09 May 2022: Clinical Research
A Nomogram Model for Evaluating the Risk of Lymph Node Metastasis in cT2-cT4N0M0 Gastric Cancer Population
Zibo Zhang1ABCDEF, Yu Liu1AE*, Guohong Ma1BE, Jixuan Su1BDOI: 10.12659/MSM.935696
Med Sci Monit 2022; 28:e935696
Abstract
BACKGROUND: Neoadjuvant chemotherapy is an important treatment for advanced gastric cancer, but it has been unclear whether neoadjuvant chemotherapy is closely related to lymph node metastasis. Therefore, based on the disease characteristics of the cT2-cT4N0M0 gastric cancer population, this study established a nomogram prediction model of lymph node metastasis risk in this gastric cancer population to help clinicians optimize clinical decision-making.
MATERIAL AND METHODS: We analyzed the data of 336 patients with advanced gastric cancer with CT imaging stage of cT2-cT4N0M0 admitted to the Third Department of the Fourth Hospital of Hebei Medical University from 2015 to 2021. Combined with the results of univariate and multivariate logistic regression analysis, 7 indicators were selected to establish a nomogram prediction model. The calibration curves, ROC curves, and decision curves were drawn against the nomogram model using R language.
RESULTS: The results showed that the AUC value of the model and the external validation data set were 0.925 and 0.911, respectively. The P value of the Hosmer-Lemeshow test for the internal validation dataset was 0.082, and the P value of Hosmer-Lemeshow test for the external validation dataset was 0.076.The decision curve results showed that when the threshold probability was 0.1-0.9, this model could benefit patients by predicting the risk of lymph node metastasis in patients with advanced gastric cancer, and formulating appropriate treatment schemes accordingly.
CONCLUSIONS: This nomogram has shown good discrimination and fit, and can also be combined with imaging examination to screen the populations suitable for neoadjuvant chemotherapy, avoid the risk of misdiagnosis of N staging to the greatest extent, and to assist clinicians to optimize clinical decision-making.
Keywords: nomogram, Advanced Gastric Cancer, lymph node metastasis, neoadjuvant chemotherapy, Humans, Lymph Nodes, Neoadjuvant Therapy, nomograms, Stomach Neoplasms
Background
According to the 2018 Global Cancer Statistics Report, approximately 1.03 million people are newly diagnosed annually with gastric cancer worldwide, accounting for 5.7% of new cancer cases, and it is the fifth most common cancer and the third leading cause of cancer-associated death worldwide [1]. China has a relatively high incidence of gastric cancer. In 2015, about 403 000 people developed gastric cancer, and the incidence and mortality ranked second and third in the spectrum of cancer incidence and mortality in China, respectively [2]. Despite a higher detection rate of early gastric cancer in China than before, patients with advanced gastric cancer still account for 70.8% of all gastric cancer cases [3,4]. Currently, the mainstay of treatment for those patients includes surgery+chemotherapy or preoperative neoadjuvant therapy+surgery+postoperative chemotherapy. A large number of clinical studies have shown that lymph node metastasis in patients with advanced gastric cancer is an independent risk factor for evaluating their prognosis. According to the
A nomogram is a chart that integrates multiple predictive indicators based on multivariate regression analysis to predict related clinical outcomes or the occurrence probability of a certain type of event. It has been applied to prediction of the related risks of gastric cancer, esophageal cancer, and breast cancer [7–9]. As for the current published literature at home and abroad, most risk prediction models of gastric cancer are based on patients with early gastric cancer. However, prediction models are not available for assessing the risk of lymph node metastasis based on those with advanced gastric cancer. Therefore, to fill this gap, we constructed a nomogram prediction model for the risk of lymph node metastasis for this population.
Material and Methods
RESEARCH SUBJECTS:
A retrospective study was conducted on 336 patients with stage cT2-cT4N0M0 advanced gastric cancer by preoperative CT imaging who were treated in the Third Department of the Fourth Hospital of Hebei Medical University from 2015 to 2021. Among them, the clinical data of 236 patients were classified as an internal validation set, which was used to establish a prediction model and for internal validation. The clinical data of the remaining 100 patients were classified as an external validation set, which was used for external validation of the prediction model. All patients had primary advanced gastric cancer without distant metastasis or other tumors. None of the patients received neoadjuvant treatment prior to surgery. Radical gastric cancer resection and D2 lymph node dissection were performed in accordance with guidelines of gastric cancer treatment proposed by the Japanese Gastric Cancer Association (JGCA). Ethics statement: The study was approved by the Institutional Review Board of the Fourth Hospital of Hebei Medical University. All patients provided written informed consent.
General data of patients were collected for constructing a nomogram model, including sex and age; and imaging reports and laboratory data were collected with respect to patient’s tumor size, tumor location, depth of invasion, degree of differentiation, histological type (Lauren classification), presence of ulcers, presence of vascular tumor thrombus, immunohistochemical results, tumor markers, and other indicators. Tumor size was measured as the largest diameter of the tumor, and tumor sites included cardia and stomach fundus, gastric body, and gastric antrum; depth of invasion included muscularis propria (T2), subserosal layer (T3), serosal layer (T4a) and invasion of adjacent organs (T4b). Histological type was based on the Lauren classification (intestinal, diffuse, and mixed type). Immunohistochemistry included positive rate of Ki67 antigen, and tumor markers included carcinoembryonic antigen, carbohydrate antigen 19-9, carbohydrate antigen 72-4, and alpha-fetoprotein.
STATISTICAL ANALYSIS:
SPSS 23.0 was used for statistical analysis of the data, and continuous variables are expressed as M±SD. Categorical variables in the 2 groups were compared using the chi-square test. The continuous variables were all tested for normal distribution, and the variables showing the normal distribution were compared and analyzed by independent-sample
In this study, the model was internally validated using 1000 repetitions of bootstrap sample corrections, and the model was validated externally by the data of the external validation set. To evaluate the calibration of the model, the calibration curve of the internal validation data set and the external validation data set of the model was plotted. The calibration curve was the comparison between the actual risk and the predicted risk. The closer the curve was to the diagonal, the smaller the prediction error. The Hosmer-Lemeshow test was performed on the model to evaluate the goodness of fit (GOF) between the predicted value of the model and the actual value. P value >0.05 indicated no significant difference between the predicted value and the actual value, suggesting good fit of the model. The receiver operator characteristic (ROC) curve of the internal and external validation set was plotted and the area under the ROC curve (AUC) was calculated to evaluate the accuracy of the model’s prediction results. AUC of 0.5–0.7 indicated a low accuracy, AUC of 0.7–0.9 indicated moderate accuracy, and AUC of >0.9 indicated a high accuracy. The decision curve analysis (DCA) method [10] was used to develop the decision curve of the 2 data sets to evaluate the degree of patient benefit under different positive thresholds. The abscissa of DCA was the threshold probability. When the estimated value of the predictive model reached a certain value, the probability of lymph node metastasis of patient i was designated as Pi; when Pi reached a certain threshold (denoted as Pt), the value was regarded as positive. The ordinate of DCA was the net benefit (NB), which was defined as [the number of true positives - the number of false positives×Pt/(1-Pt)]/sample number. The DCA curve could be obtained by plotting NB against Pt.
