20 November 2020: Clinical Research
The Relationship Between Chronic Obstructive Pulmonary Disease and Cerebral Small Vessel Disease Assessed by Magnetic Resonance Imaging: A Case-Control Study from a Single Center in Beijing, China
Wei Qin1ABCDE, Jiangmei Yin2BCDF, Lei Yang1BCF, Shuna Yang1BF, Yue Li1CE, Xuanting Li1EG, Wenli Hu1E*DOI: 10.12659/MSM.925703
Med Sci Monit 2020; 26:e925703
Abstract
BACKGROUND: Chronic obstructive pulmonary disease (COPD) and cerebral small vessel disease (CSVD) reportedly share similar risk factors and pathogenesis. However, the relationship between these 2 diseases is not clear. This study aimed to investigate the association between COPD and CSVD.
MATERIAL AND METHODS: Patients with stable COPD and matched healthy control participants were recruited for this study. Clinical characteristics were collected based on medical history, serological tests, brain magnetic resonance imaging, and pulmonary function tests. Individual CSVD imaging markers (white matter hyperintensities [WMH], enlarged perivascular space [EPVS], and brain atrophy) were assessed to determine their severity. Logistic analysis was used to test the relationship between CSVD markers and COPD.
RESULTS: Significant differences in WMH, basal ganglia EPVS (BG-EPVS), and centrum semiovale EPVS (CSO-EPVS) were found between COPD and control groups (P<0.001). Logistic analysis showed that COPD was a risk factor for WMH (odds ratio [OR]=2.467, 95% confidence interval [95% CI]: 1.550–3.927, P<0.001), while it was a protective factor for BG-EPVS (OR=0.391, 95% CI: 0.246–0.621, P<0.001) and CSO-EPVS (OR=0.053, 95% CI: 0.021–0.138, P<0.001). Among patients in the COPD group, duration of COPD was a risk factor for WMH (P<0.001) and BG-EPVS (P=0.047, 0.013, 0.746), while there was no significant correlation between the COPD grade and the severity of WMH and BG-EPVS (P>0.05).
CONCLUSIONS: A significant correlation exists between COPD and imaging markers of CSVD, including WMH, BG-EPVS, and CSO-EPVS. In addition, the severity of WMH and BG-EPVS is positively related to the duration of COPD, suggesting that COPD may be a risk factor for CSVD.
Keywords: Cerebral Small Vessel Diseases, Leukoencephalopathies, Pulmonary Disease, Chronic Obstructive, basal ganglia, Case-Control Studies, Glymphatic System, Logistic Models, Magnetic Resonance Imaging, Multivariate Analysis, Severity of Illness Index, white matter
Background
Cerebral small vessel disease (CSVD) is a general term that refers to a group of pathological processes involving arterioles and their distal branches, capillaries, and venules in the brain [1]. The imaging markers of CSVD are small infarction, lacuna, white matter hyperintensities (WMH), enlarged perivascular space (EPVS), cerebral microbleeds (CMBs), and brain atrophy [2]. It has been reported that 25% of ischemic strokes are caused by CSVD [3]. Furthermore, recent studies have shown that CSVD may led to cognitive dysfunction, gait abnormalities, affective disorders, decreased ability of daily living, and other poor outcomes [4]. Exploring the risk factors for CSVD and its pathogenesis, which is still unclear, is extremely important.
Chronic obstructive pulmonary disease (COPD) is a chronic disease characterized by persistent respiratory symptoms and airflow limitation. It can further develop into pulmonary heart disease and respiratory failure, which is one of the main causes of death worldwide [5]. Previous studies have shown that COPD is an independent risk factor for ischemic stroke [6]. In addition, COPD and CSVD share similar risk factors, such as age and smoking, and they have homogeneous pathophysiological mechanisms, such as inflammatory response and oxidative stress [7]. However, the association between COPD and individual CSVD markers is not clear. In order to diagnose, manage, and prevent the 2 diseases more effectively, we conducted this study to examine the association between the duration and severity of COPD and the individual CSVD markers and thereby explore the relationship between COPD and CSVD.
Material and Methods
STUDY POPULATION:
A total of 125 inpatients with stable COPD in Beijing Chao-Yang Hospital, Capital Medical University were recruited from January 2016 to September 2017, and 130 healthy individuals were selected for the control group, with age, sex, and education matching. Enrollment criteria for the COPD group included (1) a definite diagnosis of COPD; (2) COPD in a stable stage; and (3) ability to cooperate with various examinations for the collection of clinical information. Enrollment criteria for the control group included (1) being a healthy individual matched to the COPD group in terms of age, sex, and education; and (2) having the ability to cooperate with various examinations for the collection of clinical information. Exclusion criteria included having (1) other severe lung diseases (e.g., bronchial asthma, bronchiectasis, lung cancer, tuberculosis, restrictive ventilatory disorders); (2) severe medical diseases (e.g., kidney failure, liver disease, cardiac insufficiency, tumors); (3) nervous system disorders (e.g., white matter lesions caused by demyelination, metabolism, poisoning, infection, and other nonvascular factors; brain tumors; non-small-artery occlusion strokes; Parkinson disease; brain trauma); (4) a contraindication for magnetic resonance imaging (MRI) examination or an inability to complete the head MRI scan; and (5) an unstable condition that required close monitoring. Demographics and clinical history of all participants were recorded, including age, sex, hypertension, diabetes mellitus, hyperlipidemia, smoking, alcoholism, history of stroke or transient ischemic attack, and history of coronary heart disease. In addition, all participants underwent brain MRI and laboratory tests, and all COPD patients also underwent pulmonary function tests.
LABORATORY INSPECTION:
After participants fasted overnight (fasting for more than 8 h), anterior cubital venous blood was collected and placed in an EDTA vacuum tube. Laboratory indicators such as glycosylated hemoglobin, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, creatinine, erythrocyte sedimentation rate, alkaline phosphatase, and C-reactive protein were measured using HITACHI7170S automatic biochemical analyzer (Hitachi, Yokohama, Japan).
PULMONARY FUNCTION ASSESSMENTS:
The COPD patients were tested for forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC) with a JAEGER MasterScreen Body plethysmograph (JAEGER, Wurzburg, Germany). FEV1/FVC less than 70% after bronchodilator inhalation confirmed the presence of COPD. The severity of COPD was graded based on the percentage of FEV1 after inhalation of bronchodilator according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) classification [5]: GOLD 1, mild, FEV1 ≥80% of the predicted value; GOLD 2, moderate, 50% ≤FEV1 <80% of the predicted value; GOLD 3, severe, 30% ≤FEV1 <50% of the predicted value; and GOLD 4, very severe, FEV1 <30% of the predicted value.
MRI PROTOCOL AND ASSESSMENTS:
Brain MRI was performed using a 3.0 T scanner (Siemens, Berlin, Germany). Sequences included T1-weighted (T1-W), T2-weighted (T2-W), diffusion-weighted imaging, and fluid-attenuated inversion recovery (FLAIR). All imaging data were evaluated by 2 neurologists who were blind to the clinical data. If their evaluations differed, a third neurologist was also asked to evaluate the data. The magnetic resonance acquisition sequence parameters were as follows: axial T1-W (repetition time, 2000 ms; echo time, 9.2 ms; flip angle, 130°), axial T2-W (repetition time, 4500 ms; echo time, 93 ms; flip angle, 120°), axial diffusion-weighted imaging (repetition time, 3300 ms; echo time, 91 ms; flip angle, 90°), and coronal FLAIR (repetition time, 8000 ms; echo time, 86 ms; flip angle, 130°). All sequences had a 5-mm slice thickness and a 1.5-mm interslice gap.
WMH are defined by hyperintense areas on FLAIR and T2-W sequences and isointense or hypointense areas on T1-W sequences compared with normal brain tissue, excluding brain hemorrhage and encephala edema. Periventricular WMH (PWMH) and deep WMH (DWMH) were separately scored on the Fazekas scale [8]. Scores for PWMH were as follows: 0=absence; 1=“caps” or pencil-thin lining; 2=smooth “halo”; and 3=irregular PWMH extending into the deep white matter (Figure 1). Scores for DWMH were as follows: 0=absence; 1=punctate foci; 2=beginning confluence of foci; and 3=large confluent areas (Figure 2). WMH was rated based on the total score: 0–2, mild; 3–4, moderate; and 5–6, severe.
