05 November 2021: Clinical Research
Association Between Ascites and Clinical Findings in Patients with Acute Pancreatitis: A Retrospective Study
Quan-Xiang Zeng1EFG, Zhen-Hua Wu1EFG, Dong-Liang Huang1B, Ye-Sheng Huang1B, Hao-Jie Zhong2ACDEF*DOI: 10.12659/MSM.933196
Med Sci Monit 2021; 27:e933196
Abstract
BACKGROUND: Complications are the most important outcome determinants for acute pancreatitis (AP). We designed this single-center retrospective study to evaluate the clinical findings (complications, disease severity, and outcomes) of 218 patients with AP and to identify variables associated with ascites.
MATERIAL AND METHODS: We extracted clinical data from consecutive patients with AP and divided them into 2 groups based on presence or absence of ascites. We compared disease severity, complications, and outcomes between groups.
RESULTS: We analyzed data from 218 patients with AP (43 with ascites and 175 without it). The patients with ascites had a more severe disease (higher incidence of pancreatic inflammation [90.70% vs 68.57%; P=0.003], higher modified computed tomography severity index score [2.00 (0.00-2.00) vs 4.00 (4.00-6.00); P<0.001], higher incidence of moderate/severe AP [53.49% vs 13.14%; P<0.001]) and poorer outcomes (higher incidence of ventilation [6.98% vs 0.57%; P=0.025] and vasopressor use [4.65% vs 0%; P=0.038], and longer hospital stays [10.00 (7.00-13.00) vs 8.00 (5.00-10.00); P=0.007]) than those without ascites. Moreover, patients with ascites also displayed a higher risk for pancreatic fluid collection (odds ratio [OR]=9.206; 95% confidence interval [CI], 2.613-32.447; P<0.001), renal failure (OR=5.732; 95% CI, 1.025-32.041; P=0.024), respiratory failure (OR=6.242; 95% CI, 1.034-37.654; P=0.029), and pleural effusion (OR=5.186; 95% CI, 1.381-19.483; P<0.001) than those without ascites.
CONCLUSIONS: The findings from the experience of a single center of patients with AP showed that pancreatic fluid collections, renal failure, respiratory failure, and pleural effusion were associated with the development of ascites.
Keywords: Ascites, Pancreatitis, Risk Factors, Comorbidity, Female, Humans, Male, Severity of Illness Index
Background
Acute pancreatitis (AP) is the second most common cause of prolonged total hospital stay and the fifth leading cause of in-hospital deaths [1]; and, its incidence has increased [1,2]. Local and systemic complications (including pancreatic fluid collection, pancreatic necrosis, renal failure, respiratory failure, and cardiovascular failure) occur frequently in patients with AP [3]. These complications – especially organ failure – are the most important determinants of patient outcomes [4]; AP mortality is approximately 3% in patients without complications, 15% in those with pancreatic necrosis, and up to 35% in those with persistent organ failure [5]. In addition, a population-based cohort on patients with AP showed that the median time from the onset of systemic complications to death was as short as 3 days [6]. Moreover, the mortality rate due to complications is high even after the first 2 weeks [4]. Because complications are not always obvious in the early stage of the disease, understanding the risk factors of AP complications would be of great value in helping clinicians to prevent, identify, and carry out timely management of these complications [7].
Ascites is caused by leakage of pancreatic secretions into the peritoneum [8] and damage of capillary walls and plasma extravasation [9], and it occurs in up to 38.5% of patients with AP [10]. Ascitic fluid contains an abundance of pancreatic proteases, pancreatic lipases, and inflammatory cytokines that are highly toxic and lethal [11] and also contribute to the development of intra-abdominal hypertension [10]; thus, ascites is an independent predictor of the severity and poor prognosis of AP [12]. Although complications govern the outcome and mortality in patients with AP, limited data on the association between pancreatic ascites and AP complications exists [13]. Ascites can be easily detected in the diagnostic images of early-stage AP. Discerning whether ascites is associated with AP complications may improve prompt detection and management of those and thereby reduce complication-related mortalities. This retrospective study identified 218 patients diagnosed with AP at a single center and evaluated their clinical manifestations (including complications, disease severity, and outcomes), comparing those in patients with and without ascites.
Material and Methods
ETHICS STATEMENT:
The Institutional Review Board of Maoming People’s Hospital, Maoming, China approved this study (No. 2020MI-141-01). The requirement for informed consent was waived by the Institutional Review Board on account of the retrospective study design.
PARTICIPANTS:
Data from all adult (≥18 years) inpatients with AP at Maoming People’s Hospital from January, 2015 to December, 2019 were eligible for inclusion in the study. We excluded data from patients with a history of pancreatitis, those with ascites caused by liver or kidney disease, those with carcinoma, pregnant patients, and those with incomplete medical information.
DATA COLLECTION:
We extracted the following data from electronic medical records of the eligible patients: demographic characteristics, smoking status, alcoholism status, medical history, etiology, complications, treatment, outcomes, imaging findings, and laboratory parameters (including white blood cell and platelet counts, serum levels of amylase, calcium, alanine aminotransferase, aspartate transaminase, total bilirubin, triglycerides, blood glucose, hematocrit, creatinine, and blood urea nitrogen).
DEFINITIONS:
Alcoholism was defined as a daily alcohol consumption >30 g/day in men and >20 g/day in women. Hypocalcemia was defined as serum calcium levels <2.12 mmol/L [14]. Ascites was diagnosed in patients with peritoneal fluid around the sublienal space and among intestinal loops by ultrasonography or computed tomography (CT) [15]. The diagnosis of acute pancreatitis required the presence of 2 of the 3 following criteria: (1) characteristic epigastric abdominal pain; (2) elevation of serum amylase or lipase levels to more than 3 times the upper normal limit; and, (3) abdominal imaging findings consistent with the diagnosis [16]. The severity of AP was classified based on the revised Atlanta criteria: mild (no organ failure or local or systemic complications), moderately severe (organ failure resolving within 48 h and/or local or systemic complications without persistent organ failure [<48 h]) and severe (persistent organ failure [>48 h]) [16]. Local (pancreatic fluid collections and pancreatic necrosis) and systemic complications (renal failure, circulatory failure, and respiratory failure) of AP were diagnosed as follow: (1) Pancreatic fluid collection was diagnosed based on the presence of a homogeneous collection of dense fluid adjacent to the pancreas without evidence of pancreatic necrosis and confirmed by CT [17]. (2) Pancreatic necrosis was diagnosed based on the presence of a heterogeneous and non-liquid density of varying degrees in different pancreas locations confirmed by CT [17]. (3) Renal failure was defined as a serum creatinine >177 μmol/L [18]. (4) Circulatory failure was defined as systolic blood pressure < 90 mmHg [18]. (5) Respiratory failure was defined as PaO2 <60 mmHg [18]. Pleural effusion was diagnosed as blunting of the costophrenic or cardiophrenic angle confirmed by CT [19]. Pancreatic inflammation was defined as focal or diffuse enlargement of the pancreas with or without intrinsic pancreatic inflammatory changes [20]. We calculated the Charlson Comorbidity Index [21] and modified CT severity index (CTSI) as published [20]. Briefly, we assigned a score of 1, 2, 3, or 6 to 19 different medical condition categories (such as myocardial infarct, lymphoma, and metastatic solid tumor) to add them all up and obtain an overall Charlson Comorbidity Index score [21]. We assessed the modified CTSI based on the severity of 3 indicators (pancreatic inflammation, pancreatic necrosis, and extra-pancreatic complications). Using this modified index, we categorized the AP severity as mild (0–2 points), moderated (4–6 points), or severe (8–10 points) [20].
STATISTICAL ANALYSIS:
We used SPSS (IBM Corp., Armonk, NY, USA) to analyze the statistical data. Continuous data are presented as means±standard deviations for normally distributed variables, and medians and interquartile ranges for non-normally distributed variables. We compared continuous variables using the t test for normally distributed variables or Mann-Whitney U test for non-normally distributed variables between patients with and without ascites. Categorical data are presented as frequencies and proportions, and we compared them using the chi-squared test between 2 groups. To assess the associations between ascites and severity, complications, and outcomes of AP, we performed a multivariate logistic regression analysis with a backward conditional method, and a multivariable linear regression (with patients without ascites as the reference group) to adjust for potential confounders (ie, age, sex, hypocalcemia, blood glucose level, and white blood cell count) [22,23]. We expressed results as odds ratios (ORs) with 95% confidence intervals (CIs) and beta with standard errors (SE). A two-sided P value <0.05 was considered to indicate statistical significance.
Results
PATIENT CHARACTERISTICS:
Based on the inclusion and exclusion criteria, we analyzed data from 218 patients with AP. During the course of the disease, 43 patients (19.72%) developed ascites (41 diagnosed by CT and 2 by ultrasonography). Table 1 summarizes the findings of a comparison of the characteristics between the patients with and without ascites. We found no significant intergroup differences with respect to demographics, comorbidities, or AP etiology. In addition, laboratory results were similar between the 2 groups of patients, except for hypocalcemia (Table 2).
DISEASE SEVERITY AND COMPLICATIONS IN PATIENTS WITH AND WITHOUT ASCITES:
In terms of disease severity, patients with AP and ascites showed a higher incidence of pancreatic inflammation detected by CT scan (90.70% vs 68.57%; P=0.003), higher modified CTSI scores (2.00 [0.00–2.00] vs 4.00 [4.00–6.00]; P<0.001) and a higher incidence of moderate/severe AP (53.49% vs 13.14%; P<0.001) than the patients without ascites. However, the incidence of SIRS was similar in the 2 groups (Table 3).
In terms of local complications, patients with ascites showed a higher incidence of pancreatic fluid collection than those without ascites (46.51% vs 8.57%; P<0.001). Pancreatic necrosis was almost absent in both groups (Table 3).
In terms of systemic complications, renal failure (11.90% vs 1.86%; P=0.011), respiratory failure (9.30% vs 1.71%; P=0.041), and pleural effusion (30.23% vs 6.86%; P<0.001) were significantly higher in the ascites group than in the other group, while the incidence of circulatory failure was similar in the 2 groups (4.65% vs 2.29%; P=0.742; Table 3).
OUTCOMES IN PATIENTS WITH AND WITHOUT ASCITES:
Outcomes such as the incidences of use of ventilation (6.98% vs 0.57%; P=0.025) and vasopressors (4.65% vs 0%; P=0.038) were significantly higher and the hospital stays (10.00 [7.00–13.00] vs 8.00 [5.00–10.00]; P=0.007) were significantly longer in the ascites group than in the no-ascites group. In addition, the patients with AP and ascites had a higher incidence of intensive care unit (ICU) admission (6.98% vs 1.14%), although the difference was not significant (P=0.054). Mortality was rare in both groups (Table 4).
MULTIVARIATE LOGISTIC AND MULTIVARIABLE LINEAR REGRESSION ANALYSES:
We applied a multivariate logistic regression analysis to assess the associations between ascites and disease severity, complications, and outcomes of AP. Patients with ascites presented higher risks of pancreatic inflammation (OR=5.332; 95% CI, 1.207–23.576; P=0.004), moderate/severe AP (OR=60.531; 95% CI, 13.798–265.461; P<0.001), pancreatic fluid collection (OR=9.206; 95% CI, 2.613–32.447; P<0.001), renal failure (OR=5.732; 95% CI, 1.025–32.041; P=0.024), respiratory failure (OR=6.242; 95% CI, 1.034–37.654; P=0.029), pleural effusion (OR=5.186; 95% CI, 1.381–19.483; P<0.001), use of ventilation (OR=12.900; 95% CI, 1.551–107.260; P=0.029), and ICU admission (OR=8.108; 95% CI, 1.168–56.279; P=0.032; Table 5) than the patients without ascites.
Our multivariable linear regression analysis showed that patients with AP and ascites also had a higher modified CTSI than the patients without ascites (beta=3.072, SE=0.225; P<0.001; Table 6).
Discussion
In this study, we found that patients with AP and ascites had increased risks for more severe disease and worse prognoses than the patients without ascites. More importantly, ascites was also a risk factor for local and systemic complications of AP, including pancreatic fluid collection, renal failure, respiratory failure, and pleural effusion.
The incidence of ascites in patients with AP in our study was 19.72%, which is similar to the incidence reported by Maringhini et al (18%) [12]. Maringhini et al reported that ascites is an accurate independent predictor of AP severity [12]. Jayanta et al also revealed that patients with AP who develop ascites have significantly higher severity scores, more need for interventions, and greater mortality than patients without ascites [10]. Consistent with those results, we found that patients with ascites showed higher risks of moderate/severe AP, and need for ICU admission and ventilation, and higher modified CTSIs than patients without ascites. Ascites can occur in the early stages of AP and can be easily detected by imaging and even physical examinations; therefore, it is of great value to predict AP progression [11].
Although ascites is a sign of severity for systemic inflammatory conditions, whether it can predict AP complications is poorly understood. A previous study found an association between pancreatic ascites and the development of a pseudocyst (a delayed and local AP complication), which usually occurred more than 4 weeks after onset of the pancreatitis [1,12]. Moreover, Jayanta et al also revealed that patients with pancreatic ascites showed higher incidences of local and systemic complications (including pancreatic necrosis, acute lung injury, acute kidney injury, and shock) than patients without ascites [10]. However, that study missed many clinical data (such as alcohol and smoking status, medical history, and laboratory results) important to clarify the association between ascites and AP complications. To overcome these limitations, we compared clinical data between patients with and without ascites, adjusted the potential AP complications’ confounders, and confirmed that ascites was a risk factor for local (pancreatic fluid collection) and systemic complications (renal failure, respiratory failure and pleural effusion) of AP in our cohort.
