01 October 2021: Animal Studies
Electroacupuncture Inhibits Myelin Sheath Injury in the Internal Capsule After Focal Cerebral Infarction in Rats Through the Nogo-A/NgR Signaling Pathway
Wenqing Dong1BCDEF, Huachun Miao1BCD, Huaibin Li1AFG, Feng Wu1ABCDEFG*DOI: 10.12659/MSMBR.933253
Med Sci Monit Basic Res 2021; 27:e933253
Abstract
BACKGROUND: Ischemic stroke is usually accompanied by white matter damage. The effect of electroacupuncture (EA) on ameliorating white matter damage is still unclear. The purpose of this study was to explore the precise mechanism of EA in treating ischemic white matter.
MATERIAL AND METHODS: In this study, 40 Sprague-Dawley rats were randomly divided into 4 groups: normal group, the sham-operated group, model group, and EA group. The stroke model was established by right middle cerebral artery occlusion, and EA was performed 24 h after the operation for 30 min per day. After 14 days of treatment, brain tissue samples were collected. Hematoxylin and eosin and Luxol fast blue staining were used to observe the changes of white matter damage in the internal capsule (IC). The expression levels of myelin basic protein (MBP), Nogo-A, and Nogo-A receptor (NgR) were detected by immunohistochemistry and western blot.
RESULTS: Compared with the sham-operated group, the model group had decreased expression of MBP and significantly increased expression of Nogo-A and NgR (P<0.05). Compared with the model group, the IC damage was alleviated in the EA group. Immunohistochemistry and western blot analysis showed that EA significantly increased the expression of MBP in white matter (P<0.05) and downregulated the expression levels of Nogo-A and NgR (P<0.05).
CONCLUSIONS: The results of this study indicate that EA can inhibit the expression of Nogo-A/NgR and promote myelin sheath regeneration.
Keywords: cerebral infarction, Electroacupuncture, Internal Capsule, Nogo Proteins, Animals, Brain Ischemia, Myelin Proteins
Background
Stroke is among the most serious diseases that threaten human health worldwide [1]. The 2019 Global Burden of Disease Study showed that the majority of stroke survivors experience disability and dysfunction and require long-term rehabilitation [2]. Stroke can be divided into 2 types, ischemic and hemorrhagic, and ischemic lesions account for approximately 80% of natural strokes [3,4].
Magnetic resonance imaging studies have shown that the internal capsule (IC) is particularly vulnerable to cerebral ischemia, and injury of the posterior limb of the IC through the corticospinal tract (CST) is associated with motor impairment in ischemic stroke [5,6]. CST is the major white matter (WM) axonal pathway that facilitates neural communication between the brain and limbs and is a major source of motor impairment, with microstructural damage and axonal degeneration occurring in the CST of patients within 1 year after ischemic stroke. Nearly 90% of ischemic stroke patients show middle cerebral artery occlusion (MCAO) leading to hemiplegia or hemiparesis [7]. In patients with cerebral anterior circulation occlusion after a stroke, the microstructural integrity of the specific CST is disrupted in the posterior limb of the IC, resulting in direct white matter injury (WMI) or local edema [8].
Acupuncture is an important part of traditional Chinese medicine, and it has been widely used to treat clinical disorders including poststroke dysfunction [9]. Electroacupuncture (EA) is an extended treatment strategy of acupuncture that shows high efficacy and safety for acute ischemic stroke [10,11]. In recent years, many studies have corroborated the clinical efficacy of EA in rehabilitation of limb functions after a stroke [12,13]. Animal experiments have shown that EA at the Baihui and Zusanli acupoints can reduce the infarct volume induced by ischemia and improve motor, sensory, and cognitive functions [14,15]. In addition, EA was found to cause gene expression differences in neurotrophins/receptors, growth factors/receptors, transcription factors, and inflammatory factors; inhibit autophagy and apoptosis-related proteins; and enhance functional connectivity of the brain [16,17]. However, the mechanisms through which EA improves poststroke WMI have not been fully elucidated and warrant further study.
Nogo-A/Nogo-A receptor (NgR) signaling is one of the earliest and most broadly studied myelin-related inhibitors discovered so far; NgR is a high-affinity receptor for Nogo-A, oligodendrocyte myelin glycoprotein, and myelin-associated glycoprotein [18,19]. Nogo-A/NgR plays an important role in the pathogenesis of ischemic stroke. Studies have confirmed that Nogo-A/NgR mediates inhibition signals and can prevent axon regeneration, germination, and plasticity in the central nervous system (CNS) [20–22]. Therefore, it may be a potential therapeutic target for WMI after stroke.
In this study, a rat model of cerebral ischemia was established by the Longa Zea method to investigate the potential molecular protective mechanisms of EA in ischemic WMI. Our study showed that the neuroprotective effect of EA on ischemic WMI was associated with the inhibition of Nogo-A/NgR pathway overexpression.
Material and Methods
ANIMALS:
A total of 40 adult male Sprague-Dawley rats (230±20 g) were purchased from Qinglongshan Animal Experimental Center (animal certificate no.: scxk (Hu) 2018-0004). Animals were housed in a room under routine care conditions and had free access to food and water. This study conformed to the recommendations and protocols for the use of laboratory animals in China. All surgeries were performed under sodium pentobarbital anesthesia, and every effort was made to minimize animal suffering.
ANIMAL GROUPING:
Before surgery, rats were randomly divided into the following experimental groups: normal group (normal), sham-operated group (sham), model group (MCAO), and electroacupuncture group (EA). The normal group did not receive any treatment. The sham-operated group underwent blood vessel and nerve separation without filament insertion. Each group contained 10 rats [14].
