rhein+honokiol – rhein and honokiol treatment group.
Figure 1. The effect of rhein and honokiol on serum amylase level in mice with AP. (* P<0.05 and ** P<0.01 compared with the control group, # P<0.05 and ## P<0.01 compared with the model group). Con – control group; AP – AP model group; AP+rhein – rhein treatment group; AP+honokiol – honokiol treatment group; AP+rhein+honokiol – rhein and honokiol treatment group. 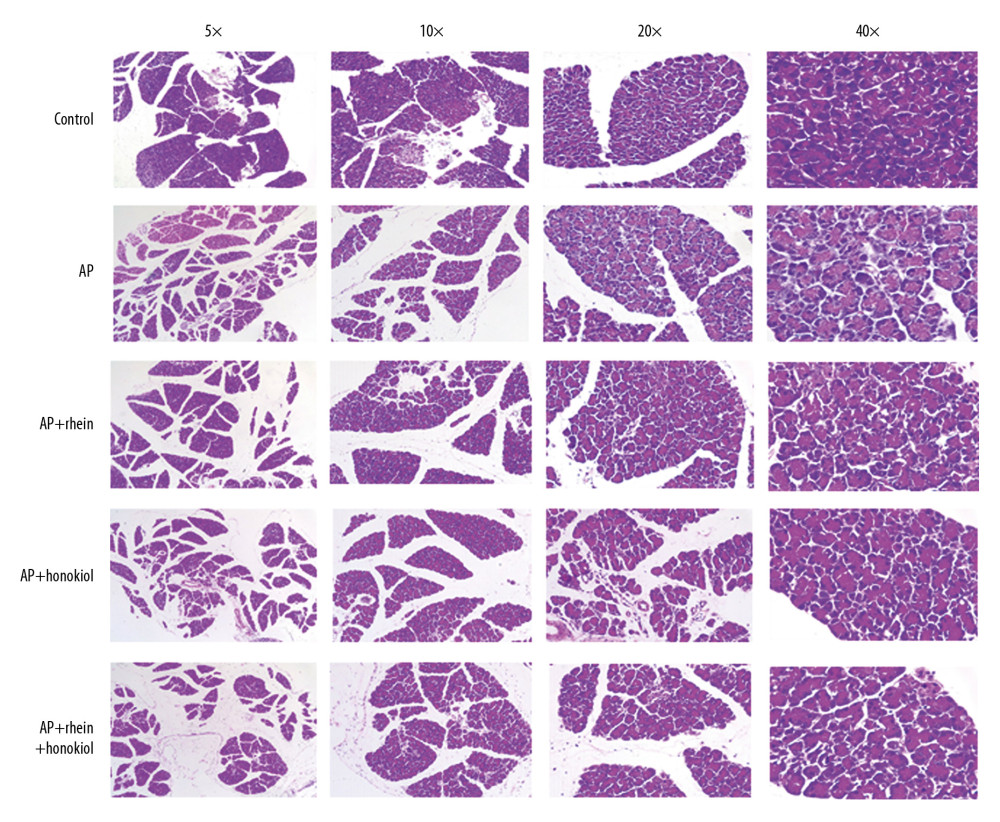 Figure 2. Pathological changes of the pancreas in H&E staining. Con – control group; AP – AP model group; AP+rhein – rhein treatment group; AP+honokiol – honokiol treatment group; AP+rhein+honokiol – rhein and honokiol treatment group.
