01 December 2021: Clinical Research
A Retrospective Observational Study of the Association Between Plasma Levels of Interleukin 8 in 42 Patients with Sepsis-Induced Myocardial Dysfunction at a Single Center Between 2017 and 2020
XiaoYing Chen1ABC, Xian Liu1BE, RuiAn Dong1BE, Dan Zhang1AG*, Shu Qin2AEGDOI: 10.12659/MSM.933065
Med Sci Monit 2021; 27:e933065
Abstract
BACKGROUND: This retrospective, observational study from a single center aimed to evaluate the association between complement (C)3 and C4, lymphocytes markers CD4 and CD8, and the interleukins IL-1β, IL-2R, IL-6, IL-10, and IL-8 in patients with sepsis-induced myocardial dysfunction (SIMD) and a reduced left ventricular ejection fraction (LVEF) of < 50%.
MATERIAL AND METHODS: Patients with sepsis from July 2017 to December 2020 were divided into a SIMD group (42 patients) and NO-SIMD group (214 patients). