15 November 2021: Clinical Research
Factors Influencing Sodium Valproate Serum Concentrations in Patients with Epilepsy Based on Logistic Regression Analysis
Xiaobu Lan12ACE*, Kai Mo12ADG, Li Nong12CEF, Yi He12BCD, Yuhong Sun12BDOI: 10.12659/MSM.934275
Med Sci Monit 2021; 27:e934275
Abstract
BACKGROUND: We aimed to explore the risk factors that affect the serum concentration of sodium valproate (VPA-Na) in patients with epilepsy and to provide references for the rationale of the use of VPA-Na.
MATERIAL AND METHODS: The enzyme-multiplied immunoassay technique was used to determine the serum VPA-NA concentrations of 109 patients, and the results were retrospectively analyzed and summarized. A multivariate logistic regression model was used to analyze substandard serum VPA-Na concentrations.
RESULTS: Fifty-six patients (51.38%) treated with VPA-Na tablets were within the effective treatment range of 50-100 μg/mL, while 53 patients (48.62%) were out of the treatment range. The results indicated that the standard-reaching rate of serum drug concentration in the juvenile group was higher than that in the adult and elderly groups; the standard-reaching rates of serum drug concentrations in the low-dose group and the intermediate-dose group were lower than that in the high-dose group; and the standard-reaching rate of serum drug concentration in the group receiving carbapenems in combination was lower than that in the non-combination group; all differences were statistically significant. The combination with carbapenems and enzyme inducers was an independent risk factor for VPA-Na serum concentration below the target level in hospitalized patients.
CONCLUSIONS: To improve clinical efficacy and reduce the occurrence of adverse reactions, there is a need for therapeutic drug monitoring of VPA-Na. Moreover, individual administration should be implemented when VPA-Na tablets are used in the treatment of epilepsy because of the significant fluctuation in VPA-Na blood concentration.
Keywords: Drug Monitoring, Epilepsy, Valproic Acid, Adolescent, Age Factors, Aged, 80 and over, Anticonvulsants, Child, Child, Preschool, Dose-Response Relationship, Drug, Female, Humans, Infant, Male, young adult
Background
Valproic acid (VPA), which is prepared as an injection, oral solution, sustained-release tablet, and ordinary tablet, is widely used to treat seizures, bipolar disorder, migraine, and other psychiatric illnesses or neuropathies [1]. Its mechanism of action involves the interruption of γ-aminobutyric acid (GABA) transferase decomposition, which causes an increase in the concentration of GABA in the brain and inhibits neuronal excitement by weakening the neuronal response to N-methyl-D-aspartic acid. Therapeutic drug monitoring of VPA is a key aspect of the drug treatment of epilepsy because the therapeutic window of VPA is relatively narrow and there are many factors that affect the serum drug concentration. The current reference treatment range of VPA for epilepsy recommended by existing guidelines is 50 to 100 mg/L [2,3]. When the serum drug concentration is lower than required for treatment, the symptoms of epilepsy are not well controlled, and when the concentration is exceeded, the risk of adverse drug reactions increases, including those of the digestive system, nervous system, and hematological system [4]. This study aimed to provide an individualized reference for rational clinical drug use based on the monitoring of clinical therapeutic drugs to explore the influence of various factors on the serum concentration of VPA. We collected relevant clinical data of patients treated with sodium valproate (VPA-Na) and analyzed them by logistic regression analysis.
Material and Methods
GENERAL INFORMATION:
This study protocol was reviewed and approved by the Ethics Committee of the First People’s Hospital of Nanning. Data were collected on 109 hospitalized patients who received oral VPA-Na medication and serum concentration monitoring in a class-A tertiary hospital in Guangxi from January 2018 to December 2019. Collected data included basic patient characteristics (sex, age), drug use information (dosage, dosage form, combination of drugs), and liver and kidney function, measured by alanine transaminase (ALT), aspartate transaminase (AST) albumin, creatinine, urea, uric acid, and cystatin C levels.