Figure 2. Pathological changes of the pancreas in H&E staining. Con – control group; AP – AP model group; AP+rhein – rhein treatment group; AP+honokiol – honokiol treatment group; AP+rhein+honokiol – rhein and honokiol treatment group. 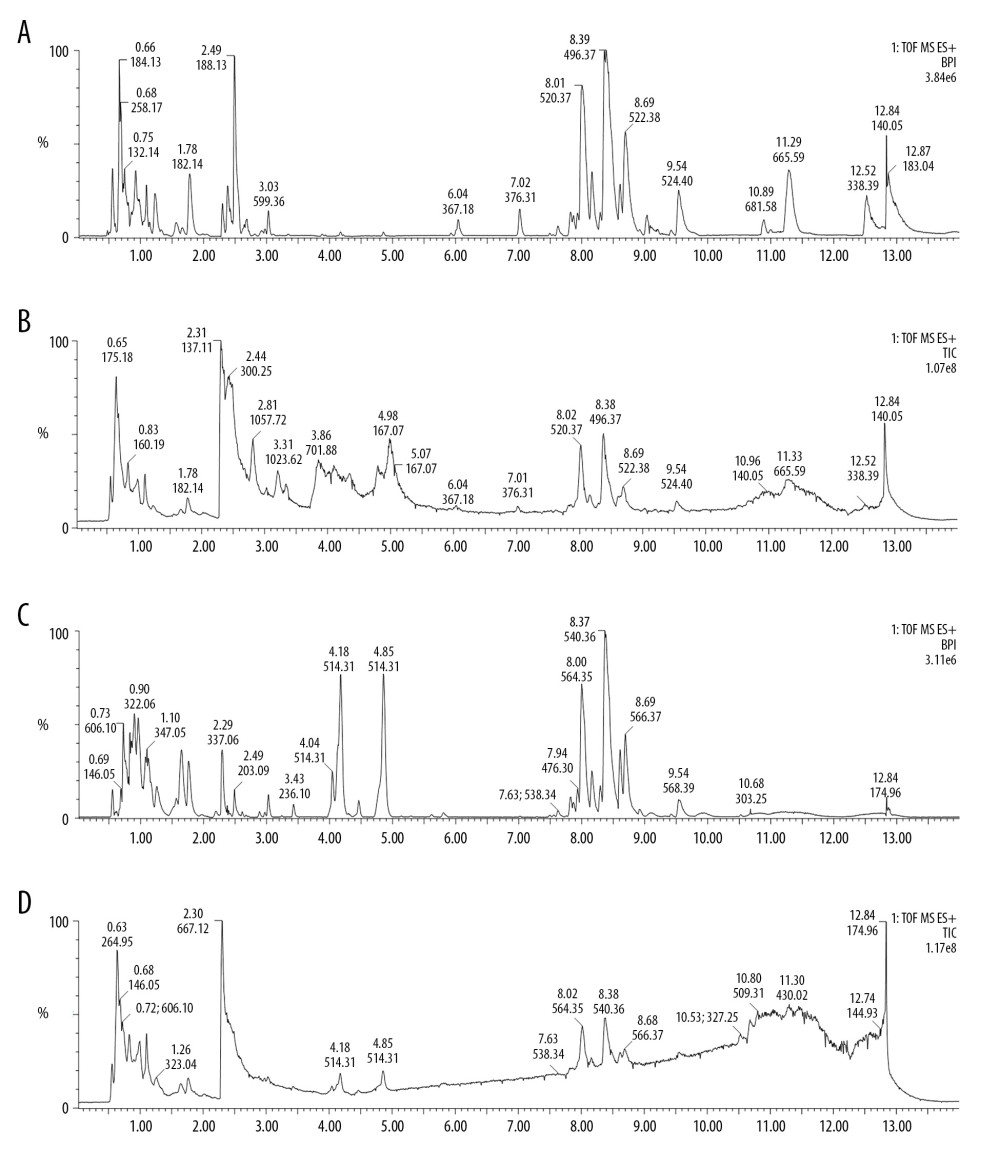 Figure 3. Base peak intensity (BPI) and total ion chromatogram (TIC) of the 3 groups. (A) BPI for the positive ions; (B) TIC for the positive ions; (C) BPI for the negative ions; (D) TIC for the negative ions.