Enlarged perivascular spaces (EPVS) were defined as lesions surrounding the cerebral vessels that had a round, oval, or linear shape; a maximum diameter of <3 mm; smooth delineated contours; and a similar signal to cerebral spinal fluid (without a hyperintense loop around the lesions on T2 FLAIR) [9]. Basal ganglia EPVS (BG-EPVS) were rated according to the number of lesions in the slice containing the greatest number of EPVS: degree 1, <5 EPVS; degree 2, 5–10 EPVS; degree 3, >10 EPVS but still countable; and degree 4, innumerable EPVS (Figure 3). Centrum semiovale EPVS (CSO-EPVS) were defined as follows: degree 1, <10 EPVS in the total white matter; degree 2, >10 EPVS in the total white matter and <10 in the slice containing the greatest number of EPVS; degree 3, 10–20 EPVS in the slice containing the greatest number of EPVS; and degree 4, >20 EPVS in the slice containing the greatest number of EPVS (Figure 4).
Brain atrophy refers to the presence of lower brain volume from a variety of causes, excluding a specific macroscopic focal injury such as brain trauma or cerebral infarction. The global cortical atrophy scale (GCA-scale) was used to assess the severity of brain atrophy: grade 0, no cortical atrophy; grade 1, mild, opening of sulci; grade 2, moderate, volume loss of gyri; and grade 3, severe, “knife blade” atrophy [10].
STATISTICAL ANALYSIS:
Continuous variables were tested for normality using the Kolmogorov-Smirnov test, and the data are presented as mean±SD for normally distributed variables and medians (interquartile range) for nonnormally distributed variables. Categorical variables are presented as absolute numbers and percentages. The comparison for continuous variables that conformed to the normal distribution was analyzed by
ETHICS STATEMENT:
This study was approved by the Ethics Committee of Beijing Chao-Yang Hospital, Capital Medical University. The Declaration of Helsinki was followed during the implementation process, and all participants agreed to participate in our study with written informed consent.
Results
CHARACTERISTICS OF PARTICIPANTS:
The results from the baseline data comparison are shown in Table 1. There were statistical differences in hypertension (P<0.001), diabetes mellitus (P<0.001), stroke history (P<0.001), smoking history (P<0.001), erythrocyte sedimentation rate (P=0.012), and C-reactive protein (P<0.001) between the COPD and control groups.
HIGHER WMH AND EPVS SCORES AMONG PATIENTS WITH COPD:
The individual markers of CSVD were compared between the COPD group and the control group, and significant differences were found in WMH (P<0.001), BG-EPVS (P=0.001), and CSO-EPVS (P<0.001). The extent of brain atrophy (P=0.537) was not significantly different between the 2 groups (Table 2).
Multivariate logistic regression analysis revealed that COPD was associated with WMH (P<0.001), BG-EPVS (P<0.001), and CSO-EPVS (P<0.001). In addition, COPD was a risk factor for WMH aggravation (OR=2.467, 95% CI: 1.550–3.927), but it appeared to be a protective factor for BG-EPVS (OR=0.391, 95% CI: 0.246–0.621) and CSO-EPVS (OR=0.053, 95% CI: 0.021–0.138) (Table 3).
WMH SEVERITY AND COPD DURATION:
The 125 patients with COPD were divided into mild, moderate, and severe WMH groups based on Fazekas scales. As shown in Table 4, there were significant differences in age (P=0.002), sex (P=0.009), stroke history (P=0.003), COPD grade (P=0.034), and COPD duration (P<0.001) between the mild, moderate, and severe WMH groups. Next, the association between the severity of WMH and the severity and duration of COPD was analyzed. After adjusting for age, sex, and stroke history, multivariate logistic regression analysis showed a significant association between the severity of WMH and the duration of COPD (P<0.001), with the severity of WMH increasing with the duration of COPD. However, there was no significant correlation between COPD grade and WMH severity (Table 5).
BG-EPVS SEVERITY AND COPD DURATION:
Based on the BG-EPVS scores, the patients with COPD were divided into 4 groups: BG-EPVS 1, BG-EPVS 2, BG-EPVS 3, and BG-EPVS 4. As shown in Table 6, there was a significant difference in stroke history (P=0.048) between the 4 groups. Multivariate logistic regression demonstrated that the duration of COPD (P=0.047, 0.013, 0.746) was a risk factor for the severity of BG-EPVS, after adjusting for stroke (Table 7). No correlation was found between COPD grade and the severity of BG-EPVS.
Discussion
Many studies have investigated the correlation of COPD with WMH and brain atrophy. Several cross-sectional studies have shown an independent correlation between COPD and WMH [11–13]. A population-based prospective cohort study suggested that COPD patients without dementia were more likely to develop severe PWMH compared with the general population [11]. Another community-based study showed that lung function was associated with cognitive decline and subcortical atrophy after controlling for sex, age, and smoking, and that men with chronic respiratory diseases were more likely to develop DWMH [12]. Dodd et al. [13] suggested that compared with control subjects, patients with nonhypoxic COPD had decreased white matter integrity and increased white matter lesion volume, which could contribute to cognitive dysfunction. They also found that COPD patients had higher WMH scores than the control group, which was consistent with the previous studies. Further, logistic regression also showed that COPD was a risk factor for WMH. The authors then analyzed the association between the severity of WMH and the grade and duration of COPD. Their results showed that the severity of WMH was positively correlated with the duration of COPD after adjusting for age, sex, and stroke, suggesting that the severity of WMH increased with prolonged COPD duration. However, Dodd et al. [13] found no significant association between the severity of WMH and the COPD grade, which may have been related to the retrospective design and small sample size.
A Chinese retrospective case-control study showed that COPD patients had decreased gray matter density in the limbic and paralimbic structures compared with controls [14]. In the COPD patients, gray matter density in damaged regions had a significant positive correlation with arterial oxygen saturation and a negative correlation with disease duration. COPD patients exhibited impaired microstructural integrity, mainly in the visual cortex of the occipital lobe, the posterior parietal lobe, as well as the temporal lobe. In addition, a German study using voxel-based morphometric analysis of MRI aimed to measure differences in generalized cortical degeneration and regional gray matter between 30 moderate-severe COPD patients and 30 matched control participants [15]. No generalized cortical degeneration was found in the COPD group, but gray matter was decreased in the posterior cingulate cortex (whole-brain analysis), anterior and midcingulate cortex, hippocampus, and amygdala (regions-of-interest analyses). In our study, brain atrophy was assessed using the whole cerebral cortical atrophy scale. However, there was no significant correlation between COPD and brain atrophy. This result may be related to the selection of a whole-brain scale for this study, and it may also be related to the small sample size.
Currently, few studies have examined the relationship between COPD and EPVS. In the current study, the association between COPD and EPVS was assessed, and the results showed that the patients with COPD had lower BG-EPVS and CSO-EPVS compared with the control group. After adjusting for stroke, the duration of COPD was found to be a risk factor for the severity of BG-EPVS. However, since the lungs provide oxygen to the blood, impairment of the lungs would be expected to affect the structure and function of the brain in a negative way [7]. Previous studies have also largely suggested that patients with COPD have higher rates of EPVS and brain lesions [7,11,15]. These conflicting results may be related to the different brain regions studied. Previous studies have tended to investigate the incidence of EPVS and lesions in the whole brain or the whole deep brain. In contrast, the study on the incidence of EPVS in the current study focused on the centrum semiovale and basal ganglia. We did not find previous studies on centrum semiovale in patients with COPD. However, the amplitude of low-frequency fluctuations in basal ganglia of COPD patients was previously reported to be abnormally changed [16], and this finding may have some as yet unknown relationship with the results of the current study. In addition, the rate of hypertension in the healthy control group was significantly higher than that in the COPD group in the current study, which would obviously also have some impact on the results. If COPD is indeed a protective factor for BG-EPVS and CSO-EPVS, as shown in our results, then the pathophysiological mechanisms of these disorders still need further study.
