08 March 2022: Review Articles
A Review of the Potential Roles of Antioxidant and Anti-Inflammatory Pharmacological Approaches for the Management of Mild-to-Moderate Symptomatic COVID-19
Serafino Fazio1AEF*, Flora Affuso2AEF, Paolo Bellavite3AEFDOI: 10.12659/MSM.936292
Med Sci Monit 2022; 28:e936292
Abstract
ABSTRACT: In the past 2 years, the coronavirus disease 2019 (COVID-19) pandemic has driven investigational studies and controlled clinical trials on antiviral treatments and vaccines that have undergone regulatory approval. Now that the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and its variants may become endemic over time, there remains a need to identify drugs that treat the symptoms of COVID-19 and prevent progression toward severe cases, hospitalization, and death. Understanding the molecular mechanisms of SARS-CoV-2 infection is extremely important for the development of effective therapies against COVID-19. This review outlines the key pathways involved in the host response to SARS-CoV-2 infection and discusses the potential role of antioxidant and anti-inflammatory pharmacological approaches for the management of early mild-to-moderate COVID-19, using the examples of combined indomethacin, low-dose aspirin, omeprazole, hesperidin, quercetin, and vitamin C. The pharmacological targets of these substances are described here for their possible synergism in counteracting SARS-CoV-2 replication and progression of the infection from the upper respiratory airways to the blood, avoiding vascular complications and cytokine and bradykinin storms.
Keywords: Anti-Inflammatory Agents, Non-Steroidal, COVID-19, Flavonoids, Hesperidin, Indomethacin, Quercetin, Anti-Inflammatory Agents, Antioxidants, Antiviral Agents, Endemic Diseases, Host Microbial Interactions, Humans, Pharmacological Phenomena, SARS-CoV-2, COVID-19 Drug Treatment
Background
The severe acute respiratory syndrome, caused by SARS-CoV-2, leads to a wide spectrum of multi-organ pathologies, known as coronavirus disease 2019 (COVID-19). While there is obviously a great deal of pharmacological research going on, no specific definitive drugs are currently available for treating low-to-moderate-risk COVID-19. Patients admitted to hospitals in the most advanced countries receive intensive care, but the problem of lack of specific care mainly concerns the care provided in the early stages of the disease and therefore home care.
The Italian Ministry of Health and the Italian Medicines Agency (AIFA) have issued guidelines for the home management of patients with COVID-19, which follow the most up-to-date acquisitions of evidence-based medicine (https://www.aifa.gov.it/aggiornamento-sui-farmaci-utilizzabili-per-il-trattamento-della-malattia-covid19). Low-risk patients are defined there by the absence of increased risk factors (eg, neoplastic pathologies or immunosuppression) and on the basis of the following characteristics: a) flu-like symptoms (eg, rhinitis, cough without breathing difficulties, myalgia, headache); b) absence of dyspnea and tachypnea (documenting, whenever possible, the presence of an SpO2 >92%); c) fever ≤38°C or >38°C, for less than 72 h; d) gastro-enteric symptoms (in the absence of dehydration and/or multiple diarrheal discharges); e) asthenia, ageusia/dysgeusia/anosmia. In such patients, lacking a widely approved therapeutic strategy and pending evidence-based guidance, the health authorities suggest a “watchful waiting” or “monitoring” of clinical evolution and the use of symptomatic drugs, such as paracetamol or non-steroidal anti-inflammatory agents (NSAIDs), unless there are specific contraindications. However, lack of treatment in the first 72 h or limiting it to symptomatic medications could be risky in many patients, whose disease is destined to progress to more severe forms [1–4]. Indeed, many patients have a significant increase in D-dimer levels, an indicator of thrombosis, which correlates with a worse prognosis [5,6]. It is now well known that the virus, on entering the blood stream, can bind itself to platelet receptors, leading to hyper-aggregation and micro-thrombi [7]. Some patients develop a condition of severe pneumonia, with reduced oxygen saturation often associated with systemic inflammation, activation of intravascular coagulation, thrombosis, and multiple-organ failure [8].
Actually, some therapies have already been approved for early treatment of COVID-19 in “high risk patients”, namely, Remdesivir, Molnupiravir, Paxlovid (Nirmatrelvir and Ritonavir) [9–11], and monoclonal antibodies (Bamlanivimab and Etesevimab, Casirivimab and Imdevimab, and, recently, Bebtelovimab) [12–14]. Among these drugs, only Molnupiravir and Paxlovid can be administered at home, while Remdesivir and monoclonal antibodies have to be administered in a hospital setting. In particular, among the monoclonal antibodies, only Bebtelovimab is specific for the Omicron variant, and Casirivimab is also partially active on this variant. However, according to the aforementioned guidelines and recent updates (https://www.salute.gov.it/portale/nuovocoronavirus/dettaglioComunicatiNuovoCoronavirus.jsp?lingua=italiano&id=5858), home therapies with monoclonal antibodies or with antivirals are indicated only for subjects with COVID-19 of recent onset, who present risk factors for the development of severe forms of the disease.
Paracetamol is an analgesic and antipyretic drug widely used in Italy to reduce fever and pains due to viral diseases. Although this drug is considered very safe, there are 2 aspects that may cast doubt on its usefulness in the case of COVID-19. The first is that it has a strong analgesic and antipyretic power, but has little anti-inflammatory effect. Therefore, intervention aimed solely at lowering the body temperature does not seem useful, or at least is not of primary importance. The second reason that would argue against the use of paracetamol is the fact that it is metabolized also by consumption of glutathione and could worsen oxidative stress [15–17]. This type of biochemical change may lower antiviral protection [18] or worsen the course of the disease, particularly in patients with liver dysfunction [19–21].
Moreover, with regard to the choice of the most suitable anti-inflammatory drugs, among the dozens available at the moment there is no criterion based on randomized studies, although a preliminary network pharmacology and molecular docking study seems to preferentially suggest indomethacin or rofecoxib as candidates for having a better impact, not only on the symptoms but also on the course of the disease [22]. Even regarding the most widely used drugs worldwide, such as ivermectin and hydroxychloroquine, there are diverging opinions and the meta-analyses are not conclusive [23–27].
In our previous study [6] we have shown that prompt treatment with different drugs featuring synergistic action mechanisms, which included anti-inflammatory drugs, acetyl salicylic acid with antiplatelet dosage, omeprazole, and a food supplement recently made available in Italy, comprising hesperidin, quercetin and vitamin C, produced a clear reduction of hospitalizations, symptom duration, and other important outcomes in patients with mild-to-moderate COVID-19.
Our retrospective study [6] confirmed the importance of early treatment by comparing therapy outcomes in 2 cohorts of patients (treated within 72 h vs later), but its aim was not to compare the efficacy of one treatment versus another. However, the study, despite being observational and retrospective, showed that this combination of drugs, used within the first 72 h from the beginning of symptoms, produced no hospitalizations, important reduction of symptom duration, and best outcomes of the disease. The aim of the current paper is, therefore, to more comprehensively review the pharmacological bases of drugs and food supplements which could counteract the main known pathophysiological alterations in this disease. This study could provide the rationale for endorsing a clinical trial comparing this multitherapy approach and other drugs that can be used in the initial phase of COVID-19 disease.
The COVID-19 pandemic has driven investigational studies and controlled clinical trials on antiviral treatments and vaccines that have undergone regulatory approval. However, there remains a need to identify pharmacological approaches to treat symptomatic COVID-19. This review outlines the key pathways involved in host response to SARS-CoV-2 infection and discusses the potential role of antioxidant and anti-inflammatory pharmacological approaches for the management of mild-to-moderate symptomatic COVID-19, such as combined indomethacin, low-dose aspirin, omeprazole, hesperidin, quercetin, and vitamin C. From the complex syndrome of COVID-19, we will consider only the aspects that seem most important as targets for pharmacological regulation as expressed in our multitherapy approach hypothesis.
Characteristics of COVID-19 in Its Earliest Stages
The pharmacological rationale for certain remedies can only be based on the pathophysiology of the disease itself, which we briefly summarize below in its essential stages, and on the well-known action mechanisms of the drugs already widely used for other indications.