Figure 3. Base peak intensity (BPI) and total ion chromatogram (TIC) of the 3 groups. (A) BPI for the positive ions; (B) TIC for the positive ions; (C) BPI for the negative ions; (D) TIC for the negative ions. 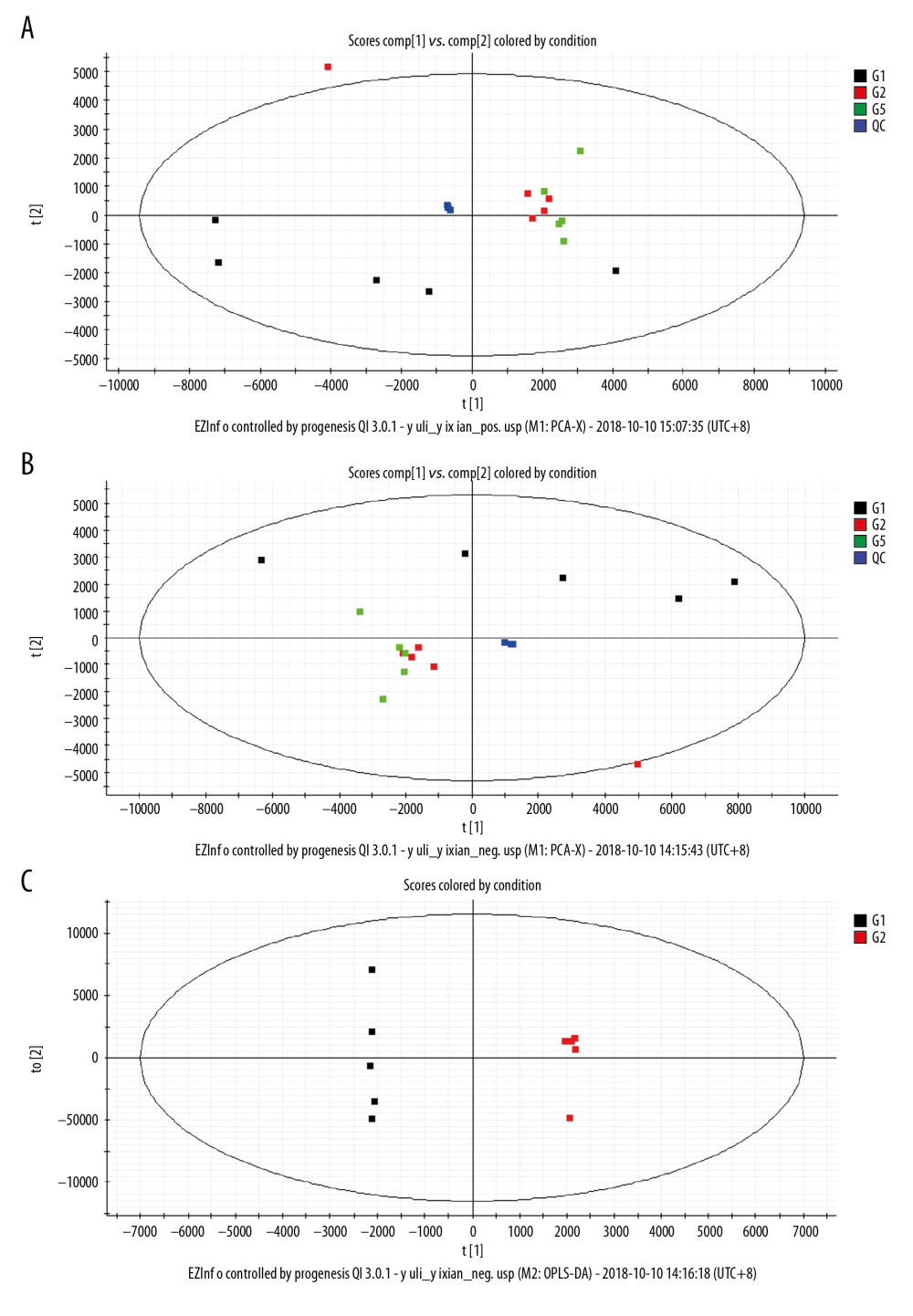 Figure 4. Principal component analysis (PCA) score scatter plot: (A) positive ions; (B) negative ions; (C) OPLS-DA scatter plot.