At present, the precise mechanism for the relationship between CSVD and COPD is not well established. COPD patients have been reported to have significantly higher levels of C-reactive protein, fibrinogen, leukocytes, and tumor necrosis factor-α than controls, suggesting the existence of a continuous systemic inflammatory response in COPD patients [17]. In addition to being in a state of inflammatory response, patients with COPD also remain in a state of chronic hypoxemia. Hypoxemia and insufficient blood perfusion can aggravate brain ischemic damage [18]. Cerebral blood flow will increase physiologically to maintain the oxygen demand of brain when the blood oxygen content is reduced [19]. Periventricular white matter is at the junction area of the cerebral artery. COPD patients are in a state of chronic hypoxemia, which can result in hypoperfusion of white matter. This may be a potential mechanism by which COPD is more likely to cause WMH. Furthermore, COPD is associated with a paradoxical response of cerebral vessels, which is possibly due to endothelial dysfunction [20]. Extensive data support a relationship between systemic inflammation and adverse outcomes both in stroke patients and in experimental stroke models. Experimental models of stroke and comorbidities have shown that systemic inflammatory conditions exacerbate brain damage via cerebrovascular inflammation, blood-brain barrier damage, encephala edema, and excitotoxicity [21]. Systemic inflammation can activate microglia to induce cyclooxygenase-2-dependent neuroinflammation, leading to an increase in superoxide anion production and causing oxidative stress [22]. In addition, systemic inflammation and oxidative stress promote cerebral vascular dysfunction and platelet hyperactivity, which increase the susceptibility to thrombotic events and are independent risk factors for ischemic stroke [23]. Therefore, the interaction of inflammation, oxidative stress, vascular endothelial dysfunction, and blood-brain barrier damage may play important roles in the mechanisms underpinning the link between COPD and CSVD. This hypothesis provides a direction for future clinical prevention and treatment of patients with COPD complicated by CSVD.
Some limitations of the current study include the retrospective collection of clinical data and the small sample size. The results are not as representative as those of a large-sample multicenter prospective study would be. In future work, we plan to conduct prospective research to obtain more accurate and representative conclusions.
Conclusions
In summary, our study found a significant correlation between COPD and imaging markers of CSVD including WMH, BG-EPVS, and CSO-EPVS. In addition, the severity of WMH and BG-EPVS was positively related to the duration of COPD, suggesting that COPD may be a risk factor for CSVD.
Figures
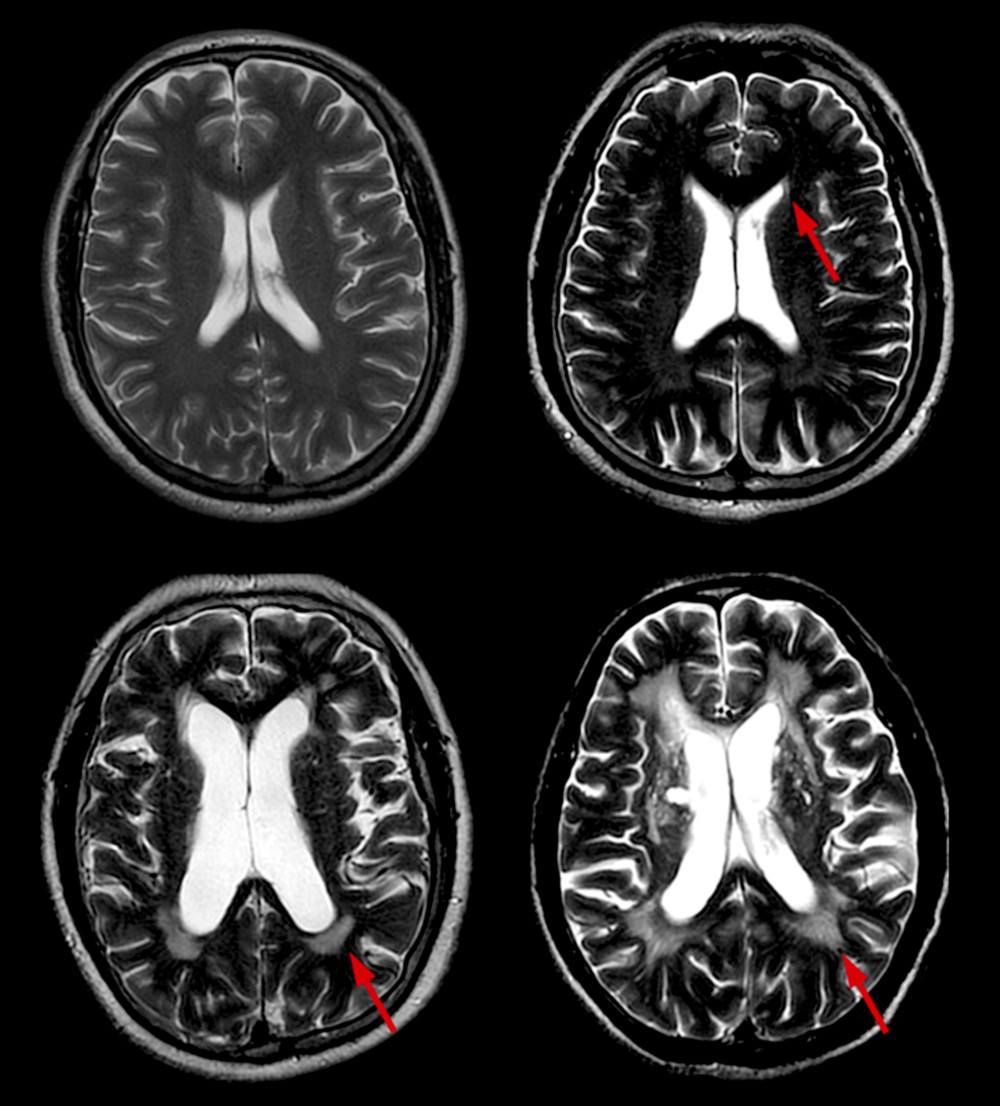 Figure 1. Different scores of PWMH. Scores of PWMH: (A) 0 score=absence; (B) 1=“caps” or pencil-thin lining; (C) 2=smooth “halo”; and (D) 3=irregular PWMH extending into the deep white matter. PWMH –periventricular white matter hyperintensities.
