26 August 2020: Articles 
Pulmonary Vein Stenosis and Pulmonary Hypertension Following a Catheter-Based Radiofrequency Ablation for Atrial Fibrillation: A Case Report
Unusual clinical course, Challenging differential diagnosis, Unusual or unexpected effect of treatment, Diagnostic / therapeutic accidents
Christopher A. Thomas1ABCDEF, Karla J. Cruz Morel2ABCDEF, Mohan N. Viswanathan3BE, Vinicio A. de Jesus Perez1ABCDEFG*DOI: 10.12659/AJCR.924709
Am J Case Rep 2020; 21:e924709
Abstract
BACKGROUND: Pulmonary vein (PV) stenosis is a rare condition characterized by progressive luminal size reduction of one or more pulmonary veins (PVs), which can increase postcapillary pressure resulting in shortness of breath, cough, hemoptysis, and pulmonary hypertension (PH). The diagnosis of PV stenosis requires a high degree of suspicion. PV stenosis is a rare but recognized complication of catheter-based radiofrequency ablation (RFA) for atrial fibrillation (AF).
CASE REPORT: We present a case of a 78-year-old man who underwent a surgical MAZE procedure followed by catheter-based RFA to treat AF. He subsequently developed shortness of breath, exercise limitation, and PH. The patient was ultimately diagnosed with PV stenosis, which was a sequela of the RFA and the cause of his PH. The patient was treated by stenting of his PV, with improvement in his exercise capacity and PH. Follow-up imaging showed improved pulmonary blood flow and reduced pulmonary pressures.
CONCLUSIONS: We conclude that PV stenosis should be high in the differential as the cause of dyspnea in patients with PH and a previous history of RFA for AF management. Early recognition and treatment can prevent complete occlusion of the affected PV and lead to an improvement in the patient’s symptoms and quality of life.
Keywords: Atrial Fibrillation, Dyspnea, Pulmonary Veins, Stents, Catheter Ablation, catheters, Hypertension, Pulmonary, Quality of Life, Stenosis, Pulmonary Vein
Background
Pulmonary vein (PV) stenosis is a rare condition characterized by progressive luminal size reduction of one or more pulmonary veins (PVs), which can increase postcapillary pressure resulting in shortness of breath, persistent cough, hemoptysis [1], and pulmonary hypertension (PH). Prior to the modern medical era, most cases of PV stenosis were related to congenital heart disease or the result of extrinsic compression from mediastinal processes such as sarcoidosis or malignancy [1]. Although it remains a rare entity, PV stenosis is increasingly recognized as a complication of catheter-based radiofrequency ablation (RFA) and MAZE procedures that are done to treat atrial fibrillation (AF). The diagnosis of PV stenosis after RFA is challenging, even with the availability of common imaging modalities such as transthoracic echocardiogram (TTE) and computed tomography angiography (CTA). As such, clinicians must have a high index of suspicion for PV stenosis, and always consider it in the differential diagnosis for patients with new dyspnea and a history of RFA of the PVs.
Case Report
A 78-year-old man with atrial fibrillation (AF) and symptomatic bradycardia requiring pacemaker placement was referred to the pulmonary hypertension (PH) clinic for worsening dyspnea on exertion. In 2014, he noticed a decrease in his exercise tolerance secondary to AF and mitral regurgitation. He underwent minimally invasive mitral valve repair with annuloplasty and a surgical MAZE procedure in 2015. Unfortunately, his AF and associated symptoms recurred 6 months later.
The recurrent AF prompted referral to the electrophysiology clinic. At the time of this evaluation, the patient underwent a TTE, which showed normal biventricular function, no significant valvular abnormalities, and a normal right ventricular systolic pressure (RVSP). He also had a CTA with left atrial mapping, which showed patent left and right PVs as well as normal pulmonary arteries. On pacemaker interrogation, the patient was found to have rising AF burden, which was felt to be the etiology of his progressive symptoms.
