30 August 2020: Articles 
Amyloid Variant of Central Odontogenic Fibroma in the Mandible: A Case Report and Literature Review
Rare disease
Wataru Kakuguchi1ABCDEF*, Yoshiyuki Nakamichi1E, Tetsuya Kitamura2BCDEDOI: 10.12659/AJCR.925165
Am J Case Rep 2020; 21:e925165
Abstract
BACKGROUND: Odontogenic fibroma is a rare mesenchymal odontogenic tumor. It can be classified as central odontogenic fibroma (COF) or peripheral odontogenic fibroma (POF) based on clinical features. There are several variants of COF, including amyloid, ossifying, and giant cell. It grows slowly and exhibits painless cortical expansion of the jawbone. Radiographically, COF appears as a radiolucent unilocular or multilocular lesion with well-defined borders. COF may be associated with unerupted or displaced teeth and root resorption.
CASE REPORT: A 35-year-old man was referred to our hospital for submandibular swelling. Panoramic radiography and contrast-enhanced computed tomography revealed a unilocular area of bone resorption with a well-defined border and equal enhancement from the canine to first molar on the right side of the mandible. Root resorption of the first premolar and root separation of the first and second premolars were also evident. The lesion was asymptomatic. Histopathological examination of a biopsy of the lesion was suggestive of OF. Enucleation of the tumor, curettage of the bone around the tumor, and extraction of the premolar were then performed. Histopathological examination of the tumor showed fibrous connective tissue with inactive-looking odontogenic epithelial islands and strands, amyloid deposit, intercalation of Langerhans cells into the tumor epithelium, and no calcification. The final diagnosis of amyloid variant of COF was made. The postoperative course is going well.
CONCLUSIONS: Herein we describe and discuss the clinical, radiological, and pathological features of the amyloid variant of COF. This report will enhance understanding of this extremely rare variant.
Keywords: Amyloid, Fibroma, Odontogenic Tumors, Mandibular Diseases, Langerhans cells, Gingival Neoplasms, Mandible, Radiography, Panoramic
Background
Odontogenic fibroma (OF) is a rare mesenchymal odontogenic tumor [1,2]. A small change has been made to the World Health Organization (WHO) subclassification of OF. In the 2017 WHO classification, OF was defined as a rare neoplasm of mature fibrous connective tissue, with variable amounts of inactive-looking odontogenic epithelium with or without evidence of calcification [1,2].
OF can be classified as central odontogenic fibroma (COF) or peripheral odontogenic fibroma (POF), based on clinical features. Surgical excision is the standard treatment for COF [3]. COF occurs in the jawbone and is believed to be derived from one of the mesenchymal components of the tooth germ, either the dental follicle, the dental papilla, or the periodontal ligament [3]. It grows slowly and results in painless cortical expansion of the jawbone. Radiographically, COF appears as a radiolucent unilocular or multilocular entity with well-defined borders. The unilocular type, the majority of which are small, resembles a unilocular ameloblastoma or odontogenic cyst. The multilocular type, which tends to be comparatively large, resembles ameloblastoma or odontogenic myxoma [3,4]. In some instances, COF can exhibit mixed radiolucency/radiopacity, with poorly defined or diffuse borders [4]. COF may be associated with unerupted or displaced teeth and root resorption [4–6].
There are several variants of COF, including amyloid, ossifying, and giant cell [7]. In the current case, there were many amyloid deposits in the stroma of the COF. Therefore, it was diagnosed as the amyloid variant or amyloid/dendritic cell-associated OF [5,7]. This variant, which is characterized by amyloid deposits and intercalation of Langerhans cells into the epithelial elements, is rare [7]. Herein we describe and discuss the characteristic features of the amyloid variant, in conjunction with a review of the literature in this area. The report will enhance understanding of this extremely rare variant of COF.