Results
GENERAL CHARACTERISTICS OF PATIENTS:
In this study, a total of 236 patients with advanced gastric cancer were included as an internal validation set, including 45.76% (108/236) patients with positive lymph node metastasis. The total number of lymph nodes cleaned in the internal validation set was 6294, and the average number of lymph nodes cleaned was 27 (20~33). A total of 100 patients with advanced gastric cancer were included as an external validation set, including 50% (50/100) of patients with positive lymph node metastasis. The total number of lymph nodes cleaned in the external validation set was 2577, and the average number of lymph nodes cleaned was 26 (19~32). However, there was no significant difference in the positive rate of lymph node metastasis between the 2 sets (P>0.05). The characteristics of clinical data of patients in the 2 validation sets are shown in Table 1.
UNIVARIATE AND MULTIVARIATE LOGISTIC REGRESSION ANALYSIS:
Univariate logistic regression analysis was conducted to screen the risk factors for positive lymph node metastasis in patients with advanced gastric cancer. The results showed that tumor size, tumor site, depth of invasion, degree of differentiation, Larune classification, presence of ulcers, presence of vascular tumor thrombus, and the positive rates of Ki67 antigen were all related to lymph node metastasis in advanced gastric cancer (P<0.05), as shown in Table 2. Multivariate logistic regression analysis of the above indicators showed that tumor site, degree of differentiation, depth of invasion, presence or absence of vascular tumor thrombus, and positive rate of Ki67 antigen were independent risk factors for lymph node metastasis in advanced gastric cancer (P<0.05), as shown in Table 3.
CONSTRUCTION OF THE NOMOGRAM OF THE PREDICTION MODEL:
Although the multivariate logistic regression analysis of this study showed that tumor size and Lauren classification were not independent risk factors for lymph node metastasis in advanced gastric cancer, both indicators were included in the model construction according to the clinical characteristics of advanced gastric cancer. Therefore, the indicators, including tumor site, tumor size, degree of differentiation, depth of invasion, presence or absence of vascular tumor thrombus, positive rate of Ki67 antigen, and Lauren classification, were selected to construct a prediction model for lymph node metastasis of advanced gastric cancer, as shown in Figure 1.
INTERNAL AND EXTERNAL VALIDATION OF THE NOMOGRAM OF THE PREDICTION MODEL:
The ROC curve of the model was drawn, and the results showed that the AUC values of the model and the external validation data set were 0.925 and 0.911, respectively (95%CI: 0.884–0.959;95%CI: 0.838–0.959). The above results demonstrated that the model had good accuracy, as shown in Figures 2 and 3. A calibration curve suggested that the predicted probability of this model was in good agreement with the actual probability, as shown in Figures 4 and 5. The P value of Hosmer-Lemeshow test for the internal validation dataset was 0.082 (Figures 6), and the P value of the Hosmer-Lemeshow test for the external validation dataset was 0.076 (Figure 7), indicating good GOF of the model. The decision curve results showed that when the threshold probability was 0.1–0.9; this model could benefit patients with respect to predicting the risk of lymph node metastasis in patients with advanced gastric cancer and formulating appropriate treatment schemes accordingly.
Discussion
Lymph node metastasis of gastric cancer is the major route of metastasis for gastric cancer, and is also the major risk factor for recurrence in patients with gastric cancer [11]. Currently, relevant experts have reached a consensus that a patient population with certain TNM stage of gastric cancer can significantly benefit from preoperative neoadjuvant chemotherapy. However, due to the limitations of CT imaging for staging, some people who are suitable for neoadjuvant chemotherapy have no access to appropriate treatment measures. Therefore, we constructed a nomogram prediction model based on the tumor site, tumor size, degree of differentiation, depth of invasion, presence or absence of vascular tumor thrombus, positive rate of Ki67 antigen, and Lauren classification for predicting lymph node metastasis in advanced gastric cancer to help clinicians provide rational treatment for patients with stage cT2-cT4N0M0 advanced gastric cancer by preoperative imaging.
This study found that the tumor site was an independent risk factor for lymph node metastasis, and the rate of lymph node metastasis was higher in the gastric antrum than in other sites. Yokota et al [12] reported that the tumor site was closely related to lymph node metastasis, which was also an independent prognostic factor for patients with gastric cancer. Qin et al [13] showed that the lymph node metastasis rate in the lower part of the stomach was 63.1%, which was higher than that in other sites, but the results were not significantly different. Hence, it still remains controversial whether there is an inevitable connection between the tumor site and lymph node metastasis. However, based on the results of this study, we still incorporated this indicator into this model.
Currently, most research results at home and abroad [14–16] have shown that the depth of invasion, degree of differentiation, and vascular tumor thrombus are independent risk factors for lymph node metastasis, which are consistent with our findings. A number of studies have reported that patients with deep tumor invasion have a significantly higher risk of lymph node metastasis [17]. The results of this study showed that the lymph node metastasis rate in stage T4 gastric cancer was 67.5%, which was higher than that in stages T2 and T3. It was found that as the depth of tumor infiltration increased, tumor tissue invaded the perigastric lymphatic system of the serosal layer, leading to increased risk of lymph node metastasis. In addition, in this study, the proportion of poorly differentiated gastric cancer in the positive lymph node group was relatively higher (66.7%), and the proportion of differentiated gastric cancer in the negative lymph node group was relatively higher (43.75%). In the positive lymph node group, the lymph node metastasis rate was higher in poorly differentiated gastric cancer than in moderately differentiated and well-differentiated gastric cancer, showing an overall negative correlation and suggesting significant differences. In addition, based on the results of large-scale data research, several scholars have found that when the lymphatic and blood vessel invasion indicates a higher risk of lymph node metastasis, suggesting that the patient may have systemic metastasis [18]. Currently, the related techniques can be used to detect the presence of tumor thrombi in larger blood vessels before surgery; however, it remains unclear whether tumor thrombi are present in smaller blood vessels.
