16 March 2022: Clinical Research
Effect of Cerebral Microbleeds on Cognitive Function and Quality of Life in Parkinson Disease
Qixiong Qin1ABCDEF, Hengming Wan2C, Danlei Wang1B, Jingyi Li1B, Qingmei Yang1B, Jingwei Zhao1B, Zheng Xue1ADFG*DOI: 10.12659/MSM.935026
Med Sci Monit 2022; 28:e935026
Abstract
BACKGROUND: This study aimed to investigate the risk factors and patterns of cerebral microbleeds (CMBs) in Parkinson disease (PD) and the impact of CMBs on cognitive function and quality of life (QoL).
MATERIAL AND METHODS: Patients with PD that underwent susceptibility-weighted imaging were recruited and divided into CMB-free, lobar-CMB, deep-CMB, and mixed-CMB groups according to CMB location. Motor function (MDS-UPDRS III), cognitive abilities (MoCA, MMSE), and QoL (PDQ-39) were compared among groups. The risk factors for CMBs in patients with PD and the association between CMBs and cognition and QoL were analyzed using multivariable logistic regression models and linear regression models.
RESULTS: Among the 209 patients with PD, 42 (20.1%) had CMBs. Lobar, deep, and mixed CMBs were observed in 15 (35.7%), 17 (40.5%), and 10 (23.8%) patients, respectively. A higher frequency of hypertension was independently associated with deep CMBs (odds ratio [OR]=4.379, 95% CI: 1.405-13.643, P=0.011). The deep-CMB and mixed-CMB groups had lower MoCA scores and MMSE scores than the CMB-free group, especially in domains of naming, attention, and orientation (P<0.05). Additionally, the presence of CMBs was associated with lower MMSE (R²=0.140, β=-0.301, P<0.001) and MoCA (R²=0.104, β=-0.289, P<0.001) and higher PDQ-39 (R²=0.052, β=0.227, P<0.05) scores, while the association between CMBs and PDQ-39 disappeared after adjustment of MMSE or MoCA as a covariate.
CONCLUSIONS: The results suggest that hypertension was associated with the occurrence of deep CMBs. Comorbidity with CMBs may impair cognitive function and indirectly reduce the QoL in patients with PD.
Keywords: Cerebral Small Vessel Diseases, Cognition, Parkinson Disease, Quality of Life, Brain, Cerebrovascular Circulation, Female, Follow-Up Studies, Humans, Magnetic Resonance Imaging, Male, Neuropsychological Tests
Background
Parkinson disease (PD) is the second most common neurodegenerative disorder after Alzheimer disease [1]. Cognitive impairment is one of the most severe non-motor symptoms of PD, and up to 80% of patients eventually develop dementia, which significantly affects the prognosis and quality of life (QoL) of patients with PD [2]. Although the prevalence of cognitive dysfunction is high in patients with PD, the underlying pathophysiology is still unclear.
Cerebral microbleeds (CMBs) are a subclinical lesion of cerebral parenchyma caused by microvascular lesions in the brain, which present as homogenous hypointense lesions on imaging [3]. Previous studies showed that CMBs affect cognition in healthy older adults and patients with ischemic stroke or Alzheimer disease [4–6]. Recently, CMBs were found in approximately 15% to 20% of patients with PD, especially those with dementia [7,8]. However, a study found that although CMBs were not rare in patients with PD, the comorbidity with CMBs did not affect various cognitive domains [9]. Thus, it remains controversial whether CMBs affect the cognitive function of patients with PD. The controversy may be related to the use of different magnetic resonance imaging (MRI) scanner types and MRI sequences to evaluate CMBs. Moreover, the assessment scales of cognitive function and the location of CMBs may also be important reasons for the inconsistent results.
Susceptibility-weighted imaging (SWI) that provides image contrast enhancement based on the T2-weighted gradient-echo has a unique display ability for detecting CMBs with a diameter of 2 to 5 mm, which cannot be detected by computed tomography and conventional MRI [10,11]. Based on the detection of CMBs by SWI, the purpose of the present study was to (1) explore the risk factors for CMBs at different locations, (2) explore the cognitive function and QoL in patients with PD with CMBs at different locations, and (3) explore the association of CMB burden and cognitive function and QoL. Our study may help clarify the potential correlation between CMBs and cognition and QoL in patients with PD and the value of appropriate management of CMBs in the treatment of PD.