The patient underwent a catheter-based RFA to treat the AF in February 2016. The procedure included focal impulse and rotor modulation (FIRM) mapping, with ablation of localized sources of AF in the posterior wall of the left atrium (LA) outside the right PVs as well as the anterior ridge of the LA. FIRM mapping was chosen based on the results of the CONFIRM trial, which showed that using FIRM mapping in addition to conventional RFA leads to increased rates of successfully treated AF in patients with persistent AF [2]. The procedure also included circumferential PV isolation with mitral and roofline and cavo-tricuspid isthmus ablation. The procedure went well, with no AF or symptoms noted post operatively.
A year after the RFA, the patient experienced progressive worsening dyspnea and intermittent lightheadedness, with no other symptoms. On physical exam, he was normotensive, with a regular heart rate of 80 beats per minute and oxygen saturation of 96% on room air. A complete physical exam was unremarkable. His only medication was rivaroxaban for AF. Pulmonary function tests did not show any abnormalities. The TTE was remarkable for an RVSP of 47 mmHg and mild right ventricular enlargement, indicative of PH. The patient refused right heart catheterization. A ventilation/perfusion (V/Q) scan showed a single sizeable mismatched perfusion defect in the left upper lung (Figure 1). A CTA of the chest revealed complete occlusion of the left superior PV (Figure 2A), moderate (30–40%) narrowing of the orifice of the left inferior PV (Figure 3A), and diminutive/near completely occluded of the left upper lobe pulmonary arteries.
Given the patient’s symptomatic burden, he was referred to another institution for PV stenting. First, the left inferior PV was engaged with a 6 Fr multipurpose catheter, and angiography revealed a stenosis at the ostium. A 0.035 Amplatz extra stiff guidewire was used to thread a catheter into the left inferior PV. A significant pressure gradient was noted across the stenosis, so a 10×19 mm Genesis (bare metal) stent was inserted into the left inferior PV.
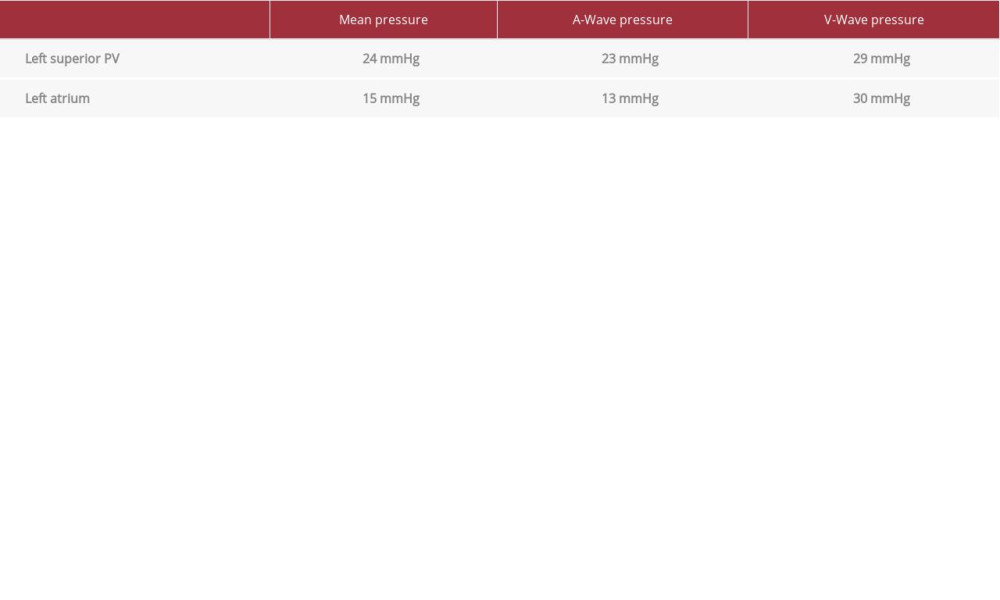
Angiography of the left superior PV revealed significant ostial stenosis with collateral vessels (see Table 1 for pressures). Once again, a 0.035 Amplatz extra stiff guidewire was used to deploy a 10×19 mm Genesis (bare metal) stent at 8 atm. The initial deployment resulted in very good stent expansion with sufficient ostial coverage. The patient was noted to have a small pericardial effusion after the procedure, and he was observed in the cardiac care unit overnight. The effusion was stable, and he was discharged home the next day. Clopidogrel was added to rivaroxaban after the stenting procedure, which was to be continued indefinitely.