To improve the accuracy of this model, we included an immunohistochemical index – the positive rate of Ki67 antigen, which generally refers to the percentage of positively stained cells in the nuclei of cancer cells. Ki67 antigen is a nuclear proliferation antigen that participates in cell cycle regulation and cell proliferation, and is considered a reliable tumor marker. Its increase indicates strong proliferation activity and high invasiveness of tumor cells, and it is considered to be related to the poor clinical outcomes of various malignant tumors. The results of this study showed that positive rate of Ki67 antigen was an independent risk factor for lymph node metastasis, and that there was a positive correlation between them. Tzanakis et al [19] found that the higher the positive rate of Ki67 antigen, the higher the proportion of metastatic lymph nodes in the total number of lymph nodes dissected. A study showed that as the positive rate of lymph nodes increased, the positive rate of Ki67 antigen also increased significantly; the positive rate of Ki67 >50% was associated with the poor prognosis of patients [20]. Another meta-analysis also showed that in gastric cancer patients with lymph node metastasis, the positive rate of Ki67 antigen was significantly increased, suggesting that the positive rate of Ki67 antigen could reveal the risk of lymph node metastasis of gastric cancer [21].
In addition to the above indicators, tumor size and Lauren classification are also included in this prediction model. Numerous studies have shown that tumor size and Lauren classification are independent risk factors for lymph node metastasis. A meta-analysis by Zhao et al [22] has indicated that tumor size is significantly related to lymph node metastasis. Patients with larger tumors have a higher risk of lymph node metastasis than those with smaller tumors. Korean scholars Khalayleh et al [23] found that patients with gastric tumors <4 cm in diameter had a lower risk of lymph node metastasis than those with gastric tumors >4 cm in diameter. Chen et al [24] also reported that the risk of lymph node metastasis was the highest in patients with tumors >4 cm in diameter. This study showed that the average tumor size was 4.09 cm and 3.0 cm in the positive and negative lymph node positive groups, respectively, which was consistent with the above conclusion. Lauren classification currently refers to the revised classification proposed by Carneiro. Sui et al [25] found that patients with diffuse type had extremely high lymph node metastasis, and the degree of lymph node metastasis was significantly higher than in patients with intestinal type. A clinical study based on a large database in Western countries indicated that the survival rate of patients with diffuse type was significantly lower than that of those with intestinal type, which was related to the poor adhesion of diffuse gastric cancer cells, which is prone to spread as well as to poor differentiation [26]. Due to the small number of cases in this study, the results showed that tumor size and Lauren classification were related to lymph node metastasis, which, however, were not independent risk factors. After comprehensive consideration of the research results of previous scholars and the biological characteristics of gastric tumors, tumor size and Lauren classification were still included into this prediction model.
To the bet of our knowledge, this nomogram is the first model based on preoperative imaging for a patient population with stage cT2-cT4N0M0 advanced gastric cancer, to screen those who are suitable for neoadjuvant chemotherapy by predicting the risk of lymph node metastasis. After being validated by a variety of statistical methods, this nomogram showed good discrimination and GOF. The DCA curve was drawn to determine the range of risk threshold that benefited patients, thus improving the accuracy of the prediction model. This study has several limitations. First, the sample size was small and there was a lack of multi-center studies based on medical records. Second, presence or absence of small vascular tumor thrombi could not be confirmed by currently available techniques. Third, the blood biochemical indicators of patients were not included, to explore their relationship with lymph node metastasis. Finally, due to the lack of postoperative survival data of patients, this model failed to predict the prognostic survival rate of patients with advanced gastric cancer.
Conclusions
This nomogram showed excellent performance, and it can also be combined with imaging examination to screen the populations suitable for neoadjuvant chemotherapy, avoid the risk of misdiagnosis of N staging to the greatest extent, and to assist clinicians to optimize clinical decision-making.
Figures
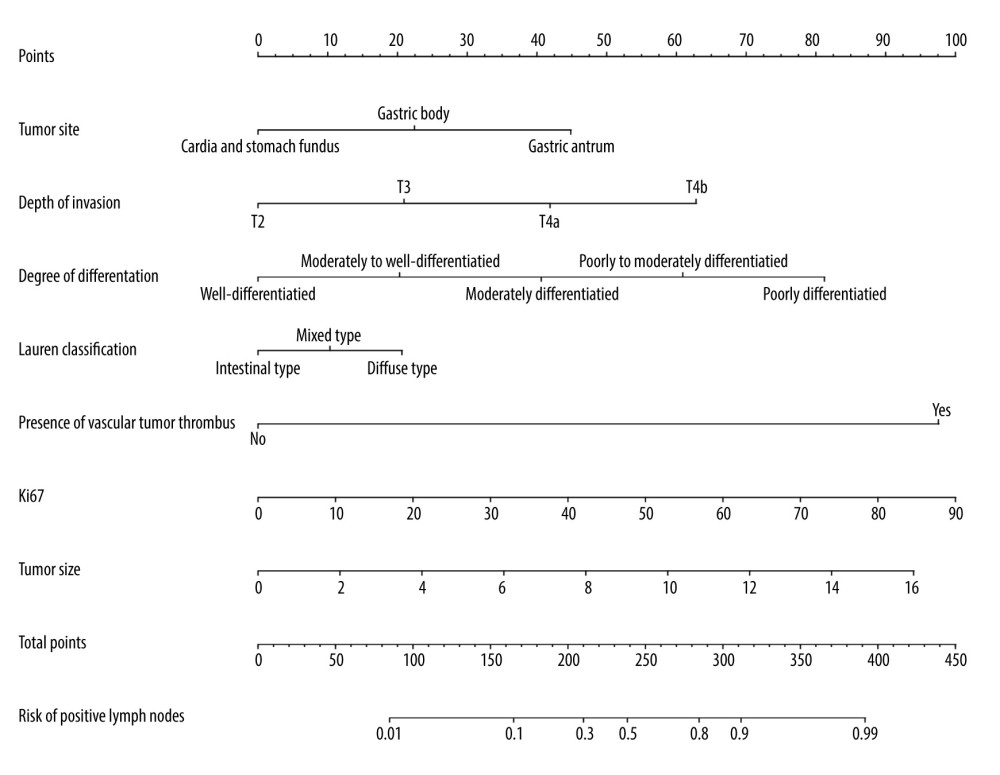 Figure 1. A nomogram model for predicting positive lymph node metastasis in advanced gastric cancer.
