11 May 2022: Clinical Research
Consistency Analysis of 3 Detection Systems for Measuring Serum C-Reactive Protein
Xueyan Chen1AEF, Chuanyihong Huang1AEF, Weichuan Zhong1BCD, Suwen Qi2BCD, Ting Huang3BCD, Zihua Yang4AE*DOI: 10.12659/MSM.935171
Med Sci Monit 2022; 28:e935171
Abstract
BACKGROUND: C-reactive protein (CRP) is an important clinical indicator. There are many methods and instruments for CRP measurement, and therefore the consistency of CRP values measured between instruments needs to be evaluated. This study aimed to compare the consistency of 3 serum CRP detection systems using turbidimetry.
MATERIAL AND METHODS: The consistency of CRP measured by 3 instruments, the Mindray BC-5390, Mindray BC-6800, and Johnson Vitros5600, was evaluated, and the consistency of blood routine measurement between the BC-5390 and BC-6800 was also evaluated. Pearson correlation analysis was used to evaluate the correlation of different instrument’s test results (R, correlation coefficient). The consistency of instruments was assessed by Passing-Bablok analysis and weighted Deming analysis.
RESULTS: CRP data and route blood test data from 847 patients were used for analysis. The results showed that there were differences in the CRP values measured by the Mindray BC5390, Mindray BC6800, and Johnson Vitros5600 (χ²=78.573, P<0.001). The CRP measurement results of the BC5390 analyzer were consistent with those of the BC6800 analyzer (R=0.994, P<0.001) and Vitros5600 analyzer (R=0.983, P<0.001). However, there was a constant deviation in the CRP values measured by the BC-6800 and Vitros5600 analyzer (R=0.994, P<0.001). In the measurement of routine blood laboratory tests, the BC5390 analyzer and BC6800 analyzer were found to be interchangeable.
CONCLUSIONS: This study analyzed the consistency of CRP detection by 3 instruments, the Mindray BC-5390, Mindray BC-6800, and Johnson Vitros5600, and may provide a reference for the selection of CRP detection instruments.
Keywords: C-Reactive Protein, Cross-Sectional Studies, Equipment and Supplies, Blood Cell Count, Hematologic Tests, Hematology, Humans, Reproducibility of Results
Background
C-reactive protein (CRP) is an acute-phase reactive protein produced by the liver and is present in blood plasma [1]. After tissue injury, the blood CRP rises rapidly, with a CRP level that is 1000 times higher than the baseline level within 24 h [2,3]. This phenomenon makes the blood CRP level an early indicator of infection or inflammatory diseases, as well as a universal biomarker for many diseases [4–8]. Therefore, the detection of CRP levels in the blood of patients is essential for the diagnosis, monitoring, and intervention of disease.
The analytical methods for assessing CRP levels should have the characteristics of a lower sample volume, sensitivity, good selectivity, rapidity, and trustworthiness. A variety of techniques have been reported for the in vitro detection of CRP, such as turbidimetry based assays (immunity transmission turbidity and immune scatter turbidity), enzyme-linked immunosorbent assay (ELISA), chemiluminescent, fluorescent, and electrochemical assays [9–13]. The ability of these methods to test the concentration of CRP is different: the turbidimetry-based assays can detect CRP in μg mL−1, while the methods of ELISA, chemiluminescent, fluorescent, and electrochemical assays can detect CRP in fg mL−1 [9]. For the measurement of CRP, different detection systems, reagents, and method principles can cause differences in detection results. Testing institutions commonly have different types of CRP testing instruments, and the consistency of different test results needs to be evaluated. In addition, the instrument should be easy to implement and use widely in clinical practice.
The purpose of this study was to compare the consistency of 3 serum CRP detection systems, the Mindray BC-5390, Mindray BC-6800, and Johnson Vitros5600, and select the CRP detection instrument most suitable for widespread use in clinical practice.
Material and Methods
STUDY DESIGN AND POPULATION:
This was a cross-section study, and all the patients were recruited from October 2020 to February 2021 in the People’s Hospital of Longhua Shenzhen Hospital. All included patients were tested for CRP and routine blood laboratory values using 3 different instruments. This study was approved by the Institutional Review Board of the People’s Hospital of Longhua Shenzhen Hospital and written informed consent was obtained from all patients.
SAMPLE HANDLING:
For CRP testing, the Mindray BC-5390 and BC-6800 analyzers use whole blood samples and the Johnson Vitros5600 analyzer use serum samples. Two blood samples were collected from all patients under fasting conditions. For testing with the Mindray analyzers, 2 mL of blood was collected in a K2-EDTA anticoagulant test tube; for the Johnson Vitros5600 analyzer, 3 to 5 mL of blood was collected in a test tube without anticoagulant. The blood sample without anticoagulant was allowed to stand for 30 min and then centrifuged at a speed of 3500 r/min for 10 min; the serum was then taken for testing. If samples could not be tested in time, the protocol was to store them in an environment at 2°C to 8°C and test as soon as possible; however, the blood samples from all patients were measured in time.
INSTRUMENTS:
Three instruments were used to analyze the blood samples: the Mindray BC-5390 and Mindray BC-6800 (Mindray, Shenzhen, China), and the Johnson Vitros5600 (Ortho-Clinical Diagnostics, Inc., Raritan, NJ, USA). All reagents used were those matched with the instruments. All operations were carried out in strict accordance with the instrument and kit instructions. In addition, samples for each patient were tested by the 3 instruments. The sample volumes used by the different instruments during the test were as follows: (1) Mindray BC-5390 (sample type, whole blood; single blood routine model, 20 μL; single CRP model, 20 μL; blood routine combined blood routine model, 35 μL); (2) Mindray BC-6800 (sample type, whole blood; routine blood manual model, 150 μL; routine blood automatic model, 200 μL; CRP model, 20 μL); (3) Johnson Vitros5600 (sample type, serum; CRP model, 41 μL).