Material and Methods
PATIENT SELECTION:
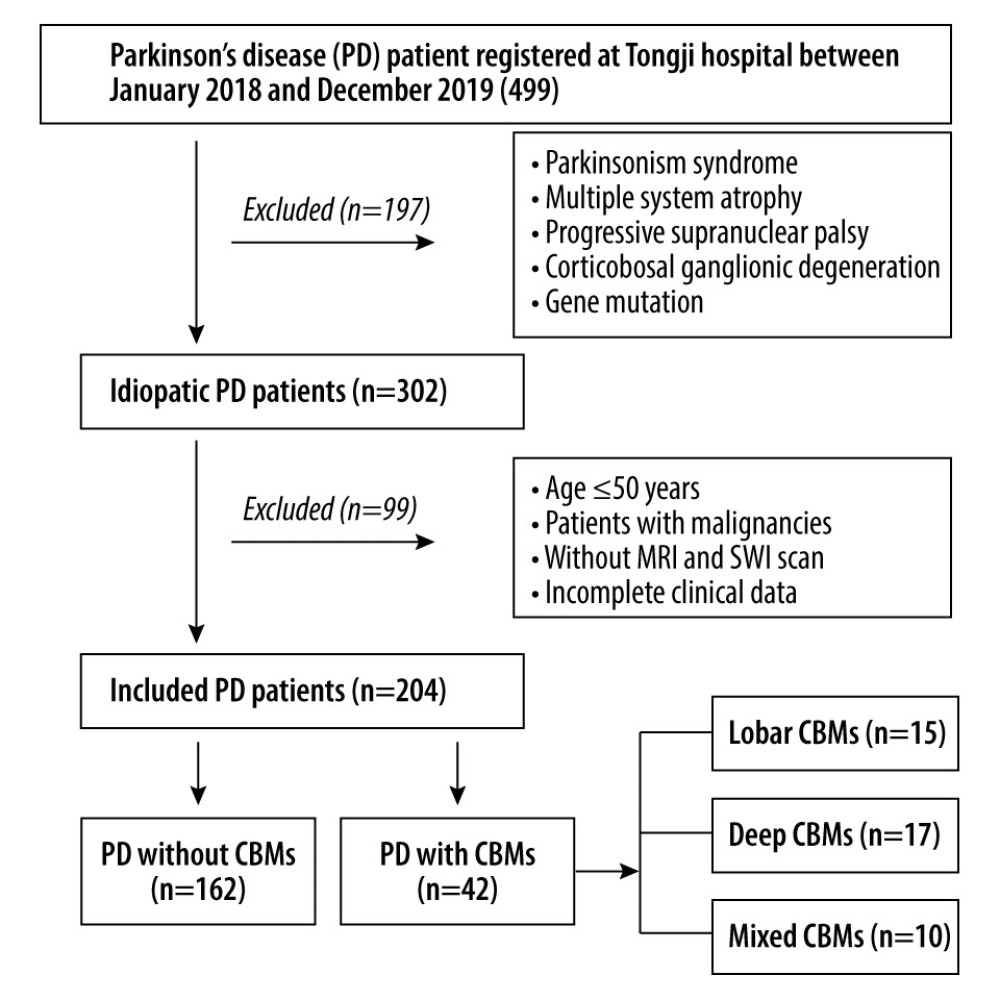
We retrospectively included patients with PD whose age of onset was >50 years and who underwent SWI in the Department of Neurology of Tongji Hospital from January 2018 to December 2019. PD was diagnosed according to the Movement Disorder Society (MDS) Clinical Diagnostic Criteria for PD [12]. Patients with parkinsonian syndrome, hereditary PD, multiple system atrophy, progressive supranuclear palsy, or corticobasal ganglionic degeneration were excluded. Patients with encephalitis, epilepsy, traumatic brain injury, cancer, or severe psychiatric illness were also excluded. Moreover, patients with a contraindication to MRI scan or incomplete medical records were excluded. To avoid the potential influence of gene mutation, patients with an onset age ≤50 years were excluded. The patient screening process is depicted in Figure 1. Our study was approved by the Institutional Review Board of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (TJ-IRB20211117).
SAMPLE SIZE ESTIMATION:
PASS 15 Power Analysis and Sample Size Software (2017) (NCSS, LLC, Kaysville, Utah, USA, ncss.com/software/pass.) were used to estimate the sample size of the study population. The incidence of CMBs in patients with PD was reported as approximately 16% in the Chinese population [13]. With α=0.05, 2-tailed, and a power of 80%, 392 patients with PD were needed in our study. However, as shown in Figure 1, based on the inclusion and exclusion criteria, 204 idiopathic patients with PD were enrolled between January 2018 and December 2019. Therefore, we ultimately required 135 patients according to n=n0/(1+n0/N) (n=final sample size, N=total population, n0=sample size calculated by PASS).
MRI DATA ACQUISITION AND CMB ASSESSMENT:
All patients with PD were examined using 3.0-T MRI devices (Discovery MR750, GE Medical Systems, Milwaukee, WI, USA) at Tongji Hospital, Tongji Medical College. The FuncTool software accompanying the 3.0-T MRI devices was used to correct and process the images.
CMBs were homogenous hypointense lesions on T2*-weighted or SWI MRI images, with a diameter of approximately 2 to 10 mm [14]. The location and number of CMBs were determined by 2 trained neurologists who were blinded to the patients’ clinical data, based on the MRI acquired. According to anatomical location, CMBs were classified as lobar, deep, and mixed CMBs (including deep and lobar). The typical characteristics of CMBs in different locations are shown in Supplementary Figure 1.
CLINICAL ASSESSMENT:
Demographic data, including age, sex, education, age at onset, disease duration, vascular risk factors, including hypertension, diabetes, and smoking, and histories of ischemic stroke and coronary heart disease, were recorded. Plasma levels of cholesterol, triglycerides, low-density lipoprotein (LDL), high-density lipoprotein, homocysteine, and uric acid during hospitalization were also recorded. Orthostatic hypotension was defined as a 20-mm Hg drop in systolic blood pressure or a 10-mm Hg drop in diastolic blood pressure within 3 min from lying to standing position, with or without postural symptoms. Levodopa equivalent daily dosage (LEDD) was calculated as described in a previous study [15].