Figure 1. A nomogram model for predicting positive lymph node metastasis in advanced gastric cancer. 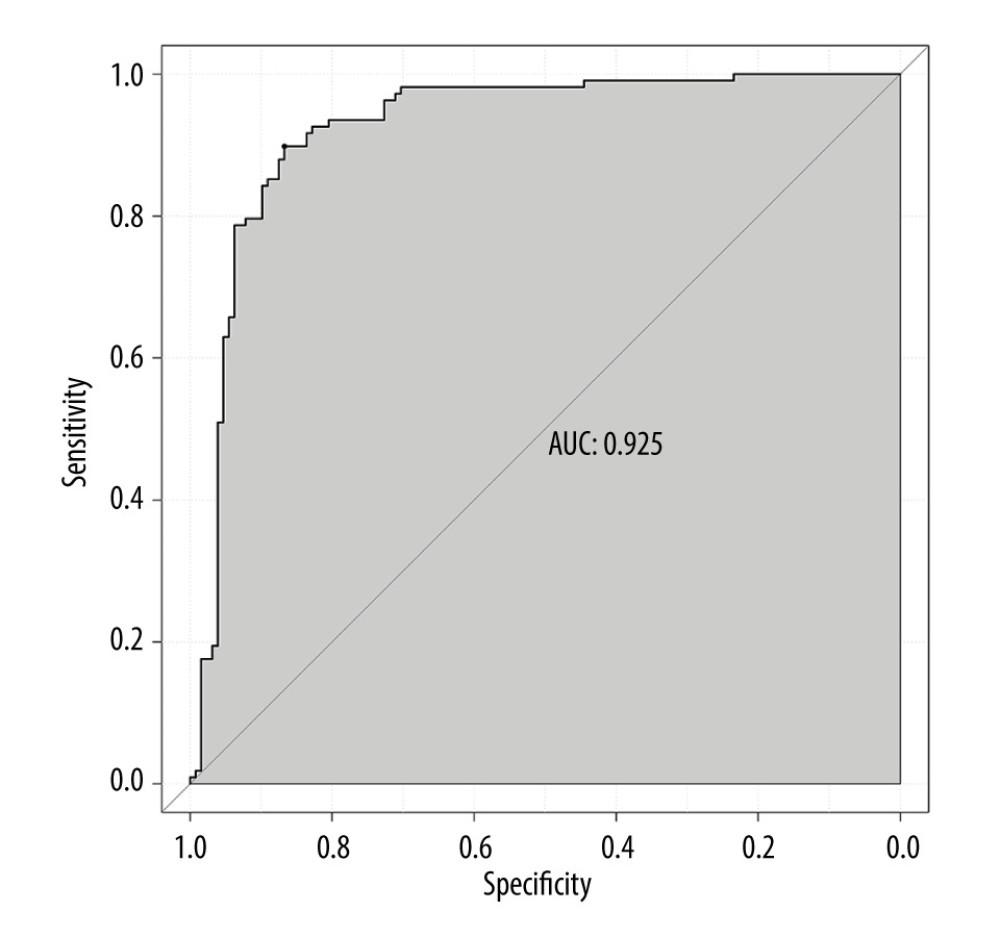 Figure 2. ROC curve of internal validation data set.
Figure 2. ROC curve of internal validation data set. 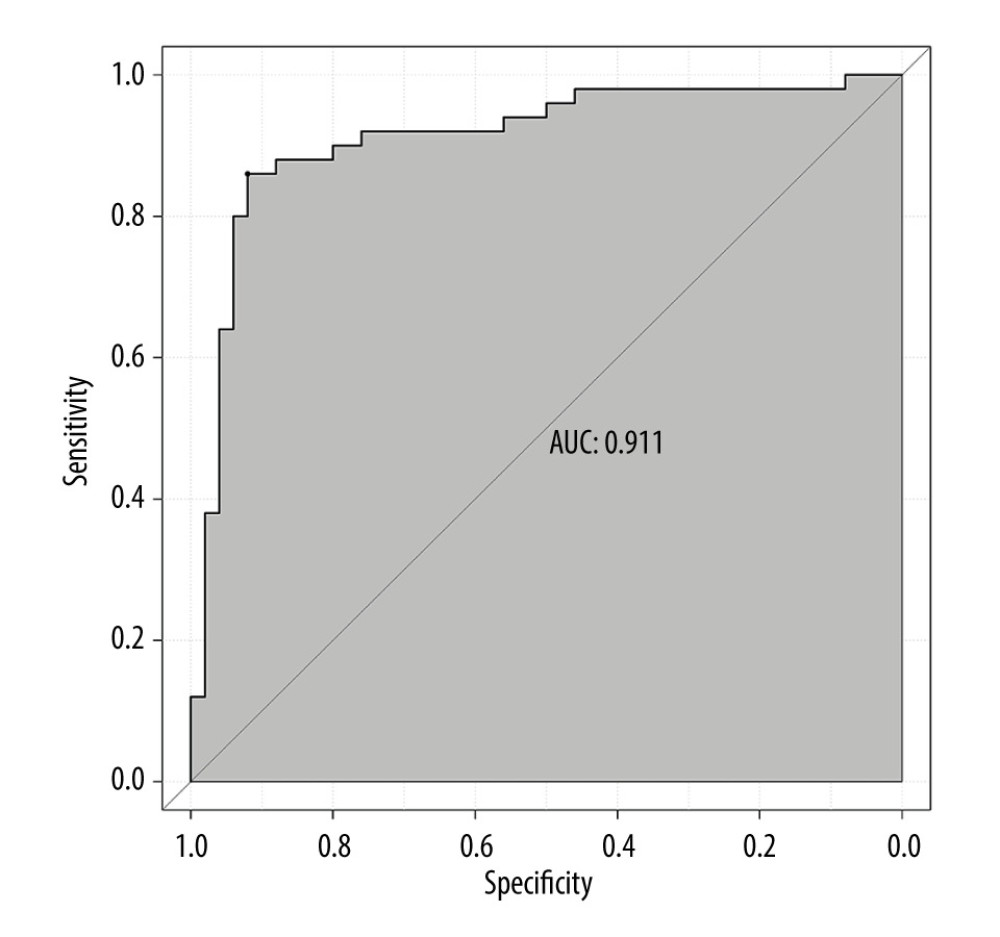 Figure 3. ROC curve of external validation data set.