Figure 4. Principal component analysis (PCA) score scatter plot: (A) positive ions; (B) negative ions; (C) OPLS-DA scatter plot. 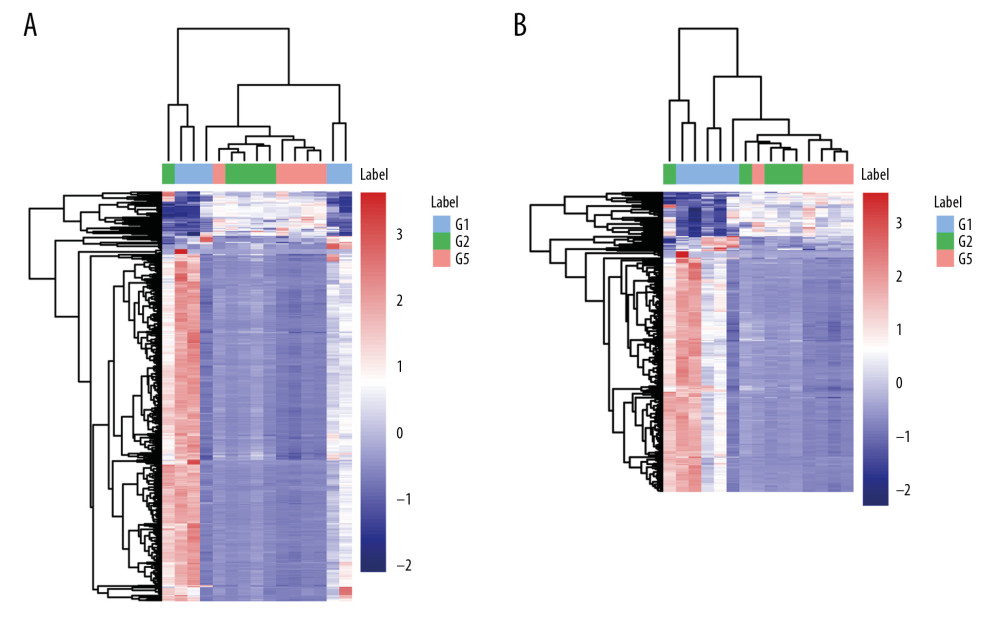 Figure 5. Heat map plots of upregulated and downregulated metabolites. (A) Positive ions; (B) negative ions.
Figure 5. Heat map plots of upregulated and downregulated metabolites. (A) Positive ions; (B) negative ions. 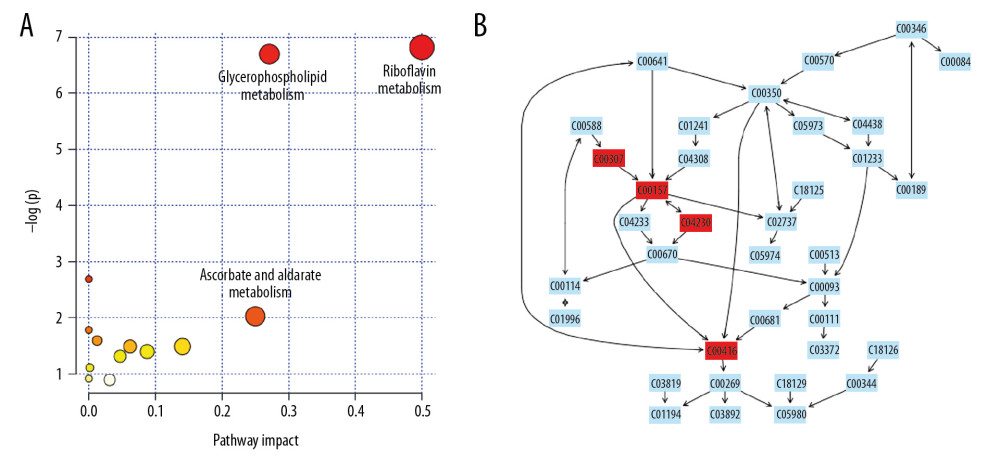 Figure 6. Summary of pathway analysis with MetaboAnalyst 3.0: (A) negative ions; (B) glycerophospholipid metabolism pathway.