The patient’s dyspnea improved dramatically, and he was able to resume his normal activities, including swimming. A repeat CTA showed patent stents in the left superior (Figure 2B) and inferior PVs (Figure 3B), as well as patent pulmonary arteries. A repeat TTE demonstrated a decrease in the RVSP to 31, with normal right ventricular size and function. A V/Q scan performed a few days after the stenting showed improved flow to the left lung (left lung 18%
Discussion
Our patient developed shortness of breath and exercise limitation about a year after undergoing a catheter-based RFA to treat AF. Symptoms of PV stenosis are nonspecific and can vary widely between patients depending on a number of factors, including lesion severity, time course, the response of the pulmonary vasculature, and other host factors [3]. Therefore, a high suspicion for PV stenosis is required to make the diagnosis in order to prevent further stenosis and parenchymal lung damage. Historically, PV stenosis occurred following RFA in as many as 15% of cases [4], with a small subset of these patients acquiring severe, symptomatic PV stenosis. As RFA techniques have improved over the last 2 decades, the incidence of PV stenosis following RFA has decreased [1]. In a recent large cohort study of over 10 000 patients who underwent RFA for AF, severe PV stenosis (defined as >70% narrowing of the PV lumen) was diagnosed in 0.5% of patients [5]. PV stenosis occurs more commonly in the left-sided PVs, a consequence of the small diameter of the left inferior PV and the cranial position of the left superior PV with the steep coumadin ridge leading to ablation sites closer to the ostium [6]. Typically, symptomatic patients with PV stenosis present about 3–6 months after RFA [7].
Radiographically, PV stenosis can present as unilateral pulmonary edema, pleural effusion, or infiltrates mimicking pneumonia. Transesophageal echocardiogram is more specific than TTE for the diagnosis of PV stenosis. Echocardiographic measurements of PV diameter and peak flow velocity are useful in the diagnosis of PV stenosis. An abnormal monophasic continuous high-velocity color signal in the LA originating at the ostia of PVs can be suggestive of PV stenosis [8]. A diameter of 0.3–0.8 cm (normal 0.9–1.2 cm) and peak flow velocity from 1.1 to 1.5 m/s (normal 0.4–0.7 m/s) suggest the diagnosis of PV stenosis, with the latter providing the best separation between normal and stenosed PVs [9]. Lung scintigraphy can show several degrees of V/Q mismatch, which can be due to pulmonary embolism or PV stenosis [10].
CTA is an excellent test to show the detailed anatomy of the PVs and has the advantage of providing proper spatial resolution and a 3-dimensional data set within a short scanning time. CT imaging findings can wax and wane depending on factors that transiently alter pulmonary venous pressures, and assessment of a severely stenosed vein can be limited by slow or incomplete opacification [11]. MRI can be reliable in depicting the stenotic lesions and the related perfusion abnormalities; however, it is limited by the long acquisition times and motion and respiration artifacts. Although noninvasive imaging can be sufficient for the diagnosis of PV stenosis, pulmonary angiography remains the goal standard test. Angiography can be performed by pulmonary artery injections or via transseptal access, which allows for optimal opacification of the veins and measurements of pressure gradients across stenotic lesions for direct hemodynamic assessment of the stenosis.
PV balloon angioplasty and stent placement are both options for the management of PV stenosis [12]. In several studies, the rate of restenosis has been shown to be lower with stenting than with balloon angioplasty [6,12]. In one case series of 56 patients with RFA-induced PV stenosis, stenting was found to be 95.8% successful, with significantly improved pressure gradients, vessel caliber, and symptoms [13]. Bare metal stents (which were used in our patient) have been shown to have a lower rate of restenosis than drug eluting stents [14]. In addition to instent stenosis, other complications of PV interventions include PV rupture, stroke, and phrenic nerve injury, which are all exceedingly rare [5]. Our patient did not experience any complications of his PV stenting, and despite an incomplete resolution of the mismatched defects on V/Q scan, he had a dramatic improvement in his exercise capacity. The PH resolved on TTE, and the previously seen filling defect in the left upper lobe pulmonary artery resolved on CT, suggesting that these findings were due to the PV stenosis. The mismatched defect on V/Q scan persisted after the stenting procedure, but the overall perfusion to the left lung improved, which also suggests that the original findings were due to PV stenosis and not a pulmonary embolism.