When the virus meets the infected person’s mucous membranes, it remains there for a few (3–4) days in a paucisymptomatic phase. If not intercepted by specific IgA, the SARS-CoV-2 virus enters the cells through the binding of the spike glycoproteins (S) to the angiotensin-converting enzyme-2 (ACE2) receptor [28,29], with the involvement of the cellular serine proteases transmembrane serine protease 2 (TMPRSS2) and/or the cathepsin system. The ACE2 receptor, which also has a fundamental enzymatic function in blood pressure homeostasis, is expressed in the lungs and many other tissues of the body, which explains the involvement of many organs and the systemic nature of the disease when the virus spreads [30–35]. Furthermore, the cysteine protease cathepsin L plays a role in the preparation of the spike protein of the virus and the internalization of the virus in the host cells [36].
After internalization, the coding sequences of the viral RNA are translated into a polyprotein, 1ab, which then undergoes a proteolytic process to form a series of non-structural proteins and, eventually, a replication complex. The main enzyme that performs this proteolytic transformation is the 3-chymotrypsin-like protease (3Clpro), or main protease (Mpro), which is the major target of antiviral drugs. Once viruses have entered the cells and started the replication process, they multiply exponentially, causing cell, tissue, and organ damage, which, together with the body’s inflammatory reactions, generates a broad range of local and systemic symptoms.
Since SARS-CoV-2 initially infects the upper respiratory tract, mucosal immunity and secretory IgA appear to be crucial in the local immune response and in preventing the spread of the virus into the host organism. However, time is needed for the immune response to develop and the mucous membranes and salivary glands need to be preserved as much as possible from virus damage. The oral cavity is an important reservoir of SARS-CoV-2, and saliva is involved in viral transmission [37,38]. Available data indicate that the oral cavity is an active site of infection [39]. A growing body of evidence suggests that patients with COVID-19 experience various oral health problems such as dry mouth, blisters on mucous membranes, rashes, necrosis of the lips, and loss of taste and smell [40]. Furthermore, interactions between oral, pulmonary, and intestinal microbes appear to occur dynamically, so a dysbiotic oral microbial community could influence respiratory and gastrointestinal diseases [39].
Viral infections also cause an increase of reactive oxygen species (ROS) production, which is revealed by a decrease in the antioxidant capacity of biological fluids and an increased presence in the serum of derivatives from the oxidation of lipids and proteins [41]. Oxidative stress has been reported in hepatitis B [42], hepatitis C [43], influenza [44], and SARS-CoV-2 [16,45,46] infections. The phenomenon is particularly worrying when it occurs in elderly people, who already have a reduced antioxidant capacity [47,48], which could reduce the efficiency of the immune system [49] and modify the expression of the membrane receptors [50,51]. Indeed, severity and risk of mortality from COVID-19 have been associated with age [48]. This mechanism could be involved in the receptor interaction because the oxidation of SH residues of proteins can increase the affinity of the spike protein for ACE2 and thus facilitate infection [50]. A clinical study has confirmed the existence of oxidative stress, reporting that subjects with COVID-19 at an early stage had lower plasma free thiol concentrations than in healthy subjects [52]. A decrease of glutathione would lead to a decrease of antioxidant defenses and favor the entry of viruses into the cells [15,50]. The use of antioxidant therapies based on natural substances, supplements, and vitamins has also been proposed in an extensive review by Fratta Pasini et al [53].
As the virus spreads through the body, with its cytotoxic effects, a systemic reaction develops, which is responsible for most clinical symptoms. Excess immune and inflammatory reactions can lead to a cytokine storm, multiple-organ failure, coagulation disorders, and eventually to autoimmune phenomena [54–57]. Inflammation seems to be involved in viral replication in cytomegalovirus (HCMV) infection: PGE2 stimulates the activity of the major immediate early promoter that controls the synthesis of viral regulatory proteins, which are essential for HCMV replication [58,59]. IL1beta and PGE2 significantly increase the expression of ACE2 and TMPRSS2 in gingival fibroblasts, thus facilitating virus entry [60]. Histamine released by mast cells, by increasing IL-1 production, can also amplify the inflammatory process in lung infected with SARS-CoV-2 [61]. The dual role of inflammation, both defensive and facilitating infection [62], makes the choice of anti-inflammatory drug type a particularly delicate one, possibly explaining the discrepancies in results in different patients and at different stages of the disease.
A particularly important aspect of circulatory pathophysiology concerns the renin-angiotensin system (RAS), which is responsible for controlling blood pressure and hydro-electrolyte balances, as well as certain inflammatory and coagulation mechanisms. The relationship among the virus, the immune system, and the renin-angiotensin system is a very complex one. Physiologically, ACE2 plays a role in regulating these 3 systems that could potentially be involved in the pathogenesis of COVID-19: the renin-angiotensin system, promoting cardiovascular instability; the coagulation system, leading to thromboembolism; and the kinin-kallikrein system, resulting in acute inflammatory pulmonary edema [63–68]. Angiotensin II-mediated activation of ATR1 receptors can, in turn, reinforce prolonged inflammation in the lungs [69]. Furthermore, the physiological clearance of bradykinin by ACE2 is missing and bradykinin-mediated inflammation likely precipitates respiratory and circulatory complications [70] until vicious cycles are established in the form of a so-called “bradykinin storm” [71–73].
Prescribed Drugs and Food Supplements
Based on the above, it appears that a rational approach to COVID-19 must be based on the use of various synergistic remedies, in order to best “cover” the greatest number of pathophysiological, immunological, and biochemical disorders involved in the disease: blocking or delaying virus replication, regulating inflammatory reactions, reducing the risk of thromboembolic complications, and limiting the potentially harmful oxidative stress. It is not the purpose of this paper to systematically review all the pharmacological proposals made to date for the treatment of COVID-19, so we will focus on the substances we used in a recent cohort study [6]. The suggested drugs and food supplements, supposedly endowed with the above-mentioned features, are the following: a) Indomethacin as a NSAID with additional antiviral activity; b) aspirin at low doses, to prevent platelet aggregation; c) omeprazole as a gastric protector; and d) hesperidin, quercetin, and vitamin C with antioxidant, anti-inflammatory, and possible antiviral activity. Below, we briefly describe the pharmacological properties of the substances used in relation to their potential and/or supposed effects on COVID-19 disease progression.
Indomethacin as a Promising NSAID
While inflammation is normally seen as a defensive phenomenon, in COVID-19 it is likely to play a pathogenic role even in the early stages, helping the virus in its replication rather than fighting it. Therefore, intervening with anti-inflammatory agents in the early stages could be a very reasonable therapeutic option.
The importance of early treatment with non-steroidal anti-inflammatory drugs (NSAIDs) in the treatment of patients with COVID-19 has been suggested by several authors [74–76]. Although no conclusive evidence is available for or against the use of NSAIDs, observational studies suggest that the use of selective COX-2 inhibitors, together with other drugs, can reduce the frequency of hospitalization, although it does not reduce the duration of symptoms [3]. In particular, indomethacin is an old and inexpensive anti-inflammatory drug used for headache and arthritis [77,78], and it can combat cough [79,80], which is a major cause of the spread of infection.
Moreover, this drug also has other interesting properties. A recent network pharmacology approach identified 3 target proteins associated with the renin-angiotensin system imbalance caused by SARS-CoV-2 (MAPK8, MAPK10, and BAD) and showed that indomethacin can reduce excessive inflammation by inactivating target proteins [22]. It has also been hypothesized that indomethacin can counteract the proinflammatory effects of bradykinin, thus reducing the COVID-19-induced symptoms induced by bradykinin network activation, such as dry cough and musculoskeletal pains [80]. It has also been shown that indomethacin together with resveratrol can improve the disease [81]. Indomethacin also reduces the levels of interleukin-6 and tumor necrosis factor alpha, which, by increasing during the disease, lead to some of its detrimental consequences [82].
The particular interest of indomethacin, among the multiple NSAIDs available, is related to the fact that this drug has direct antiviral properties against several viruses, including cytomegalovirus, herpes virus 6, and hepatitis B virus [59,83,84], SARS-COV-1 [85], and, recently, SARS-CoV-2, without cytotoxic effects [86,87]. According to Amici [88], in a vesicular stomatitis infection model, indomethacin activated PKR (double-stranded RNA-dependent protein kinase), resulting in the phosphorylation of elF2α and, in turn, interrupting the translation of the viral protein and inhibiting viral replication.