STROKE MODEL:
In this experiment, we applied the MCAO model. The rats were anesthetized by intraperitoneal injection of 1.5% pentobarbital sodium (2 mL/kg). They were then fixed on the operating table in the supine position and routinely disinfected with iodophor. The right common carotid artery, external carotid artery, and internal carotid artery (ICA) were bluntly exposed through a median cervical incision, and the vagus nerve was carefully protected from injury. The proximal end of the ICA was ligated, and the ICA was inserted into the MCAO filament (Beijing Cinonech Co. Ltd, A2-2432, China), matching an insertion depth of about (18.5±0.5) mm, such that the head end of the filament passed through the beginning of the middle cerebral artery to reach the anterior cerebral artery. The ICA was then ligated with suture to fix the MCAO filament and prevent bleeding. The incision was sutured routinely. For the sham-operated group, the procedure was the same except that the common carotid artery, external carotid artery, and ICA on the right side were only separated bluntly without filament insertion and artery occlusion. The stimulation and damage of the vagus nerve was also avoided.
ELECTROACUPUNCTURE:
The rats in the EA group received acupuncture therapy 24 h after MCAO surgery. Acupuncture needles (Hua Tuo, 0.30×25 mm, China) were used for stimulation. The selected acupoints were Baihui (GV20, parietal bone center, oblique needling 2 mm) and left Zusanli (ST36, below the knee joint, 5 mm below the fibular head, straight needling 7 mm). Dense-sparse wave modulation at 1 mA, 2 Hz/10 Hz was introduced using a Hua Tuo brand EA device (Suzhou Medical Supplies Factory Co. Ltd., SDZ-III, China) for 30 min a day at 2: 00 PM, lasting for 2 weeks.
HEMATOXYLIN AND EOSIN STAINING:
The tissue sections of the specific right IC region were evaluated by hematoxylin and eosin staining (Biosharp Life Science Co. Ltd, BL735B, China) to observe the pathological changes in each group. Paraffin sections, 5 μm thick, were successively put into xylene and ethanol gradients for dewaxing and stained with hematoxylin solution for 8 min and then rinsed with distilled water for 1 min and differentiated with 1% HCl-alcohol for several seconds. After being washed with phosphate-buffered saline, slices were successively immersed in 70%, 85%, and 95% gradient ethanol for dehydration, followed by eosin staining for 1 min. The pathological morphology of the right IC was distinguished under a light microscope at ×400 magnification.
LUXOL FAST BLUE STAINING:
Luxol fast blue staining (LFB, Leagene Biotechnology, DK0009, China) was used to identify the myelin sheath changes in the right IC of rats in each group. Paraffin sections at a thickness of 5 μm were conventionally dewaxed, immersed in LFB staining solution, and incubated at 60°C for 3 h. Slides were then washed with 95% ethanol and rinsed in distilled water to remove excess dye. Tissue sections were placed in differentiation solution and 70% ethanol for 10–15 s to stain. The differentiation steps were repeated until a sharp contrast between the corpus callosum and cortex developed, and the sections were then dehydrated with ethanol. The images were collected using a microscope at ×400 magnification, and the average optical density (equal to integrated optical density divided by area) of myelin staining in the right IC was analyzed by Image-Pro Plus 6.0 software.
IMMUNOFLUORESCENCE STAINING:
The paraffin sections were dewaxed in xylene and hydrated through an ethanol gradient. The microwave method was carried out for antigen retrieval; that is, sections were boiled in a 10 mM sodium citrate buffer at pH 6.0 for 15 min. Endogenous peroxidase was inactivated by 3% H2O2 for 10 min, and nonspecific antibody binding was then blocked by 5% goat serum for 30 min. The sections were incubated overnight at 4°C with primary antibodies: myelin basic protein (MBP, 1: 100), Nogo-A (1: 100), and NgR (1: 100). The sections were then incubated in horseradish peroxidase (HRP) anti-rabbit IgG secondary antibody, and 3,3′-diaminobenzidine (DAB; TIANGEN Biotech, PA110, China) was used to develop staining in the sections. Ischemic IC was selected and average optical density was analyzed using Image-Pro Plus software.
WESTERN BLOTTING:
IC tissues were isolated from the ischemic side of the brain on ice, and neutral RIPA lysis buffer with 1% phenylmethylsulfonyl fluoride was added to completely lyse the tissues. The tissues were placed on ice for 40 min, and then centrifuged at 12000 rpm and 4°C for 30 min to obtain the supernatant. The supernatant was added to 5× protein loading buffer at a ratio of 4: 1, denatured in boiling water for 8 min, and stored at −80°C. Protein homogenate samples (80 μg) were loaded onto a gel, and after SDS-PAGE, transfer of the separated proteins to polyvinylidene fluoride membranes was done at 110 V for 100 min. The membrane was then blocked with 5% skimmed milk powder (0.1% Tris-buffered saline with Tween [TBST]) at room temperature for 2 h. Membranes were incubated with antibodies against MBP (Affinity, DF6539, 1: 1000, China), Nogo-A (Abcam, ab62024, 1: 1000, UK), NgR (Affinity, DF13593, 1: 1000, China), and GAPDH (Affinity, AF7021, 1: 1000, China), respectively, overnight at 4°C. After being washed 3 times (10 min each time) with TBST on a decolorizing shaker at room temperature, membranes were incubated with HRP-labeled goat anti-rabbit IgG (H+L) secondary antibodies (Beyotime Biotechnology, A0208, 1: 1000, China) for 60 min at room temperature, then washed 3 times as described above. The positive proteins combined with antibodies were visualized by enhanced chemiluminescence kit (Beyotime Biotechnology, P0018AS, China) and Amersham Imager 600 system. The bands were then quantified by ImageJ software.