There are different possible explanations for our findings. First, ascites can be caused by the leakage of pancreatic fluid; however, pancreatic proteases and lipases, and inflammatory cytokines from ascitic fluid can also aggravate the inflammatory exudate of the pancreas, which may further contribute to pancreatic fluid collection. Second, ascites accumulation always leads to elevated intra-abdominal pressure, known as intra-abdominal hypertension (IAH) [24]. IAH impairs the venous return from the peripheral circulation to the right heart, which subsequently decreases the cardiac outflow and reduces arterial perfusion, thereby directly compressing the renal parenchyma [25]. Thus, IAH may result in renal function impairment and even renal failure. In addition, IAH also decreases the functional residual capacity of the lungs and the thoracic wall compliance by transmitting the pressure to the thorax [25]. Under these circumstances, acute respiratory distress syndrome and respiratory failure may occur, and ventilation may be needed [26]. Third, lung injury and pleurisy caused by ascites can also promote pleural effusions [27]. However, our data did not show a significant association between ascites and pancreatic necrosis and circulatory failure. This might be due to the limited sample size and the low incidence of these complications in our cohort.
Although complications account for the poor prognoses and most deaths in patients with AP, they often remain undetected, and the time from the occurrence of a systemic complication to the death of the patient can be short [4,28]. Some interventions against pancreatic ascites have decreased the prevalence of new complications and have improved the outcomes of patients with AP [13]. For example, an experimental study showed that abdominal paracentesis drainage may ameliorate the illness as well as complications in rats with severe AP by removing various proinflammatory mediators, such as interleukin-1β and tumor necrosis factor-α [29]. Moreover, clinical studies also have shown that removal of pancreatic ascites by peripancreatic percutaneous catheter drainage significantly decreases the incidence of complications and subsequently improves prognoses [30,31]. Therefore, clinicians should monitor the occurrence of AP complications in patients with ascites and provide early medical and surgical interventions (such as percutaneous drainage) to reduce the mortality of these patients.
Our study has some limitations. First, information about the volume and characteristics (including white blood cell counts, albumin levels, and bacterial culture) of the ascites was limited due to the retrospective nature of the study. Thus, we fell short of assessing the association between ascites characteristics (such as its volume) and AP complications. Second, although we analyzed the clinical data to our best abilities, we did not consider genetic factors, such as mutations of SPINK1, IL-1b, IL-10, which greatly influence the risk, etiology, and severity of AP [32]. Third, this was a single-center study with a small cohort. Hence, our conclusions should be interpreted with caution, and large-scale prospective clinical observations are needed to validate our findings.
Conclusions
The findings from the experience of a single center of patients with AP showed that pancreatic fluid collections, renal failure, respiratory failure, and pleural effusion were associated with the development of ascites.
Tables
Table 1. Patient characteristics.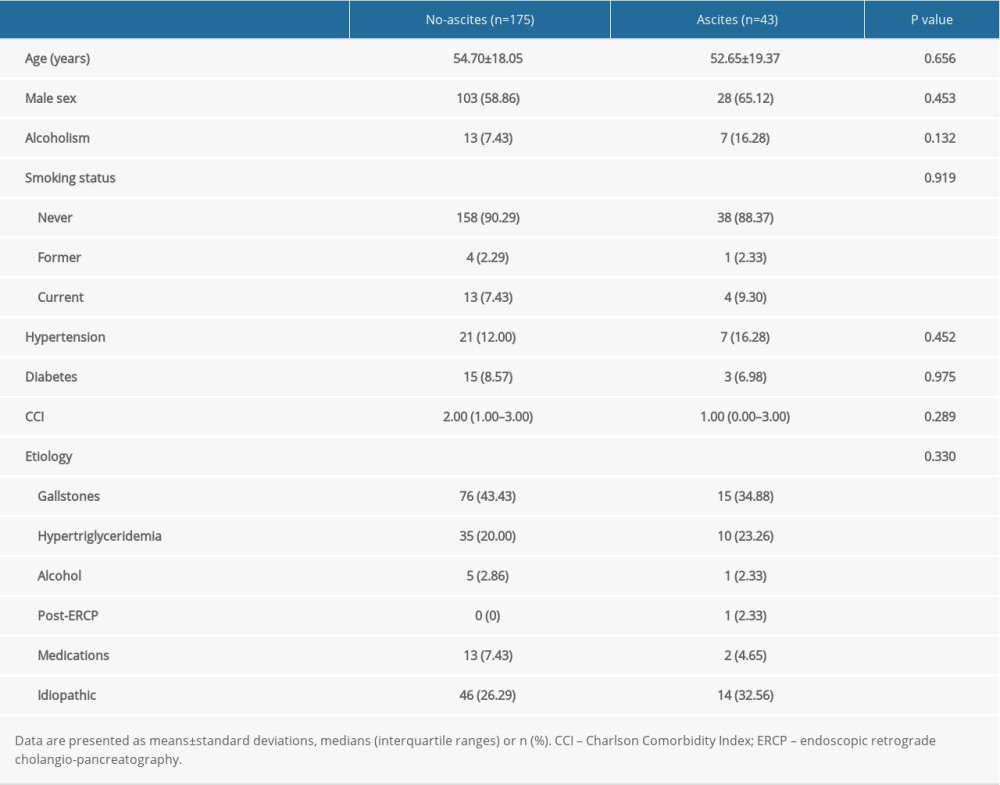 Table 2. Laboratory variables in patients with or without ascites.