Figure 6. Summary of pathway analysis with MetaboAnalyst 3.0: (A) negative ions; (B) glycerophospholipid metabolism pathway. 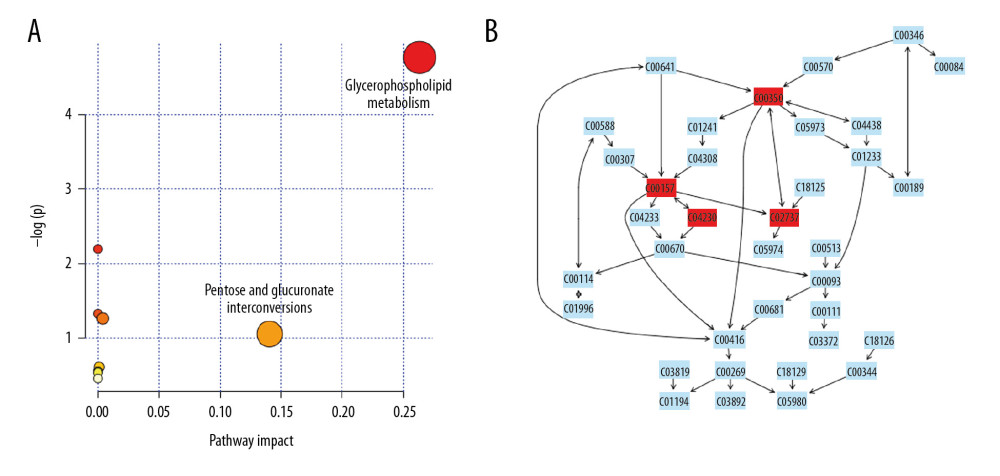 Figure 7. Summary of pathway analysis with MetaboAnalyst 3.0: (A) positive ions; (B) glycerophospholipid metabolism pathway.
Figure 7. Summary of pathway analysis with MetaboAnalyst 3.0: (A) positive ions; (B) glycerophospholipid metabolism pathway. References
1. Li N, Tian Y, Wang C, Protective effect of Lai Fu Cheng Qi decoction on severe acute pancreatitis-induced myocardial injury in a rat model: Exp Ther Med, 2015; 9(4); 1133-40
2. Yadav D, Lowenfels AB, The epidemiology of pancreatitis and pancreatic cancer: Gastroenterology, 2013; 144(6); 1252-61
3. Heinrich S, Schäfer M, Rousson V, Clavien PA, Evidence-based treatment of acute pancreatitis: A look at established paradigms: Ann Surg, 2006; 243(2); 154-68
4. Andersson R, Andersson B, Haraldsen P, Incidence, management and recurrence rate of acute pancreatitis: Scand J Gastroenterol, 2004; 39(9); 891-94
5. Chen H, Li F, Jia JG, Effects of Traditional Chinese Medicine on intestinal mucosal permeability in early phase of severe acute pancreatitis: Chin Med J (Engl), 2010; 123(12); 1537-42
6. Duraipandiyan V, Baskar A, Ignacimuthu A: Asian Pac J Trop Dis, 2012; 2; S517-23
7. Antonisamy P, Agastian P, Kang CW: Saudi J Biol Sci, 2019; 26(1); 96-104
8. Zheng J, Fan R, Wu HQ, Directed self-assembly of herbal small molecules into sustained release hydrogels for treating neural inflammation: Nat Commun, 2019; 10(1); 1604
9. Zhao X, Li J, Zhu S, Rhein induces a necrosis-apoptosis switch in pancreatic acinar cells: Evid Based Complement Alternat Med, 2014; 2014 404853
10. Yu C, Qi D, Sun JF, Rhein prevents endotoxin-induced acute kidney injury by inhibiting NF-κB activities: Sci Rep, 2015; 5; 11822
11. Zhao J, Li G, Xiong W, Protective effects of rhubarb in rats with acute pancreatitis and the role of its active compound rhein on mitochondria of exocrine cells: Evid Based Complement Alternat Med, 2018; 2018 7321352
12. Tsang SW, Zhang H, Lin C, Rhein, a natural anthraquinone derivative, attenuates the activation of pancreatic stellate cells and ameliorates pancreatic fibrosis in mice with experimental chronic pancreatitis: PLoS One, 2013; 8(12); e82201
13. Woodbury A, Yu SP, Wei L, García P, Neuro-modulating effects of honokiol: A review: Front Neurol, 2013; 4; 130
14. Lee J, Jung E, Park J, Anti-inflammatory effects of magnolol and honokiol are mediated through inhibition of the downstream pathway of MEKK-1 in NF-κB activation signaling: Planta Med, 2005; 71(4); 338-43
15. Chen YJ, Tsai KS, Chan DC, Honokiol, a low molecular weight natural product, prevents inflammatory response and cartilage matrix degradation in human osteoarthritis chondrocytes: J Orthop Res, 2014; 32(4); 573-80