Figure 3. ROC curve of external validation data set. 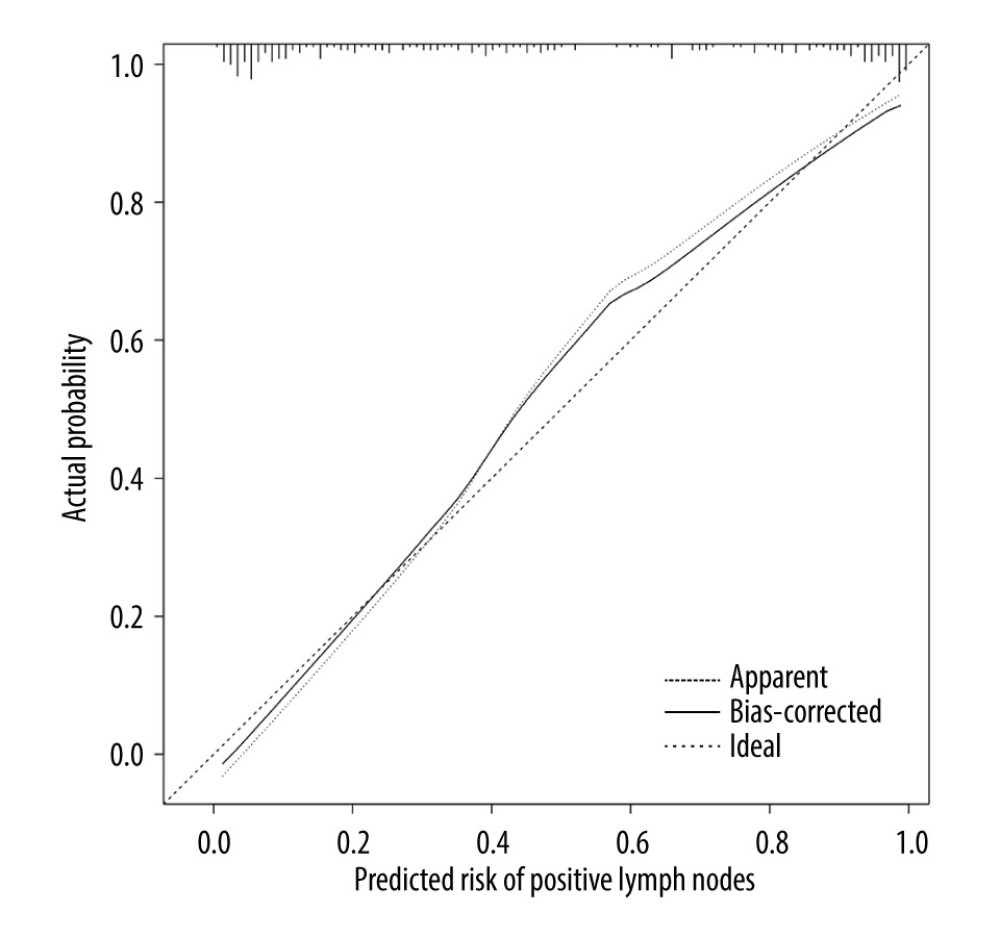 Figure 4. Calibration curve of internal validation data set.
Figure 4. Calibration curve of internal validation data set. 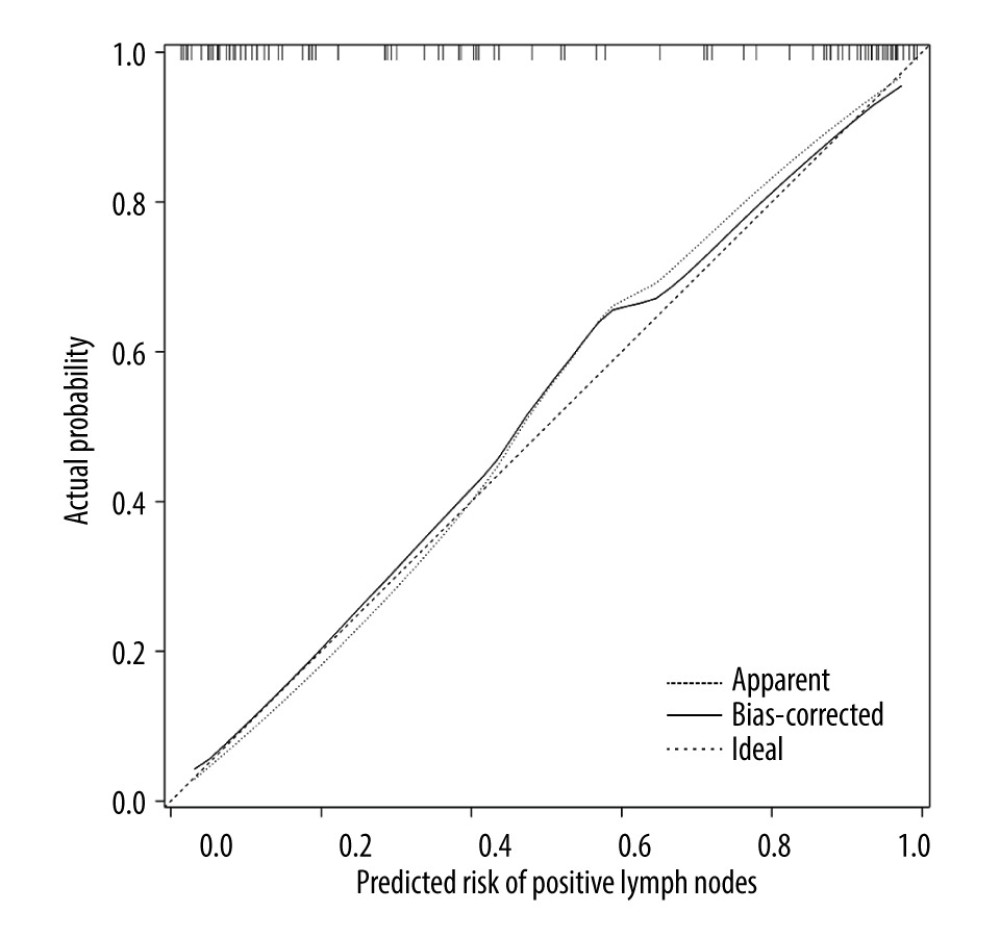 Figure 5. Calibration curve of external validation data set.
Figure 5. Calibration curve of external validation data set. 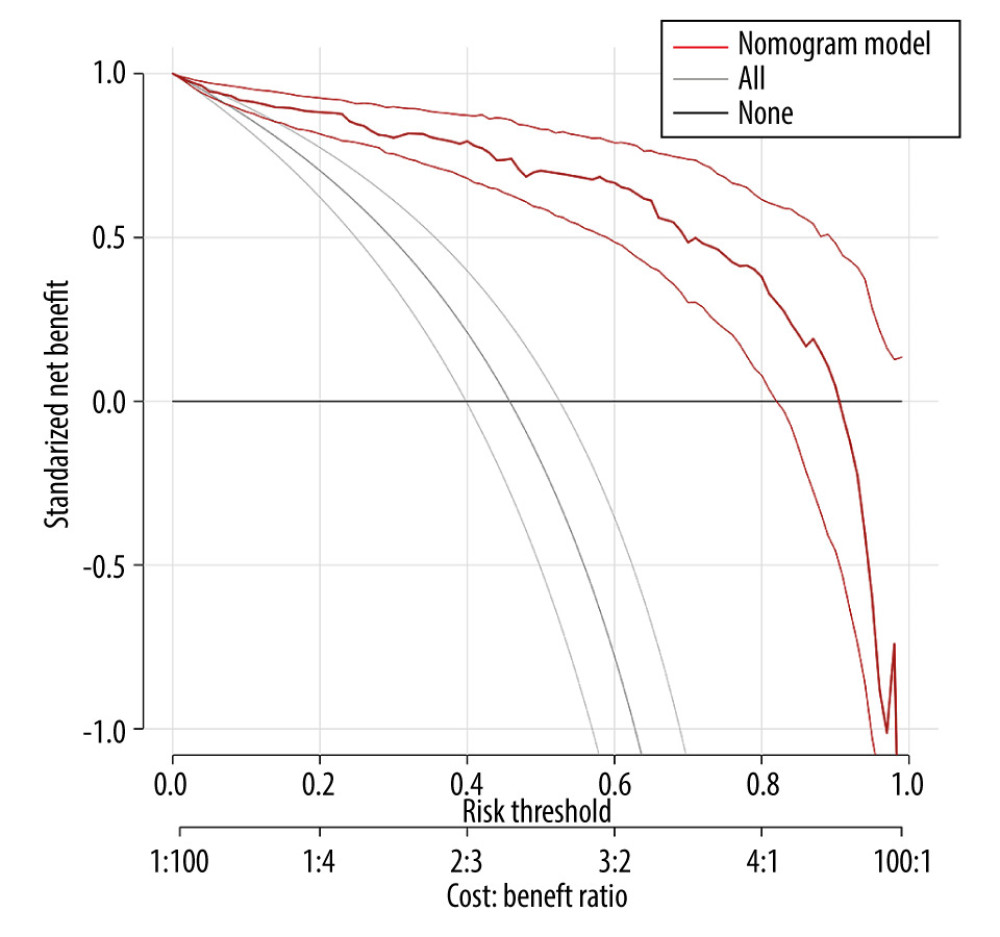 Figure 6. DCA curve of internal validation data set.