Table 2. Laboratory variables in patients with or without ascites.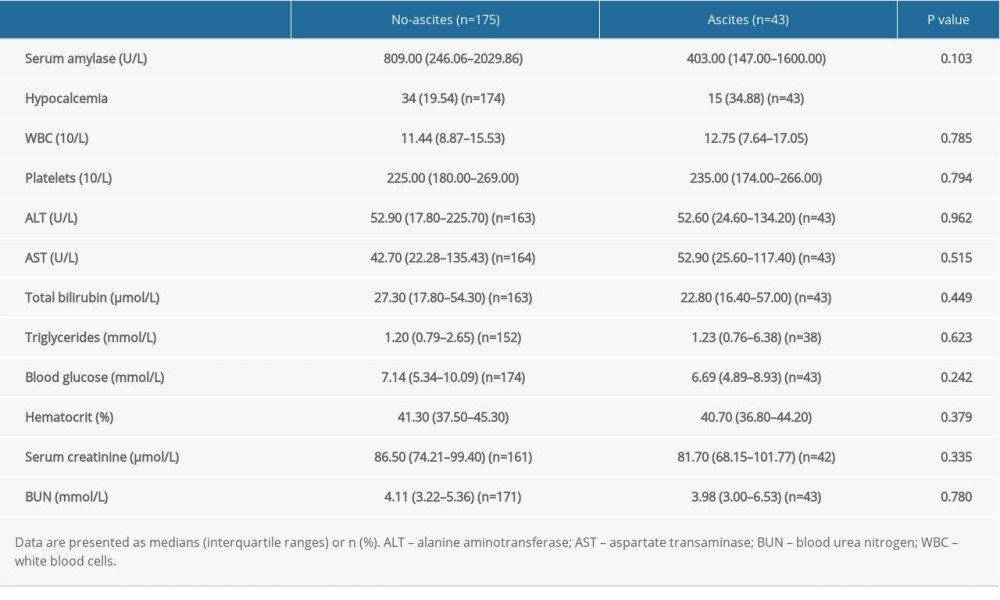 Table 3. Disease severity and complication-related variables in patients with or without ascites.
Table 3. Disease severity and complication-related variables in patients with or without ascites.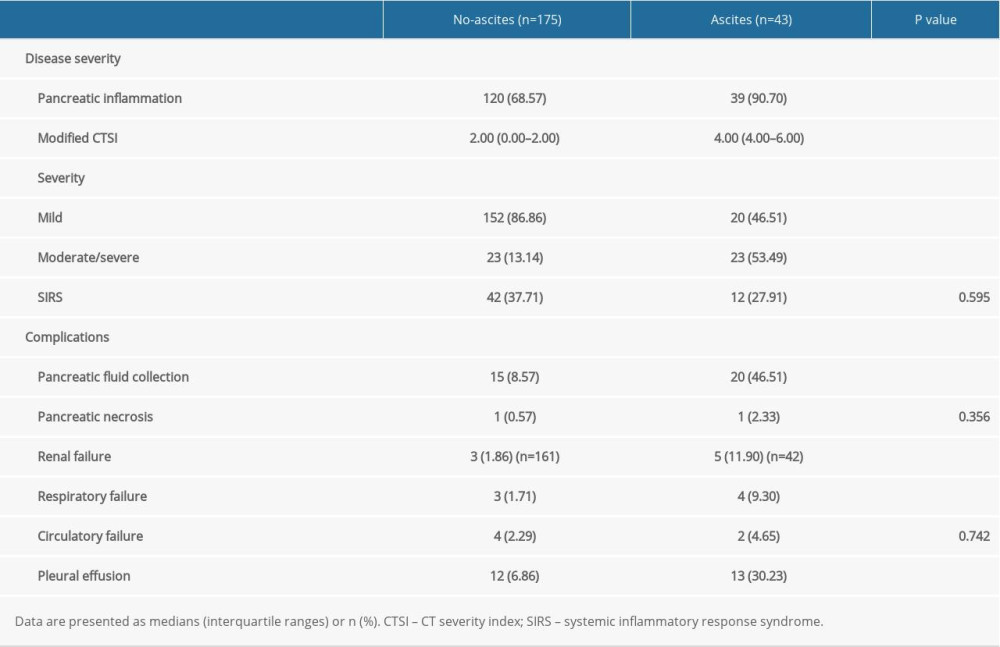 Table 4. Outcomes of patients with or without ascites.