16. Munroe ME, Arbiser JL, Bishop GA, Honokiol, a natural plant product, inhibits inflammatory signals and alleviates inflammatory arthritis: J Immunol, 2007; 179(2); 753-63
17. Li Z, Dong H, Li M, Honokiol induces autophagy and apoptosis of osteosarcoma through PI3K/Akt/mTOR signaling pathway: Mol Med Rep, 2018; 17(2); 2719-23
18. Weng T, Honokiol attenuates the severity of acute pancreatitis-associated lung injury by acceleration of acinar cell apoptosis: Crit Care, 2011; 15(Suppl 1); 254
19. Wei J, Xie G, Ge S, Metabolic transformation of DMBA-induced carcinogenesis and inhibitory effect of salvianolic acid B and breviscapine treatment: J Proteome Res, 2012; 11(2); 1302-16
20. Yuan L, Zhu L, Zhang Y, Effect of Da-Cheng-Qi decoction for treatment of acute kidney injury in rats with severe acute pancreatitis: Chin Med, 2018; 13; 38
21. Huang JH, He D, Chen L, GC-MS based metabolomics strategy to distinguish three types of acute pancreatitis: Pancreatology, 2019; 19(5); 630-37
22. Xia Q, Jiang JM, Gong X, Experimental study of Tong Xia purgative method in ameliorating lung injury in acute necrotizing pancreatitis: World J Gastroenterol, 2000(1); 115-18
23. Gibbs GE, Ivy AC, Early histological changes following obstruction of pancreatic ducts in dogs; correlation with serum amylase: Proc Soc Exp Biol Med, 1951; 77(2); 251-54
24. Jin H, Zhu B, Liu X, Metabolic characterization of diabetic retinopathy: An (1)H-NMR-based metabolomic approach using human aqueous humor: J Pharm Biomed Anal, 2019; 174; 414-21
25. Mazur-Bialy AI, Majka A, Wojtas L, Strain-specific effects of riboflavin supplementation on zymosan-induced peritonitis in C57BL/6J, BALB/c and CBA mice: Life Sci, 2011; 88(5–6); 265-71
26. Rose P, Fraine E, Hunt LP, Dietary antioxidants and chronic pancreatitis: Hum Nutr Clin Nutr, 1986; 40(2); 151-64
27. van Meer G, Voelker DR, Feigenson GW, Membrane lipids: Where they are and how they behave: Nat Rev Mol Cell Biol, 2008; 9(2); 112-24
28. Zhan B, Wen S, Lu J, Identification and causes of metabonomic difference between orthotopic and subcutaneous xenograft of pancreatic cancer: Oncotarget, 2017; 8(37); 61264-81
29. Chandra D, Pawan N, Krutika P, Acute lipotoxicity regulates severity of biliary acute pancreatitis without affecting its initiation: Am J Pathol, 2014; 184(6); 1773-84
30. Irma K, Antanas G, Johannes C, Fatty acids of erythrocyte membrane in acute pancreatitis patients: World J Gastroenterol, 2013(34); 91-97
31. Stevens T, Berk MP, Lopez R, Lipidomic profiling of serum and pancreatic fluid in chronic pancreatitis: Pancreas, 2012; 41(4); 518-22
32. Zhang Y, He W, He C, Large triglyceride-rich lipoproteins in hypertriglyceridemia are associated with the severity of acute pancreatitis in experimental mice: Cell Death Dis, 2019; 10(10); 728
33. Gao S, Keith RH, Xujing W, Gualtiero C, Cross tissue trait-pathway network reveals the importance of oxidative stress and inflammation pathways in obesity-induced diabetes in mouse: PLoS One, 2012; 7(9); e44544