STATISTICAL ANALYSIS:
Data were analyzed in SPSS 18.0 and expressed as the mean±standard deviation. One-way analysis of variance (ANOVA) was conducted to test the differences among multiple groups and the least significant difference (LSD) method was performed subsequently to compare each group.
Results
HEMATOXYLIN AND EOSIN STAINING:
The histopathology showed that WM fibers in the IC of the normal group and sham-operated group were closely arranged and the nuclei were interspersed normally, no significant difference was observed between the 2 groups (P>0.05). The IC of the MCAO group showed increased tissue damage, including significantly increased vesicles, loosely arranged nuclei, partial nuclear fragmentation, and blurring of nuclei (P<0.05). Compared with the MCAO group, EA rats had normal-appearing tissue structure and the area of vesicles was decreased, and the cells had a proper nucleus distribution and clear nuclei (P<0.05). No statistically significant difference was found between the EA group and the sham-operated group (P>0.05) (Figure 1A, 1C).
LFB STAINING:
LFB staining was used to evaluate the morphological changes of myelin in the IC. As shown in Figure 1B, the myelinated nerve fibers in the normal group and sham-operated group showed blue staining in the IC, whereas blue-stained fibers were rarely observed in the IC of MCAO-injured rats, as shown by a decrease in the average optical density, indicating a massive loss of myelinated axons. In contrast, the average optical density of the EA group was increased. LFB staining showed that EA could enhance the blue staining of the MCAO group and partially reduce the loss of IC myelin sheath induced by MCAO (P<0.05). However, the average optical density for the EA group was not significantly different from that for the sham-operated group (P>0.05) (Figure 1B, 1D).
MBP PROTEIN EXPRESSION:
MBP protein was detected to evaluate myelin sheath integrity in the IC of WM. As expected, MBP immunoreactivity was strong in the normal and sham-treated rats, which indicated the integrity of the WM. Compared with the sham-operated group, the model group had weak MBP immunoreactivity within the IC and the average optical density of MBP was significantly reduced after MCAO on the 14th day (P<0.05), reflecting the injury of the myelin sheath. At the same time, we examined the expression of MBP after MCAO by western blot analysis. The results indicated that the MBP protein levels were significantly decreased after MCAO (P<0.05) in the model group compared with the sham-operated group. Compared with the MCAO group, MBP protein in the EA group was significantly increased, indicating that remyelination had occurred after 14 days of EA treatment (P<0.05). However, no significant difference was found in the expression of MBP between the sham-operated group and the EA group (P>0.05) (Figures 2, 3).
NOGO-A AND NGR PROTEIN EXPRESSION:
To confirm whether the Nogo-A/NgR pathway was involved in the effect of EA on MCAO, Nogo-A and NgR protein expression levels were examined through immunohistochemical staining and western blotting. The immunohistochemical staining results suggested that no significant difference existed between the normal group and the sham-operated group (P>0.05). Compared with the sham-operated group, the MCAO group had significantly increased protein expression of Nogo-A/NgR (P<0.05); however, compared with the MCAO group, the protein expression of Nogo-A/NgR significantly decreased in the EA group (P<0.05). The western blot results indicated that no statistically significant difference existed between the normal group and the sham-operated group (P>0.05), but Nogo-A/NgR expression increased in the MCAO group compared with the sham-operated group (P<0.05), and the relative expression levels of Nogo-A/NgR were significantly reduced in the EA group compared with those in the MCAO group (P<0.05). The expression of Nogo-A and NgR did not differ between the sham-operated and EA groups (P>0.05) (Figures 2, 3).
Discussion
The study of WM damage after stroke should be prioritized because WM has less collateral circulation and a smaller blood supply than gray matter, and ischemic stroke can therefore rapidly and profoundly damage WM. In the pathophysiological cascade of WM damage after stroke, glutamate and ATP cause inflammation and oxidative stress, which ultimately lead to oligodendrocyte death, axonal demyelination, structural damage to the WM, and neurobehavioral impairment [23,24]. The IC carries CST from the primary sensorimotor and premotor cortex [25]. Focal posterior IC infarction induces long-lasting motor deficits associated with inflammation, axonal degeneration, oligodendrocyte loss, and impaired differentiation in the infarct area [26,27]. Acupuncture therapy has been found to accelerate cerebral WM perfusion and integrity and to promote the recovery of neurological function in rats with bilateral carotid artery occlusion [28]. To better simulate the clinical process of ischemic cerebral infarction, we inserted filaments into the middle cerebral artery of rats to generate focal ischemia.
EA, a form of acupuncture, is an important treatment in traditional Chinese medicine. After the occurrence of cerebral infarction, cerebral ischemia quickly leads to a reduction in regional cerebral blood flow. Notably, EA stimulation of the forepaw in aged rats can substantially increase blood flow in cortical brain tissue [29]. The best treatment time to improve cerebral circulation should be within 2–3 days after onset, but particularly at 24 h, when apoptosis and brain edema reach a peak after cerebral ischemia-reperfusion injury [30]. Typically, acupuncture intervention within 2 days of a stroke is the best time to achieve early recovery of motor function [31]. Therefore, the time point chosen in this study was 24 h after MCAO, thus revealing the possible role of EA in the occurrence and progression and development of acute cerebral ischemia. In addition, EA has the advantages of strong intensity, stable frequency, and easy quantification [32,33]. In this study, we selected Baihui and Zusanli (GV20, ST36), which are common acupoints that demonstrate significant therapeutic effects [34,35]. According to the theory of traditional Chinese medicine, GV20 is a governing vessel, whose function is to collect Qi around the body. After stimulation of GV20, local Qi is dispersed and energizes the whole body. ST36 is rich in Qi and blood and has the ability to modulate the function of the whole body. Once the Qi and blood are abundant, the body can be activated again. In addition, Xu et al [36] showed that acupuncture and EA at the GV20 and ST36 acupoints in cerebral ischemia/reperfusion injury rats significantly reduced the expression of the proinflammatory matrix metalloproteinase2 and aquaporin 4, and significantly it was also shown to relieve inflammation-related brain edema [37].