Table 4. Outcomes of patients with or without ascites.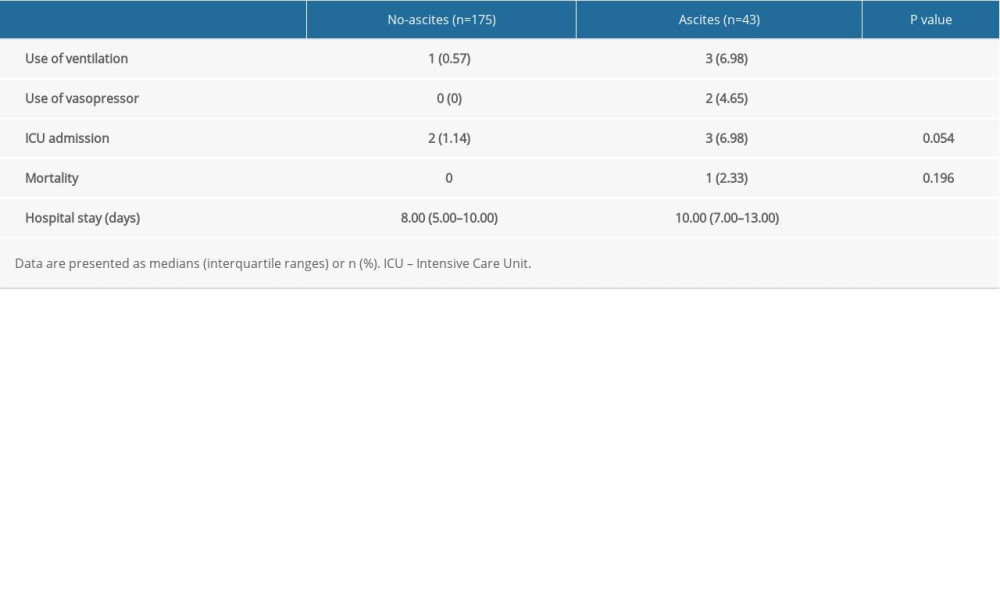 Table 5. Logistic regression analyses of ascites as a risk factor for severe acute pancreatitis and its complications.
Table 5. Logistic regression analyses of ascites as a risk factor for severe acute pancreatitis and its complications.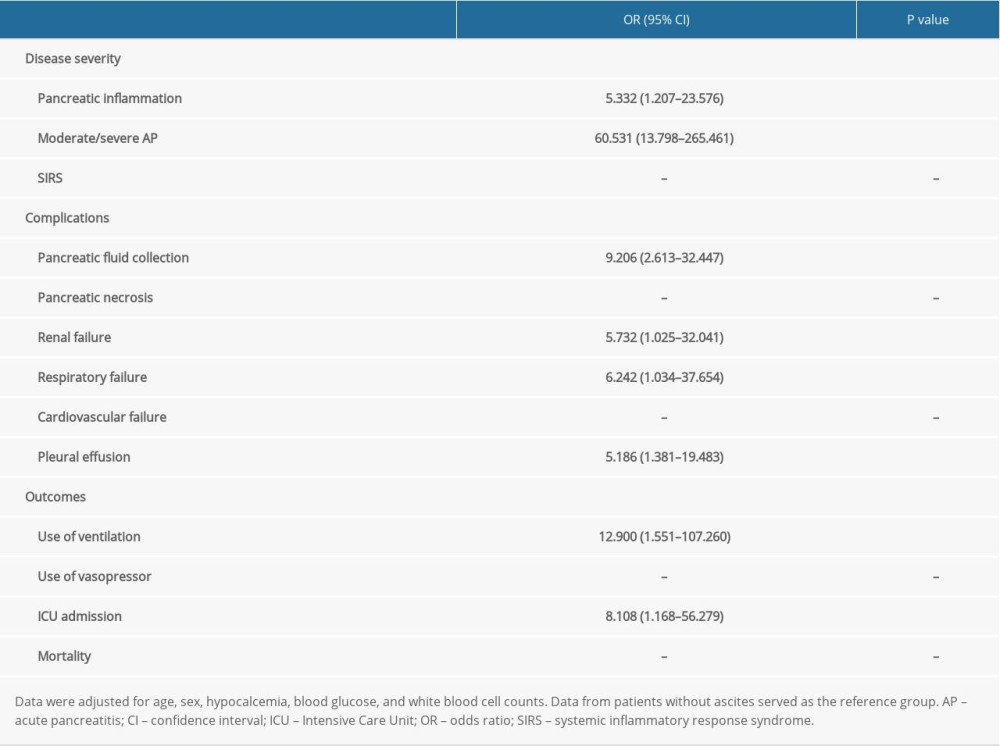 Table 6. Multivariate linear regression analysis for ascites as a predictor of severe acute pancreatitis.