INCLUSION CRITERIA:
The patients met the diagnostic criteria for epilepsy in the “Guidelines for Clinical Diagnosis and Treatment - Epilepsy Volume” (2015 revised edition). After the patients had taken 5 to 6 doses of VPA-Na, blood samples were collected within the following 30 min.
EXCLUSION CRITERIA:
Patients were excluded from the study for incomplete clinical medical records; poor compliance with the prescribed medications; steady-state concentration not reached; blood sampling monitoring after the patients took VPA-Na; serum concentration monitoring not performed; and pregnancy or lactation.
INSTRUMENTS AND REAGENTS:
The following instruments and reagents were used: VPA detection kit (Siemens, USA) and Viva-E automatic biochemical analyzer (Siemens, USA).
METHODS:
After the VPA-Na serum concentration reached a steady state in patients treated with VPA-Na by the oral route, 5 mL of fasting venous blood was collected before the patients took the medication the next morning. Blood samples were centrifuged at 4000 rpm to collect the serum. The drug concentration of VPA-Na was determined by enzyme-multiplied immunoassay with the Viva-E analysis system. The treatment window of VPA-Na ranged from 50 to 100 mg/L. If the result was within the treatment window, it was classified as reaching standard requirements; otherwise, it was classified as failing to meet standard requirements.
STATISTICAL ANALYSIS:
Data with a normal distribution were shown as mean±standard deviation, while non-normally distributed data were represented by median of the interquartile range (IQR, P25, P75), and the means of each group were compared. The independent samples were analyzed using the
Results
GENERAL DATA:
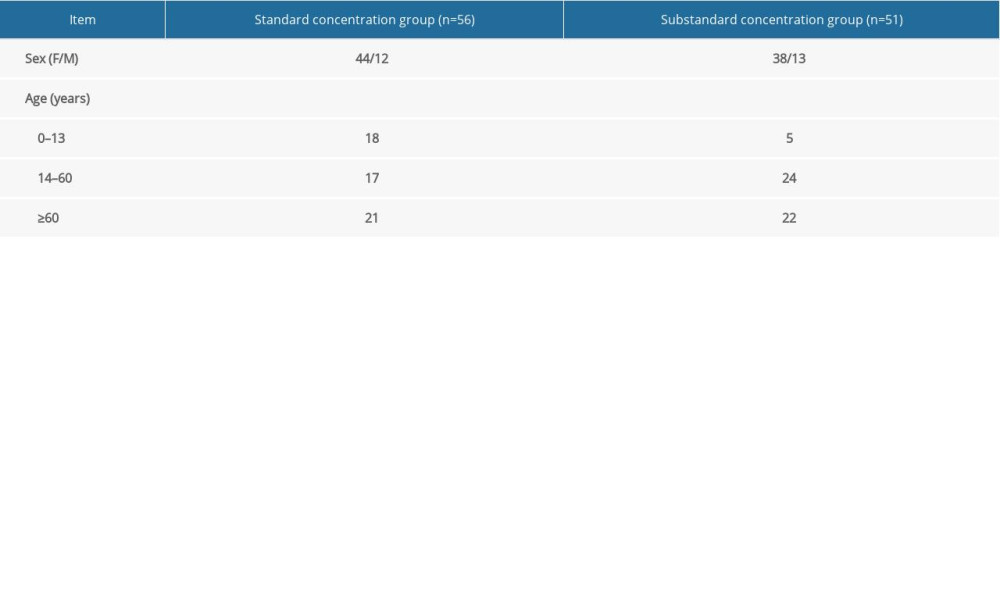
Therapeutic drug monitoring data were collected from 109 patients, including 83 male patients and 26 female patients. The patients’ ages ranged from 3 months to 91 years, with an average age of 47.46±29.29 years. The daily dose of the patients was 0.2 to 1.8 g, so that the average serum concentration of VPA-Na was 52.47±26.26 μg/mL. The serum drug concentration of 56 patients (51.38%) was within the reference range; the serum drug concentration of 51 patients (46.79%) was below the lower limit of the reference value; and the remaining 2 patients had serum concentrations above the upper limit of the reference value (Table 1).
CLINICAL DATA:
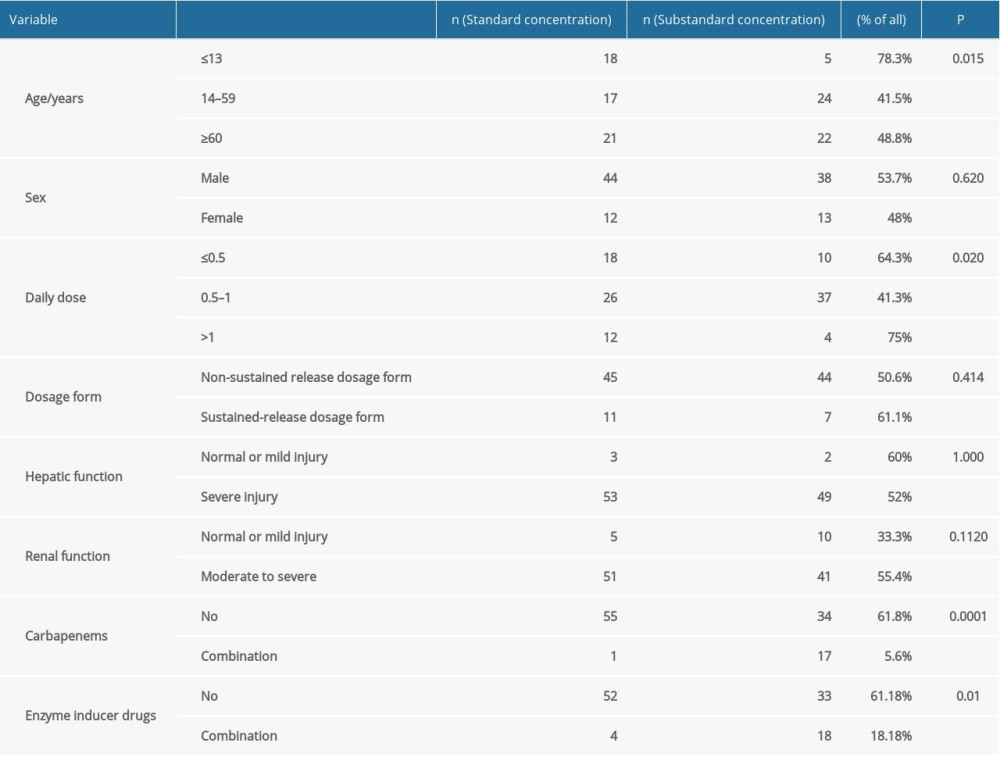
First, we carried out univariate analysis. To facilitate the discussion and accurate conclusions, the data of the 2 patients exceeding the upper limit of the reference value were eliminated, and the chi-squared test was used to calculate the difference of the standard-reaching rate under the influence of a single factor. The chi-squared test results showed no significant differences in the standard-reaching rate of serum drug concentrations in groups divided by sex, dosage form, liver function, kidney function, and combined enzyme inducer. However, there were significant differences in the standard-reaching rate of serum drug concentration in other groups, such as the juvenile and high-dose groups. The standard-reaching rate in the juvenile group was higher than that in the mature group and elderly group; the rate was lower in the low-dose group and intermediate-dose group than in the high-dose group; and in the group receiving carbapenems in combination, the standard-reaching rate was lower than that in the non-combination group (Table 2).
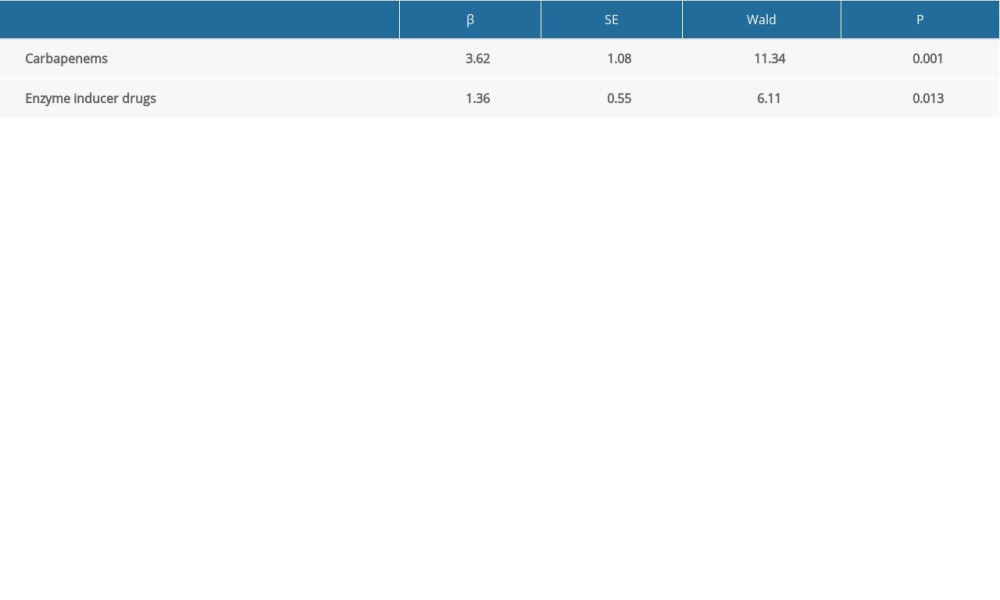
The second part of the study used multiple logistic regression to analyze VPA-Na serum concentrations lower than the standard. According to the results of the influence of a single factor and after excluding the data of the 2 patients whose concentrations exceeded the upper limit of the reference value, the variables mentioned above were analyzed by binary logistic regression. We showed that the combination of carbapenems and enzyme inducers was an independent risk factor for VPA-Na serum concentration below the target level (P<0.05). The results indicated a goodness of fit of 0.882 by the Hosmer-Lemeshow test (Table 3).
Discussion
LIMITATIONS AND PROBLEMS:
There were some limitations in our study. First, the sample size was relatively small, with only 2 patients having serum drug concentration greater than the upper limit of the treatment window, which led us to study only the factors leading to substandard concentration in the multivariate regression analysis. Second, the therapeutic effects and toxicities of VPA-Na were affected by the target receptors, effector pathways, absorption, metabolism, and polymorphisms of transporter-related genes [17,18], but the polymorphisms of genes [19] were not included in this study. Studies [20,21] have shown that the genetic polymorphisms of CYP450ABCB1 and UGT genes are significantly related to the serum concentration of epilepsy patients treated with VPA-Na. Third, the effective therapeutic concentration of VPA-Na remains controversial; in this study, 50 to 100 μg/mL was considered the target value. However, some studies have shown that the type of disease onset should be considered in the selection of effective therapeutic concentrations because sometimes patients’ conditions could be well controlled even with the concentration lower than 50 μg/mL, whereas some patients can need excessive drug concentration to control the disease, but with careful monitoring of liver function and routine blood parameters.
Conclusions
Considering the serum concentration in hospitalized patients is often lower than the standard concentration, clinical pharmacists may benefit from our study by adjusting the serum concentration of VPA-Na. For patients with a low dose or combined use of an enzyme inducer, a dose increase can be used to reach the standard drug concentration. Meanwhile, it is necessary to continuously monitor drug concentrations after the adjustment of the medication regimen to avoid great fluctuations. When possible, patients using non-sustained-release dosage forms should switch to sustained-release dosage forms. For patients who must be fed nasally, oral liquids or plain tablets are recommended, as grinding can destroy the special structure of the sustained-release tablets. The combined use of carbapenems should be avoided as much as possible. If the combined use of carbapenems is necessary, clinical pharmacists should select drugs other than VPA-Na, according to the type and frequency of seizure attacks.