The severity and stage of motor symptoms were assessed with the Unified Parkinson’s Disease Rating Scale Part III (UPDRS III) and Hoehn and Yahr scale (HY) during the off state. Cognitive function was evaluated by the Mini Mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA) [16]. The Hamilton Anxiety Rating Scale (HAMA), Hamilton Depression Rating Scale (HAMD), REM Sleep Behavior Disorder Screening Questionnaire (RBDSQ), and Epworth Sleepiness Scale (ESS) were used to evaluate anxiety, depression, REM sleep behavior disorder (RBD), and daytime sleepiness, respectively. The Parkinson’s Disease Quality of Life Questionnaire, consisting of 39 items (PDQ-39), was used to evaluate QoL [20]. The PDQ-39 scores and subdomain scores were standardized and shown as a summary index. A higher PDQ-39 score indicates a worse QoL.
STATISTICAL ANALYSIS:
Statistical analysis was performed using SPSS version 26.0 (IBM Corp, Armonk, NY, USA). Continuous variables are presented as means and standard deviations or medians and interquartile ranges. Categorical variables are presented as frequencies (%). For comparison among the 4 groups, the chi-squared test was used for categorical variables, the 1-way ANOVA test or Kruskal-Wallis test was used for continuous variables, and the Bonferroni correction method was used for multiple comparisons. Multiple-adjusted logistic regression models were used to explore the risk factors for CMBs. Variables with
Results
CHARACTERISTICS OF PARTICIPANTS:
The general characteristics of patients are shown in Table 1. Of the 204 patients with PD, 42 (20.59%) had CMBs. According to the location of CMBs, patients were divided into a CMB-free group (n=162), lobar-CMB group (n=15), deep-CMB group (n=17), and mixed-CMB group (n=10). The groups did not show differences in sex, age, educational level, disease duration, and LEDD. Compared to that in the CMB-free group, the frequency of hypertension was higher in the lobar-CMB group (22.2% vs 46.7%), deep-CMB group (22.2% vs 52.9%), and mixed-CMB group (22.2% vs 30.0%). Further, a history of ischemic stroke was more common in the deep-CMB group (3.1% vs 17.6%) and mixed-CMB group (3.1% vs 10.0%). In addition, LDL-C levels were higher in the deep-CMB group than in the CMB-free group (3.41±3.27 mmol/L vs 2.47±0.81 mmol/L).
RISK FACTORS FOR CMB IN DIFFERENT REGIONS IN PATIENTS WITH PD:
The associations between hypertension, ischemic stroke, and LDL-C levels and CMBs were analyzed using multivariable-adjusted logistic regression models, and the results are shown in Supplementary Table 1. A higher frequency of hypertension was correlated with lobar CMBs and deep CMBs in model 1, while the association was not significant in models 2 and 3 in the lobar-CMB group. A history of ischemic stroke was associated with deep CMBs in models 1 and 2, while the correlation was not significant in model 3. LDL-C levels were not associated with any CMBs in patients with PD.
COMPARISON OF MOTOR SYMPTOMS AND NON-MOTOR SYMPTOMS AMONG GROUPS:
In cognitive function, the MMSE and MoCA scores were significantly lower in the deep-CMB group and mixed-CMB group than in the CMB-free group (Table 2). The subdomain score of naming and orientation in the mixed-CMB group and attention in the deep-CMB group were lower than in the CMB-free group (all P<0.05). Regarding motor function, rigidity scores differed among the 4 groups (P=0.030), while no difference was found between groups in multiple-adjusted comparisons. There was no difference in HY stage, HAMD, HAMA, RBDSQ, ESS scores, and other subdomains of the UPDRS-II and MoCA among the 4 groups.
ASSESSMENT OF QOL AMONG GROUPS:
QoL assessed by the PDQ-39 is shown in Table 3 and Supplementary Figure 2. QoL was more impaired in patients with CMBs than in those without CMBs (mixed-CMB group >lobar-CMB group >deep-CMB group >CMB-free group). The subdomain score of mobility, activity of daily living, and cognition were significantly different between groups (P=0.035, P=0.023, and P=0.037, respectively). Multiple-adjusted comparisons showed that only activity of daily living remained different between the mixed-CMB and CMB-free groups (50.41±21.73 vs 28.02±20.39, P<0.05).
ASSOCIATION BETWEEN CMBS AND COGNITION AND QOL:
Linear regression analysis showed that the presence of CMBs was associated with lower MMSE (R2=0.140, β=−0.301, P<0.001) and lower MoCA (R2=0.104, β=−0.289, P<0.001) scores in patients with PD after adjustment for sex, age, education levels, disease duration, and LEDD. Regarding QoL, although the presence of CMBs was correlated with more severe mobility (R2=0.033, β=0.181, P=0.011), activity of daily living (R2=0.041, β=0.202, P=0.007), and cognition (R2=0.038, β=0.195, P=0.009), the significance disappeared (P=0.188, P=0.159, P=0.346, respectively) after adjustment for sex, age, education levels, disease duration, LEDD, UPDRS-III, and MMSE or MoCA (Table 4). Additionally, there were significant associations between the PDQ-39 summary index and MMSE (R2=0.130, β=−0.310, P<0.001) and MoCA (R2=0.107, β=−0.257, P=0.000) scores after adjustment for the above variables.
Discussion
The present study demonstrated that CMBs were found in 20.59% of patients with PD. Higher frequency of hypertension was an independent risk factor for the occurrence of deep CMB but not lobar CMB. Patients with CMBs had worse cognitive function in the domains of naming, orientation, and attention. Although after adjustment for MMSE or MoCA, the association between CMBs and QoL was not significant, QoL was independently correlated with cognition. These results suggest that CMB might be an important contributor to cognitive dysfunction and an indirect damaging factor for QoL in patients with PD.