Molecular docking studies have suggested that indomethacin is a potential main protease antagonist of SARS-COV-2 [74] and is able to downregulate genes of interest for virus fusion (ACE2 and TMPRSS2), as well as other genes involved in the same pathways [89]. Direct evidence for the antiviral efficacy of indomethacin, but not of aspirin, against SARS-CoV-2 was provided in cellular models and in vivo in an infected canine model [90], suggesting that the effect is independent of anti-inflammatory action. A very recent review has shown, with system biology-based modeling, that the action of indomethacin against SARS-CoV-2 depends on the fact that prostaglandin synthetase is related to the action of some structural proteins, so that the inhibition of the former blocks the function of the latter, which are involved in the replication of viral RNA [91].
Finally, another peculiar aspect of the action of indomethacin is that it reduces bradykinin, which is linked to RAS imbalance and is responsible for important symptoms such as generalized pain, myalgia, and dry cough [80]. The specific involvement of bradykinin metabolism in the disease also depends on the fact that the enzyme ACE2 is involved in the degradation of bradykinin [92,93], and the acute phases of the inflammatory process in COVID-19 has been also defined as “bradykinin storm” [71–73,94]. Some studies have also shown that indomethacin has a favorable immunomodulatory role in the treatment of COVID-19 [86,95,96].
Low-Dose Aspirin
Low-dose aspirin has been widely used as an antithrombotic agent. Its use in SARS-CoV-2 infection could help reduce or dampen platelet hyper-aggregation, which may be caused by the binding of the virus spike to platelet ACE2 receptors, and it could be used to prevent thrombosis during the early phase of COVID-19 [97,98].
According to Rizk et al [99], aspirin would be indicated for early-stage COVID-19 because it interrupts a vicious cycle between platelet aggregation caused by the virus spike, activation and degranulation of neutrophils, coagulation, and immune-thrombosis. Others have also proposed a solid treatment option with aspirin – in addition to indomethacin, diclofenac, and celecoxib – to deactivate the inflammasome and modulate the overproduction of proinflammatory cytokines [76].
A Gastric Protector with Possible Antiviral Action: Omeprazole
The use of a gastric protector could be considered to prevent possible gastrointestinal damage due to NSAIDs [100,101]. Among these, omeprazole was chosen because, in addition to its known ability to protect against gastro-duodenal lesions caused by NSAIDs, it was proposed as a molecule capable of inhibiting the Mpro of SARS-CoV-2 by binding to its C-terminal domain [102].
Another interesting work that could support the rationale for the use of omeprazole in COVID-19 concerns the inhibition of SARS-CoV-2 internalization through ACE2 [103]. In contrast to this evidence, in renal cells omeprazole increased the expression of ACE2, but not TMPRSS2 [104]. How much the different effects of omeprazole on different cells are involved in the clinical and pharmacological treatment of COVID-19 should be the subject of further studies.
Flavonoids and Vitamin C
Taking into account the importance of oxidative stress in the dynamics of viral diseases and in particular in SARS-CoV-2 infection [105], it is proposed to include in the therapeutic scheme a food supplement based on flavonoids and vitamin C. These substances are endowed with antioxidant power and could also play a regulatory role in thrombotic mechanisms [45,48,106–108].
Hesperidin is the glycosidic flavonoid most common in citrus fruits and exerts its action by enhancing cellular antioxidant defenses through the ERK/Nrf2 pathway [109]. In addition, hesperidin has a mild anti-inflammatory action by suppressing the production of cytokines by inhibiting the activation of the NF-κB signaling cascade [110]. Hesperidin can reduce replication of the influenza virus [111,112] and the SARS virus [113]. Several lines of research have allowed us to repurpose hesperidin as a suitable candidate for blocking the interaction of SARS-CoV-2 with ACE2 receptors [114–118] or for inhibiting its replication [119–121]. Through its anti-inflammatory activity, hesperidin inhibits the secretion of proinflammatory cytokines such as INF-gamma and IL-2, thus reducing the possibility of a cytokine storm [110,122–125]. The high safety of hesperidin after oral intake has been declared by FASEB (Federation of American Societies of Experimental Biology), at the request of the FDA [110], and has been confirmed by animal [125,126] and clinical [127] toxicity studies.
Quercetin is a carbohydrate-free flavonoid that is most abundant in vegetables and fruits and is the most-studied phytochemical when it comes to assessing the biological effects of flavonoids [128,129]. Quercetin also acts as a free radical scavenger, donating 2 electrons to oxidized species which are reduced. Colunga Biancatelli et al argued that this antioxidant activity of quercetin could be exploited in the treatment of COVID-19 in synergy with vitamin C [130]. In fact, vitamin C has the ability to protect the flavonol molecule, recycling its oxidized quinonic form after the scavenger action on free radicals. As its action mechanisms, quercetin directly inhibits the coronavirus major protease [131,132] and the inflammasome NLR family pyrin domain containing 3 (NLRP3) in macrophages [133]. Interestingly, a molecular docking study showed that hesperidin and quercetin interact with the SARS-CoV-2 enzyme Mpro on different amino acids of its activation site, suggesting that they have synergistic antiviral actions [134].
A prospective randomized controlled open-label study suggested quercetin supplementation at an early stage of COVID-19. The results of this study have shown a reduction in the frequency and duration of hospitalizations, the need for non-invasive oxygen therapy, progression to intensive care units, and the number of deaths [135]. A further study has shown that quercetin, in the bioavailable form of phytosome, resulted in a greater likelihood of early benign disease resolution in subjects with COVID-19 [136].
It is also interesting that, in mouse models, quercetin has a protective effect against gastric [137] and intestinal epithelial injury [138] induced by high doses of indomethacin, suggesting that in our proposed multitherapy, quercetin may have a further protective effect, besides omeprazole.
The Combined Therapy
The main aim of a therapy in the early stage of the disease is to prevent spread of the virus and to reduce the risk of excessively pathological reactions. A reduction of morbidity and mortality for COVID-19 has been described with a prompt intervention based on the use of different associations of drugs, as compared with no treatment [2] or delayed treatment [6].
In theory, the ideal therapy should include drugs that block the virus on the mucous membranes, preventing it from entering the bloodstream and spreading to vital organs for long enough to allow the affected person to form enough antibodies, particularly mucosal secretory IgA, to control and render the virus harmless. The first humoral responses to SARS-CoV-2 are dominated by IgA antibodies, which appear to have greater neutralizing power than IgG. After natural infection, secretory and neutralizing IgA are found in the saliva for a longer time, at least 2–3 months after the symptoms [139], but immune responses to vaccination do not develop an adequate mucosal IgA response [140]. Furthermore, it is known that the dimeric form of IgA, which is found in response to the virus in all mucosal secretions, in both the respiratory and intestinal tracts, is more potent against SARS-CoV-2 than both the monomeric form of IgG and monomeric IgA [141,142]. Furthermore, it is possible that mucosal immunity could be exploited for beneficial diagnostic, therapeutic, or prophylactic purposes [143].
To this end, drugs with antiviral action should be used at the first symptoms of the disease, which, by interfering with the virus binding to the host’s cellular ACE2 receptors, prevent further invasion of the blood stream and vital organs, thus giving the infected person time to form sufficient neutralizing levels of secretory IgA. Nutraceutical compounds, such as flavonoids, which dissolve slowly in the oral cavity, could be of considerable importance in protecting the mucous membranes of the salivary glands and tonsils, which are the primary lymphatic organs involved in defending against infection [144–146]. The defense could be direct, by preventing the virus binding to abundant receptors in the oral mucosa, but also indirect, because flavonoids could protect the mucosal epithelial cells [147] and periodontal tissue [148–151] from oxidative stress and thus promote phagocytosis and secretory IgA formation (epithelial function).
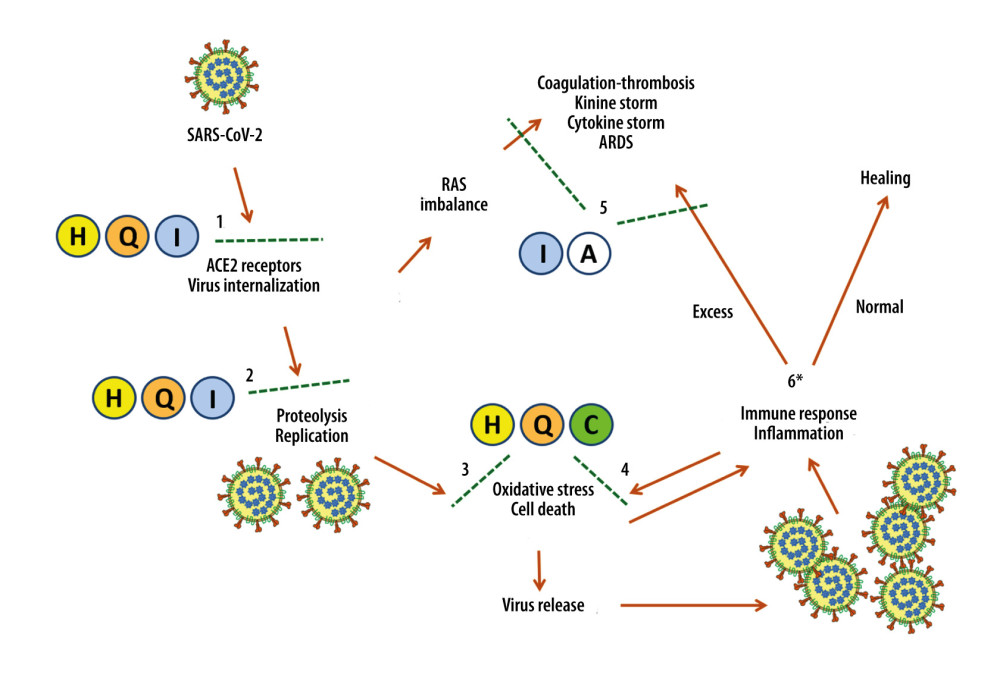
Based on these considerations and our results, the main substances used in this study appear to have synergistic antiviral actions when administered early at the onset of symptoms (Figure 1, steps no. 1–2) [74,81,87,120,152–155]. In addition, hesperidin, quercetin, and vitamin C counter oxidative stress, namely the SARS-COV-2 cytotoxic effects (Figure 1, step no. 3) [45,46,115,130,156–160], and the action of phagocytes activated NADPH oxidase (Figure 1, step no. 4) [161,162]. Finally, the known anti-inflammatory properties of indomethacin and aspirin may help prevent complications associated with excessive inflammatory and immune reactions (Figure 1, step no. 5).
Figure 1 also shows a “bifurcation” point (asterisk at step no. 6), which is very important in determining the evolution of the disease – whether the patient is heading toward recovery or is likely to have clinically serious complications.
A multi-drug approach is also crucial to limit the possibility of developing strains resistant to a single drug that may have shown great efficacy in early applications. The same strategy led to the victory against HIV [163]. Our approach involves the use of indomethacin and 2 flavonoids that act both on the spike protein/ACE2 receptor binding and on the major protease that is a key step in viral replication.
Perspectives
The evidence presented in this review could help design suitable clinical trials for the therapy of early stages of COVID-19. Any antiviral will need to be administered as soon as possible at the onset of symptoms, because once the virus has entered the bloodstream in significant quantities, it will rapidly initiate platelet micro-thrombosis, with subsequent worsening, respiratory failure, and, in some cases, the triggering of an excessive immune response and cytokine storm. Therefore, to verify the efficacy of multi-drug approaches, it will not suffice to examine the efficacy of the individual components, but it will be necessary to carry out comparative studies between different formulations applied to patients at comparable stages of disease and baseline characteristics. To do this, it would be useful to carry out studies in which the basic combination therapy could be modified by the addition of a new component or the subtraction of any of the existing ones. A model of this approach was followed by Ravichandran et al [164], who formed 2 groups given the same multitherapy and differing only in that one was given paracetamol and the other indomethacin. Ideally, this type of comparative, or “non-inferiority”, research between different treatment models could be carried out in randomized trials, although the organizational difficulties would be considerable and double blinding seems objectively difficult to achieve.
Another aspect is the pharmacological convenience of combating a viral disease with a wide range of active ingredients targeting various mechanisms of virus infectivity and possibly acting synergistically. Recently, Suter et al showed that early anti-inflammatory treatment of outpatients with mild-to-moderate COVID-19 could reduce the risk of hospitalization and related costs by 90%, although it did not achieve a faster recovery from symptoms [3], probably due to the lack of an antiviral and antioxidant component, or the use of NSAIDs with suboptimal antiviral defense capabilities.
It would certainly be useful to carry out robust prospective randomized studies to confirm the results of our preliminary research, carried out with a retrospective observational design [6]. The importance of identifying drug combinations for multiple targets, addressing different stages of COVID-19 and different pathophysiological changes, has also been emphasized by others [165]. This approach envisages that, from an experimental point of view, multicenter studies can be initiated on large numbers of patients treated with different combinations of drugs and supplements, but following a common protocol of recruitment, data collection, and analysis, to compare the different options in the field by means of multivariate analysis. In such a complex viral disease, which is likely to be progressive, and in the absence of an effective and decisive drug, there are obviously several therapeutic proposals, which mostly consist of various drug combinations. It is essential that the results of the various approaches can be compared, so that the most suitable choices for each stage of the disease can be progressively specified and clarified on the basis of hard evidence.
The critical point of disease progress described in Figure 1 (step no. 6) is influenced by the subject’s general state of health, and hence by age and the possible presence of unfavorable genetic factors, comorbidities, or metabolic disorders, such as diabetes, hypertension, or dyslipidemia. Therefore, these potentially confounding factors should be controlled in the protocol of any comparative multicenter study of different treatment approaches. Equally important will be to take note of the delay of initiation of therapy compared to the onset of symptoms, as this factor seems crucial in determining outcome [2–4,6].
Our model of treatment does not present a “magic formula”, but a working hypothesis based on common and proven safe drugs, although obviously the specific contraindications of each of the substances mentioned here must be taken into account. Moreover, it should be stressed that we present a basic scheme and deliberately do not deal with clinical complications and hospital treatment, which require additional drugs.
Conclusions
While waiting for a resolving drug, the complexity of the dynamic alterations of the COVID-19 disease, particularly the double role (defensive and offensive) of the immunological and inflammatory mechanisms, requires a multitherapy approach that affects the different targets. Indomethacin, aspirin, hesperidin, quercetin, vitamin C, and omeprazole are presented as a set of safe and inexpensive substances with synergistic effects on the early stages of infection, viral replication, cytotoxicity, oxidative stress, and excess inflammation. This approach could be compared, through controlled clinical trials, with other combinations of potentially useful drugs.