WM tracts transmit action potentials through myelin, thereby supporting important communication between neurons. Axonal injury delivers retrograde signals of injury to neuronal cell bodies, which leads to selective neuronal damage and may induce selective neuronal degeneration over time [38]. After a stroke, reduced WM integrity can quickly occur in the primary lesion location, or it may be a delayed and distant consequence of retrograde and/or retrograde axonal degeneration [39]. Our results suggested that EA ameliorated WMI after stroke, with a decrease in the vesicle area and a reduction in the degree of myelin damage after EA intervention. Meanwhile, the pathological changes of white matter at the histological level were close to those in the sham-operated group, indicating that EA alters the damage in IC tissue with cerebral ischemia. The interaction between WM fibers depends on the interaction between MBP expressed by oligodendrocytes [40]. MBP is a multifunctional protein involved in maintaining myelin compact structure by regulating myelin sheath formation around axons of some nerve fibers through complex signal transduction mechanisms [41]. In this study, we evaluated myelin damage by analyzing the expression of MBP. MCAO decreased the expression of MBP in the ischemic IC, indicating that myelin loss is a pathological hallmark of WM in ischemic stroke. Early treatment with EA increased the expression of MBP after ischemic injury, and relative to the sham-operated group, the expression level of MBP was close to normal, suggesting that 14 days of EA could alleviate myelin damage and axonal stimulation in ischemic WM.
The repairability of CNS in the peri-infract area after stroke is limited. Dingman et al [42] found that focal WM stroke mice had a profound increase in oligodendrocyte progenitor cells 24 h after ischemia, which was sustained for up to 7 days, but these new cells did not survive to maturity after WM stroke. To further determine the protective effect of EA on myelin fibers in ischemic WM, we employed immunohistochemistry and western blot methods to detect the expression of Nogo-A and NgR. Nogo-A is considered one of the most important inhibitors of axonal growth in CNS, and it is mainly expressed by oligodendrocytes [43]. The Nogo-A region of Nogo-66 binds to NgR1 to inhibit axonal regeneration [44,45]. Wang et al [46] observed that Nogo-A was continuously highly expressed in oligodendrocytes in the ipsilateral ventroposterior nucleus within 4 weeks after distal MCAO and continuously expressed in oligodendrocytes, and meanwhile it was also distributed around the damaged axons and dendrites. Although previous studies have found that Nogo-A plays a regulatory role in neurite growth inhibition, neuronal homeostasis, precursor migration, plasticity, and neurodegeneration [47], its therapeutic mechanism in the process of MCAO ischemic WMI has not been described. Nogo-A knockout significantly decreased the levels of Nogo-A and NgR1 in the hippocampus and cortex of cerebral infarction area, increased the expression of the anti-apoptotic gene
Conclusions
In this study, the MCAO method was used in a rat model to determine the important role of EA in the repair of myelin after a stroke. We found that EA can accelerate the recovery of infarcted brain tissue and neurological function 24 h after stroke. Furthermore, EA promotes the formation of myelin in damaged IC by regulating the Nogo-A/NgR signaling pathway. However, the recovery of myelin induced by EA is a complex process that involves many factors. We plan to have a sham acupuncture group in future experiments and for further investigation. This study provides a potential molecular mechanism for the clinical efficacy of traditional Chinese medicine in the treatment of cerebral infarction.
Figures
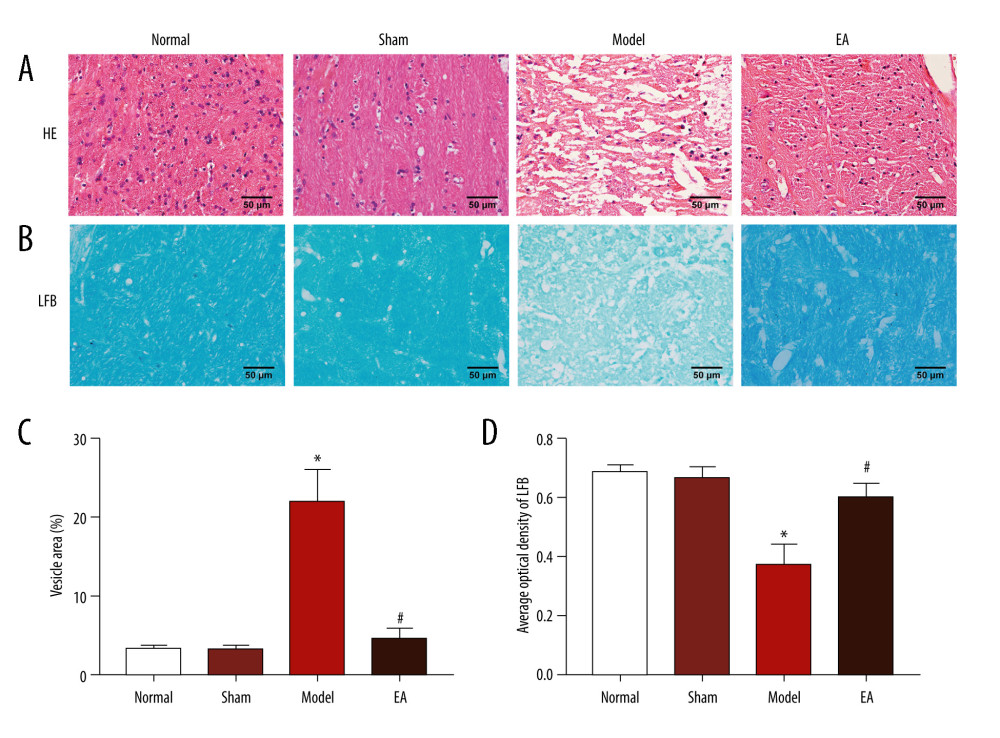 Figure 1. Effects of electroacupuncture (EA) on histopathological changes in the internal capsule (IC) of middle cerebral artery occlusion (MCAO) rats. (A) Micrographs of hematoxylin and eosin (HE) staining in the IC. (B) Micrographs of Luxol fast blue (LFB) staining in the IC. (C) Quantification of the vesicle area. (D) Quantification of the average optical density of myelin sheath. Scale bars: 50 μm, magnification: ×400, values are expressed as mean±standard deviation (n=5), * P<0.05 compared with sham-operated group and # P<0.05 compared with model group.