The CMB pattern depends on the underlying pathological mechanisms [17]. Lobar CMBs are related to cerebral amyloid angiopathy involving the leptomeningeal and cortical arteries, while deep or infratentorial CMBs can be related to hypertensive microangiopathy that damages small perforating arteries in the deep gray matter [18,19]. In the present study, a higher frequency of hypertension was identified as an independent risk factor for deep CMBs. These results were partially consistent with the study by Yamashiro et al, in which hypertension, orthostatic hypotension, and a history of ischemic stroke were associated with CMBs [20]. However, there were no significant differences in orthostatic hypotension and history of ischemic stroke between patients with and without CMBs in our study. This was probably due to the strict exclusion criteria of the present study, which excluded patients with parkinsonian syndrome caused by ischemic stroke. Future research with long-term 24-h blood pressure monitoring may help clarify the relationship between orthostatic hypotension and CMB occurrence.
Although several studies suggested that CMBs were not related to general cognitive function, the most recent studies have shown CMBs affect cognitive performance in healthy older adults and patients with ischemic stroke or Alzheimer disease [21]. Importantly, CMBs have been found to be more common in patients with PD with dementia than in those without dementia [4,22], and comorbidity with CMBs can impair cognitive performance and motor function in patients with PD [23,24]. Moreover, location-specific analysis in healthy elderly patients showed that strictly lobar CMBs were associated with a decline in global cognition, executive function, and memory domains, while deep and mixed CMBs were not associated with cognitive decline [25,26]. In the present study, deep CMBs had a negative impact on attention-related cognition, and mixed CMBs damaged naming- and orientation-related cognitive domains, suggesting that the effect of CMBs on cognition of patient with PD may be different from that of healthy elderly patients. Although the exact mechanism underlying the impact of CMBs in cognitive impairment in patients with PD remains unclear, histopathological studies have suggested that the presence of CMBs indicates extensive damage to arteries and pathological changes such as glial cell proliferation or neuronal cell necrosis. This ultimately leads to the destruction of nerve conduction bundles related to cognition in the white matter [27,28]. Hence, we speculated that CMBs might impair cognitive function in patients with PD by disturbing the connection of nerve conduction bundles.
QoL is considered a vital outcome indicator in patients with PD [29]. A broad list of motor and non-motor symptoms was shown to be important contributors to QoL in patients with PD [30,31]. In addition, cognitive impairment and severe depression were significantly correlated with poor QoL [32–34]. However, there is a lack of evidence on the relationships between the presence of CMBs and QoL. In the present study, we found that the presence of CMBs was associated with QoL in patients with PD, especially in the domains of mobility, activity of daily living, and cognition, while the significant association of CMBs with QoL disappeared after adjusting MMSE or MoCA scores as a covariate. Because patients with PD have more severe cognitive disorders, we speculated that CMBs might indirectly affect the QoL of patients with PD by impairing cognitive function.
There are several limitations to our study. First, as a single-center retrospective study, there was an inevitable selection bias because not all hospitalized patients underwent SWI. Moreover, almost half of the patients with PD were excluded because they did not meet the inclusion and exclusion criteria. Second, although subgroup analysis of CMBs was done in our study, the number of patients with CMBs was relatively small; thus, the risk factors of CMBs and the correlation between CMBs and cognition and QoL in PD need further investigation. Third, we excluded patients with early-onset PD; therefore, the results may not represent the frequency of CMB-positive patients in the overall PD population. A multicenter, larger-population, and longitudinal long-term follow-up study is needed to confirm our findings and clarify the impact of CMBs on cognitive function and QoL in patients with PD.
Conclusions
In conclusion, the present study demonstrated that CMBs were common in patients with PD and hypertension associated with deep CMBs. The presence of CMBs affected cognitive performance, particularly in the domains of naming, orientation, and attention, thus affecting the QoL of patients with PD. Clinically, hypertension management in patients with PD may be essential to reduce the incidence of CMBs, thereby reducing CMB-associated impairment of cognitive performance and QoL in patients with PD.