Figure 6. DCA curve of internal validation data set. 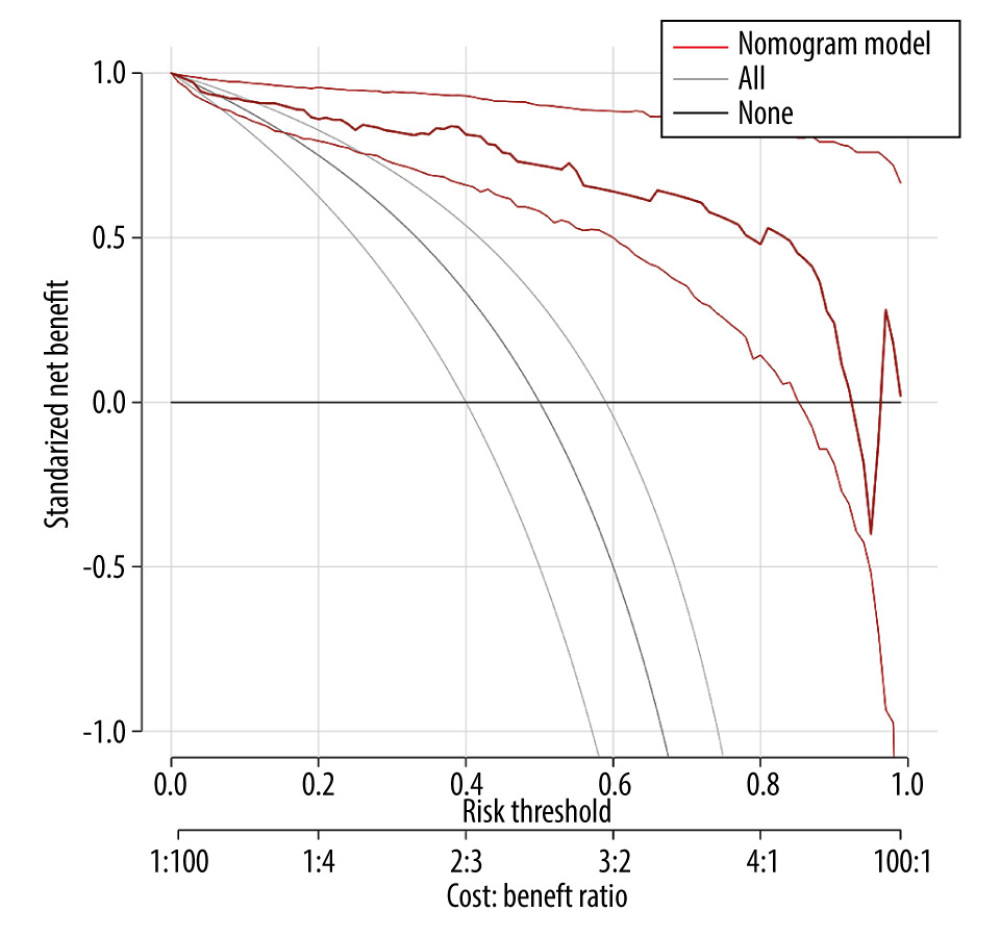 Figure 7. DCA curve of external validation data set.
Figure 7. DCA curve of external validation data set. Tables
Table 1. General information of patients in the 2 validation sets.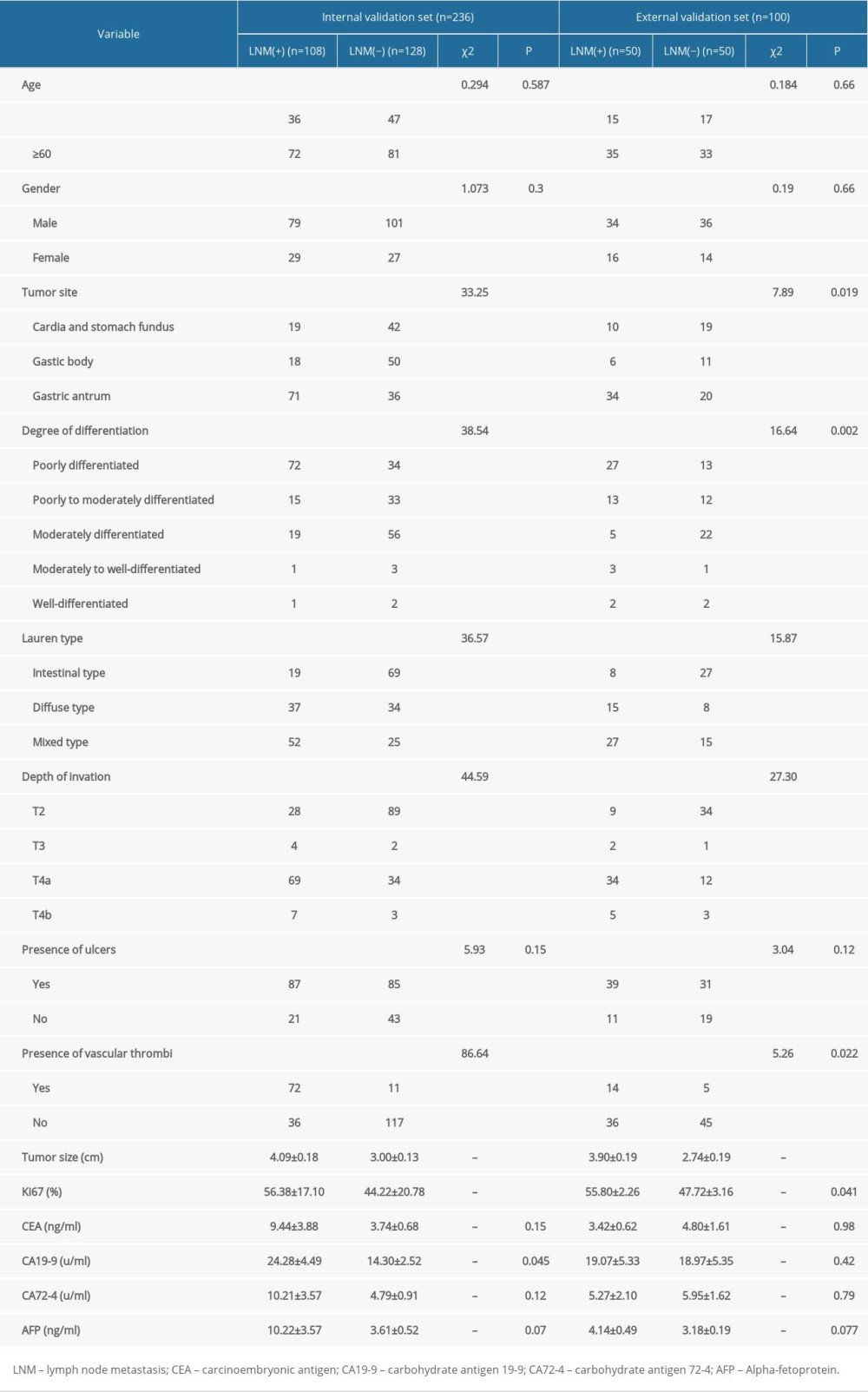 Table 2. Univariate analysis of lymph node metastasis in advanced gastric cancer in the internal validation data set.