DATA COLLECTION:
Test data of all patients were collected, and the following variables were extracted: CRP levels, white blood cells, neutrophils (×109/L), lymphocytes (×109/L), monocytes (×109/L), eosinophils (×109/L), basophils (×109/L), neutrophils (%), lymphocytes (%), monocytes (%), eosinophils (%), basophils (%), red blood cells (RBC), hemoglobin, hematocrit, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), RBC distribution width-coefficient of variation (RDW-CV), RBC distribution width-standard deviation (RDW-SD), platelets, plateletcrit, mean platelet volume (MPV), and platelet distribution width.
STATISTICAL ANALYSIS:
Statistical comparisons between combinations of 2 instruments (Mindray BC-5390 analyzer, Mindray BC-6800 analyzer, and Johnson Vitros5600 analyzer) were performed using Pearson correlation analysis, Passing-Bablok analysis, and weighted Deming analysis. Pearson correlation analysis was used to evaluate the correlation of the different instruments’ test results (R, correlation coefficient). The consistency analysis of the results was evaluated by Passing-Bablok analysis and weighted Deming analysis, and the proportional and constant systematic errors were evaluated by the slope and intercept, with 95% confidence intervals (95% CI) [14]. The bias and 95% agreement limit of CRP value were evaluated by Bland-Altman analysis and calculated as the mean difference between the instruments. Effect analysis was used to test the consistency of the indicator direction. For the comparison of routine blood indicators, the Shapiro test was used to test the normality of measurement data. Continuous variables with a normal distribution were displayed as mean±SD, and the t test was used for comparisons between 2 groups; non-normally distributed measurement data were represented by a median and interquartile range, and the Wilcoxon rank test was used between 2 groups. All statistical analyses were 2-sided, and R software (version 4.0.3) was used to perform the analysis. P<0.05 was considered to indicate statistical significance.
Results
COMPARISON OF CRP VALUES DETERMINED BY BC-5390, BC-6800, AND VITROS5600:
Test information was initially collected from 1058 patients. There were 201 patients with missing CRP values due to failure to automatically upload instrument test data to the hospital laboratory information system, and the BC5390 analyzer was updating and debugging the new system, including 182 cases using BC-5390, 17 cases using the BC-6800, and 3 cases using Vitros5600. In addition, the CRP values measured by the BC-5390 and BC-6800 were 0 in 4 cases and 6 cases, respectively. After excluding patients with missing CRP values and CRP values of 0, data from a total of 847 patients were included to compare the consistency of CRP values measured by the BC-5390, BC-6800, and Vitros5600.
Results of comparing the CRP values tested by the 3 analyzers showed a statistical difference in values (χ2=78.573, P<0.001). The results of the Wilcoxon test showed that the differences between every combination of 2 analyzers were statistically significant (all P<0.001). The detailed results are shown in Figure 1.
COMPARISON OF CONSISTENCY OF CRP VALUES MEASURED BY BC-5390, BC-6800, AND VITROS5600:
The CRP values measured by the Mindray BC-5390 analyzer were compared with those measured by the Mindray BC-6800 analyzer (Figure 2). Pearson correlation analysis showed that the correlation coefficient of the CRP values measured by the 2 instruments was 0.994 (P<0.001). The Passing-Bablok analysis yielded the equation y=0.999(95% CI: 0.989–1.011)x+0.565(95% CI: 0.305–0.824), in which the slope range contained 1. The weighted Deming analysis yielded the equation y=1.011(95% CI: 1.005–1.029)x+0.406(95% CI: −0.0937–0.459), in which the slope and intercept were close to 1 and equal to 0, respectively. The Bland-Altman test showed that the mean bias between the 2 different instruments’ tests was −1.34 mg/L. These results indicated that the CRP values measured by the BC-5390 and BC-6800 had a certain deviation, but they were still relatively consistent.
The consistency analyses of CRP between the Mindray BC-6800 analyzer and Johnson Vitros5600 analyzer are shown in Figure 3. The correlation coefficient of the CRP value measured by the 2 instruments was 0.994 (P<0.001) in Pearson correlation analysis. The Passing-Bablok analysis yielded the equation y=1.081(95% CI: 1.063–1.098)x–0.826(95% CI: −1.811–[−0.165]), in which the slope was close to 1 and the intercept was −0.826. The weighted Deming analysis yielded the equation y=1.005(95% CI: 0.982–1.028)x+1.152(95% CI: 0.366–1.957), in which the slope range contained 1 and the intercept was 1.152. In the Bland-Altman analysis, the mean bias between the 2 instruments’ tests was −4.31 mg/L. These results showed that there was a constant deviation in the CRP values measured by Mindray BC-6800 and Johnson Vitros5600.
The comparison results of the Mindray BC-5390 analyzer and Johnson Vitros5600 analyzer are shown in Figure 4. Pearson correlation analysis found that the correlation coefficient of the CRP values measured by the 2 instruments was 0.983 (P<0.001). The Passing-Bablok analysis yielded the equation y=1.088(95% CI: 1.066–1.109)x–0.310(95% CI: −1.068–0.475), in which the slope and intercept were close to 1 and equal to 0. The weighted Deming analysis yielded the equation y=1.025(95% CI: 0.996–1.052)x+1.306(CI: 0.372–2.217), in which the slope range contained 1. The Bland-Altman test indicated that the mean bias between the 2 different instrument tests was −5.66 mg/L. These results showed that the CRP test results between the Mindray BC-5390 and Johnson Vitros5600 were more consistent than those between other instruments.
In addition, there were differences in test cost and test time between the Mindray analyzers and Johnson Vitros5600 analyzer. The use of the Johnson Vitros5600 analyzer inspection costs $6.60/time, while Mindray analyzers (BC-5390 and BC-6800) costs $5.60/time. In terms of length of test time, the Johnson Vitros5600 analyzer takes 9 min/time, while the Mindray analyzer takes 3 min/time. Furthermore, the Johnson Vitros5600 analyzer requires centrifugation for 10 min/time before testing. In summary, the test cost and time of the Johnson Vitros5600 analyzer were higher than that of the Mindray analyzers.
ANALYSIS OF THE CONSISTENCY OF BC-5390 AND BC-6800 IN ROUTINE BLOOD INDICATORS:
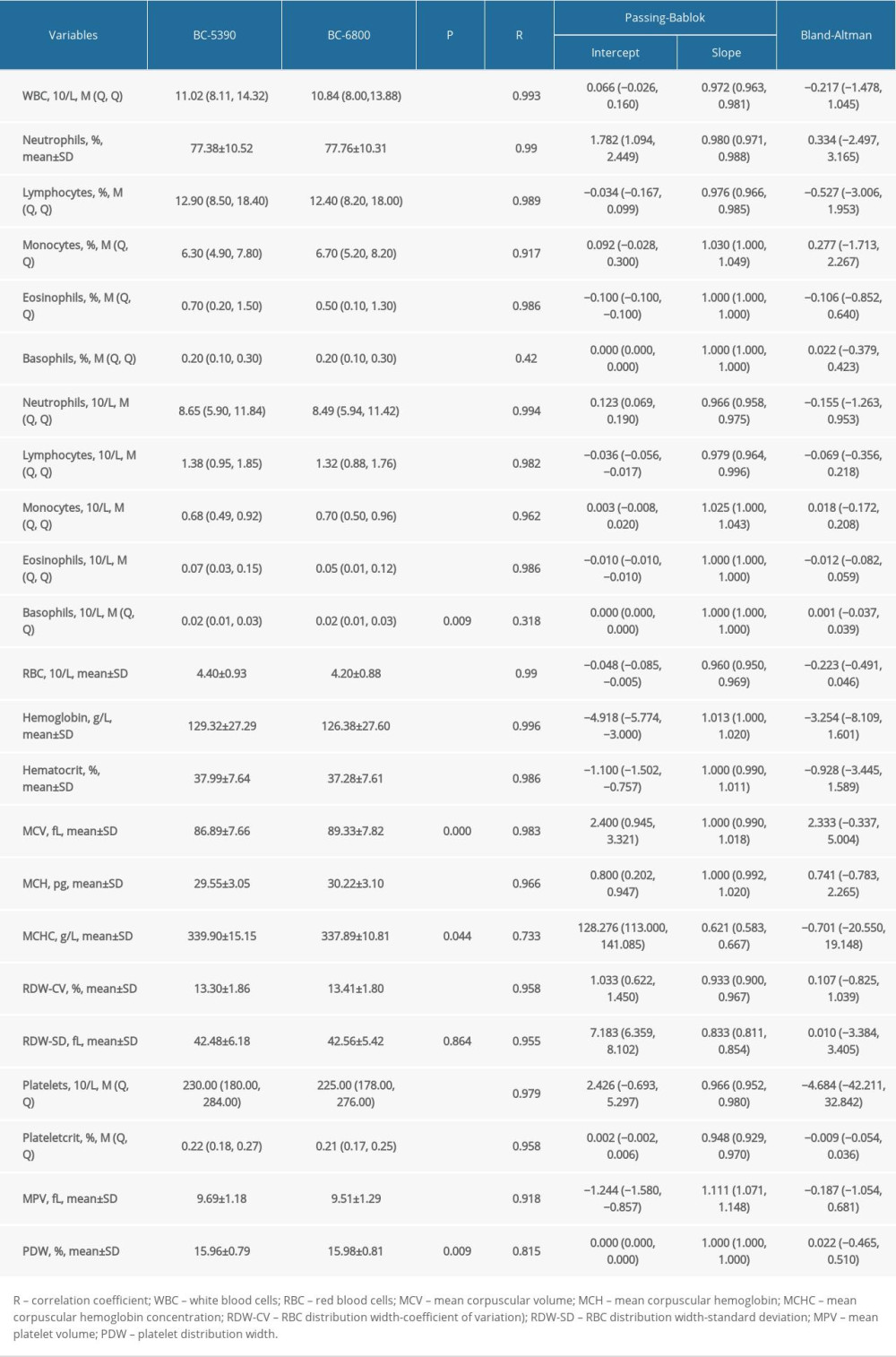
The blood laboratory results from 847 patients were used to compare the consistency of the Mindray BC-5390 and BC-6800 in routine blood indicators. The results of difference analysis showed that there were statistically significant differences in all indicators except RDW-SD (P<0.05). The summary results of the Pearson correlation analysis, Passing-Bablok analysis, and Bland-Altman test indicated that the BC-5390 and BC-6800 had deviations in the measurement of hemoglobin, MCV, MCHC, RDW-CV, RDW-SD, and MPV (Table 1).
The consistency of the indicator direction was evaluated by effect analysis, as shown in Figure 5. The results showed that the distribution of the BC6800 and BC5390 analyzers in the 3 directions of low, normal, and high measured values were in the same direction (on the same side of the dot). The directions of the BC6800 and BC5390 were relatively consistent, indicating that the 2 instruments are interchangeable.