Tables
Table 1. Clinical characteristics of patients with PD with or without cerebral microbleeds.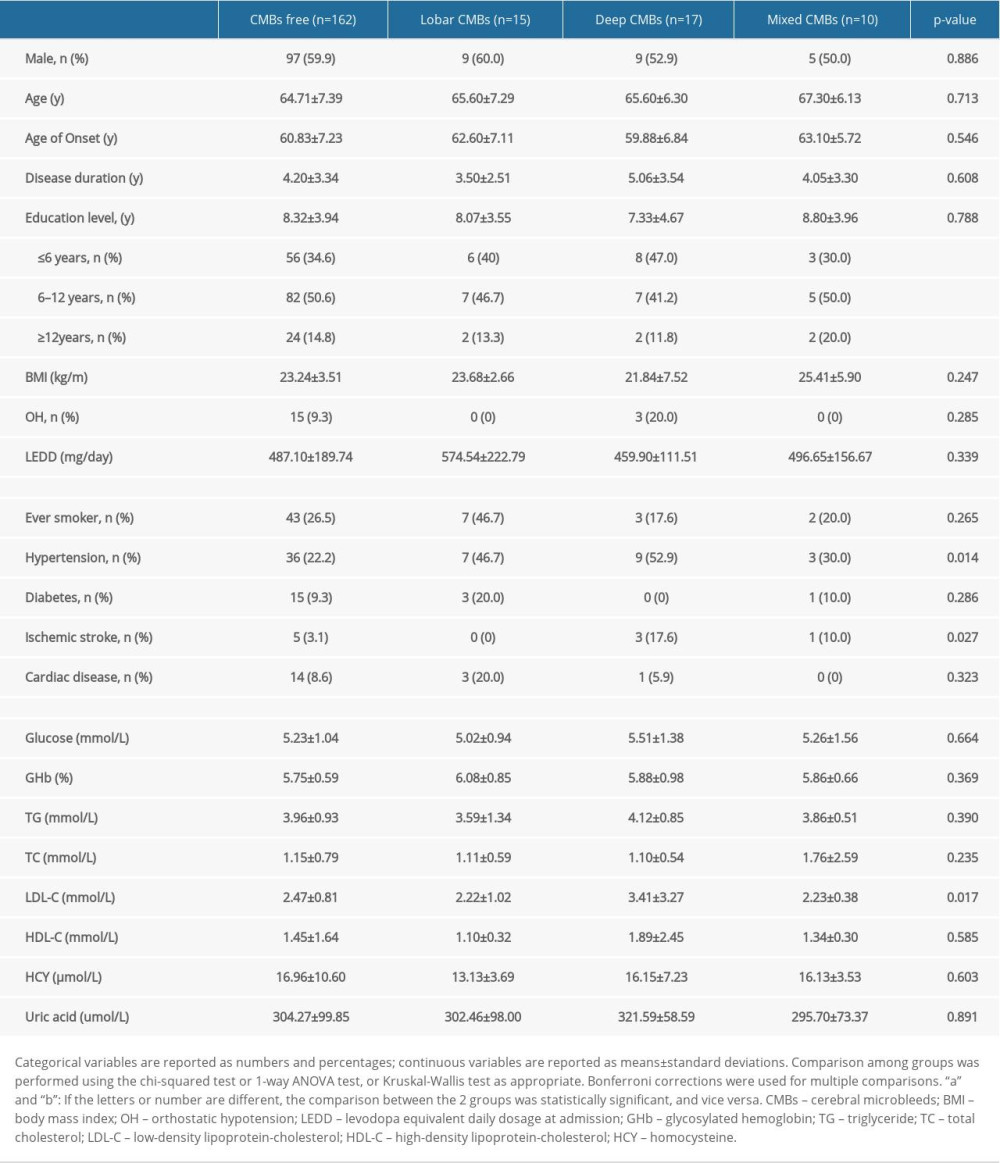 Table 2. Motor symptoms and non-motor symptoms of participants.
Table 2. Motor symptoms and non-motor symptoms of participants.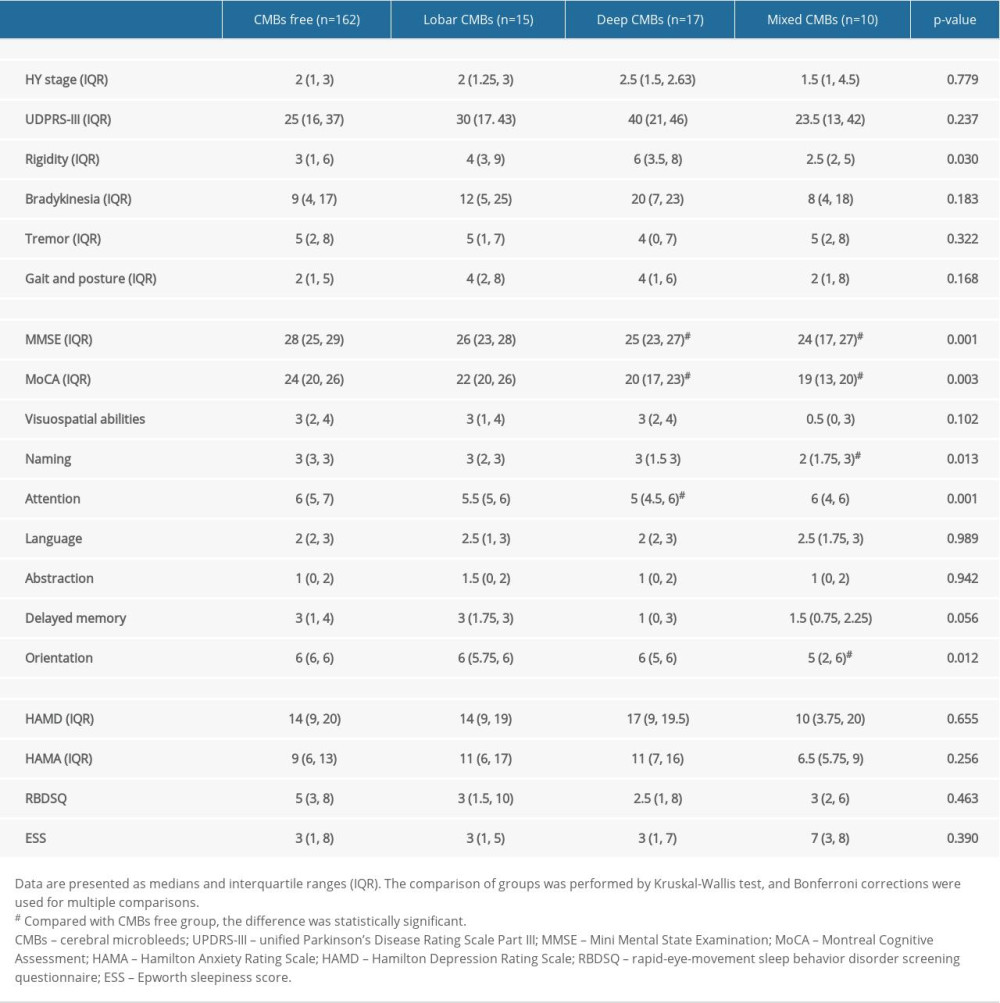 Table 3. Quality of life assessment in patients with different locations of cerebral microbleeds.