Table 2. Univariate analysis of lymph node metastasis in advanced gastric cancer in the internal validation data set.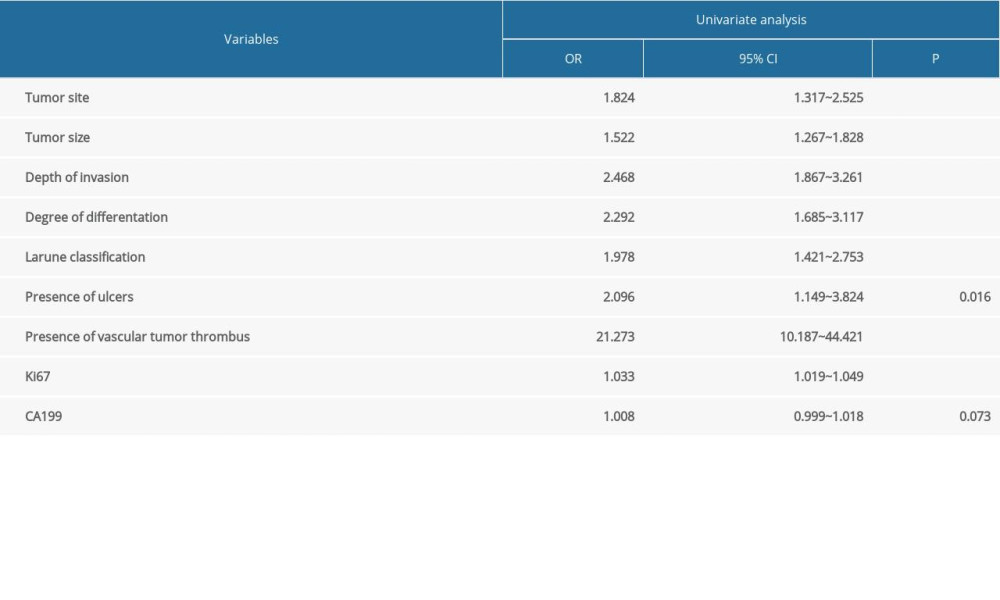 Table 3. Multivariate analysis of lymph node metastasis in advanced gastric cancer in the internal validation data set.
Table 3. Multivariate analysis of lymph node metastasis in advanced gastric cancer in the internal validation data set.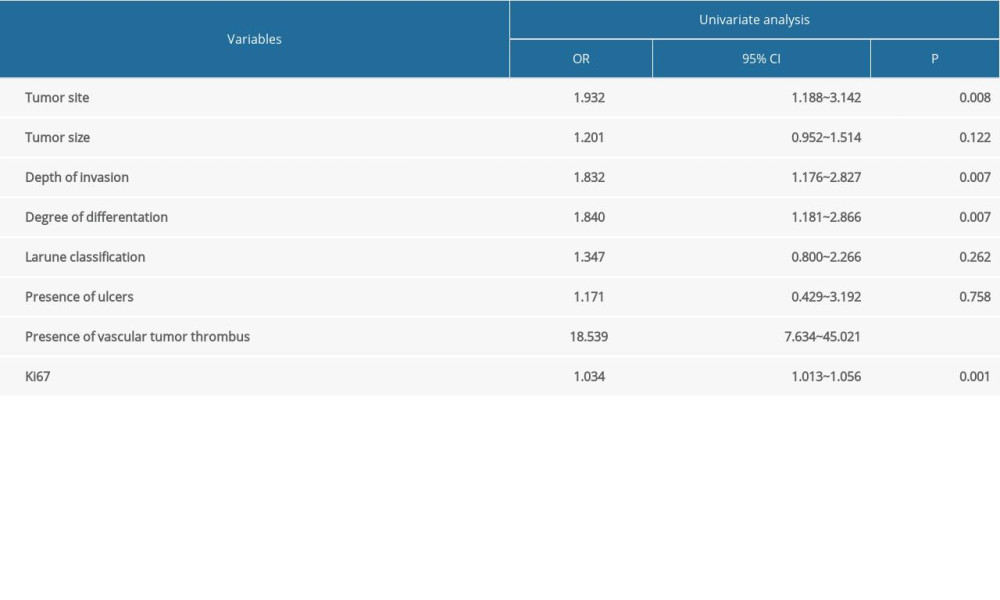
References
1. Bray F, Ferlay J, Soerjomataram I, Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries: Cancer J Clin, 2018; 68(6); 394-424
2. Chen W, Zheng R, Baade P, Cancer statistics in China, 2015: Cancer J Clin, 2016; 66(2); 115-32
3. Wang YK, Li ZY, Shan F, Diagnosis and treatment status of early gastric cancer in China – enlightenment from data from China Gastrointestinal Tumor Surgery Union: Chin J Gastrointest Surg, 2018; 21(2); 168-74
4. Miao RL, Li ZY, Ji JF, Analysis of the status and development trend of early gastric cancer diagnosis and treatment in China based on the relevant data of China Gastrointestinal Tumor Surgery Union: Chin J Prac Sur, 2019; 39(05); 419-23
5. Liu GQ, Yan L, Gao R, The application value of 1.5T magnetic resonance diffusion weighted imaging in the diagnosis of lymph node metastasis of gastric cancer: Shaanxi Medical Journal, 2019; 48(4); 456-458
6. , Standards for Diagnosis and Treatment of Gastric Cancer (2018 Edition): E-Journal of Comprehensive Cancer Therapy, 2019; 5(01); 55-82
7. Semenkovich TR, Yan Y, Subramanian M, A clinical nomogram for predicting node-positive disease in esophageal cancer: Ann Surg, 2021; 273(6); e214-21
8. Zheng ZF, Lu J, Wang W, Development and external validation of a simplified nomogram predicting individual survival after R0 resection for gastric cancer: An international, multicenter study: Ann Surg Oncol, 2018; 25(8); 2383-90
9. Lin S, Mo HN, Li YQ, Development and validation of a nomogram for predicting survival of advanced breast cancer patients in China: Breast, 2020; 53; 172-80
10. Vickers AJ, Holland F, Decision curve analysis to evaluate the clinical benefit of prediction models: Spine J, 2021; 21(10); 1643-48
11. Saka M, Katai H, Fukagawa T, Recurrence in early gastric cancer with lymph node metastasis: Gastric Cancer, 2008; 11(4); 214-18
12. Yokota T, Ishiyama S, Saito T, Lymph node metastasis as a significant prognostic factor in gastric cancer: A multiple logistic regression analysis: Scand J Gastroenterol, 2004; 39(4); 380-84
13. Qin HL, Lin CH, The relationship between clinicopathological characteristics of gastric cancer and lymph node metastasis: Chin J Prac Sur, 2001; 21(7); 415-17
14. Pang BR, Zhu ZL, Li CPredictive factors for lymph node metastasis in patients with poorly differentiated early gastric cancer: Zhonghua Wei Chang Wai Ke Za Zhi, 2019; 22(5); 446-50 [in Chinese]
15. Yang QC, Feng SW, Liu HB, Analysis of clinicopathology and lymph node metastasis risk in 315 patients with early gastric cancer based on the standards of the World Health Organization: Chinese Journal of Digestion, 2018; 38(12); 800-5
16. Li GT, Yan L, Yu WW, Analysis of risk factors related to lymph node metastasis of gastric cancer and their clinical predictive value: Chin J Gen Surg, 2020; 29(4); 412-19
17. Zhang C, Ning F, Zeng X, Lymphovascular invasion as a predictor for lymph node metastasis and a prognostic factor in gastric cancer patients under 70 years of age: A retrospective analysis: Int J Surg, 2018; 53; 214-20
18. Fujikawa H, Koumori K, Watanabe H, The clinical significance of lymphovascular invasion in gastric cancer: In Vivo, 2020; 34(3); 1533-39
19. Tzanakis NE, Peros G, Karakitsos P, Prognostic significance of p53 and Ki67 proteins expression in Greek gastric cancer patients: Acta Chir Belg, 2009; 109(5); 606-11