Discussion
CRP is an acute-phase inflammatory protein, which shows increased expression under inflammatory conditions. The routine determination of CRP in clinical situations is based on analyzers, ELISA, and lateral flow assays [9]. In clinical practice, CRP levels can be measured by different types of instruments, and it is necessary to assess the consistency of the different instruments. This study evaluated the consistency of 3 instruments to measure CRP. The results showed that the CRP values measured by the Mindray BC5390, Mindray BC6800, and Johnson Vitros5600 were different. The CRP measurement results of the Mindray BC5390 analyzer were consistent with those of the Mindray BC6800 and Johnson Vitros5600 analyzers. However, there was a constant deviation in the CRP values measured by the Mindray BC-6800 and Johnson Vitros5600 analyzer. Furthermore, the Mindray analyzers consumed less time and cost in testing than the Johnson Vitros5600 analyzer. For the measurement of routine blood tests, the BC5390 analyzer and BC6800 analyzer were found to be interchangeable.
The available evidence indicates that CRP is highly expressed in rheumatoid arthritis, certain cardiovascular diseases and infections, and other inflammatory diseases [6,15,16]. Transcriptional induction of the CRP gene occurs primarily in liver cells in response to increased levels of inflammatory cytokines, such as interleukin-6 [17]. CRP is an acute-phase protein, and therefore, its plasma concentrations change significantly in the presence of inflammatory disease [18,19]. During bacterial infections, the maximum concentration of serum CRP levels can increase by up to 1000 times that of baseline levels [20]. However, when the stimulation ends, the CRP value decreases exponentially for 18 to 20 h [21]. Because of these characteristics, CRP has become an important biomarker in clinical practice [22–25].
There are more than 10 detection methods for CRP, of which turbidimetry-based assays and ELISA are the most widely used in clinical practice [9,26,27]. Clinical chemistry analyzers based on turbidimetric principles are used in large clinical settings, while commercially approved ELISA kits are preferred in dispersed and remote settings [9]. In the present study, the Mindray BC-5390, Mindray BC-6800, and Johnson Vitros5600 analyzers were based on the principle of the turbidimetric method to detect CRP value [10,28]. The difference was that the Mindray analyzers used immunity transmission turbidity, while the Johnson Vitros5600 analyzer used immune scatter turbidity. Different detection systems, reagents, and methodological principles have different detection results for the same item. Our results showed that the CRP measurement results of the Mindray BC-5390 analyzer were consistent with the results of the Mindray BC-6800 and Johnson Vitros5600 analyzers. However, the CRP values measured by the Mindray BC-6800 and Johnson Vitros5600 analyzer had a constant deviation. The difference between the 2 analyzers may have been related to a certain difference in the performance of the different instruments. Furthermore, there are also reports on the use of the Mindray BC-6800 analyzer outside of China, such as in Poland [29], Spain [30], Korea [31], and Italy [32].
In the large-scale clinical practice environment, many factors should be considered in the test instrument, such as the price of the instrument, accuracy, test time, test cost, and the difficulty of sample acquisition. The results of this study indicated that the Mindray analyzers required less time and cost to test CRP values than the Johnson Vitros5600 analyzer. In terms of sample detection, the Mindray analyzers used whole blood for testing, and almost no manual intervention was required, while the Johnson Vitros5600 analyzer used serum, which required blood samples to be processed before measurement. In addition, the Johnson Vitros5600 analyzer (41 μL serum) consumed more blood when measuring CRP, while the Mindray analyzers (20 μL whole blood) used less blood, and can test routine blood values and CRP at the same time. For patients with difficulty in taking a blood sample, such as infants, the Mindray analyzers may be more suitable for detection in these populations.
Conclusions
The consistency of 3 serum CRP analyzers using turbidimetry was analyzed. The measurement of CRP by the Mindray BC5390 analyzer was consistent with that of the Mindray BC6800 and Johnson Vitros5600 analyzers. A constant deviation was observed in the CRP values measured by the Mindray BC-6800 and Johnson Vitros5600 analyzer. The Mindray analyzers required less time and cost in CRP measurement than the Johnson Vitros5600 analyzer. In addition, the BC5390 analyzer and BC6800 analyzer were found to be interchangeable for the measurement of routine blood values.
Figures
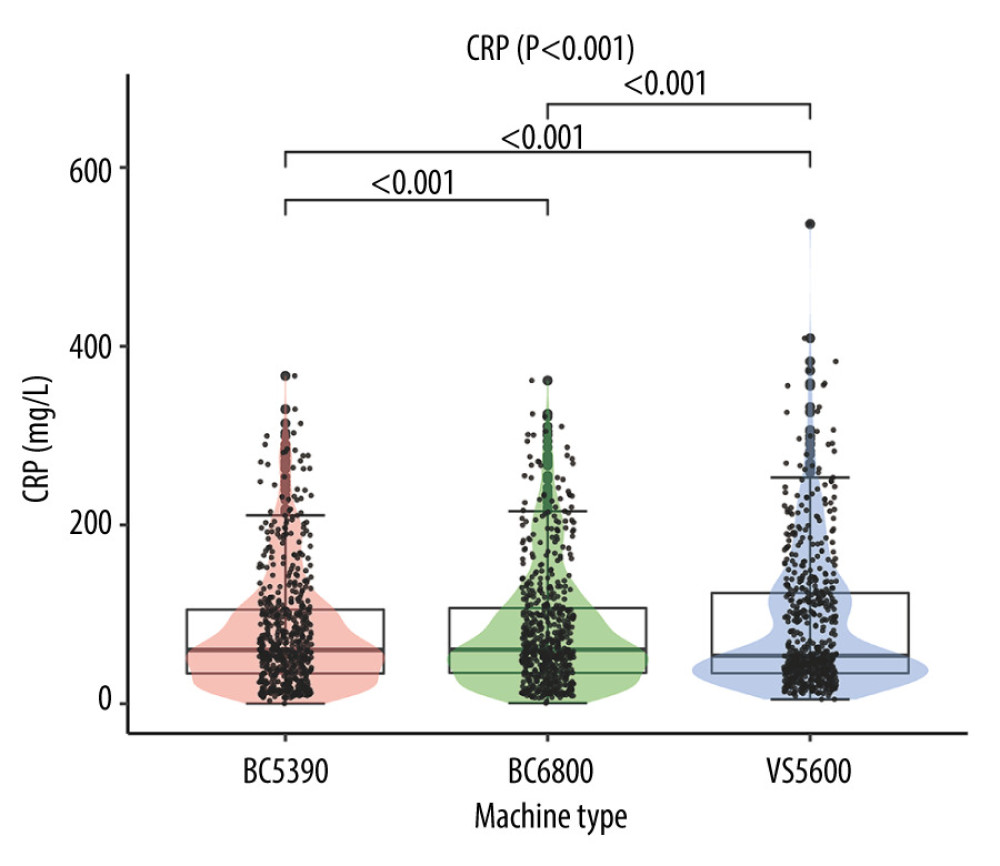 Figure 1. Difference analysis of C-reactive protein levels tested by the Mindray BC-5390, Mindray BC-6800, and Johnson Vitros5600.