References
1. McCullough PA, Vijay K, SARS-CoV-2 infection and the COVID-19 pandemic: A call to action for therapy and interventions to resolve the crisis of hospitalization, death, and handle the aftermath: Rev Cardiovasc Med, 2021; 22(1); 9-10
2. Alexander PE, Armstrong R, Fareed G, Early multidrug treatment of SARS-CoV-2 infection (COVID-19) and reduced mortality among nursing home (or outpatient/ambulatory) residents: Med Hypotheses, 2021; 153; 110622
3. Suter F, Consolaro E, Pedroni S, A simple, home-therapy algorithm to prevent hospitalisation for COVID-19 patients: A retrospective observational matched-cohort study: EClinical Medicine, 2021; 2021; 100941
4. Consolaro E, Suter F, Rubis N, A home-treatment algorithm based on anti-inflammatory drugs to prevent hospitalization of patients with early COVID-19: A matched-cohort study (Cover 2): MedRxiv, 2021; 2021; 21264298
5. Fazio S, Tufano A, de Simone G, Sustained high D-dimer in outpatients who have recovered from mild to moderate coronavirus disease 2019 (COVID-19): Semin Thromb Hemost, 2022; 48(1); 115-17
6. Fazio S, Bellavite P, Zanolin E, Retrospective study of outcomes and hospitalization rates of patients in italy with a confirmed diagnosis of early COVID-19 and treated at home within 3 days or after 3 days of symptom onset with prescribed and non-prescribed treatments between November 2020 and August 2021: Med Sci Monit, 2021; 27; e935379
7. Bellavite P, Renin-angiotensin system, SARS-CoV-2 and hypotheses about adverse effects following vaccination: EC Pharmacology and Toxicology, 2021; 9(4); 1-10
8. Bonaventura A, Vecchie A, Dagna L, Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19: Nat Rev Immunol, 2021; 21(5); 319-29
9. Rommasi F, Nasiri MJ, Mirsaiedi M, Antiviral drugs proposed for COVID-19: Action mechanism and pharmacological data: Eur Rev Med Pharmacol Sci, 2021; 25(11); 4163-73
10. Vegivinti CTR, Evanson KW, Lyons H, Efficacy of antiviral therapies for COVID-19: A systematic review of randomized controlled trials: BMC Infect Dis, 2022; 22(1); 107
11. Okoli GN, Rabbani R, Al-Juboori A, Antiviral drugs for coronavirus disease 2019 (COVID-19): A systematic review with network meta-analysis: Expert Rev Anti Infect Ther, 2022; 20(2); 267-78
12. Kreuzberger N, Hirsch C, Chai KL, SARS-CoV-2-neutralising monoclonal antibodies for treatment of COVID-19: Cochrane Database Syst Rev, 2021; 9; CD013825
13. Kow CS, Ramachandram DS, Hasan SS, The use of neutralizing monoclonal antibodies and risk of hospital admission and mortality in patients with COVID-19: A systematic review and meta-analysis of randomized trials: Immunopharmacol Immunotoxicol, 2022; 44(1); 28-34
14. Lin WT, Hung SH, Lai CC, The impact of neutralizing monoclonal antibodies on the outcomes of COVID-19 outpatients: A systematic review and meta-analysis of randomized controlled trials: J Med Virol, 2022 [Online ahead of print]
15. Sestili P, Fimognari C, Paracetamol-induced glutathione consumption: Is there a link with severe COVID-19 illness?: Front Pharmacol, 2020; 11; 579944
16. Silvagno F, Vernone A, Pescarmona GP, The role of glutathione in protecting against the severe inflammatory response triggered by COVID-19: Antioxidants (Basel), 2020; 9(7); 624
17. Pergolizzi JV, Varrassi G, Magnusson P, COVID-19 and NSAIDS: A narrative review of knowns and unknowns: Pain Ther, 2020; 9(2); 353-58
18. Dattilo M, The role of host defences in COVID-19 and treatments thereof: Mol Med, 2020; 26(1); 90
19. Bertolini A, van de Peppel IP, Bodewes FAJA, Abnormal liver function tests in patients with COVID-19: Relevance and potential pathogenesis: Hepatology, 2020; 72(5); 1864-72
20. Piano S, Dalbeni A, Vettore E, Abnormal liver function tests predict transfer to intensive care unit and death in COVID-19: Liver Int, 2020; 40(10); 2394-406
21. Marin-Duenas I, Vega J, Carrillo-Ng H, Alteration in liver function tests among patients hospitalized for COVID-19: A multicentric study in Peru: Rev Gastroenterol Peru, 2021; 41(2); 86-93
22. Oh KK, Adnan M, Cho DH, Network pharmacology approach to decipher signaling pathways associated with target proteins of NSAIDs against COVID-19: Sci Rep, 2021; 11(1); 9606
23. Scotto Di Vetta M, Morrone M, Fazio S, COVID-19: Off-label therapies based on mechanism of action while waiting for evidence-based medicine recommendations: World J Meta-Anal, 2020; 8(3); 173-77
24. Ayipo YO, Yahaya SN, Alananzeh WA, Pathomechanisms, therapeutic targets and potent inhibitors of some beta-coronaviruses from bench-to-bedside: Infect Genet Evol, 2021; 93; 104944
25. Hill A, Garratt A, Levi J, Meta-analysis of randomized trials of ivermectin to treat SARS-CoV-2 infection: Open Forum Infect Dis, 2021; 8(11); ofab358
26. Cruciani M, Pati I, Masiello F, Ivermectin for prophylaxis and treatment of COVID-19: a systematic review and meta-analysis: Diagnostics (Basel), 2021; 11(9); 1645
27. Amani B, Khanijahani A, Amani B, Hydroxychloroquine plus standard of care compared with standard of care alone in COVID-19: A meta-analysis of randomized controlled trials: Sci Rep, 2021; 11(1); 11974
28. Wang Q, Zhang Y, Wu L, Structural and functional basis of SARS-CoV-2 entry by using human ACE2: Cell, 2020; 181(4); 894-904
29. Lan J, Ge J, Yu J, Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor: Nature, 2020; 581(7807); 215-20
30. Ni W, Yang X, Yang D, Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19: Crit Care, 2020; 24(1); 422
31. Gupta A, Madhavan MV, Sehgal K, Extrapulmonary manifestations of COVID-19: Nat Med, 2020; 26(7); 1017-32
32. Xu H, Zhong L, Deng J, High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa: Int J Oral Sci, 2020; 12(1); 8
33. Nuzzo D, Picone P, Potential neurological effects of severe COVID-19 infection: Neurosci Res, 2020; 158; 1-5
34. Watzky M, de DM, Letessier A, Saint-Ruf C, Miotto B, Assessing the consequences of environmental exposures on the expression of the human receptor and proteases involved in SARS-CoV-2 cell-entry: Environ Res, 2020; 195; 110317
35. Lamers MM, Beumer J, van d, SARS-CoV-2 productively infects human gut enterocytes: Science, 2020; 369(6499); 50-54
36. Zhao MM, Yang WL, Yang FY, Cathepsin L plays a key role in SARS-CoV-2 infection in humans and humanized mice and is a promising target for new drug development: Signal Transduct Target Ther, 2021; 6(1); 134
37. Huang N, Perez P, Kato T, Integrated single-cell atlases reveal an oral SARS-CoV-2 infection and transmission axis: medRxiv, 2020; 2020; 20219089
38. Herrera D, Serrano J, Roldan S, Sanz M, Is the oral cavity relevant in SARS-CoV-2 pandemic?: Clin Oral Investig, 2020; 24(8); 2925-30
39. Xiang Z, Koo H, Chen Q, Potential implications of SARS-CoV-2 oral infection in the host microbiota: J Oral Microbiol, 2020; 13(1); 1853451
40. Coke CJ, Davison B, Fields N, SARS-CoV-2 infection and oral health: Therapeutic opportunities and challenges: J Clin Med, 2021; 10(1); 156
41. Khomich OA, Kochetkov SN, Bartosch B, Ivanov AV, Redox biology of respiratory viral infections: Viruses, 2018; 10(8); 392
42. Zhang X, Wu X, Hu Q, Mitochondrial DNA in liver inflammation and oxidative stress: Life Sci, 2019; 236; 116464
43. Bhargava A, Raghuram GV, Pathak N, Occult hepatitis C virus elicits mitochondrial oxidative stress in lymphocytes and triggers PI3-kinase-mediated DNA damage response: Free Radic Biol Med, 2011; 51(9); 1806-14