References
1. Patsalos PN, Spencer EP, Berry DJ, Therapeutic drug monitoring of antiepileptic drugs in epilepsy: A 2018 Update: Ther Drug Monit, 2018; 40(5); 526-48
2. Collins-Yoder A, Lowell J, Valproic acid: Special considerations and targeted monitoring: J Neurosci Nurs, 2017; 49(1); 56-61
3. Verrotti A, Lattanzi S, Brigo F, Zaccara G, Pharmacodynamic interactions of antiepileptic drugs: From bench to clinical practice: Epilepsy Behav, 2020; 104(Pt A); 106939
4. Perucca E, Hebdige S, Frigo G, Interaction between phenytoin and valproic acid: Plasma protein binding and metabolic effects: Clin Pharm Therap, 1980; 28(6); 779-89
5. Borowicz-Reutt K, Czuczwar S, Rusek M, Interactions of antiepileptic drugs with drugs approved for the treatment of indications other than epilepsy: Expert Rev Clin Pharmacol, 2020; 13(12); 1329-45
6. Al-Quteimat O, Laila A, Valproate interaction with carbapenems: Review and recommendations: Hosp Pharm, 2020; 55(3); 181-87
7. Mancl EE, Gidal BE, The effect of carbapenem antibiotics on plasma concentrations of valproic acid: Ann Pharmacother, 2009; 43(12); 2082-87
8. Wu CC, Pai TY, Hsiao FY, The effect of different carbapenem antibiotics (ertapenem, imipenem/cilastatin, and meropenem) on serum valproic acid concentrations: Ther Drug Monit, 2016; 38(5); 587-92
9. Huang CR, Lin CH, Hsiao SC, Drug interaction between valproic acid and carbapenems in patients with epileptic seizures: Kaohsiung J Med Sci, 2017; 33(3); 130-36
10. Wen ZP, Fan SS, Du C, Drug-drug interaction between valproic acid and meropenem: A retrospective analysis of electronic medical records from neurosurgery inpatients: J Clin Pharm Ther, 2017; 42(2); 221-27
11. Guo HL, Jing X, Sun JY, Valproic acid and the liver injury in patients with epilepsy: An update: Curr Pharm Design, 2019; 25(3); 343-51
12. Zhang H, Zhang WF, Li YC, Correlations between UGT2B7*2 gene polymorphisms and plasma concentrations of carbamazepine and valproic acid in epilepsy patients: Brain Dev-Jpn, 2018; 40(2); 100-6
13. Chen Y, Cai SY, Wang JW, Xu MJ, Valproic acid-induced histone acetylation suppresses CYP19 gene expression and inhibits the growth and survival of endometrial stromal cells: International J Mol Med, 2015; 36(3); 725-32
14. Kudin A, Mawasi H, Eisenkraft A, Mitochondrial liver toxicity of valproic acid and its acid derivatives is related to inhibition of α-lipoamide dehydrogenase: Int J Mol Sci, 2017; 18(9); 1912
15. Mei SH, Feng WX, Zhu LT, Effect of CYP2C19, UGT1A8, and UGT2B7 on valproic acid clearance in children with epilepsy: A population pharmacokinetic model: Eur J Clin Pharmacol, 2018; 74(8); 1029-36
In Press
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
06 Mar 2024 : Clinical Research
Prevalence and Variation of Medical Comorbidities in Oral Surgery Patients: A Retrospective Study at Jazan ...Med Sci Monit In Press; DOI: 10.12659/MSM.943884
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952