Figure 1. Different scores of PWMH. Scores of PWMH: (A) 0 score=absence; (B) 1=“caps” or pencil-thin lining; (C) 2=smooth “halo”; and (D) 3=irregular PWMH extending into the deep white matter. PWMH –periventricular white matter hyperintensities. 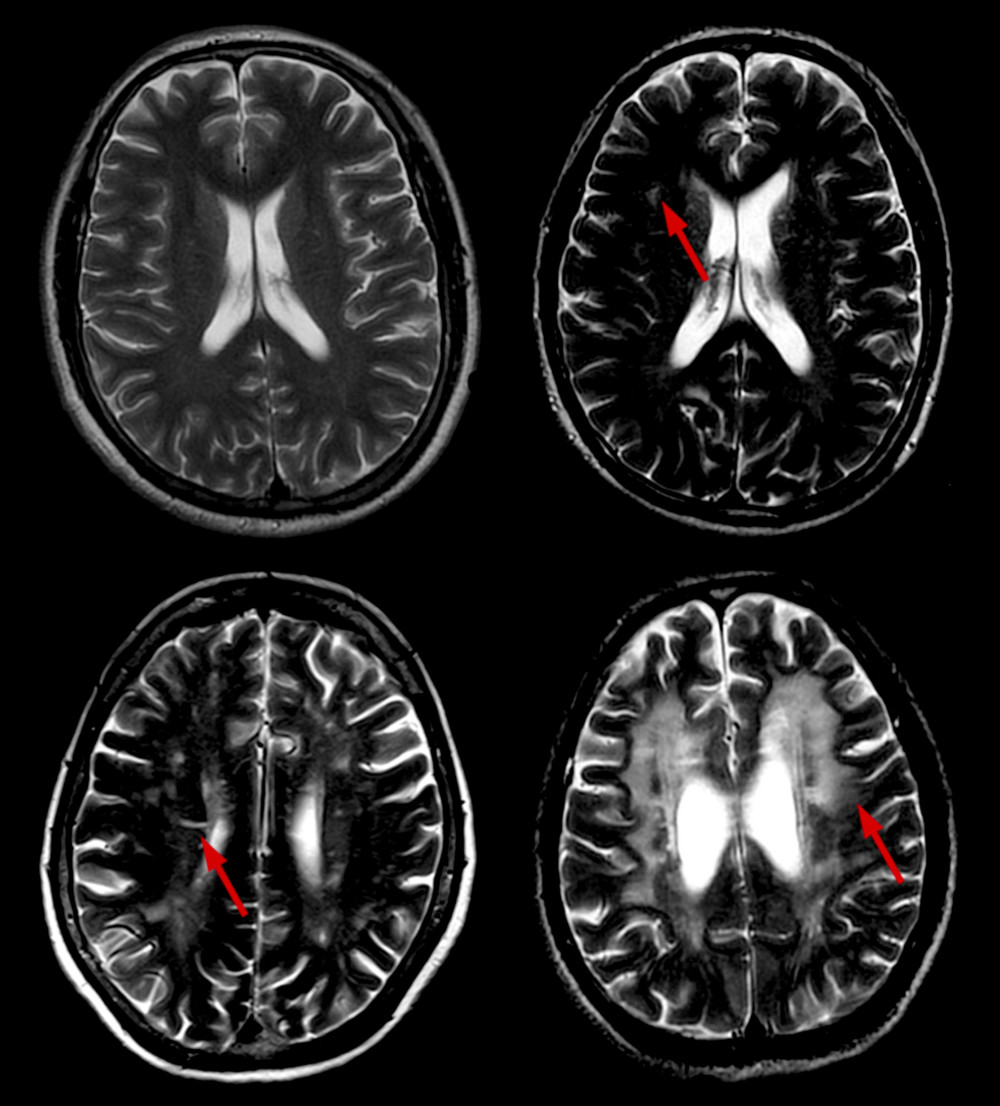 Figure 2. Different scores of DWMH. Scores of DWMH: (A) 0=absence; (B) 1=punctate foci; (C) 2=beginning confluence of foci; and (D) 3=large confluent areas. DWMH – deep white matter hyperintensities.
Figure 2. Different scores of DWMH. Scores of DWMH: (A) 0=absence; (B) 1=punctate foci; (C) 2=beginning confluence of foci; and (D) 3=large confluent areas. DWMH – deep white matter hyperintensities. 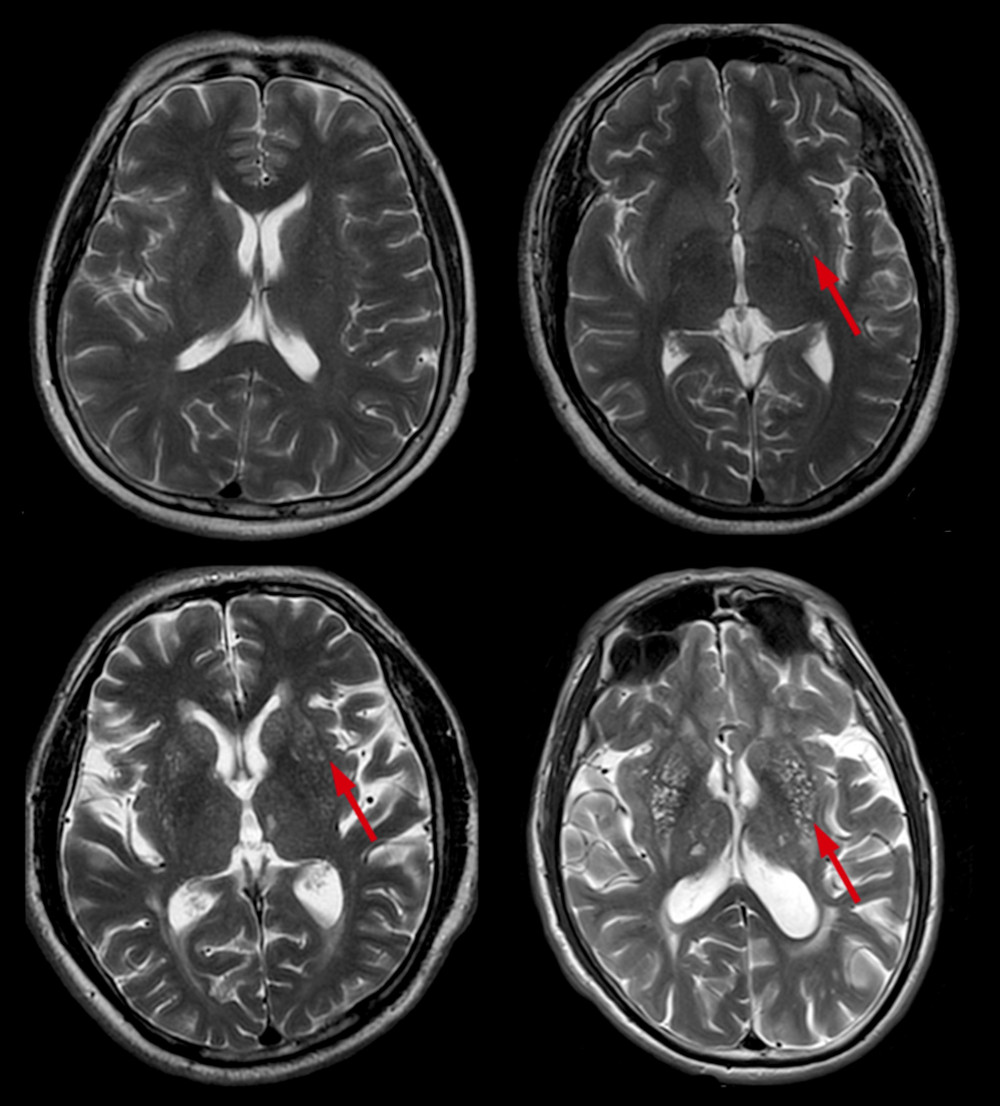 Figure 3. Different scores of BG-EPVS. Scores of BG-EPVS: (A) degree 1, <5 EPVS; (B) degree 2, 5–10 EPVS; (C) degree 3, >10 EPVS but still countable; and (D) degree 4, innumerable EPVS. BG – basal ganglia; EPVS – enlarged perivascular space.