44. Kido H, Indalao IL, Kim H, Energy metabolic disorder is a major risk factor in severe influenza virus infection: Proposals for new therapeutic options based on animal model experiments: Respir Investig, 2016; 54(5); 312-19
45. Saleh J, Peyssonnaux C, Singh KK, Edeas M, Mitochondria and microbiota dysfunction in COVID-19 pathogenesis: Mitochondrion, 2020; 54; 1-7
46. Suhail S, Zajac J, Fossum C, Role of oxidative stress on SARS-CoV (SARS) and SARS-CoV-2 (COVID-19) infection: A review: Protein J, 2020; 39(6); 644-56
47. Keles ES, Mild SARS-CoV-2 infections in children might be based on evolutionary biology and linked with host reactive oxidative stress and antioxidant capabilities: New Microbes New Infect, 2020; 36; 100723
48. Delgado-Roche L, Mesta F, Oxidative stress as key player in severe acute respiratory syndrome coronavirus (SARS-CoV) infection: Arch Med Res, 2020; 51(5); 384-87
49. Trujillo JA, Croft NP, Dudek NL, The cellular redox environment alters antigen presentation: J Biol Chem, 2014; 289(40); 27979-91
50. Hati S, Bhattacharyya S, Impact of thiol-disulfide balance on the binding of COVID-19 spike protein with angiotensin-converting enzyme 2 receptor: ACS Omega, 2020; 5(26); 16292-98
51. Dalan R, Bornstein SR, El-Armouche A, The ACE-2 in COVID-19: Foe or Friend?: Horm Metab Res, 2020; 52(5); 257-63
52. van Eijk LE, Tami A, Hillebrands JL, Mild coronavirus disease 2019 (COVID-19) is marked by systemic oxidative stress: A pilot study: Antioxidants (Basel), 2021; 10(12); 2022
53. Fratta Pasini AM, Stranieri C, Cominacini L, Mozzini C, Potential role of antioxidant and anti-inflammatory therapies to prevent severe SARS-Cov-2 complications: Antioxidants (Basel), 2021; 10(2); 272
54. Cavalli E, Bramanti A, Ciurleo R, Entangling COVID-19 associated thrombosis into a secondary antiphospholipid antibody syndrome: Diagnostic and therapeutic perspectives (Review): Int J Mol Med, 2020; 46(3); 903-12
55. Lyons-Weiler J, Pathogenic priming likely contributes to serious and critical illness and mortality in COVID-19 via autoimmunity: J Transl Autoimmun, 2020; 3; 100051
56. Ehrenfeld M, Tincani A, Andreoli L, Covid-19 and autoimmunity: Autoimmun Rev, 2020; 19(8); 102597
57. Shoenfeld Y, Corona (COVID-19) time musings: Our involvement in COVID-19 pathogenesis, diagnosis, treatment and vaccine planning: Autoimmun Rev, 2020; 19(6); 102538
58. Zhu H, Cong JP, Yu D, Inhibition of cyclooxygenase 2 blocks human cytomegalovirus replication: Proc Natl Acad Sci USA, 2002; 99(6); 3932-37
59. Schroer J, Shenk T, Inhibition of cyclooxygenase activity blocks cell-to-cell spread of human cytomegalovirus: Proc Natl Acad Sci USA, 2008; 105(49); 19468-73
60. Sena K, Furue K, Setoguchi F, Noguchi K, Altered expression of SARS-CoV-2 entry and processing genes by Porphyromonas gingivalis-derived lipopolysaccharide, inflammatory cytokines and prostaglandin E2 in human gingival fibroblasts: Arch Oral Biol, 2021; 129; 105201
61. Conti P, Caraffa A, Tete G, Mast cells activated by SARS-CoV-2 release histamine which increases IL-1 levels causing cytokine storm and inflammatory reaction in COVID-19: J Biol Regul Homeost Agents, 2020; 34(5); 1629-32
62. Chen JS, Alfajaro MM, Chow RD, Non-steroidal anti-inflammatory drugs dampen the cytokine and antibody response to SARS-CoV-2 infection: J Virol, 2021; 95(7); e00014-21
63. Oz M, Lorke DE, Multifunctional angiotensin converting enzyme 2, the SARS-CoV-2 entry receptor, and critical appraisal of its role in acute lung injury: Biomed Pharmacother, 2021; 136; 111193
64. Sidarta-Oliveira D, Jara CP, Ferruzzi AJ, SARS-CoV-2 receptor is co-expressed with elements of the kinin-kallikrein, renin-angiotensin and coagulation systems in alveolar cells: Sci Rep, 2020; 10(1); 19522
65. Zhang S, Liu Y, Wang X, SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19: J Hematol Oncol, 2020; 13(1); 120
66. Bernard I, Limonta D, Mahal LK, Hobman TC, Endothelium infection and dysregulation by SARS-CoV-2: Evidence and caveats in COVID-19: Viruses, 2020; 13(1); 29
67. Lu J, Sun PD, High affinity binding of SARS-CoV-2 spike protein enhances ACE2 carboxypeptidase activity: J Biol Chem, 2020; 295(52); 18579-88
68. Rahman MM, Hasan M, Ahmed A, Potential detrimental role of soluble ACE2 in severe COVID-19 comorbid patients: Rev Med Virol, 2021; 31(5); 1-12
69. Rojas A, Gonzalez I, Morales MA, SARS-CoV-2-mediated inflammatory response in lungs: Should we look at RAGE?: Inflamm Res, 2020; 69(7); 641-43
70. Roche JA, Roche R, A hypothesized role for dysregulated bradykinin signaling in COVID-19 respiratory complications: FASEB J, 2020; 34(6); 7265-69
71. Polidoro RB, Hagan RS, de Santis SR, Schmidt NW, Overview: Systemic inflammatory response derived from lung injury caused by SARS-CoV-2 infection explains severe outcomes in COVID-19: Front Immunol, 2020; 11; 1626
72. Matsuishi Y, Mathis BJ, Shimojo N, Severe COVID-19 infection associated with endothelial dysfunction induces multiple organ dysfunction: A review of therapeutic interventions: Biomedicines, 2021; 9(3); 279
73. Karamyan VT, Between two storms, vasoactive peptides or bradykinin underlie severity of COVID-19?: Physiol Rep, 2021; 9(5); e14796
74. Abo Elmaaty A, Hamed MIA, Ismail MI, Computational insights on the potential of some NSAIDs for treating COVID-19: Priority set and lead optimization: Molecules, 2021; 26(12); 3772
75. Cabbab ILN, Manalo RVM, Anti-inflammatory drugs and the renin-angiotensin-aldosterone system: Current knowledge and potential effects on early SARS-CoV-2 infection: Virus Res, 2021; 291; 198190
76. Prasher P, Sharma M, Gunupuru R, Targeting cyclooxygenase enzyme for the adjuvant COVID-19 therapy: Drug Dev Res, 2021; 82(4); 469-73
77. Lucas S, The pharmacology of indomethacin: Headache, 2016; 56(2); 436-46
78. Xu L, Liu S, Guan M, Xue Y, Comparison of prednisolone, etoricoxib, and indomethacin in treatment of acute gouty arthritis: An open-label, randomized, controlled trial: Med Sci Monit, 2016; 22; 810-17
79. Fogari R, Zoppi A, Tettamanti F, Effects of nifedipine and indomethacin on cough induced by angiotensin-converting enzyme inhibitors: A double-blind, randomized, cross-over study: J Cardiovasc Pharmacol, 1992; 19(5); 670-73
80. Alkotaji M, Al-Zidan RN, Indomethacin: Can it counteract bradykinin effects in COVID-19 patients?: Curr Pharmacol Rep, 2021; 7(3); 102-6
81. Marinella MA, Indomethacin and resveratrol as potential treatment adjuncts for SARS-CoV-2/COVID-19: Int J Clin Pract, 2020; 2020; e13535
82. Russell B, Moss C, George G, Associations between immune-suppressive and stimulating drugs and novel COVID-19 – a systematic review of current evidence: Ecancermedicalscience, 2020; 14; 1022
83. Reynolds AE, Enquist LW, Biological interactions between herpesviruses and cyclooxygenase enzymes: Rev Med Virol, 2006; 16(6); 393-403
84. Bahrami H, Daryani NE, Haghpanah B, Effects of indomethacin on viral replication markers in asymptomatic carriers of hepatitis B: A randomized, placebo-controlled trial: Am J Gastroenterol, 2005; 100(4); 856-61
85. Amici C, Di CA, Ciucci A, Indomethacin has a potent antiviral activity against SARS coronavirus: Antivir Ther, 2006; 11(8); 1021-30
86. Gomeni R, Xu T, Gao X, Bressolle-Gomeni F, Model based approach for estimating the dosage regimen of indomethacin a potential antiviral treatment of patients infected with SARS CoV-2: J Pharmacokinet Pharmacodyn, 2020; 47(3); 189-98
87. Kiani P, Scholey A, Dahl TA, In vitro assessment of the antiviral activity of ketotifen, indomethacin and naproxen, alone and in combination, against SARS-CoV-2: Viruses, 2021; 13(4); 558