Figure 1. Effects of electroacupuncture (EA) on histopathological changes in the internal capsule (IC) of middle cerebral artery occlusion (MCAO) rats. (A) Micrographs of hematoxylin and eosin (HE) staining in the IC. (B) Micrographs of Luxol fast blue (LFB) staining in the IC. (C) Quantification of the vesicle area. (D) Quantification of the average optical density of myelin sheath. Scale bars: 50 μm, magnification: ×400, values are expressed as mean±standard deviation (n=5), * P<0.05 compared with sham-operated group and # P<0.05 compared with model group. 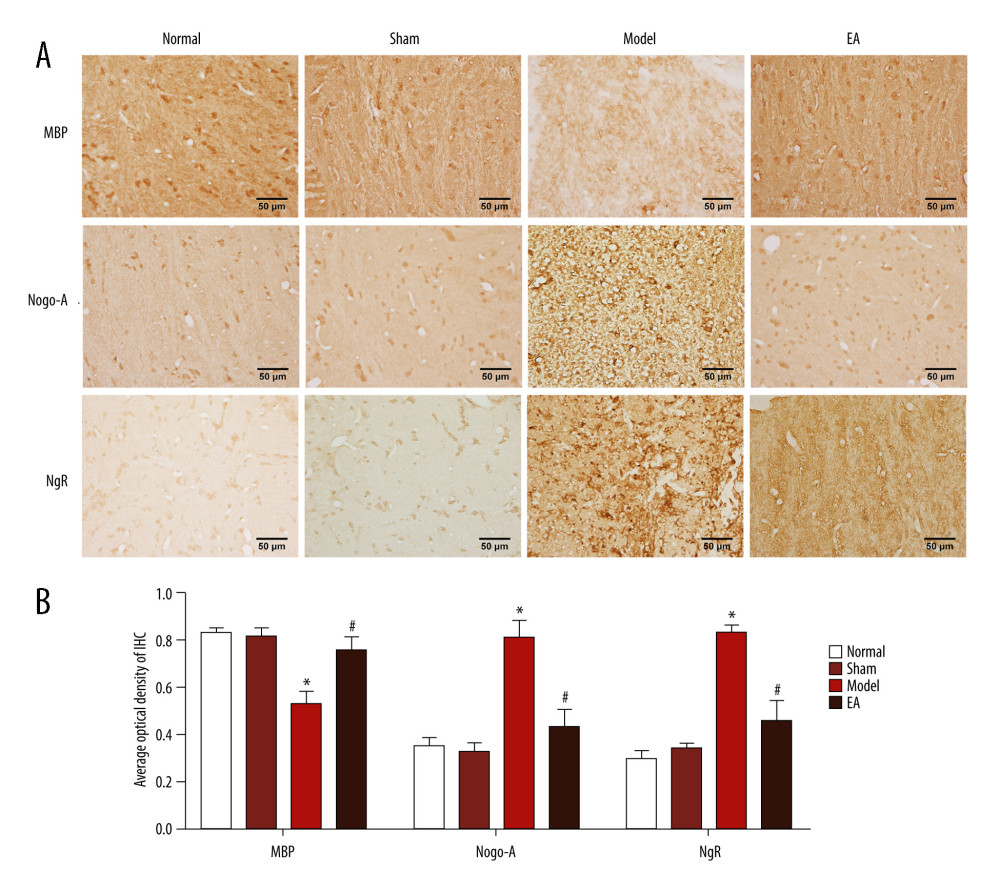 Figure 2. Effect of electroacupuncture (EA) on myelin basic protein (MBP), Nogo-A, and Nogo-A receptor (NgR) protein expression was examined through immunohistochemical staining. (A) The expression of MBP, Nogo-A, and NgR protein. (B) Quantitative results of MBP, Nogo-A, and NgR protein. Scale bars: 50 μm, magnification: ×400, values are expressed as mean±standard deviation (n=5), * P<0.05 compared with sham-operated group and # P<0.05 compared with model group.