Case Report
A 35-year-old man was referred to our hospital in 2019 due to right submandibular swelling, severe spontaneous pain, and trismus. His medical history was unremarkable. Acute right submandibular cellulitis and abscess causing apical periodontitis of the right second molar were diagnosed via panoramic radiography and contrast-enhanced computed tomography (CT). Another lesion was also evident, which exhibited bone resorption with a well-defined border and equal enhancement from the right mandibular canine to the first molar, root resorption of the right first premolar, and root separation of the first and second premolars (Figures 1, 2). It was suspected to be a solid lesion such as desmoplastic ameloblastoma, COF, or central giant cell granuloma. The lesion was asymptomatic and the patient had not noticed any signs of it prior to its detection. The first premolar had not been moved and a vital pulp reaction was elicited via electrical stimulation. The periodontal pocket was normal. The lingual attached gingiva of the first premolar was depressed, but the surface was normal (Figure 3A). Histopathological examination of a biopsy of the lesion (Figure 3B) showed fibrous connective tissue with odontogenic epithelial islands and strands, suggesting OF. Enucleation of the tumor, curettage of the bone around the tumor, and extraction of the premolar were then performed (Figure 4). Histopathological examination of the tumor showed fibrous connective tissue with inactive-looking odontogenic epithelial islands and strands, homogeneous eosinophilic globular masses, and no calcification (Figure 5A, 5B). The odontogenic epithelium was positive for cytokeratin cocktail (AE1/AE3), CK5/6, and CK19 (Figure 5C–5E), but negative for ki-67 (Figure 5F). The homogeneous eosinophilic globular masses proved to be amyloid deposit because they stained positive for Direct Fast Scarlet (DFS) and Congo red (Figure 5G–5J). Also, green birefringence was demonstrated under polarized light following Congo red staining (Figure 5J). Moreover, the Langerhans cells expressing CD1a and S-100 (Figure 5K, 5L) were observed intercalated in the tumor epithelium. The final diagnosis of amyloid variant of COF was made. The patient has since been followed up for 5 months, and his dental radiographs have revealed no recurrence of COF (Figure 6).
Discussion
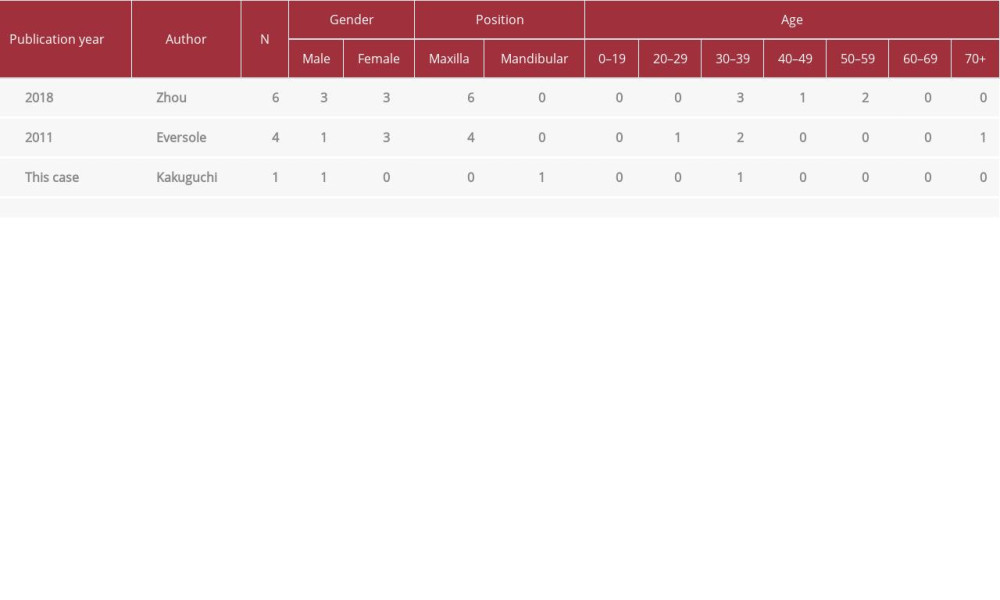
OF is a rare mesenchymal odontogenic tumor (OT) [1,2]. OTs are diagnosed in 0.74–9.56% of all oral biopsies [8–15]. COFs account for 1.21–4.85% of all OTs [8–10,14–16]. COF is rarer than POF [9]. Reports discussing the frequency of occurrence of COF in the maxilla and the mandible are contradictory, making it difficult to ascertain which jaw is more likely to be affected [4–7,12]. In the maxilla, COF occurs most frequently in the anterior region, followed by the premolar and the molar regions. In the mandible, this is reversed [6]. The incidence is highest in individuals aged 10 to 39 years [4–7]. The amyloid variant most commonly occurs in the maxilla, unlike in the current case, and is usually seen in the premolar region in patients aged 30–39 years (Table 1).