Figure 1. Difference analysis of C-reactive protein levels tested by the Mindray BC-5390, Mindray BC-6800, and Johnson Vitros5600. 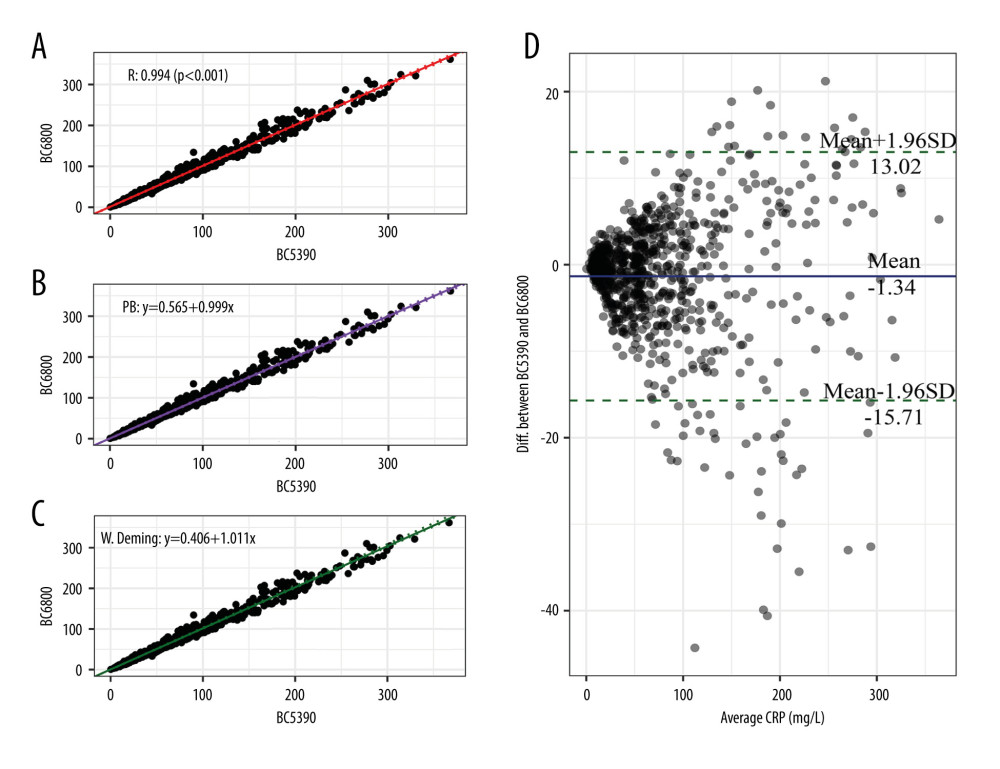 Figure 2. Consistency analysis and Bland-Altman difference plot of C-reactive protein levels between the Mindray BC-5390 and Mindray BC-6800. (A) Pearson correlation analysis; (B) Passing-Bablok analysis; (C) weighted Deming analysis; and (D) Bland-Altman test.
Figure 2. Consistency analysis and Bland-Altman difference plot of C-reactive protein levels between the Mindray BC-5390 and Mindray BC-6800. (A) Pearson correlation analysis; (B) Passing-Bablok analysis; (C) weighted Deming analysis; and (D) Bland-Altman test. 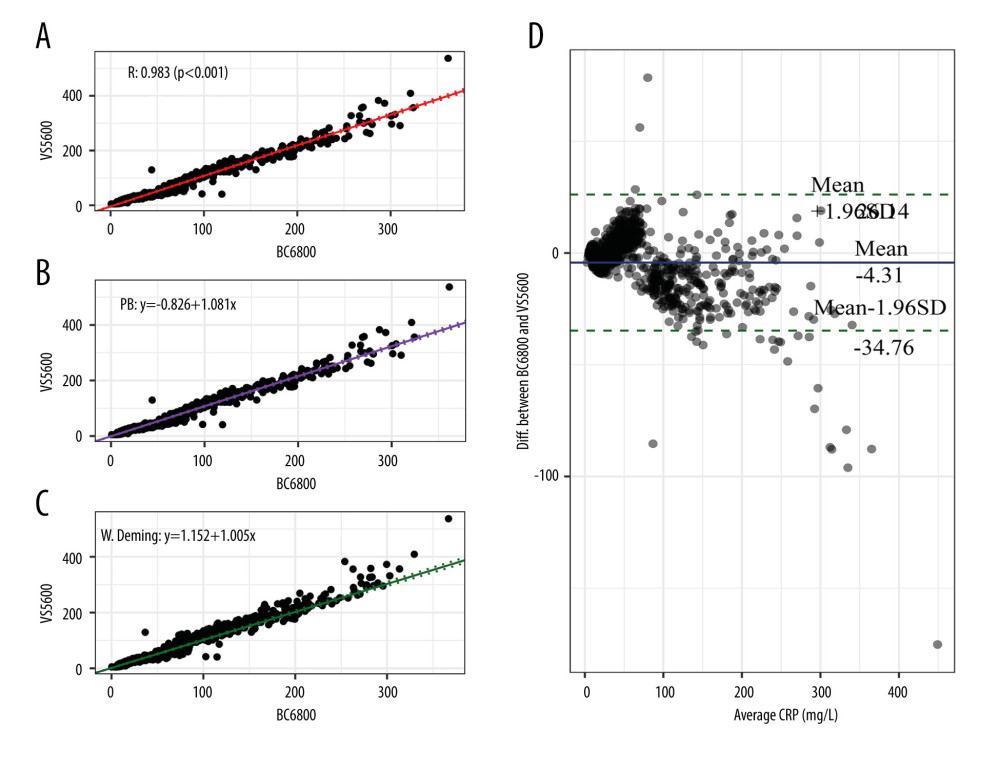 Figure 3. Consistency analysis and Bland-Altman difference plot of C-reactive protein levels between the Johnson Vitros5600 and Mindray BC-6800. (A) Pearson correlation analysis; (B) Passing-Bablok analysis; (C) weighted Deming analysis; (D) Bland-Altman test.