Figure 2. Effect of electroacupuncture (EA) on myelin basic protein (MBP), Nogo-A, and Nogo-A receptor (NgR) protein expression was examined through immunohistochemical staining. (A) The expression of MBP, Nogo-A, and NgR protein. (B) Quantitative results of MBP, Nogo-A, and NgR protein. Scale bars: 50 μm, magnification: ×400, values are expressed as mean±standard deviation (n=5), * P<0.05 compared with sham-operated group and # P<0.05 compared with model group. 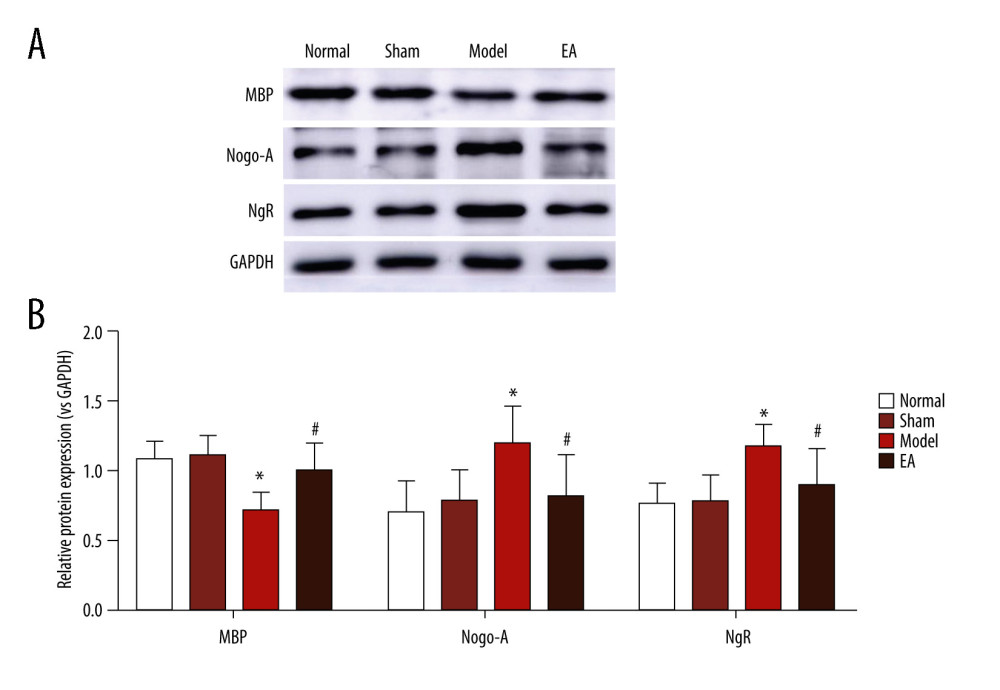 Figure 3. Effect of electroacupuncture (EA) on myelin basic protein (MBP), Nogo-A, and Nogo-A receptor (NgR) protein expression was examined through western blotting. (A) The bands of MBP, Nogo-A, NgR, and GAPDH protein. (B) Quantitative results of MBP, Nogo-A, and NgR protein. Values are expressed as mean±standard deviation (n=5), * P<0.05 compared with sham-operated group and # P<0.05 compared with model group.
Figure 3. Effect of electroacupuncture (EA) on myelin basic protein (MBP), Nogo-A, and Nogo-A receptor (NgR) protein expression was examined through western blotting. (A) The bands of MBP, Nogo-A, NgR, and GAPDH protein. (B) Quantitative results of MBP, Nogo-A, and NgR protein. Values are expressed as mean±standard deviation (n=5), * P<0.05 compared with sham-operated group and # P<0.05 compared with model group. References
1. Qiu S, Xu Y, Guidelines for acute ischemic stroke treatment: Neurosci Bull, 2020; 36; 1229-32
2. Cieza A, Causey K, Kamenov K, Global estimates of the need for rehabilitation based on the Global Burden of Disease Study 2019: A systematic analysis for the global burden of disease study 2019: Lancet, 2020; 396; 2006-17
3. Fu K, Chen M, Zheng H, Pelargonidin ameliorates MCAO-induced cerebral ischemia/reperfusion injury in rats by the action on the Nrf2/HO-1 pathway: Transl Neurosci, 2021; 12; 20-31
4. Freitas-Andrade M, Raman-Nair J, Lacoste B, Structural and functional remodeling of the brain vasculature following stroke: Front Physiol, 2020; 11; 948
5. Kerleroux B, Tomasino C, Soriano D, EASY score (Eloquent, Age and baseline SYmptoms score) for outcome prediction in patients with acute ischemic stroke: Clin Neurol Neurosurg, 2021; 205; 106626
6. Al-Dasuqi K, Payabvash S, Torres-Flores G, Effects of collateral status on infarct distribution following endovascular therapy in large vessel occlusion stroke: Stroke, 2020; 51; e193-e202
7. Haque ME, Hasan KM, George S, Longitudinal neuroimaging evaluation of the corticospinal tract in patients with stroke treated with autologous bone marrow cells: Stem Cells Transl Med, 2021; 10; 943-55
8. Berndt MT, Pürner D, Maegerlein C, Basal ganglia versus peripheral infarcts: Predictive value of early fiber alterations: Am J Neuroradiol, 2021; 42; 264-70
9. Chang Q, Lin Y, Hsieh C, Acupuncture and neuroregeneration in ischemic stroke: Neural Regen Res, 2018; 13; 573-83
10. Luo Y, Wang CZ, Hesse-Fong J, Application of Chinese medicine in acute and critical medical conditions: Am J Chin Med, 2019; 47; 1223-35
11. Zhan J, Pan R, Zhou M, Electroacupuncture as an adjunctive therapy for motor dysfunction in acute stroke survivors: A systematic review and meta-analyses: BMJ Open, 2018; 8; e017153
12. Yang K, Zhang H, Hu G, Electroacupuncture for patients with spasticity after stroke: A protocol for systematic review and meta-analysis: Medicine, 2021; 100; e24859
13. Zhang J, Zhu L, Tang Q, Electroacupuncture with rehabilitation training for limb spasticity reduction in post-stroke patients: A systematic review and meta-analysis: Top Stroke Rehabil, 2021; 28(5); 340-61
14. Wang M, Zhang M, Feng Y, Electroacupuncture inhibits neuronal autophagy and apoptosis the PI3K/AKT pathway following ischemic stroke: Front Cell Neurosci, 2020; 14; 134
15. Li Z, Yang M, Lin Y, Electroacupuncture promotes motor function and functional connectivity in rats with ischemic stroke: An animal resting-state functional magnetic resonance imaging study: Acupunct Med, 2021; 39; 146-55
16. Wang C, Yang F, Liu X, Neurotrophic signaling factors in brain ischemia/reperfusion rats: differential modulation pattern between single-time and multiple electroacupuncture stimulation: Evid Based Complement Alternat Med, 2014; 2014; 625050
17. Zhang Q, Li J, Huang S, Functional connectivity of the retrosplenial cortex in rats with ischemic stroke is improved by electroacupuncture: Acupunct Med, 2021; 39; 200-7
18. Hong J, Kang S, Sa J, Modulation of Nogo receptor 1 expression orchestrates myelin-associated infiltration of glioblastoma: Brain, 2021; 144; 636-54