COFs, including the amyloid variant, exhibit dispersed staining for CK10/13 but they are negative for CK7 and CK8/18.
Langerhans cells – which are positive for S-100, langerin, and CD1a – are commonly detected in the islands and strands of odontogenic epithelia in COF. Amyloid deposits stain positively for Congo red and DFS, and exhibit green birefringence under polarized light [5].
Amyloid deposition is known to occur in calcifying epithelial odontogenic tumors (CEOTs). Moreover, amyloid deposition was also observed in dental follicles, which are one of the origins for COF [17]. Zhou et al. [5] reported that intercalation of Langerhans cells in CEOT epithelium was no more than 2%, but in COF, it was approximately 40%. Intercalation of that Langerhans cells into odontogenic epithelium of COF has been reported in many cases [5,7,18]. Therefore, a noncalcifying variant of CEOT demonstrating amyloid deposits and inter-calation of Langerhans cells was considered to be a variant of COF [5,7]. In the current case, both amyloid deposit and inter-calation of Langerhans cells into the odontogenic epithelium were observed with immunostaining (Figure 5G–5L). However, the significance of amyloid deposition and Langerhans cell intercalation is unknown.
Eversole et al. [7] described 65 cases of OF (25 COF and 40 POF), and 4 of the cases of COF had amyloid deposits. Zhou et al. [5] described 17 cases of COF, and of them, there were 6 cases with amyloid deposits. Interestingly, all cases with amyloid deposits were located in the maxilla [5,7] (Table 1). Palatal gingival depression was detected in 5 of 6 cases in one report [5] and in 2 of 4 cases in another [7] (Table 1), although gingival depression is generally unusual in solid tumors of the jaw. In the current case, the tumor was located in the mandible, which is comparatively rare, and it exhibited gingival depression.
Surgery is the main treatment for COF that does not infiltrate around the bone and exhibits clear border radiolucency [5].
Therefore, enucleation and curettage were performed. Resection for radical surgery has also been reported [6].
The rate of recurrence of COF is reportedly up to 10% [5–7]. Higher rates of recurrence are associated with occurrence in the maxilla, cortical bone perforation, and multilocularity [5–7]. No cases of malignant transformation of COF have been reported [5,6]. In the current case, located in the mandible, there was a clear margin, no cortical bone perforation, and the lesion was unilocular. Therefore, recurrence may be unlikely in this patient.
Symptoms of COF that have been reported and evidence on clinical examination are gingival depression, loose teeth, and swelling [5]. As in the current case, COF has previously been detected incidentally via panoramic x-ray in asymptomatic patients.
Sclerosing odontogenic carcinoma (SOC) has been considered in the differential diagnosis of COF [1]. It can exhibit some histological features that are similar to those of COF [1]. The epithelial component can be conspicuous, can be highlighted using immunostaining for CK19, CK5/6, and p63, and can exhibit subtle focal CK7 positivity [1]. SOC is a cytologically bland epithelial tumor with significant stromal sclerosis, and it is characterized by aggressive infiltrative growth into muscles and nerves [2]. Clinically, SOC is an expansile mass that sometimes results in nerve symptoms. It is associated with osteolysis, variable cortical destruction, sclerotic changes, ground-glass appearance of the involved bone, a wholly or partially ill-defined border, and tooth resorption evident on panoramic x-ray [19,20]. In the current case, the border of the lesion was clear, there were no sclerotic changes or clinical symptoms. Thus, SOC was ruled out.
Conclusions
Herein we have described the clinical, radiological, and pathological features of a case of COF with amyloid deposition and intercalation of Langerhans cells into the tumor epithelium. Gingival depression is characteristic of COFs, especially those with amyloid deposition. This report and literature review will enhance understanding of the amyloid variant of COF.