Figure 3. Consistency analysis and Bland-Altman difference plot of C-reactive protein levels between the Johnson Vitros5600 and Mindray BC-6800. (A) Pearson correlation analysis; (B) Passing-Bablok analysis; (C) weighted Deming analysis; (D) Bland-Altman test. 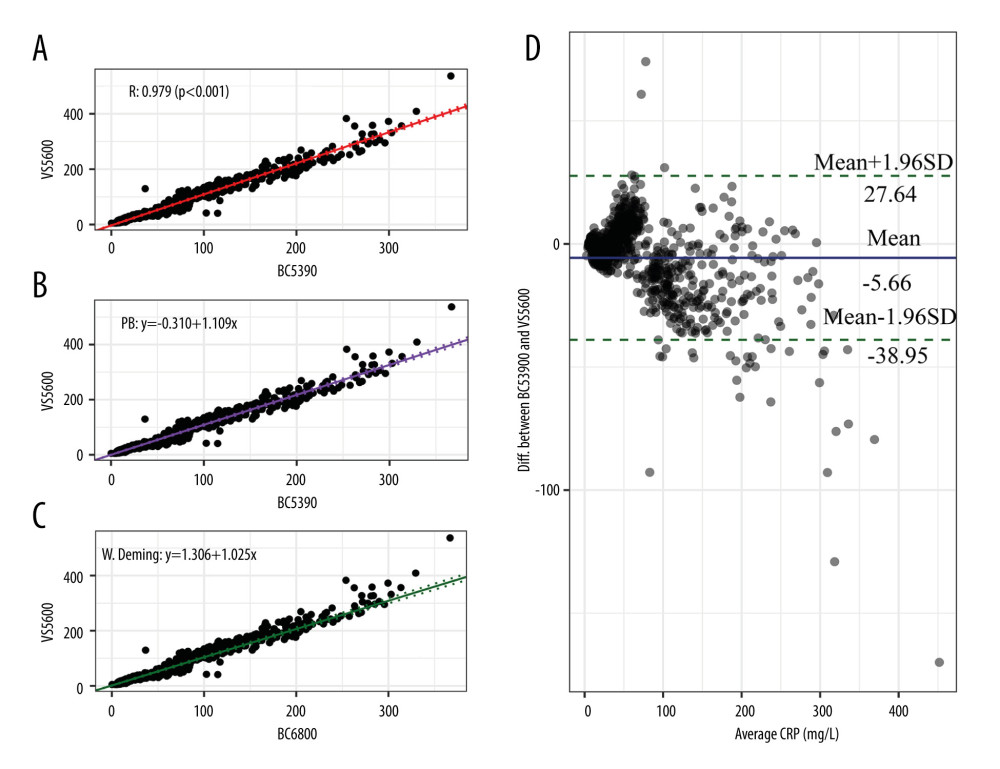 Figure 4. Consistency analysis and Bland-Altman difference plot of C-reactive protein levels between the Johnson Vitros5600 and Mindray BC-5390. (A) Pearson correlation analysis; (B) Passing-Bablok analysis; (C) weighted Deming analysis; (D) Bland-Altman test.