Figure 3. Different scores of BG-EPVS. Scores of BG-EPVS: (A) degree 1, <5 EPVS; (B) degree 2, 5–10 EPVS; (C) degree 3, >10 EPVS but still countable; and (D) degree 4, innumerable EPVS. BG – basal ganglia; EPVS – enlarged perivascular space. 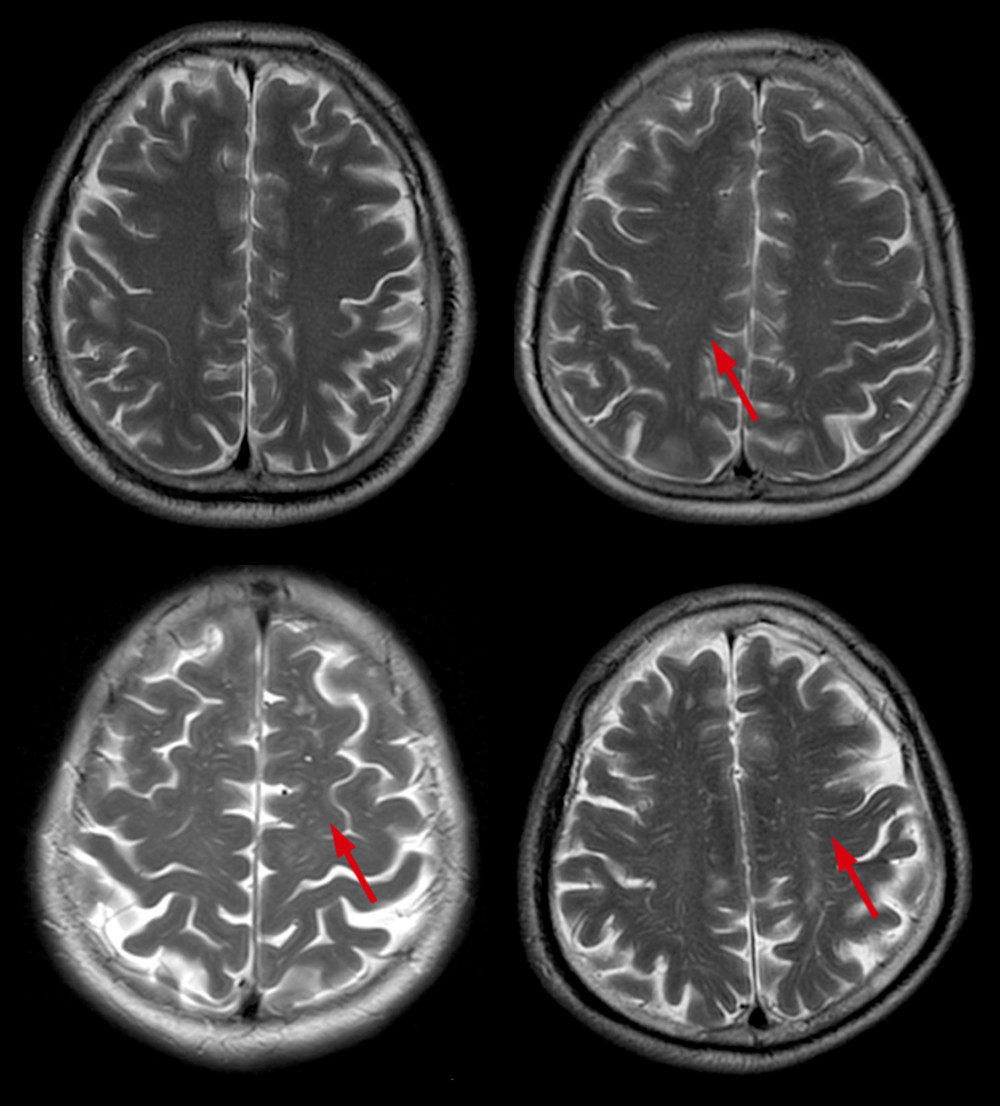 Figure 4. Different scores of CSO-EPVS. Scores of CSO-EPVS: (A) degree 1, <10 EPVS in the total white matter; (B) degree 2, >10 EPVS in the total white matter and <10 in the slice containing the greatest number of EPVS; (C) degree 3, 10–20 EPVS in the slice containing the greatest number of EPVS; and (D) degree 4, >20 EPVS in the slice containing the greatest number of EPVS. CSO – centrum semiovale; EPVS – enlarged perivascular space.
Figure 4. Different scores of CSO-EPVS. Scores of CSO-EPVS: (A) degree 1, <10 EPVS in the total white matter; (B) degree 2, >10 EPVS in the total white matter and <10 in the slice containing the greatest number of EPVS; (C) degree 3, 10–20 EPVS in the slice containing the greatest number of EPVS; and (D) degree 4, >20 EPVS in the slice containing the greatest number of EPVS. CSO – centrum semiovale; EPVS – enlarged perivascular space. Tables
Table 1. General characteristics of chronic obstructive pulmonary disease (COPD) group and control group.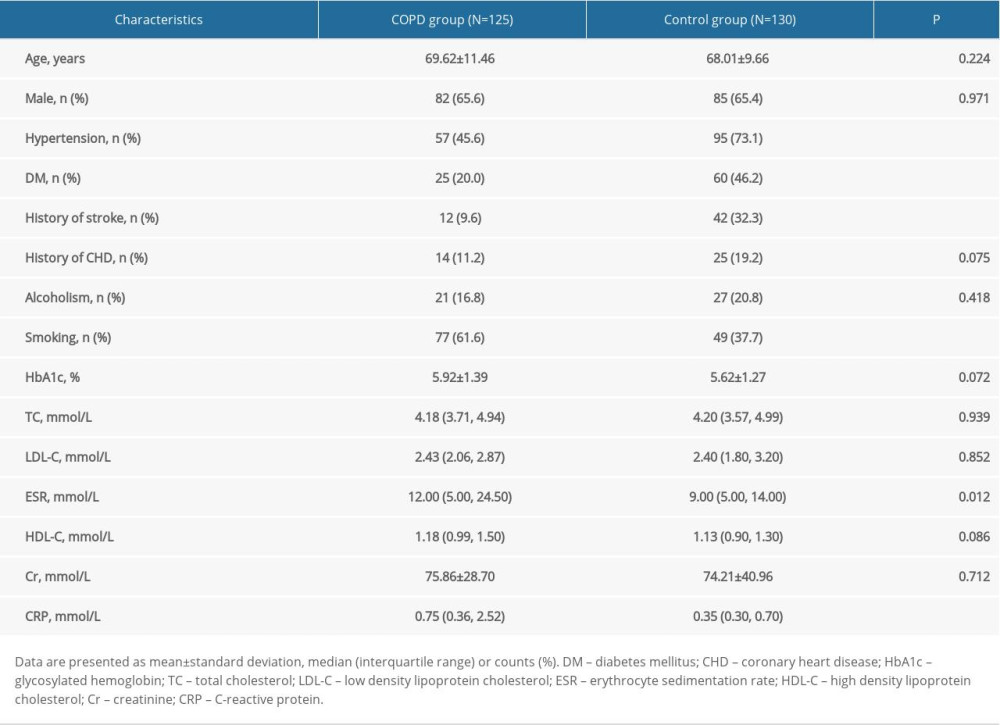 Table 2. Comparisons for individual cerebral small vessel disease (CSVD) imaging markers between chronic obstructive pulmonary disease (COPD) group and control group.
Table 2. Comparisons for individual cerebral small vessel disease (CSVD) imaging markers between chronic obstructive pulmonary disease (COPD) group and control group.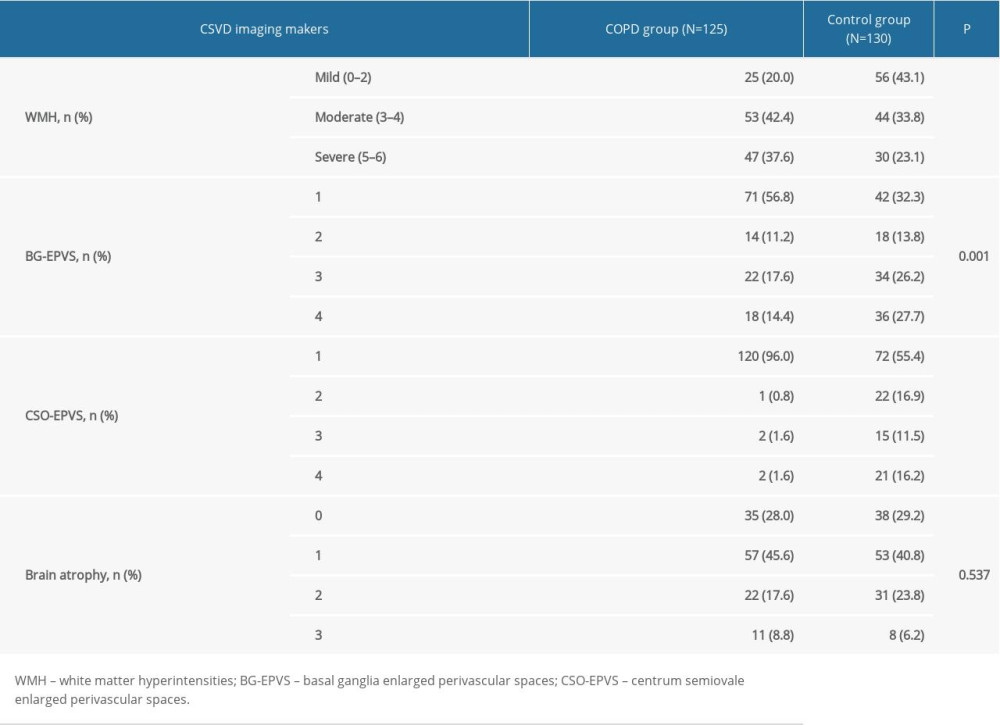 Table 3. The results of multivariate logistic regression analysis between group membership (chronic obstructive pulmonary disease [COPD] group and control group) and cerebral small vessel disease (CSVD) markers.