88. Amici C, La Frazia S, Brunelli C, Inhibition of viral protein translation by indomethacin in vesicular stomatitis virus infection: Role of eIF2alpha kinase PKR: Cell Microbiol, 2015; 17(9); 1391-404
89. Napolitano FGG, Carrella D, Gao X, di Bernardo D, Computational drug repositioning and elucidation of mechanism of action of compounds against SARSCoV-2: arXiv (preprint), 2020; 2020; 07697
90. Xu TGX, Wu Z, Selinger DW, Zhou Z, Indomethacin has a potent antiviral activity against SARS-COV-2 in vitro and canine coronavirus in vivo: BioRxiv, 2020; 2020; 017624
91. Shekhar N, Kaur H, Sarma P, Indomethacin: An exploratory study of antiviral mechanism and host-pathogen interaction in COVID-19: Expert Rev Anti Infect Ther, 2021 [Online ahead of print]
92. Garvin MR, Alvarez C, Miller JI, A mechanistic model and therapeutic interventions for COVID-19 involving a RAS-mediated bradykinin storm: Elife, 2020; 9; e59177
93. Haybar H, Maniati M, Saki N, Zayeri ZD, COVID-19: imbalance of multiple systems during infection and importance of therapeutic choice and dosing of cardiac and anti-coagulant therapies: Mol Biol Rep, 2021; 48(3); 2917-28
94. McCarthy CG, Wilczynski S, Wenceslau CF, Webb RC, A new storm on the horizon in COVID-19: Bradykinin-induced vascular complications: Vascul Pharmacol, 2020; 137; 106826
95. Ghareeb DA, Saleh SR, Nofal MS, Potential therapeutic and pharmacological strategies for SARS-CoV2: J Pharm Investig, 2021; 51(3); 281-96
96. Ho P, Zheng JQ, Wu CC, Perspective adjunctive therapies for COVID-19: Beyond antiviral therapy: Int J Med Sci, 2021; 18(2); 314-24
97. Li G, Wei W, Suo L, Low-dose aspirin prevents kidney damage in LPS-induced preeclampsia by inhibiting the WNT5A and NF-kappaB signaling pathways: Front Endocrinol (Lausanne), 2021; 12; 639592
98. Liu Q, Huang N, Li A, Effect of low-dose aspirin on mortality and viral duration of the hospitalized adults with COVID-19: Medicine (Baltimore), 2021; 100(6); e24544
99. Rizk JG, Lavie CJ, Gupta A, Low-dose aspirin for early COVID-19: Does the early bird catch the worm?: Expert Opin Investig Drugs, 2021; 30(8); 785-88
100. Massimo Claar G, Monaco S, Del Veccho Blanco C, Omeprazole 20 or 40 mg daily for healing gastroduodenal ulcers in patients receiving non-steroidal anti-inflammatory drugs: Aliment Pharmacol Ther, 1998; 12(5); 463-68
101. Bianchi Porro G, Lazzaroni M, Petrillo M, Prevention of gastroduodenal damage with omeprazole in patients receiving continuous NSAIDs treatment. A double blind placebo controlled study: Ital J Gastroenterol Hepatol, 1998; 30(1); 43-47
102. Gao J, Zhang L, Liu X, Repurposing low-molecular-weight drugs against the main protease of severe acute respiratory syndrome coronavirus 2: J Phys Chem Lett, 2020; 11(17); 7267-72
103. Tanimoto K, Hirota K, Fukazawa T, Inhibiting SARS-CoV-2 infection in vitro by suppressing its receptor, angiotensin-converting enzyme 2, via aryl-hydrocarbon receptor signal: Sci Rep, 2021; 11(1); 16629
104. Saheb Sharif-Askari N, Saheb Sharif-Askari F, Alabed M, Effect of common medications on the expression of SARS-CoV-2 entry receptors in kidney tissue: Clin Transl Sci, 2020; 13(6); 1048-54
105. Checconi P, De AM, Marcocci ME, Redox-modulating agents in the treatment of viral infections: Int J Mol Sci, 2020; 21(11); 4084
106. Mironova GD, Belosludtseva NV, Ananyan MA, Prospects for the use of regulators of oxidative stress in the comprehensive treatment of the novel coronavirus disease 2019 (COVID-19) and its complications: Eur Rev Med Pharmacol Sci, 2020; 24(16); 8585-91
107. Aviram M, Dornfeld L, Rosenblat M, Pomegranate juice consumption reduces oxidative stress, atherogenic modifications to LDL, and platelet aggregation: Studies in humans and in atherosclerotic apolipoprotein E-deficient mice: Am J Clin Nutr, 2000; 71(5); 1062-76
108. Qiao J, Arthur JF, Gardiner EE, Regulation of platelet activation and thrombus formation by reactive oxygen species: Redox Biol, 2018; 14; 126-30
109. Parhiz H, Roohbakhsh A, Soltani F, Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: An updated review of their molecular mechanisms and experimental models: Phytother Res, 2015; 29(3); 323-31
110. Haggag YA, El-Ashmawy NE, Okasha KM, Is hesperidin essential for prophylaxis and treatment of COVID-19 Infection?: Med Hypotheses, 2020; 144; 109957
111. Saha RK, Takahashi T, Suzuki T, Glucosyl hesperidin prevents influenza a virus replication in vitro by inhibition of viral sialidase: Biol Pharm Bull, 2009; 32(7); 1188-92
112. Ding Z, Sun G, Zhu Z, Hesperidin attenuates influenza A virus (H1N1) induced lung injury in rats through its anti-inflammatory effect: Antivir Ther, 2018; 23(7); 611-15
113. Lin CW, Tsai FJ, Tsai CH, Anti-SARS coronavirus 3C-like protease effects of Isatis indigotica root and plant-derived phenolic compounds: Antiviral Res, 2005; 68(1); 36-42
114. Wu C, Liu Y, Yang Y, Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods: Acta Pharm Sin B, 2020; 10(5); 766-88
115. Bellavite P, Donzelli A, Hesperidin and SARS-CoV-2: New light on the healthy function of citrus fruits: Antioxidants (Basel), 2020; 9(8); 742
116. Balmeh N, Mahmoudi S, Mohammadi N, Karabedianhajiabadi A, Predicted therapeutic targets for COVID-19 disease by inhibiting SARS-CoV-2 and its related receptors: Inform Med Unlocked, 2020; 20; 100407
117. Wang X, Yang C, Sun Y, A novel screening strategy of anti-SARS-CoV-2 drugs via blocking interaction between Spike RBD and ACE2: Environ Int, 2021; 147; 106361
118. Cheng FJ, Huynh TK, Yang CS, Hesperidin is a potential inhibitor against SARS-CoV-2 infection: Nutrients, 2021; 13(8); 2800
119. Adem S, Eyupoglu V, Sarfraz I, Identification of potent COVID-19 main protease (Mpro) inhibitors from natural polyphenols: An in silico strategy unveils a hope against CORONA: Preprints, 2020; 2020; 2020030333
120. Kandeil A, Mostafa A, Kutkat O, Bioactive polyphenolic compounds showing strong antiviral activities against severe acute respiratory syndrome coronavirus 2: Pathogens, 2021; 10(6); 758
121. Das S, Sarmah S, Lyndem S, Singha Roy A, An investigation into the identification of potential inhibitors of SARS-CoV-2 main protease using molecular docking study: J Biomol Struct Dyn, 2021; 39(9); 3347-57
122. Ahmed O, Fahim H, Mahmoud A, Eman Ahmed EA, Bee venom and hesperidin effectively mitigate complete Freund’s adjuvant-induced arthritis via immunomodulation and enhancement of antioxidant defense system: Arch Rheumatol, 2018; 33(2); 198-212
123. Qi W, Lin C, Fan K, Hesperidin inhibits synovial cell inflammation and macrophage polarization through suppression of the PI3K/AKT pathway in complete Freund’s adjuvant-induced arthritis in mice: Chem Biol Interact, 2019; 306; 19-28
124. Guazelli CFS, Fattori V, Ferraz CR, Antioxidant and anti-inflammatory effects of hesperidin methyl chalcone in experimental ulcerative colitis: Chem Biol Interact, 2021; 333; 109315
125. Li Y, Kandhare AD, Mukherjee AA, Bodhankar SL, Acute and sub-chronic oral toxicity studies of hesperidin isolated from orange peel extract in Sprague Dawley rats: Regul Toxicol Pharmacol, 2019; 105; 77-85
126. N-AY , Chapter 58 – Safety of high and long-term intake of polyphenols: Polyphenols in Human Health and Disease, 2014; 747-56, San Diego, Academic Press