19. Kuhlmann T, Remington L, Maruschak B, Nogo-A is a reliable oligodendroglial marker in adult human and mouse CNS and in demyelinated lesions: J Neuropathol Exp Neurol, 2007; 66; 238-46
20. Chang J, Yao X, Zou H, BDNF/PI3K/Akt and Nogo-A/RhoA/ROCK signaling pathways contribute to neurorestorative effect of Houshiheisan against cerebral ischemia injury in rats: J Ethnopharmacol, 2016; 194; 1032-42
21. Chen S, Wang H, Xu H, Electroacupuncture promotes axonal regrowth by attenuating the myelin-associated inhibitors-induced RhoA/ROCK pathway in cerebral ischemia/reperfusion rats: Brain Res, 2020; 1748; 147075
22. Sekine Y, Algarate PT, Cafferty WBJ, Strittmatter SM, Plexina2 and CRMP2 signaling complex is activated by Nogo-A-Liganded Ngr1 to restrict corticospinal axon sprouting after trauma: J Neurosci, 2019; 39; 3204-16
23. Wang Y, Liu G, Hong D, White matter injury in ischemic stroke: Prog Neurobiol, 2016; 141; 45-60
24. Li S, Rao JH, Lan XY, White matter demyelination predates axonal injury after ischemic stroke in cynomolgus monkeys: Exp Neurol, 2021; 340; 113655
25. Chorghay Z, Karadottir RT, Ruthazer ES, White matter plasticity keeps the brain in tune: Axons conduct while glia wrap: Front Cell Neurosci, 2018; 12; 428
26. Yamazaki R, Ohno N, Huang J, Acute motor deficit and subsequent remyelination-associated recovery following internal capsule demyelination in mice: J Neurochem, 2021; 156; 917-28
27. Blasi F, Whalen MJ, Ayata C, Lasting pure-motor deficits after focal posterior internal capsule white-matter infarcts in rats: J Cereb Blood Flow Metab, 2015; 35; 977-84
28. Ma SM, Wang L, Su XT, Acupuncture improves white matter perfusion and integrity in rat model of vascular dementia: An MRI-based imaging study: Front Aging Neurosci, 2020; 12; 582904
29. Uchida S, Kagitani F, Effect of acupuncture-like stimulation on cortical cerebral blood flow in aged rats: J Physiol Sci, 2015; 65; 67-75
30. Xing Y, Zhang M, Wang MM, The anti-apoptosis effect of single electroacupuncture treatment suppressing neuronal autophagy in the acute stage of ischemic stroke without infarct alleviation: Front Cell Neurosci, 2021; 15; 633280
31. Xu J, Pei J, Fu QH, Earlier acupuncture enhancing long-term effects on motor dysfunction in acute ischemic stroke: Retrospective cohort study: Am J Chin Med, 2020; 48; 1787-802
32. Liu L, Zhang Q, Xie HY, Differences in post-ischemic motor recovery and angiogenesis of MCAO rats following electroacupuncture at different acupoints: Curr Neurovasc Res, 2020; 17; 71-78
33. Li G, Li X, Dong J, Han Y, Electroacupuncture ameliorates cerebral ischemic injury by inhibiting ferroptosis: Front Neurol, 2021; 12; 619043
34. Xing Y, Zhang M, Li WB, Mechanisms involved in the neuroprotection of electroacupuncture therapy for ischemic stroke: Front Neurosci, 2018; 12; 929
35. Sun J, Liu Y, Zhang J, Electroacupuncture improves cerebral vasospasm and functional outcome of patients with aneurysmal subarachnoid hemorrhage: Front Neurosci, 2018; 12; 724
36. Xu H, Zhang Y, Sun H, Effects of acupuncture at GV20 and ST36 on the expression of matrix metalloproteinase 2, aquaporin 4, and aquaporin 9 in rats subjected to cerebral ischemia/reperfusion injury: PLoS One, 2014; 9; e97488
37. Wang HQ, Hou M, Li H, Effects of acupuncture treatment on motor function in patients with subacute hemorrhagic stroke: A randomized controlled study: Complement Ther Med, 2020; 49; 102296
38. Xiao G, Hinman J, Concepts and opportunities for repair in cerebral microvascular disease and white matter stroke: Neural Regen Res, 2016; 11; 1398-400
39. Petoe MA, Byblow WD, de Vries EJ, A template-based procedure for determining white matter integrity in the internal capsule early after stroke: Neuroimage Clin, 2014; 4; 695-700
40. Unal DB, Caliari SR, Lampe KJ, Engineering biomaterial microenvironments to promote myelination in the central nervous system: Brain Res Bull, 2019; 152; 159-74
41. Valdivia AO, Farr V, Bhattacharya SK, A novel myelin basic protein transcript variant in the murine central nervous system: Mol Biol Rep, 2019; 46; 2547-53
42. Dingman AL, Rodgers KM, Dietz RM, Oligodendrocyte progenitor cell proliferation and fate after white matter stroke in juvenile and adult mice: Dev Neurosci, 2019 [Online ahead of print]
43. Kurihara Y, Takai T, Takei K, Nogo receptor antagonist LOTUS exerts suppression on axonal growth-inhibiting receptor PIR-B: J Neurochem, 2020; 155; 285-99
44. Schwab M, Functions of Nogo proteins and their receptors in the nervous system: Nat Rev Neurosci, 2010; 11; 799-811