Table 3. The results of multivariate logistic regression analysis between group membership (chronic obstructive pulmonary disease [COPD] group and control group) and cerebral small vessel disease (CSVD) markers.![The results of multivariate logistic regression analysis between group membership (chronic obstructive pulmonary disease [COPD] group and control group) and cerebral small vessel disease (CSVD) markers.](https://jours.isi-science.com/imageXml.php?i=t3-medscimonit-26-e925703.jpg&idArt=925703&w=1000) Table 4. Comparison of the characteristics of chronic obstructive pulmonary disease (COPD) patients among groups according to white matter hyperintensities (WMH) burden.
Table 4. Comparison of the characteristics of chronic obstructive pulmonary disease (COPD) patients among groups according to white matter hyperintensities (WMH) burden.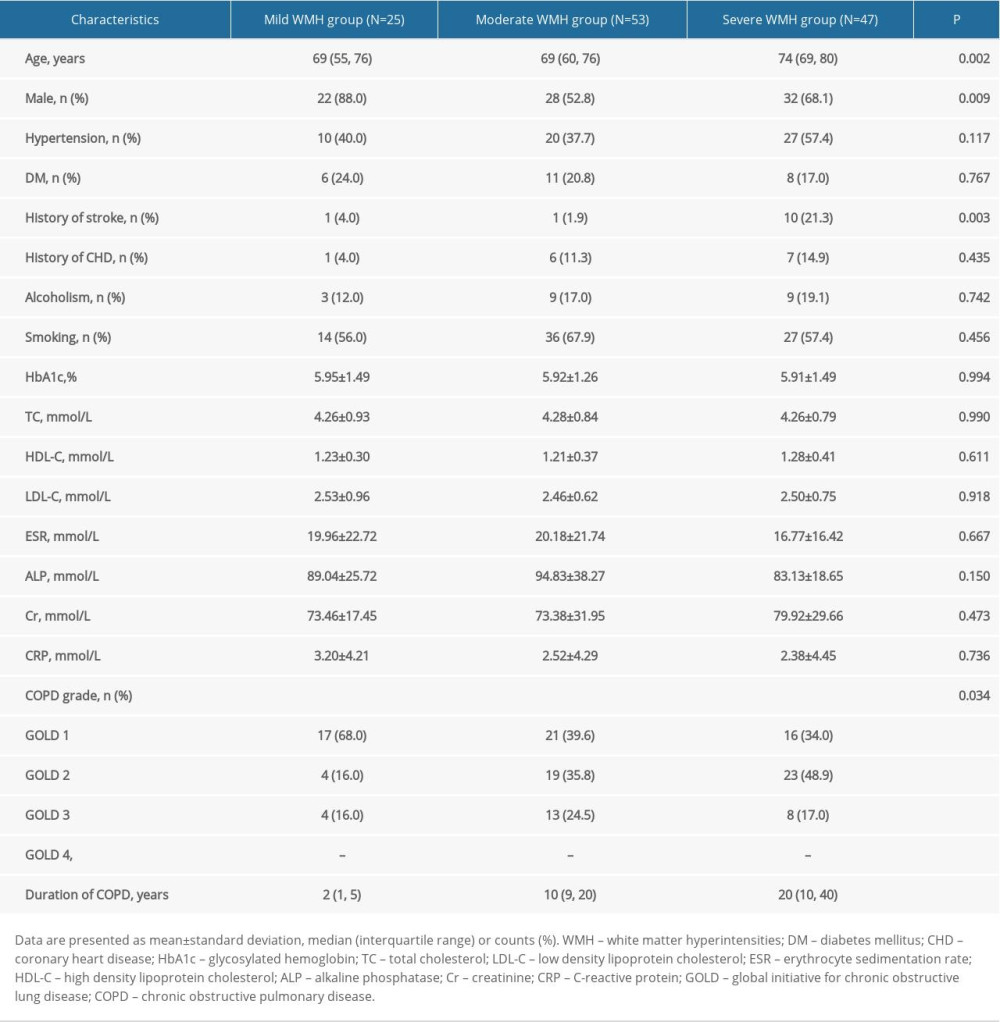 Table 5. Results of multivariate logistic regression analysis.
Table 5. Results of multivariate logistic regression analysis.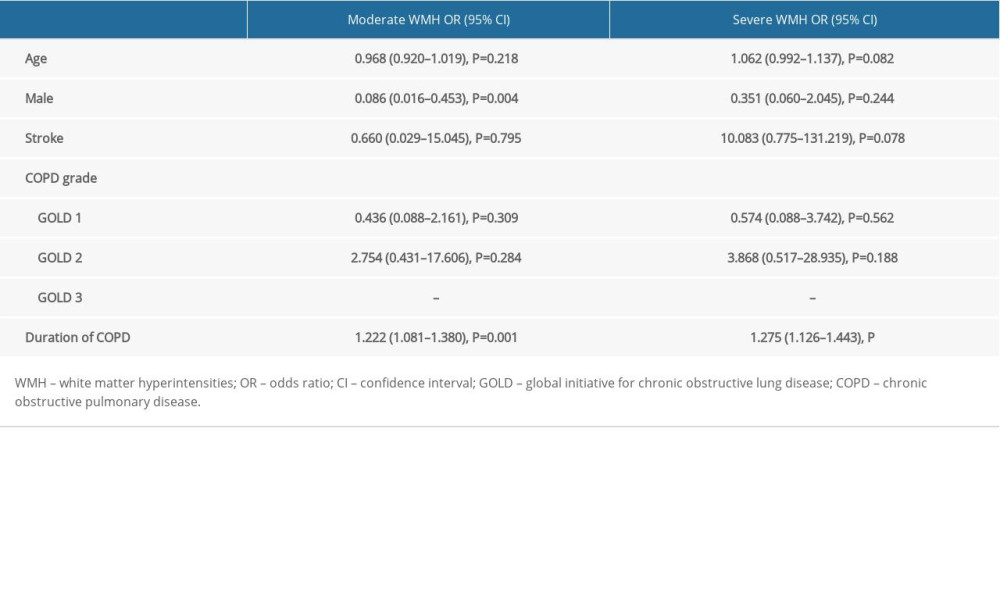 Table 6. Comparison of the characteristics of patients with chronic obstructive pulmonary disease (COPD) among groups according to basal ganglia enlarged perivascular space (BG-EPVS) burden.
Table 6. Comparison of the characteristics of patients with chronic obstructive pulmonary disease (COPD) among groups according to basal ganglia enlarged perivascular space (BG-EPVS) burden.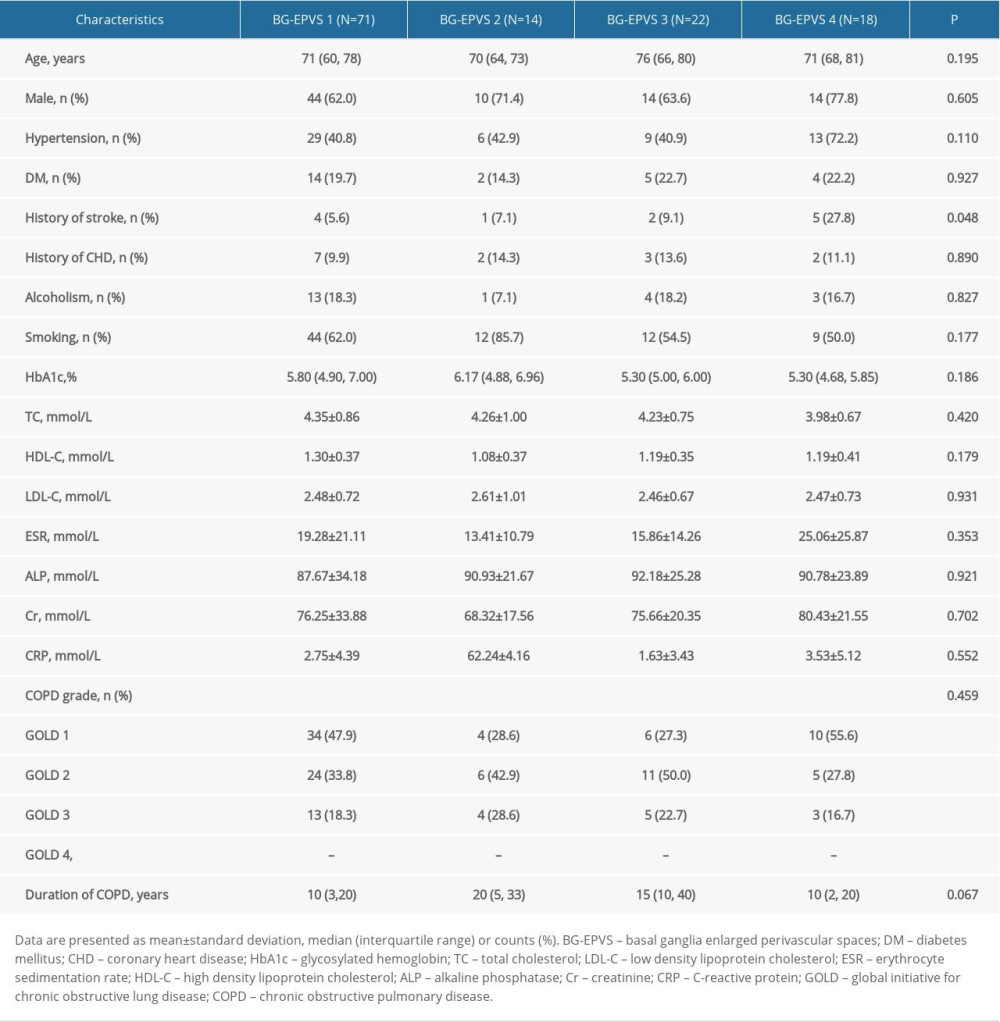 Table 7. Results of multivariate logistic regression analysis.