127. Dupuis J, Laurin P, Tardif JC, Fourteen-days evolution of COVID-19 symptoms during the third wave in non-vaccinated subjects and effects of hesperidin therapy: A randomized, double-blinded, placebo-controlled study: medRxiv, 2021; 2021; 21264483
128. Formica JV, Regelson W, Review of the biology of Quercetin and related bioflavonoids: Food Chem Toxicol, 1995; 33(12); 1061-80
129. Magar RT, Sohng JK, A review on structure, modifications and structure-activity relation of quercetin and its derivatives: J Microbiol Biotechnol, 2020; 30(1); 11-20
130. Colunga Biancatelli RML, Berrill M, Quercetin and Vitamin C: An experimental, synergistic therapy for the prevention and treatment of SARS-CoV-2 related disease (COVID-19): Front Immunol, 2020; 11; 1451
131. da Silva FMA, da Silva KPA, de Oliveira LPM, Flavonoid glycosides and their putative human metabolites as potential inhibitors of the SARS-CoV-2 main protease (Mpro) and RNA-dependent RNA polymerase (RdRp): Mem Inst Oswaldo Cruz, 2020; 115; e200207
132. Abian O, Ortega-Alarcon D, Jimenez-Alesanco A, Structural stability of SARS-CoV-2 3CLpro and identification of quercetin as an inhibitor by experimental screening: Int J Biol Macromol, 2020; 164; 1693-703
133. Saeedi-Boroujeni A, Mahmoudian-Sani MR, Anti-inflammatory potential of Quercetin in COVID-19 treatment: J Inflamm (Lond), 2021; 18(1); 3
134. Vijayakumar BG, Ramesh D, Joji A, In silico pharmacokinetic and molecular docking studies of natural flavonoids and synthetic indole chalcones against essential proteins of SARS-CoV-2: Eur J Pharmacol, 2020; 886; 173448
135. Di Pierro F, Derosa G, Maffioli P, Possible therapeutic effects of adjuvant quercetin supplementation against early-stage COVID-19 infection: A prospective, randomized, controlled, and open-label study: Int J Gen Med, 2021; 14; 2359-66
136. Di Pierro F, Iqtadar S, Khan A, Potential clinical benefits of quercetin in the early stage of COVID-19: Results of a second, pilot, randomized, controlled and open-label clinical trial: Int J Gen Med, 2021; 14; 2807-16
137. Min YS, Lee SE, Hong ST, The inhibitory effect of Quercetin-3-O-beta-D-Glucuronopyranoside on gastritis and reflux esophagitis in rats: Korean J Physiol Pharmacol, 2009; 13(4); 295-300
138. Fan J, Li BR, Zhang Q, Pretreatment of IEC-6 cells with quercetin and myricetin resists the indomethacin-induced barrier dysfunction via attenuating the calcium-mediated JNK/Src activation: Food Chem Toxicol, 2021; 147; 111896
139. Sterlin D, Mathian A, Miyara M, IgA dominates the early neutralizing antibody response to SARS-CoV-2: Sci Transl Med, 2021; 13(577); eabd2223
140. Azzi L, Dalla Gasperina D, Veronesi G, Mucosal immune response in BNT162b2 COVID-19 vaccine recipients: EBioMedicine, 2021; 75; 103788
141. Drummer HE, Van H, Klock E, Dimeric IgA is a specific biomarker of recent SARS-CoV-2 infection: medRxiv, 2021; 2021; 21259671
142. Wang Z, Lorenzi JCC, Muecksch F, Enhanced SARS-CoV-2 neutralization by dimeric IgA: Sci Transl Med, 2021; 13(577); eabf1555
143. Russell MW, Moldoveanu Z, Ogra PL, Mestecky J, Mucosal immunity in COVID-19: A neglected but critical aspect of SARS-CoV-2 infection: Front Immunol, 2020; 11; 611337
144. Yoon JG, Yoon J, Song JY, Clinical significance of a high SARS-CoV-2 viral load in the saliva: J Korean Med Sci, 2020; 35(20); e195
145. Hanege FM, Kocoglu E, Kalcioglu MT, SARS-CoV-2 presence in the saliva, tears, and cerumen of COVID-19 patients: Laryngoscope, 2021; 131(5); E1677-82
146. Savela ES, Winnett A, Romano AE, Quantitative SARS-CoV-2 viral-load curves in paired saliva and nasal swabs inform appropriate respiratory sampling site and analytical test sensitivity required for earliest viral detection: medRxiv, 2021; 2021; 21254771
147. Cao JH, Xue R, He B, Quercetin protects oral mucosal keratinocytes against lipopolysaccharide-induced inflammatory toxicity by suppressing the AKT/AMPK/mTOR pathway: Immunopharmacol Immunotoxicol, 2021; 43(5); 519-26
148. Petti S, Scully C, Polyphenols, oral health and disease: A review: J Dent, 2009; 37(6); 413-23
149. Costa CR, Amorim BR, de Magalhães P, Effects of plants ON osteogenic differentiation AND mineralization OF periodontal ligament cells: A systematic review: Phytother Res, 2016; 30(4); 519-31
150. Fordham JB, Naqvi AR, Nares S, Leukocyte production of inflammatory mediators is inhibited by the antioxidants phloretin, silymarin, hesperetin, and resveratrol: Mediators Inflamm, 2014; 2014; 938712
151. Zhang W, Jia L, Zhao B, Quercetin reverses TNFalpha induced osteogenic damage to human periodontal ligament stem cells by suppressing the NFkappaB/NLRP3 inflammasome pathway: Int J Mol Med, 2021; 47(4); 39
152. Gour A, Manhas D, Bag S, Gorain B, Nandi U, Flavonoids as potential phytotherapeutics to combat cytokine storm in SARS-CoV-2: Phytother Res, 2021; 35(8); 4258-83
153. Basu A, Sarkar A, Maulik U, Molecular docking study of potential phytochemicals and their effects on the complex of SARS-CoV2 spike protein and human ACE2: Sci Rep, 2020; 10(1); 17699
154. Russo M, Moccia S, Spagnuolo C, Roles of flavonoids against coronavirus infection: Chem Biol Interact, 2020; 328; 109211
155. Bhowmik D, Nandi R, Prakash A, Kumar D, Evaluation of flavonoids as 2019-nCoV cell entry inhibitor through molecular docking and pharmacological analysis: Heliyon, 2021; 7(3); e06515
156. Shovlin CL, Vizcaychipi MP, Vascular inflammation and endothelial injury in SARS-CoV-2 infection: The overlooked regulatory cascades implicated by the ACE2 gene cluster: QJM, 2020 [Online ahead of print]
157. Potus F, Mai V, Lebret M, Novel insights on the pulmonary vascular consequences of COVID-19: Am J Physiol Lung Cell Mol Physiol, 2020; 319(2); L277-88
158. Polonikov A, Endogenous deficiency of glutathione as the most likely cause of serious manifestations and death in COVID-19 patients: ACS Infect Dis, 2020; 6(7); 1558-62
159. Iddir M, Brito A, Dingeo G, Strengthening the immune system and reducing inflammation and oxidative stress through diet and nutrition: Considerations during the COVID-19 crisis: Nutrients, 2020; 12(6); 1562
160. Mrityunjaya M, Pavithra V, Neelam R, Immune-boosting, antioxidant and anti-inflammatory food supplements targeting pathogenesis of COVID-19: Front Immunol, 2020; 11; 570122
161. Violi F, Oliva A, Cangemi R, Nox2 activation in COVID-19: Redox Biol, 2020; 36; 101655
162. Bellavite P: Reappraisal of dietary phytochemicals for coronavirus infection: Focus on hesperidin and quercetin. Antioxidants, 2021; 473-87, London, Intechopen
163. Gill MSA, Hassan SS, Ahemad N, Evolution of HIV-1 reverse transcriptase and integrase dual inhibitors: Recent advances and developments: Eur J Med Chem, 2019; 179; 423-48
164. Ravichandran RMS, Surapaneni KM, Sukumaran SK, Indomethacin use for mild & moderate hospitalised COVID-19 patients: An open label randomized clinical trial: MedRxiv, 2021; 2021; 21261007
165. Kontoghiorghes GJ, Fetta S, Kontoghiorghe CN, The need for a multi-level drug targeting strategy to curb the COVID-19 pandemic: Front Biosci (Landmark Ed), 2021; 26(12); 1723-36
In Press
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
12 Mar 2024 : Clinical Research
Comparing Neuromuscular Blockade Measurement Between Upper Arm (TOF Cuff®) and Eyelid (TOF Scan®) Using Miv...Med Sci Monit In Press; DOI: 10.12659/MSM.943630
11 Mar 2024 : Clinical Research
Enhancement of Frozen-Thawed Human Sperm Quality with Zinc as a Cryoprotective AdditiveMed Sci Monit In Press; DOI: 10.12659/MSM.942946
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952