34. Schmahl MJ, Regan DP, Rivers AC, NMR-based metabolic profiling of urine, serum, fecal, and pancreatic tissue samples from the Ptf1a-Cre; LSL-KrasG12D transgenic mouse model of pancreatic cancer: PLoS One, 2018; 13(7); e0200658
Figures
 Figure 1. The effect of rhein and honokiol on serum amylase level in mice with AP. (* P<0.05 and ** P<0.01 compared with the control group, # P<0.05 and ## P<0.01 compared with the model group). Con – control group; AP – AP model group; AP+rhein – rhein treatment group; AP+honokiol – honokiol treatment group; AP+rhein+honokiol – rhein and honokiol treatment group.
Figure 1. The effect of rhein and honokiol on serum amylase level in mice with AP. (* P<0.05 and ** P<0.01 compared with the control group, # P<0.05 and ## P<0.01 compared with the model group). Con – control group; AP – AP model group; AP+rhein – rhein treatment group; AP+honokiol – honokiol treatment group; AP+rhein+honokiol – rhein and honokiol treatment group. Figure 2. Pathological changes of the pancreas in H&E staining. Con – control group; AP – AP model group; AP+rhein – rhein treatment group; AP+honokiol – honokiol treatment group; AP+rhein+honokiol – rhein and honokiol treatment group.
Figure 2. Pathological changes of the pancreas in H&E staining. Con – control group; AP – AP model group; AP+rhein – rhein treatment group; AP+honokiol – honokiol treatment group; AP+rhein+honokiol – rhein and honokiol treatment group. Figure 3. Base peak intensity (BPI) and total ion chromatogram (TIC) of the 3 groups. (A) BPI for the positive ions; (B) TIC for the positive ions; (C) BPI for the negative ions; (D) TIC for the negative ions.
Figure 3. Base peak intensity (BPI) and total ion chromatogram (TIC) of the 3 groups. (A) BPI for the positive ions; (B) TIC for the positive ions; (C) BPI for the negative ions; (D) TIC for the negative ions. Figure 4. Principal component analysis (PCA) score scatter plot: (A) positive ions; (B) negative ions; (C) OPLS-DA scatter plot.
Figure 4. Principal component analysis (PCA) score scatter plot: (A) positive ions; (B) negative ions; (C) OPLS-DA scatter plot. Figure 5. Heat map plots of upregulated and downregulated metabolites. (A) Positive ions; (B) negative ions.
Figure 5. Heat map plots of upregulated and downregulated metabolites. (A) Positive ions; (B) negative ions. Figure 6. Summary of pathway analysis with MetaboAnalyst 3.0: (A) negative ions; (B) glycerophospholipid metabolism pathway.
Figure 6. Summary of pathway analysis with MetaboAnalyst 3.0: (A) negative ions; (B) glycerophospholipid metabolism pathway. Figure 7. Summary of pathway analysis with MetaboAnalyst 3.0: (A) positive ions; (B) glycerophospholipid metabolism pathway.
Figure 7. Summary of pathway analysis with MetaboAnalyst 3.0: (A) positive ions; (B) glycerophospholipid metabolism pathway. In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