45. Sekine Y, Lindborg JA, Strittmatter SM, A proteolytic C-terminal fragment of Nogo-A (reticulon-4A) is released in exosomes and potently inhibits axon regeneration: J Biol Chem, 2020; 295; 2175-83
46. Wang F, Liang Z, Hou Q, Nogo-A is involved in secondary axonal degeneration of thalamus in hypertensive rats with focal cortical infarction: Neurosci Lett, 2007; 417; 255-60
47. Wang YY, Han N, Hong DJ, Zhang J, Nogo-A aggravates oxidative damage in oligodendrocytes: Neural Regen Res, 2021; 16; 179-85
48. Xie YX, Zhang M, Zhang CR, Chen F, Relationship between NogoA/NgR1/RhoA signaling pathway and the apoptosis of cerebral neurons after cerebral infarction in rats: Eur Rev Med Pharmacol Sci, 2020; 24; 295-303
49. Wang T, Wang J, Yin C, Down-regulation of Nogo receptor promotes functional recovery by enhancing axonal connectivity after experimental stroke in rats: Brain Res, 2010; 1360; 147-58
Figures
 Figure 1. Effects of electroacupuncture (EA) on histopathological changes in the internal capsule (IC) of middle cerebral artery occlusion (MCAO) rats. (A) Micrographs of hematoxylin and eosin (HE) staining in the IC. (B) Micrographs of Luxol fast blue (LFB) staining in the IC. (C) Quantification of the vesicle area. (D) Quantification of the average optical density of myelin sheath. Scale bars: 50 μm, magnification: ×400, values are expressed as mean±standard deviation (n=5), * P<0.05 compared with sham-operated group and # P<0.05 compared with model group.
Figure 1. Effects of electroacupuncture (EA) on histopathological changes in the internal capsule (IC) of middle cerebral artery occlusion (MCAO) rats. (A) Micrographs of hematoxylin and eosin (HE) staining in the IC. (B) Micrographs of Luxol fast blue (LFB) staining in the IC. (C) Quantification of the vesicle area. (D) Quantification of the average optical density of myelin sheath. Scale bars: 50 μm, magnification: ×400, values are expressed as mean±standard deviation (n=5), * P<0.05 compared with sham-operated group and # P<0.05 compared with model group. Figure 2. Effect of electroacupuncture (EA) on myelin basic protein (MBP), Nogo-A, and Nogo-A receptor (NgR) protein expression was examined through immunohistochemical staining. (A) The expression of MBP, Nogo-A, and NgR protein. (B) Quantitative results of MBP, Nogo-A, and NgR protein. Scale bars: 50 μm, magnification: ×400, values are expressed as mean±standard deviation (n=5), * P<0.05 compared with sham-operated group and # P<0.05 compared with model group.
Figure 2. Effect of electroacupuncture (EA) on myelin basic protein (MBP), Nogo-A, and Nogo-A receptor (NgR) protein expression was examined through immunohistochemical staining. (A) The expression of MBP, Nogo-A, and NgR protein. (B) Quantitative results of MBP, Nogo-A, and NgR protein. Scale bars: 50 μm, magnification: ×400, values are expressed as mean±standard deviation (n=5), * P<0.05 compared with sham-operated group and # P<0.05 compared with model group. Figure 3. Effect of electroacupuncture (EA) on myelin basic protein (MBP), Nogo-A, and Nogo-A receptor (NgR) protein expression was examined through western blotting. (A) The bands of MBP, Nogo-A, NgR, and GAPDH protein. (B) Quantitative results of MBP, Nogo-A, and NgR protein. Values are expressed as mean±standard deviation (n=5), * P<0.05 compared with sham-operated group and # P<0.05 compared with model group.
Figure 3. Effect of electroacupuncture (EA) on myelin basic protein (MBP), Nogo-A, and Nogo-A receptor (NgR) protein expression was examined through western blotting. (A) The bands of MBP, Nogo-A, NgR, and GAPDH protein. (B) Quantitative results of MBP, Nogo-A, and NgR protein. Values are expressed as mean±standard deviation (n=5), * P<0.05 compared with sham-operated group and # P<0.05 compared with model group. Most Viewed Current Articles
15 Jun 2022 : Clinical Research
Evaluation of Apical Leakage After Root Canal Obturation with Glass Ionomer, Resin, and Zinc Oxide Eugenol ...DOI :10.12659/MSMBR.936675
Med Sci Monit Basic Res 2022; 28:e936675
07 Jul 2022 : Laboratory Research
Cytotoxicity, Apoptosis, Migration Inhibition, and Autophagy-Induced by Crude Ricin from Ricinus communis S...DOI :10.12659/MSMBR.936683
Med Sci Monit Basic Res 2022; 28:e936683
01 Jun 2022 : Laboratory Research
Comparison of Sealing Abilities Among Zinc Oxide Eugenol Root-Canal Filling Cement, Antibacterial Biocerami...DOI :10.12659/MSMBR.936319
Med Sci Monit Basic Res 2022; 28:e936319
08 Dec 2022 : Original article
Use of Estimated Glomerular Filtration Rate and Urine Albumin-to-Creatinine Ratio Based on KDIGO 2012 Guide...DOI :10.12659/MSMBR.938176
Med Sci Monit Basic Res 2022; 28:e938176