Table 7. Results of multivariate logistic regression analysis.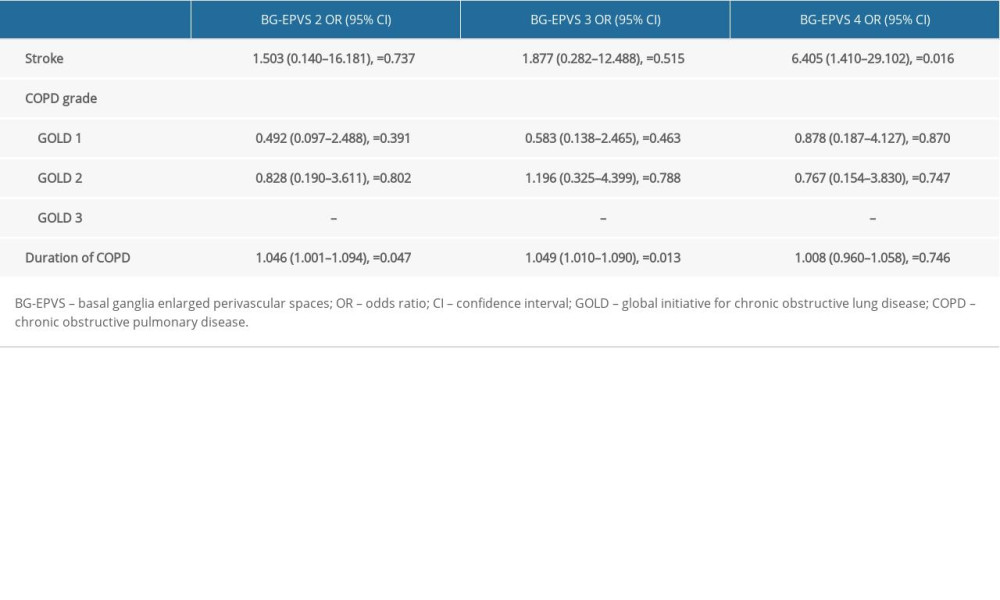
References
1. Pantoni L, Cerebral small vessel disease: From pathogenesis and clinical characteristics to therapeutic challenges: Lancet Neurol, 2010; 9(7); 689-701
2. Wardlaw JM, Smith EE, Biessels GJ, Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration: Lancet Neurol, 2013; 12(8); 822-38
3. Thompson CS, Hakim AM, Living beyond our physiological means: Small vessel disease of the brain is an expression of a systemic failure in arteriolar function: A unifying hypothesis: Stroke, 2009; 40(5); e322-30
4. Müller K, Courtois G, Ursini MV, Schwaninger M, New insight into the pathogenesis of cerebral small-vessel diseases: Stroke, 2017; 48(2); 520-27
5. Vogelmeier CF, Criner GJ, Martinez FJ, Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary: Am J Respir Crit Care Med, 2017; 195(5); 557-82
6. Fumagalli G, Fabiani F, Forte S, INDACO project: A pilot study on incidence of comorbidities in COPD patients referred to pneumology units: Multidiscip Respir Med, 2013; 8(1); 28
7. Lahousse L, Vernooij MW, Darweesh SK, Chronic obstructive pulmonary disease and cerebral microbleeds. The Rotterdam Study: Am J Respir Crit Care Med, 2013; 188(7); 783-88
8. Van Straaten EC, Fazekas F, Rostrup E, Impact of white matter hyperintensities scoring method on correlations with clinical data: The LADIS study: Stroke, 2006; 37(3); 836-40
9. Zhu YC, Tzourio C, Soumaré A, Severity of dilated Virchow-Robin spaces is associated with age, blood pressure, and MRI markers of small vessel disease: A population-based study: Stroke, 2010; 41(11); 2483-90
10. Pasquier F, Leys D, Weerts JG, Inter- and intraobserver reproducibility of cerebral atrophy assessment on MRI scans with hemispheric infarcts: Eur Neurol, 1996; 36(5); 268-72
11. Van Dijk E, Vermeer S, de Groot JC, Arterial oxygen saturation, COPD, and cerebral small vessel disease: J Neurol Neurosurg Psychiatry, 2004; 75(5); 733-36
12. Sachdev PS, Anstey KJ, Parslow R, Pulmonary function, cognitive impairment and brain atrophy in a middle-aged community sample: Dement Geriatr Cogn Disord, 2006; 21(5–6); 300-8
13. Dodd JW, Chung AW, van den Broek MD, Brain structure and function in chronic obstructive pulmonary disease: A multimodal cranial magnetic resonance imaging study: Am J Respir Crit Care Med, 2012; 186(3); 240-45
14. Zhang H, Wang X, Lin J, Grey and white matter abnormalities in chronic obstructive pulmonary disease: A case-control study: BMJ Open, 2012; 2(2); e000844
15. Esser RW, Stoeckel MC, Kirsten A, Structural brain changes in patients with COPD: Chest, 2016; 149(2); 426-34
16. Lu C, Xu W, Zeng C, Altered amplitude of low-frequency fluctuation in basal ganglia correlates to pulmonary ventilation function in COPD patients: A resting-state fMRI study: Brain Behav, 2019; 9(7); e01336
17. Gan WQ, Man SFP, Senthilselvan A, Sin DD, Association between chronic obstructive pulmonary disease and systemic inflammation: A systematic review and a meta-analysis: Thorax, 2004; 59(7); 574-80
18. Miyamoto O, Auer R, Hypoxia, hyperoxia, ischemia, and brain necrosis: Neurology, 2000; 54(2); 362-71
19. Cohen P, Alexander S, Smith TC, Effects of hypoxia and normocarbia on cerebral blood flow and metabolism in conscious man: J Appl Physiol, 1967; 23(2); 183-89
20. Geltser B, Brodskaya T, Kotelnikov V, Endothelial dysfunction of cerebral and major arteries during chronic obstructive disease: Bull Exp Biol Med, 2007; 144(6); 768-71
21. Dénes Á, Ferenczi S, Kovács KJ, Systemic inflammatory challenges compromise survival after experimental stroke via augmenting brain inflammation, blood-brain barrier damage and brain oedema independently of infarct size: J Neuroinflammation, 2011; 8(1); 164
22. Wu KL, Chan SH, Chan JY, Neuroinflammation and oxidative stress in rostral ventrolateral medulla contribute to neurogenic hypertension induced by systemic inflammation: J Neuroinflammation, 2012; 9(1); 212
23. Austin V, Crack PJ, Bozinovski S, COPD and stroke: Are systemic inflammation and oxidative stress the missing links?: Clin Sci, 2016; 130(13); 1039-50
Figures
 Figure 1. Different scores of PWMH. Scores of PWMH: (A) 0 score=absence; (B) 1=“caps” or pencil-thin lining; (C) 2=smooth “halo”; and (D) 3=irregular PWMH extending into the deep white matter. PWMH –periventricular white matter hyperintensities.