Conclusions
This patient developed shortness of breath 1 year after a catheter-based RFA procedure to treat AF. He was found to have PH due to PV stenosis. This case is unusual in that the patient presented with symptoms and PH a full year after his procedure, when typically, PV stenosis becomes symptomatic around 3 months after RFA. His symptoms and PH resolved after stenting of the left superior and inferior PV. PV stenosis should be high on the differential diagnosis in a patient with shortness of breath following a catheter-based RFA for AF. In patients with PV stenosis, early recognition can prevent complete occlusion of the affected PV and parenchymal lung damage, and treatment may lead to an improvement in the patient’s symptoms, exercise capacity, and quality of life.
Figures
References:
1.. Pazos-López P, García-Rodríguez C, Guitián-González A, Pulmonary vein stenosis: Etiology, diagnosis and management: World J Cardiol, 2016; 8(1); 81-88
2.. Narayan SM, Krummen DE, Shivkumar K, Treatment of atrial fibrillation by the ablation of localized sources: J Am Coll Cardiol, 2012; 60(7); 628-36
3.. Holmes DR, Monahan KH, Packer D, Pulmonary vein stenosis complicating ablation for atrial fibrillation: JACC Cardiovasc Interv, 2009; 2(4); 267-76
4.. Saad EB, Rossillo A, Saad CP, Pulmonary vein stenosis after radiofrequency ablation of atrial fibrillation: Functional characterization, evolution, and influence of the ablation strategy: Circulation, 2003; 108(25); 3102-7
5.. Raeisi-Giglou P, Wazni OM, Saliba WI, Outcomes and management of patients with severe pulmonary vein stenosis from prior atrial fibrillation ablation: Circ Arrhythm Electrophysiol, 2018; 11(5); e006001
6.. Schoene K, Arya A, Jahnke C, Acquired pulmonary vein stenosis after radiofrequency ablation for atrial fibrillation: JACC Cardiovasc Interv, 2018; 11(16); 1626-32
7.. Packer DL, Keelan P, Munger TM, Clinical presentation, investigation, and management of pulmonary vein stenosis complicating ablation for atrial fibrillation: Circulation, 2005; 111(5); 546-54
8.. Agrawal V, Agrawal V, Congenital pulmonary vein stenosis in an adult patient: J Am Coll Cardiol, 2014; 63(1); 83
9.. Obeid AI, Carlson RJ, Evaluation of pulmonary vein stenosis by transesophageal echocardiography: J Am Soc Echocardiogr, 1995; 8(6); 888-96
10.. Wong KK, Gruenewald SM, Larcos G, Ventilation-perfusion mismatch resulting from iatrogenic pulmonary vein stenosis after radiofrequency ablation: A case report: Clin Cardiol, 2009; 32(11); E67-70
11.. Galizia M, Renapurkar R, Prieto L, Radiologic review of acquired pulmonary vein stenosis in adults: Cardiovasc Diagn Ther, 2018; 8(3); 387-98
12.. Fender EA, Widmer RJ, Hodge DO, Severe pulmonary vein stenosis resulting from ablation for atrial fibrillation: Presentation, management, and clinical outcomes: Circulation, 2016; 134(23); 1812-21
13.. Li Y-J, Pan X, Wang C, He B, Stent implantation for severe pulmonary vein stenosis or occlusion secondary to atrial fibrillation ablation: Int J Cardiol, 2020; 301; 85-89
14.. Fink T, Schlüter M, Heeger CH, Pulmonary vein stenosis or occlusion after catheter ablation of atrial fibrillation: Long-term comparison of drug-eluting versus large bare metal stents: EP Europace, 2018; 20(10); e148-55
Figures
In Press
04 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.941835
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943042
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942578
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943801
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250