Figure 4. Consistency analysis and Bland-Altman difference plot of C-reactive protein levels between the Johnson Vitros5600 and Mindray BC-5390. (A) Pearson correlation analysis; (B) Passing-Bablok analysis; (C) weighted Deming analysis; (D) Bland-Altman test. 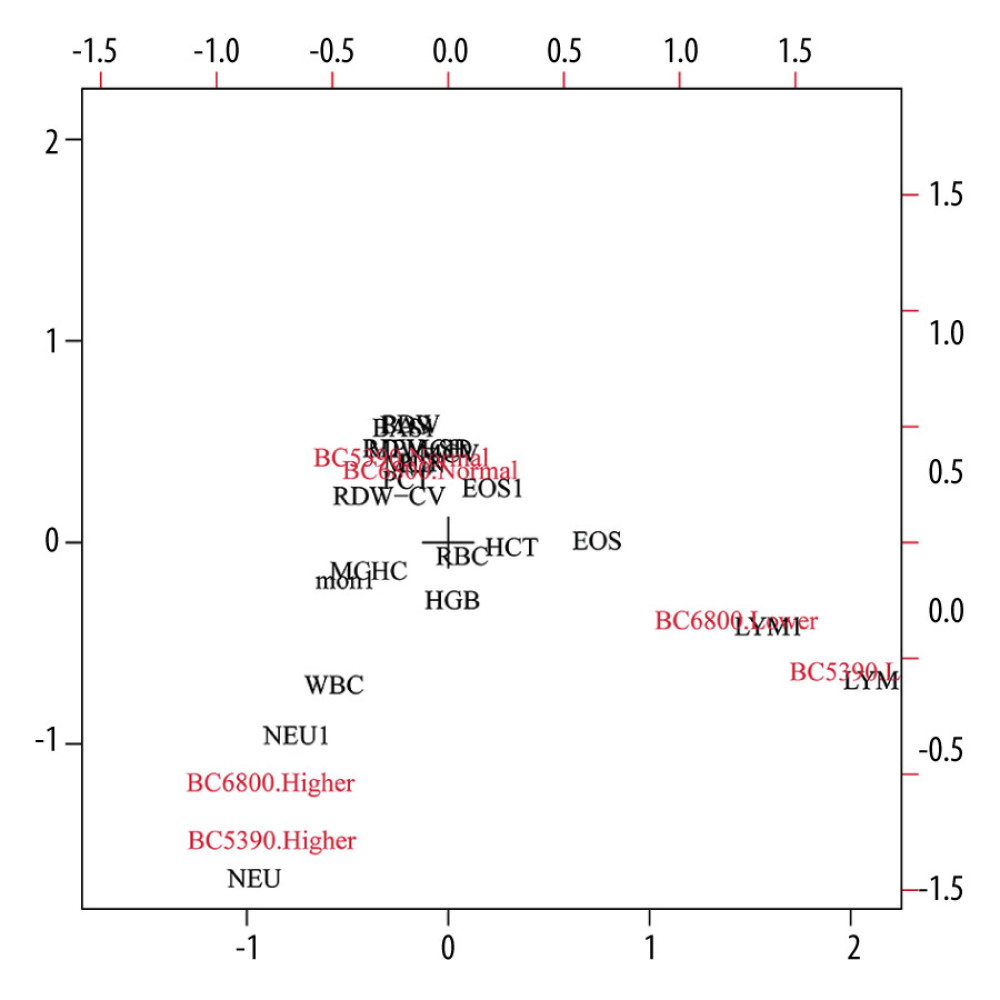 Figure 5. Consistency analysis of indicator direction between the Mindray BC-5390 and Mindray BC-6800.
Figure 5. Consistency analysis of indicator direction between the Mindray BC-5390 and Mindray BC-6800. References
1. Pepys MB, Hirschfield GM, C-reactive protein: A critical update: J Clin Invest, 2003; 111; 1805-12
2. Hage FG, Szalai AJ, C-reactive protein gene polymorphisms, C-reactive protein blood levels, and cardiovascular disease risk: J Am Coll Cardiol, 2007; 50; 1115-22
3. Sproston NR, Ashworth JJ, Role of C-reactive protein at sites of inflammation and infection: Front Immunol, 2018; 9; 754
4. Cahill LE, Bertoia ML, Aroner SA, New and emerging biomarkers in cardiovascular disease: Curr Diab Rep, 2015; 15; 88
5. Yeh ET, CRP as a mediator of disease: Circulation, 2004; 109(21 Suppl); II11-14
6. Luan YY, Yao YM, The clinical significance and potential role of c-reactive protein in chronic inflammatory and neurodegenerative diseases: Front Immunol, 2018; 9; 1302
7. Di Napoli M, Slevin M, Popa-Wagner A, Monomeric C-reactive protein and cerebral hemorrhage: From bench to bedside: Front Immunol, 2018; 9; 1921
8. Ridker PM, MacFadyen JG, Glynn RJ, Comparison of interleukin-6, C-reactive protein, and low-density lipoprotein cholesterol as biomarkers of residual risk in contemporary practice: Secondary analyses from the Cardiovascular Inflammation Reduction Trial: Eur Heart J, 2020; 41; 2952-61
9. Vashist SK, Venkatesh AG, Marion Schneider E, Bioanalytical advances in assays for C-reactive protein: Biotechnol Adv, 2016; 34; 272-90
10. Mulinganya G, Balolebwami S, Zigabe S, Evaluation of a turbidimetric C-reactive protein assay to monitor early-onset neonatal sepsis in South Kivu (Democratic Republic of the Congo): Clin Chem Lab Med, 2021; 59; 625-30
11. Islam MS, Kang SH, Chemiluminescence detection of label-free C-reactive protein based on catalytic activity of gold nanoparticles: Talanta, 2011; 84; 752-58
12. Bryan T, Luo X, Bueno PR, Davis JJ, An optimised electrochemical biosensor for the label-free detection of C-reactive protein in blood: Biosens Bioelectron, 2013; 39; 94-98
13. Bravin C, Amendola V, Wide range detection of C-Reactive protein with a homogeneous immunofluorimetric assay based on cooperative fluorescence quenching assisted by gold nanoparticles: Biosens Bioelectron, 2020; 169; 112591
14. Houyhongthong V, Nunphuak W, Sripatumtong C, Automated nucleated red blood cell count using the Mindray BC-6800 hematology analyzer: Int J Lab Hematol, 2018; 40; 611-16
15. Del Angel-Pablo AD, Buendía-Roldán I, Mejía M, Anti-HLA class II antibodies correlate with C-reactive protein levels in patients with rheumatoid arthritis associated with interstitial lung disease: Cells, 2020; 9; 691
16. Avan A, Tavakoly Sany SB, Ghayour-Mobarhan M, Serum C-reactive protein in the prediction of cardiovascular diseases: Overview of the latest clinical studies and public health practice: J Cell Physiol, 2018; 233; 8508-25
17. Boras E, Slevin M, Alexander MY, Monomeric C-reactive protein and Notch-3 co-operatively increase angiogenesis through PI3K signalling pathway: Cytokine, 2014; 69; 165-79
18. Gabay C, Kushner I, Acute-phase proteins and other systemic responses to inflammation: N Engl J Med, 1999; 340; 448-54
19. Agca R, Heslinga M, Kneepkens EL, The effects of 5-year etanercept therapy on cardiovascular risk factors in patients with psoriatic arthritis: J Rheumatol, 2017; 44; 1362-68
20. Wu Y, Potempa LA, El Kebir D, Filep JG, C-reactive protein and inflammation: Conformational changes affect function: Biol Chem, 2015; 396; 1181-97
21. Ridker PM, Clinical application of C-reactive protein for cardiovascular disease detection and prevention: Circulation, 2003; 107; 363-69
22. Poole L, Steptoe A, The combined association of depressive symptoms and C-reactive protein for incident disease risk up to 12 years later. Findings from the English Longitudinal Study of Ageing (ELSA): Brain Behav Immun, 2020; 88; 908-12