Figures
References:
1.. Soluk-Tekkeşin M, Wright JM, The World Health Organization Classification of Odontogenic Lesions: A summary of the changes of the 2017 (4th) edition: Turk Patoloji Derg, 2018; 34(1)
2.. Wright JM, Vered M, Update from the 4th Edition of the World Health Organization Classification of Head and Neck Tumours: Odontogenic and maxillofacial bone tumors: Head Neck Pathol, 2017; 11(1); 68-77
3.. Wesley RK, Wysocki GP, Mintz SM, The central odontogenic fibroma. Clinical and morphologic studies: Oral Surg Oral Med Oral Pathol, 1975; 40(2); 235-45
4.. Kaffe I, Buchner A, Radiologic features of central odontogenic fibroma: Oral Surg Oral Med Oral Pathol, 1994; 78(6); 811-18
5.. Zhou CX, Li TJ, A clinicopathologic study on central odontogenic fibroma: With special reference to amyloid variant: Oral Surg Oral Med Oral Pathol Oral Radiol, 2018; 126(6); 513-20
6.. Correa Pontes FS, Lacerda de Souza L, Central odontogenic fibroma: An updated systematic review of cases reported in the literature with emphasis on recurrence influencing factors: J Craniomaxillofac Surg, 2018; 46(10); 1753-57
7.. Eversole LR, Odontogenic fibroma, including amyloid and ossifying variants: Head Neck Pathol, 2011; 5(4); 335-43
8.. Buchner A, Merrell PW, Carpenter WM, Relative frequency of central odontogenic tumors: A study of 1,088 cases from Northern California and comparison to studies from other parts of the world: J Oral Maxillofac Surg, 2006; 64(9); 1343-52
9.. Buchner A, Merrell PW, Carpenter WM, Relative frequency of peripheral odontogenic tumors: A study of 45 new cases and comparison with studies from the literature: J Oral Pathol Med, 2006; 35(7); 385-91
10.. Daley TD, Wysocki GP, Pringle GA, Relative incidence of odontogenic tumors and oral and jaw cysts in a Canadian population: Oral Surg Oral Med Oral Pathol, 1994; 77(3); 276-80
11.. Tamme T, Soots M, Kulla A, Odontogenic tumours, a collaborative retrospective study of 75 cases covering more than 25 years from Estonia: J Craniomaxillofac Surg, 2004; 32(3); 161-65
12.. Mosqueda-Taylor A, Ledesma-Montes C, Caballero-Sandoval S, Odontogenic tumors in Mexico: A collaborative retrospective study of 349 cases: Oral Surg Oral Med Oral Pathol Oral Radiol Endod, 1997; 84(6); 672-75
13.. Ladeinde AL, Ajayi OF, Ogunlewe MO, Odontogenic tumors: A review of 319 cases in a Nigerian teaching hospital: Oral Surg Oral Med Oral Pathol Oral Radiol Endod, 2005; 99(2); 191-95
14.. Ratthapong W, Montip T, Odontogenic Tumors in Thailand: A study of 590 Thai patients: J Oral Maxillofac Surg Med Pathol, 2015; 27(4); 567-76
15.. Saghravanian N, Jafarzadeh H, Bashardoost N, Odontogenic tumors in an Iranian population: A 30-year evaluation: J Oral Sci, 2010; 52(3); 391-96
16.. Wu PC, Chan KW, A survey of tumours of the jawbones in Hong Kong Chinese: 1963–1982: Br J Oral Maxillofac Surg, 1985; 23(2); 92-102
17.. Murphy CL, Kestler DP, Foster JS, Odontogenic ameloblast-associated protein nature of the amyloid found in calcifying epithelial odontogenic tumors and unerupted tooth follicles: Amyloid, 2008; 15(2); 89-95
18.. Wu YC, Wang YP, Chang JTY, Langerhans cells in odontogenic epithelia of odontogenic fibromas: J Formos Med Assoc, 2013; 112(12); 756-60
19.. Todorovic E, Berthelet E, O’Connor R, Sclerosing odontogenic carcinoma with local recurrence: Case report and review of literature: Head Neck Pathol, 2019; 13(3); 371-77
20.. Kennedy RA, WHO is in and WHO is out of the mouth, salivary glands, and jaws sections of the 4th edition of the WHO Classification of Head and Neck Tumours: Br J Oral Maxillofac Surg, 2018; 56(2); 90-95
Figures
In Press
04 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.941835
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943042
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942578
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943801
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250