Figure 1. Different scores of PWMH. Scores of PWMH: (A) 0 score=absence; (B) 1=“caps” or pencil-thin lining; (C) 2=smooth “halo”; and (D) 3=irregular PWMH extending into the deep white matter. PWMH –periventricular white matter hyperintensities. Figure 2. Different scores of DWMH. Scores of DWMH: (A) 0=absence; (B) 1=punctate foci; (C) 2=beginning confluence of foci; and (D) 3=large confluent areas. DWMH – deep white matter hyperintensities.
Figure 2. Different scores of DWMH. Scores of DWMH: (A) 0=absence; (B) 1=punctate foci; (C) 2=beginning confluence of foci; and (D) 3=large confluent areas. DWMH – deep white matter hyperintensities. Figure 3. Different scores of BG-EPVS. Scores of BG-EPVS: (A) degree 1, <5 EPVS; (B) degree 2, 5–10 EPVS; (C) degree 3, >10 EPVS but still countable; and (D) degree 4, innumerable EPVS. BG – basal ganglia; EPVS – enlarged perivascular space.
Figure 3. Different scores of BG-EPVS. Scores of BG-EPVS: (A) degree 1, <5 EPVS; (B) degree 2, 5–10 EPVS; (C) degree 3, >10 EPVS but still countable; and (D) degree 4, innumerable EPVS. BG – basal ganglia; EPVS – enlarged perivascular space. Figure 4. Different scores of CSO-EPVS. Scores of CSO-EPVS: (A) degree 1, <10 EPVS in the total white matter; (B) degree 2, >10 EPVS in the total white matter and <10 in the slice containing the greatest number of EPVS; (C) degree 3, 10–20 EPVS in the slice containing the greatest number of EPVS; and (D) degree 4, >20 EPVS in the slice containing the greatest number of EPVS. CSO – centrum semiovale; EPVS – enlarged perivascular space.
Figure 4. Different scores of CSO-EPVS. Scores of CSO-EPVS: (A) degree 1, <10 EPVS in the total white matter; (B) degree 2, >10 EPVS in the total white matter and <10 in the slice containing the greatest number of EPVS; (C) degree 3, 10–20 EPVS in the slice containing the greatest number of EPVS; and (D) degree 4, >20 EPVS in the slice containing the greatest number of EPVS. CSO – centrum semiovale; EPVS – enlarged perivascular space. Tables
 Table 1. General characteristics of chronic obstructive pulmonary disease (COPD) group and control group.
Table 1. General characteristics of chronic obstructive pulmonary disease (COPD) group and control group. Table 2. Comparisons for individual cerebral small vessel disease (CSVD) imaging markers between chronic obstructive pulmonary disease (COPD) group and control group.
Table 2. Comparisons for individual cerebral small vessel disease (CSVD) imaging markers between chronic obstructive pulmonary disease (COPD) group and control group.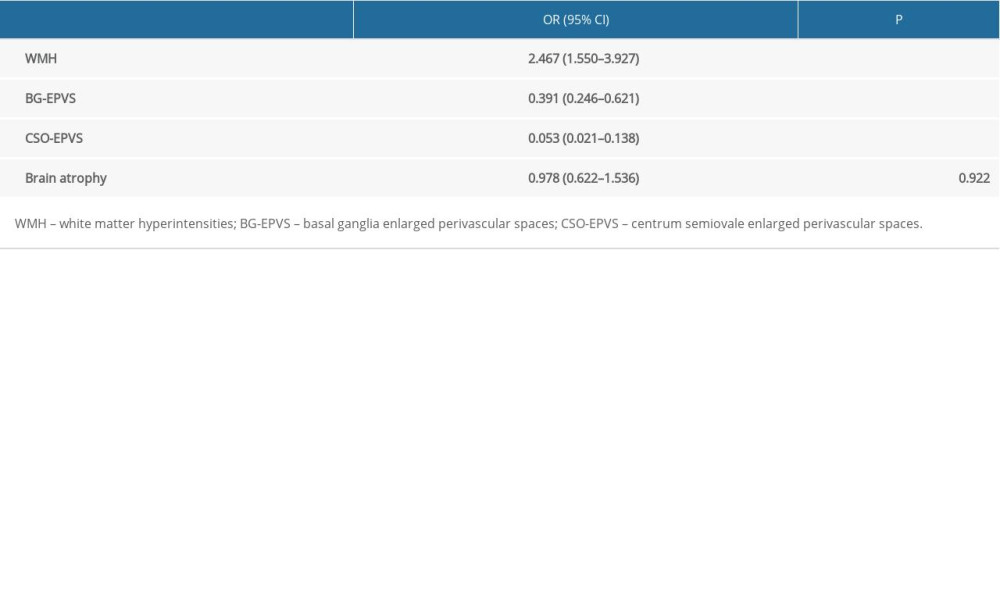 Table 3. The results of multivariate logistic regression analysis between group membership (chronic obstructive pulmonary disease [COPD] group and control group) and cerebral small vessel disease (CSVD) markers.
Table 3. The results of multivariate logistic regression analysis between group membership (chronic obstructive pulmonary disease [COPD] group and control group) and cerebral small vessel disease (CSVD) markers. Table 4. Comparison of the characteristics of chronic obstructive pulmonary disease (COPD) patients among groups according to white matter hyperintensities (WMH) burden.
Table 4. Comparison of the characteristics of chronic obstructive pulmonary disease (COPD) patients among groups according to white matter hyperintensities (WMH) burden. Table 5. Results of multivariate logistic regression analysis.
Table 5. Results of multivariate logistic regression analysis. Table 6. Comparison of the characteristics of patients with chronic obstructive pulmonary disease (COPD) among groups according to basal ganglia enlarged perivascular space (BG-EPVS) burden.
Table 6. Comparison of the characteristics of patients with chronic obstructive pulmonary disease (COPD) among groups according to basal ganglia enlarged perivascular space (BG-EPVS) burden. Table 7. Results of multivariate logistic regression analysis.
Table 7. Results of multivariate logistic regression analysis. Table 1. General characteristics of chronic obstructive pulmonary disease (COPD) group and control group.
Table 1. General characteristics of chronic obstructive pulmonary disease (COPD) group and control group. Table 2. Comparisons for individual cerebral small vessel disease (CSVD) imaging markers between chronic obstructive pulmonary disease (COPD) group and control group.
Table 2. Comparisons for individual cerebral small vessel disease (CSVD) imaging markers between chronic obstructive pulmonary disease (COPD) group and control group. Table 3. The results of multivariate logistic regression analysis between group membership (chronic obstructive pulmonary disease [COPD] group and control group) and cerebral small vessel disease (CSVD) markers.
Table 3. The results of multivariate logistic regression analysis between group membership (chronic obstructive pulmonary disease [COPD] group and control group) and cerebral small vessel disease (CSVD) markers. Table 4. Comparison of the characteristics of chronic obstructive pulmonary disease (COPD) patients among groups according to white matter hyperintensities (WMH) burden.
Table 4. Comparison of the characteristics of chronic obstructive pulmonary disease (COPD) patients among groups according to white matter hyperintensities (WMH) burden. Table 5. Results of multivariate logistic regression analysis.
Table 5. Results of multivariate logistic regression analysis. Table 6. Comparison of the characteristics of patients with chronic obstructive pulmonary disease (COPD) among groups according to basal ganglia enlarged perivascular space (BG-EPVS) burden.
Table 6. Comparison of the characteristics of patients with chronic obstructive pulmonary disease (COPD) among groups according to basal ganglia enlarged perivascular space (BG-EPVS) burden. Table 7. Results of multivariate logistic regression analysis.
Table 7. Results of multivariate logistic regression analysis. In Press
08 Mar 2024 : Clinical Research
Evaluation of Foot Structure in Preschool Children Based on Body MassMed Sci Monit In Press; DOI: 10.12659/MSM.943765
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