23. Steiner J, Frodl T, Schiltz K, Innate immune cells and C-reactive protein in acute first-episode psychosis and schizophrenia: Relationship to psychopathology and treatment: Schizophr Bull, 2020; 46; 363-73
24. Peres LC, Mallen AR, Townsend MK, High levels of C-reactive protein are associated with an increased risk of ovarian cancer: Results from the Ovarian Cancer Cohort Consortium: Cancer Res, 2019; 79; 5442-51
25. Opotowsky AR, Valente AM, Alshawabkeh L, Prospective cohort study of C-reactive protein as a predictor of clinical events in adults with congenital heart disease: Results of the Boston adult congenital heart disease biobank: Eur Heart J, 2018; 39; 3253-61
26. Zhang L, Li HY, Li W, An ELISA assay for quantifying monomeric C-reactive protein in plasma: Front Immunol, 2018; 9; 511
27. Vashist SK, Czilwik G, van Oordt T, One-step kinetics-based immunoassay for the highly sensitive detection of C-reactive protein in less than 30 min: Anal Biochem, 2014; 456; 32-37
28. Coletta G, Amendola V, Numerical modelling of the optical properties of plasmonic and latex nanoparticles to improve the detection limit of immuno-turbidimetric assays: Nanomaterials (Basel), 2021; 11; 1147
29. Ciepiela O, Kotuła I, Kierat S, A Comparison of mindray BC-6800, Sysmex XN-2000, and Beckman coulter LH750 automated hematology analyzers: A pediatric study: J Clin Lab Anal, 2016; 30; 1128-34
30. Fuster O, Andino B, Pardo A, Laiz B, Continuous ambulatory peritoneal dialysis, ascitic and pleural body fluids evaluation with the Mindray BC-6800 hematology analyzer: J Clin Lab Anal, 2018; 32; e22240
31. Lee HT, Park PW, Seo YH, Performance evaluation of Mindray CAL 8000(BC-6800 and SC-120) hematology analyzer and slidemaker/stainer: J Clin Lab Anal, 2017; 31; e22065
32. La Gioia A, Fumi M, Fiorini F, Mindray BC-6800 haematological analyser: 3D-DIFF scattergram usefulness in infectious mononucleosis diagnosis: Int J Lab Hematol, 2021; 43; 581-87
Figures
 Figure 1. Difference analysis of C-reactive protein levels tested by the Mindray BC-5390, Mindray BC-6800, and Johnson Vitros5600.
Figure 1. Difference analysis of C-reactive protein levels tested by the Mindray BC-5390, Mindray BC-6800, and Johnson Vitros5600. Figure 2. Consistency analysis and Bland-Altman difference plot of C-reactive protein levels between the Mindray BC-5390 and Mindray BC-6800. (A) Pearson correlation analysis; (B) Passing-Bablok analysis; (C) weighted Deming analysis; and (D) Bland-Altman test.
Figure 2. Consistency analysis and Bland-Altman difference plot of C-reactive protein levels between the Mindray BC-5390 and Mindray BC-6800. (A) Pearson correlation analysis; (B) Passing-Bablok analysis; (C) weighted Deming analysis; and (D) Bland-Altman test. Figure 3. Consistency analysis and Bland-Altman difference plot of C-reactive protein levels between the Johnson Vitros5600 and Mindray BC-6800. (A) Pearson correlation analysis; (B) Passing-Bablok analysis; (C) weighted Deming analysis; (D) Bland-Altman test.
Figure 3. Consistency analysis and Bland-Altman difference plot of C-reactive protein levels between the Johnson Vitros5600 and Mindray BC-6800. (A) Pearson correlation analysis; (B) Passing-Bablok analysis; (C) weighted Deming analysis; (D) Bland-Altman test. Figure 4. Consistency analysis and Bland-Altman difference plot of C-reactive protein levels between the Johnson Vitros5600 and Mindray BC-5390. (A) Pearson correlation analysis; (B) Passing-Bablok analysis; (C) weighted Deming analysis; (D) Bland-Altman test.
Figure 4. Consistency analysis and Bland-Altman difference plot of C-reactive protein levels between the Johnson Vitros5600 and Mindray BC-5390. (A) Pearson correlation analysis; (B) Passing-Bablok analysis; (C) weighted Deming analysis; (D) Bland-Altman test. Figure 5. Consistency analysis of indicator direction between the Mindray BC-5390 and Mindray BC-6800.
Figure 5. Consistency analysis of indicator direction between the Mindray BC-5390 and Mindray BC-6800. In Press
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952