20. Chen SC, Zhang CD, Xu XY, Analysis of the correlation between Ki-67 expression and recurrence and metastasis in gastric cancer tissues: Chinese Journal of Cancer Prevention and Treatment, 2014; 21(18); 1423-28
21. Ji BB, Feng K, Dong HC, Meta-analysis of the correlation between Ki67 gene expression and lymph node metastasis in gastric cancer: Inner Mongolia Medical Journal, 2019; 51(12); 1428-31
22. Zhao X, Cai A, Xi H, Predictive factors for lymph node metastasis in undifferentiated early gastric cancer: A systematic review and meta-analysis: J Gastrointest Surg, 2017; 21(4); 700-11
23. Khalayleh H, Kim YW, Yoon HM, Assessment of lymph node metastasis in patients with gastric cancer to identify those suitable for middle segmental gastrectomy: JAMA Netw Open, 2021; 4(3); e211840
24. Chen YJ, Peng HP, Mo PS, Analysis of risk factors for lymph node metastasis in patients with gastric cancer and patient prognosis: Chin J Gen Surg (Electronic Edition), 2019; 13(1); 41-43
25. Sui MH, Zhang LM, Clinical features and prognosis of intestinal and diffuse gastric cancer according to Lauren classification: Journal of Clinical and Experimental Medicine, 2013; 12(22); 1841-43
26. Tang CT, Zeng L, Yang J, Analysis of the incidence and survival of gastric cancer based on the lauren classification: A large population-based study using SEER: Front Oncol, 2020; 10; 1212
Figures
 Figure 1. A nomogram model for predicting positive lymph node metastasis in advanced gastric cancer.
Figure 1. A nomogram model for predicting positive lymph node metastasis in advanced gastric cancer. Figure 2. ROC curve of internal validation data set.
Figure 2. ROC curve of internal validation data set. Figure 3. ROC curve of external validation data set.
Figure 3. ROC curve of external validation data set. Figure 4. Calibration curve of internal validation data set.
Figure 4. Calibration curve of internal validation data set. Figure 5. Calibration curve of external validation data set.
Figure 5. Calibration curve of external validation data set. Figure 6. DCA curve of internal validation data set.
Figure 6. DCA curve of internal validation data set. Figure 7. DCA curve of external validation data set.
Figure 7. DCA curve of external validation data set. Tables
 Table 1. General information of patients in the 2 validation sets.
Table 1. General information of patients in the 2 validation sets. Table 2. Univariate analysis of lymph node metastasis in advanced gastric cancer in the internal validation data set.
Table 2. Univariate analysis of lymph node metastasis in advanced gastric cancer in the internal validation data set. Table 3. Multivariate analysis of lymph node metastasis in advanced gastric cancer in the internal validation data set.
Table 3. Multivariate analysis of lymph node metastasis in advanced gastric cancer in the internal validation data set. Table 1. General information of patients in the 2 validation sets.
Table 1. General information of patients in the 2 validation sets. Table 2. Univariate analysis of lymph node metastasis in advanced gastric cancer in the internal validation data set.
Table 2. Univariate analysis of lymph node metastasis in advanced gastric cancer in the internal validation data set. Table 3. Multivariate analysis of lymph node metastasis in advanced gastric cancer in the internal validation data set.
Table 3. Multivariate analysis of lymph node metastasis in advanced gastric cancer in the internal validation data set. In Press
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
06 Mar 2024 : Clinical Research
Prevalence and Variation of Medical Comorbidities in Oral Surgery Patients: A Retrospective Study at Jazan ...Med Sci Monit In Press; DOI: 10.12659/MSM.943884
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