Table 3. Quality of life assessment in patients with different locations of cerebral microbleeds.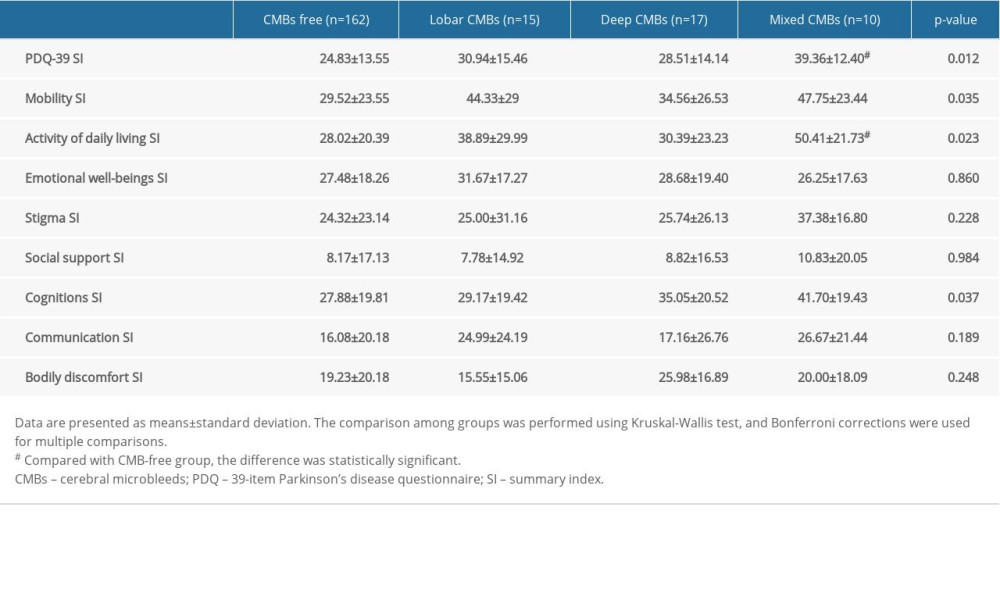 Table 4. The associations of cerebral microbleeds (CMBs) with cognition and quality of life in Parkinson disease.
Table 4. The associations of cerebral microbleeds (CMBs) with cognition and quality of life in Parkinson disease.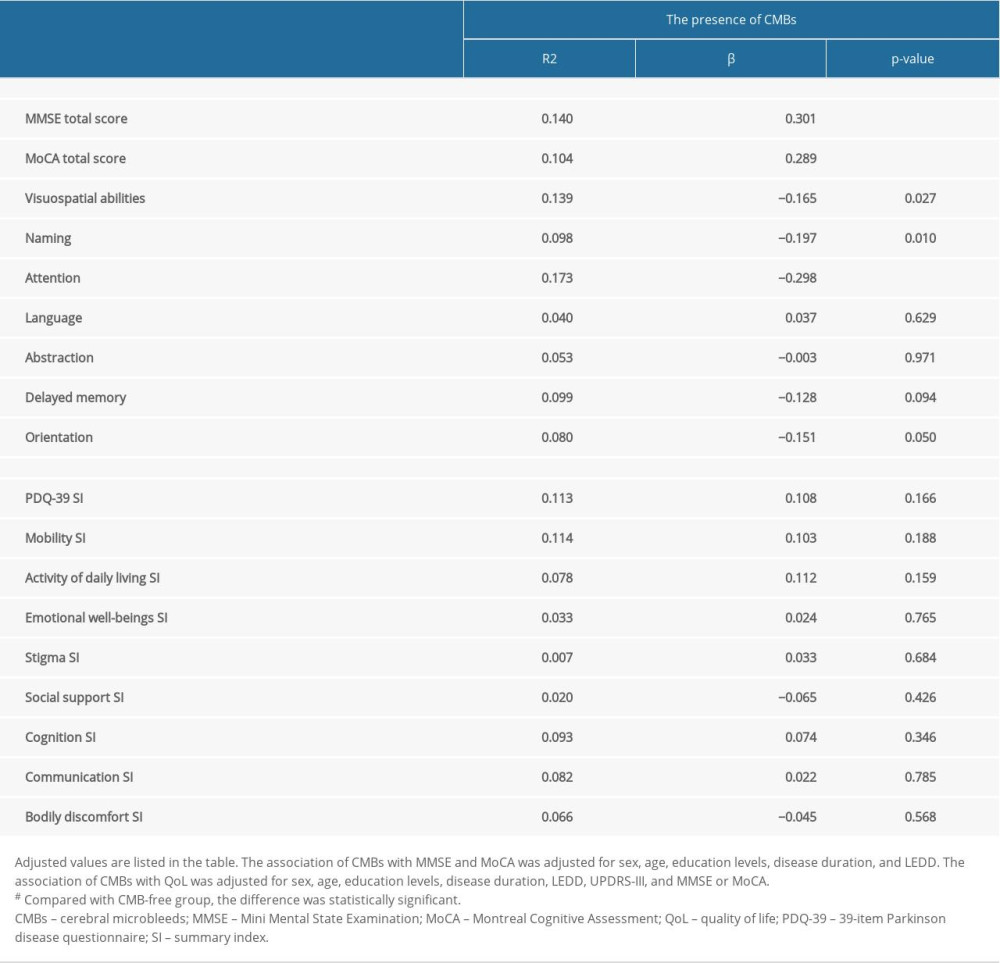 Supplementary Table 1. Identification of risk factors for cerebral microbleeds in patients with Parkinson disease.