Table 6. Multivariate linear regression analysis for ascites as a predictor of severe acute pancreatitis.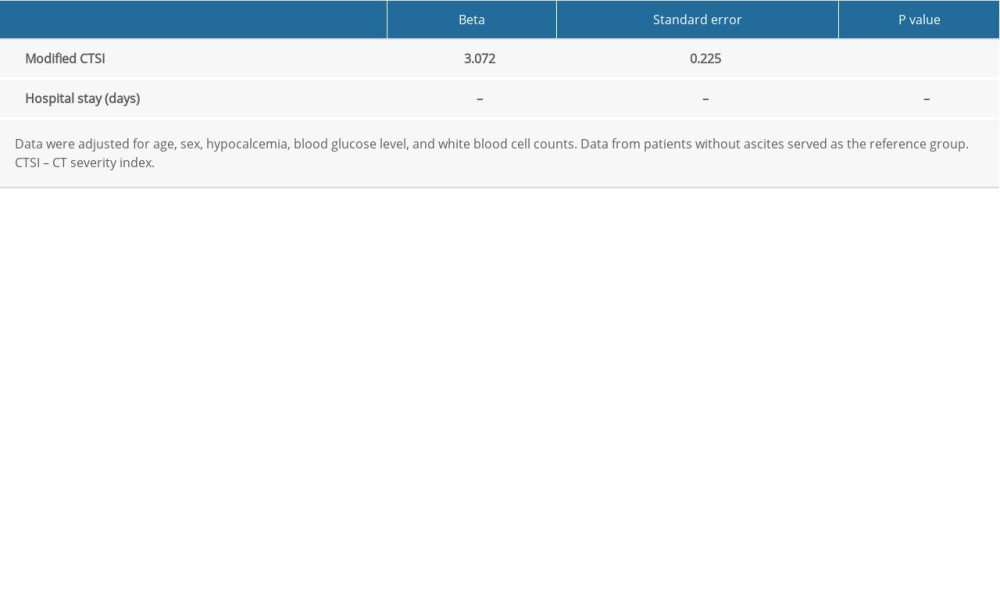
References
1. Lankisch PG, Apte M, Banks PA, Acute pancreatitis: Lancet, 2015; 386; 85-96
2. Lee PJ, Papachristou GI, New insights into acute pancreatitis: Nat Rev Gastro Hepat, 2019; 16; 479-96
3. Forsmark CE, Vege SS, Wilcox CM, Acute pancreatitis: N Engl J Med, 2016; 375; 1972-81
4. Garg PK, Singh VP, Organ failure due to systemic injury in acute pancreatitis: Gastroenterology, 2019; 156; 2008-23
5. Vivian E, Cler L, Conwell D, Acute pancreatitis task force on quality: Development of quality indicators for acute pancreatitis management: Am J Gastroenterol, 2019; 114; 1322-42
6. Mole DJ, Olabi B, Robinson V, Garden OJ, Parks RW, Incidence of individual organ dysfunction in fatal acute pancreatitis: Analysis of 1024 death records: HPB (Oxford), 2009; 11; 166-70
7. Di MY, Liu H, Yang ZY, Prediction models of mortality in acute pancreatitis in adults: A systematic review: Ann Intern Med, 2016; 165; 482-90
8. Gapp JHG, Chandra S, Pancreatic ascites: StatPearls [Internet], 2021, Treasure Island (FL), StatPearls Publishing Available from: URL:https://www.ncbi.nlm.nih.gov/books/NBK482468/
9. Li YY, Lu XY, Li XJ, Intervention of pyrrolidine dithiocarbamate and tetrandrine on cellular calcium overload of pancreatic acinar cells induced by serum and ascitic fluid from rats with acute pancreatitis: J Gastroenterol Hepatol, 2009; 24; 155-65
10. Samanta J, Rana A, Dhaka N, Ascites in acute pancreatitis: Not a silent bystander: Pancreatology, 2019; 19; 646-52
11. He WH, Xion ZJ, Zhu Y, Percutaneous drainage versus peritoneal lavage for pancreatic ascites in severe acute pancreatitis: A prospective randomized trial: Pancreas, 2019; 48; 343-49
12. Maringhini A, Ciambra M, Patti R, Ascites, pleural, and pericardial effusions in acute pancreatitis. A prospective study of incidence, natural history, and prognostic role: Dig Dis Sci, 1996; 41; 848-52
13. Bush N, Rana SS, Ascites in acute pancreatitis: clinical implications and management: Dig Dis Sci, 2021 [Online ahead of print]
14. Pepe J, Colangelo L, Biamonte F, Diagnosis and management of hypocalcemia: Endocrine, 2020; 69; 485-95
15. Farkas H, Harmat G, Kaposi PN, Ultrasonography in the diagnosis and monitoring of ascites in acute abdominal attacks of hereditary angioneurotic oedema: Eur J Gastroenterol Hepatol, 2001; 13; 1225-30
16. Banks PA, Bollen TL, Dervenis C, Classification of acute pancreatitis – 2012: Revision of the Atlanta classification and definitions by international consensus: Gut, 2013; 62; 102-11
17. Hines OJ, Pandol SJ, Management of severe acute pancreatitis: BMJ, 2019; 367; l6227
18. Babu RY, Gupta R, Kang M, Predictors of surgery in patients with severe acute pancreatitis managed by the step-up approach: Ann Surg, 2013; 257; 737-50
19. Kumar P, Gupta P, Rana S, Thoracic complications of pancreatitis: JGH Open, 2019; 3; 71-79
20. Mortele KJ, Wiesner W, Intriere L, A modified CT severity index for evaluating acute pancreatitis: improved correlation with patient outcome: Am J Roentgenol, 2004; 183; 1261-65
21. Charlson ME, Pompei P, Ales KL, MacKenzie CR, A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation: J Chronic Dis, 1987; 40; 373-83