Supplementary Table 1. Identification of risk factors for cerebral microbleeds in patients with Parkinson disease.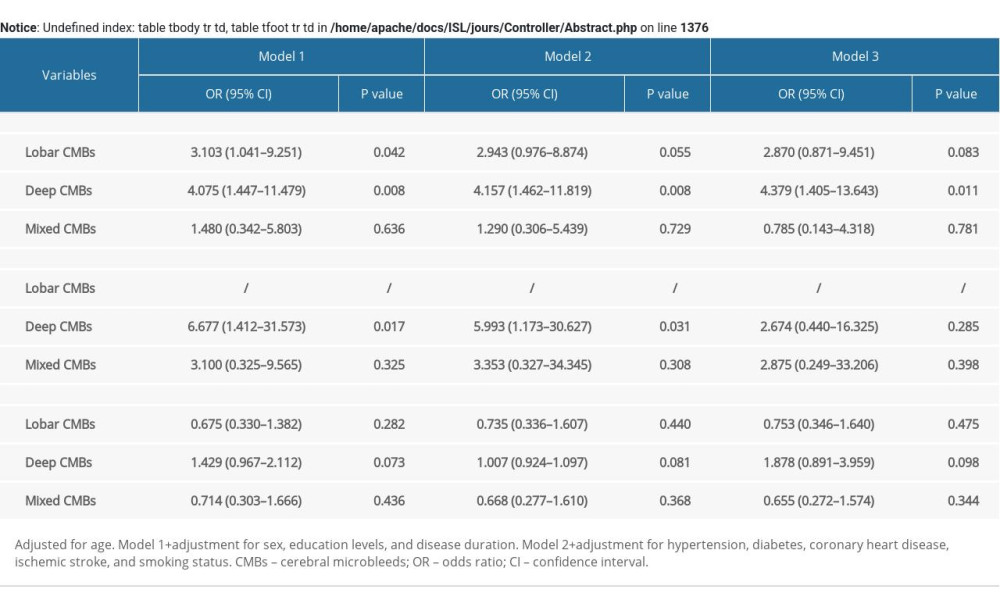
References
1. , Global, regional, and national burden of Parkinson’s disease, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016: Lancet Neurol, 2018; 17(11); 939-53
2. Hely MA, Reid WGJ, Adena MA, The Sydney multicenter study of Parkinson’s disease: The inevitability of dementia at 20 years: Mov Disord, 2008; 23(6); 837-44
3. Greenberg SM, Vernooij MW, Cordonnier C, Cerebral microbleeds: A guide to detection and interpretation: Lancet Neurol, 2009; 8(2); 165-74
4. Ham JH, Yi H, Sunwoo MK, Cerebral microbleeds in patients with Parkinson’s disease: J Neurol, 2014; 261(8); 1628-35
5. Shibata K, Sugiura M, Nishimura Y, The effect of small vessel disease on motor and cognitive function in Parkinson’s disease: Clin Neurol Neurosurg, 2019; 182; 58-62
6. Gregg NM, Kim AE, Gurol ME, Incidental cerebral microbleeds and cerebral blood flow in elderly individuals: JAMA Neurol, 2015; 72(9); 1021-28
7. Kim KJ, Bae YJ, Kim JM, The prevalence of cerebral microbleeds in non-demented Parkinson’s disease patients: J Korean Med Sci, 2018; 33(46); e289
8. Chiu WT, Chan L, Wu D, Cerebral microbleeds are associated with postural instability and gait disturbance subtype in people with Parkinson’s disease: Eur Neurol, 2018; 80(5–6); 335-40
9. Kim JH, Park J, Kim YH, Characterization of cerebral microbleeds in idiopathic Parkinson’s disease: Eur J Neurol, 2015; 22(2); 377-83
10. Shams S, Martola J, Cavallin L, SWI or T2*: Which MRI sequence to use in the detection of cerebral microbleeds? The Karolinska Imaging Dementia Study: Am J Neuroradiol, 2015; 36(6); 1089-95
11. Sepehry AA, Lang D, Hsiung GY, Prevalence of brain microbleeds in Alzheimer disease: A systematic review and meta-analysis on the influence of neuroimaging techniques: Am J Neuroradiol, 2016; 37(2); 215-22
12. Postuma RB, Berg D, Stern M, MDS clinical diagnostic criteria for Parkinson’s disease: Mov Disord, 2015; 30(12); 1591-601
13. He D, Liu CF, Chu L, The risk factors and pattern of cerebral microbleeds in Parkinson’s disease: Int J Neurosci, 2017; 127(10); 909-14
14. Gregoire SM, Chaudhary UJ, Brown MM, The Microbleed Anatomical Rating Scale (MARS): Reliability of a tool to map brain microbleeds: Neurology, 2009; 73(21); 1759-66
15. Tomlinson CL, Stowe R, Patel S, Systematic review of levodopa dose equivalency reporting in Parkinson’s disease: Mov Disord, 2010; 25(15); 2649-53
16. Nasreddine ZS, Phillips NA, Bédirian V, The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment: J Am Geriatr Soc, 2005; 53(4); 695-99
17. Poels Mariëlle MF, Vernooij Meike W, Ikram MA, Prevalence and risk factors of cerebral microbleeds: An update of the Rotterdam scan study: Stroke, 2010; 41(10 Suppl); S103-6
18. Poels Mariëlle MF, Ikram MA, van der Lugt A, Incidence of cerebral microbleeds in the general population: The Rotterdam Scan Study: Stroke, 2011; 42(3); 656-61
19. Vernooij MW, van der Lugt A, Ikram MA, Prevalence and risk factors of cerebral microbleeds: The Rotterdam Scan Study: Neurology, 2008; 70(14); 1208-14
20. Yamashiro K, Tanaka R, Hoshino Y, The prevalence and risk factors of cerebral microbleeds in patients with Parkinson’s disease: Parkinsonism Relat Disord, 2015; 21(9); 1076-81
21. Christ N, Mocke V, Fluri F, Cerebral microbleeds are associated with cognitive decline early after ischemic stroke: J Neurol, 2019; 266(5); 1091-94
22. Yang HJ, Cerebral microbleeds and the heterogeneity of Parkinson’s disease: J Korean Med Sci, 2018; 33(46); e293
23. Daida K, Tanaka R, Yamashiro K, The presence of cerebral microbleeds is associated with cognitive impairment in Parkinson’s disease: J Neurol Sci, 2018; 393; 39-44