22. Wu BU, Johannes RS, Sun X, Early changes in blood urea nitrogen predict mortality in acute pancreatitis: Gastroenterology, 2009; 137; 129-35
23. Carvalho JR, Fernandes SR, Santos P, Acute pancreatitis in the elderly: A cause for increased concern?: Eur J Gastroenterol Hepatol, 2018; 30; 337-41
24. Chen H, Li F, Sun JB, Jia JG, Abdominal compartment syndrome in patients with severe acute pancreatitis in early stage: World J Gastroenterol, 2008; 14; 3541-48
25. Mifkovic A, Skultety J, Sykora P, Intra-abdominal hypertension and acute pancreatitis: Bratisl Lek Listy, 2013; 114; 166-71
26. Malbrain ML, De laet IE, De Waele JJ, IAH/ACS: The rationale for surveillance: World J Surg, 2009; 33; 1110-15
27. Pérez S, Pereda J, Sabater L, Sastre J, Pancreatic ascites hemoglobin contributes to the systemic response in acute pancreatitis: Free radical Biol Med, 2015; 81; 145-55
28. Koutroumpakis E, Wu BU, Bakker OJ, Admission hematocrit and rise in blood urea nitrogen at 24 h outperform other laboratory markers in predicting persistent organ failure and pancreatic necrosis in acute pancreatitis: A post hoc analysis of three large prospective databases: Am J Gastroenterol, 2015; 110(12); 1707-16 [Erratum in: Am J Gastroenterol. 2016;111(8):1216]
29. Liu RH, Wen Y, Sun HY, Abdominal paracentesis drainage ameliorates severe acute pancreatitis in rats by regulating the polarization of peritoneal macrophages: World J Gastroenterol, 2018; 24; 5131-43
30. van Santvoort HC, Besselink MG, Bakker OJ, A step-up approach or open necrosectomy for necrotizing pancreatitis: N Engl J Med, 2010; 362; 1491-502
31. Wang T, Liu LY, Luo H, Intra-abdominal pressure reduction after percutaneous catheter drainage is a protective factor for severe pancreatitis patients with sterile fluid collections: Pancreas, 2016; 45; 127-33
32. Weiss FU, Laemmerhirt F, Lerch MM, Acute pancreatitis: genetic risk and clinical implications: J Clin Med, 2021; 10; 190
Tables
 Table 1. Patient characteristics.
Table 1. Patient characteristics. Table 2. Laboratory variables in patients with or without ascites.
Table 2. Laboratory variables in patients with or without ascites. Table 3. Disease severity and complication-related variables in patients with or without ascites.
Table 3. Disease severity and complication-related variables in patients with or without ascites. Table 4. Outcomes of patients with or without ascites.
Table 4. Outcomes of patients with or without ascites. Table 5. Logistic regression analyses of ascites as a risk factor for severe acute pancreatitis and its complications.
Table 5. Logistic regression analyses of ascites as a risk factor for severe acute pancreatitis and its complications. Table 6. Multivariate linear regression analysis for ascites as a predictor of severe acute pancreatitis.
Table 6. Multivariate linear regression analysis for ascites as a predictor of severe acute pancreatitis. In Press
05 Mar 2024 : Clinical Research
Muscular Function Recovery from General Anesthesia in 132 Patients Undergoing Surgery with Acceleromyograph...Med Sci Monit In Press; DOI: 10.12659/MSM.942780
05 Mar 2024 : Clinical Research
Effects of Thermal Insulation on Recovery and Comfort of Patients Undergoing Holmium Laser LithotripsyMed Sci Monit In Press; DOI: 10.12659/MSM.942836
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