24. Chen H, Wan H, Zhang M, Cerebral small vessel disease may worsen motor function, cognition, and mood in Parkinson’s disease: Parkinsonism Relat Disord, 2021; 83; 86-92
25. Zhang M, Xie B, Gao J, Distinct profiles of cognitive impairment associated with different silent cerebrovascular lesions in hypertensive elderly Chinese: J Neurol Sci, 2019; 403; 139-45
26. Gyanwali B, Lui B, Tan CS, Cerebral microbleeds and white matter hyperintensities are associated with cognitive decline in an Asian memory clinic study: Curr Alzheimer Res, 2021; 18(5); 399-413
27. Fazekas F, Kleinert R, Roob G, Histopathologic analysis of foci of signal loss on gradient-echo T2*-weighted MR images in patients with spontaneous intracerebral hemorrhage: Evidence of microangiopathy-related microbleeds: Am J Neuroradiol, 1999; 20(4); 637-42
28. Schrag M, McAuley G, Pomakian J, Correlation of hypointensities in susceptibility-weighted images to tissue histology in dementia patients with cerebral amyloid angiopathy: a postmortem MRI study: Acta Neuropathol, 2010; 119(3); 291-302
29. Zhao N, Yang Y, Zhang L, Quality of life in Parkinson’s disease: A systematic review and meta-analysis of comparative studies: CNS Neurosci Ther, 2021; 27(3); 270-79
30. Kuhlman GD, Flanigan JL, Sperling SA, Predictors of health-related quality of life in Parkinson’s disease: Parkinsonism Relat Disord, 2019; 65; 86-90
31. Santos-García D, de Deus Fonticoba T, Suárez Castro E, Quality of life and non-motor symptoms in Parkinson’s disease patients with subthreshold depression: J Neurol Sci, 2020; 418; 117109
32. Lawson RA, Yarnall AJ, Duncan GW, Cognitive decline and quality of life in incident Parkinson’s disease: The role of attention: Parkinsonism Relat Disord, 2016; 27; 47-53
33. Prell T, Teschner U, Witte OL, Current and desired quality of life in people with Parkinson’s disease: The Calman Gap Increases with Depression: J Clin Med, 2020; 9(5); 1496
34. Fan Y, Liang X, Han L, Determinants of quality of life according to cognitive status in Parkinson’s disease: Front Aging Neurosci, 2020; 12; 269
Tables
 Table 1. Clinical characteristics of patients with PD with or without cerebral microbleeds.
Table 1. Clinical characteristics of patients with PD with or without cerebral microbleeds. Table 2. Motor symptoms and non-motor symptoms of participants.
Table 2. Motor symptoms and non-motor symptoms of participants. Table 3. Quality of life assessment in patients with different locations of cerebral microbleeds.
Table 3. Quality of life assessment in patients with different locations of cerebral microbleeds. Table 4. The associations of cerebral microbleeds (CMBs) with cognition and quality of life in Parkinson disease.
Table 4. The associations of cerebral microbleeds (CMBs) with cognition and quality of life in Parkinson disease. Table 1. Clinical characteristics of patients with PD with or without cerebral microbleeds.
Table 1. Clinical characteristics of patients with PD with or without cerebral microbleeds. Table 2. Motor symptoms and non-motor symptoms of participants.
Table 2. Motor symptoms and non-motor symptoms of participants. Table 3. Quality of life assessment in patients with different locations of cerebral microbleeds.
Table 3. Quality of life assessment in patients with different locations of cerebral microbleeds. Table 4. The associations of cerebral microbleeds (CMBs) with cognition and quality of life in Parkinson disease.
Table 4. The associations of cerebral microbleeds (CMBs) with cognition and quality of life in Parkinson disease. Supplementary Table 1. Identification of risk factors for cerebral microbleeds in patients with Parkinson disease.
Supplementary Table 1. Identification of risk factors for cerebral microbleeds in patients with Parkinson disease. In Press
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952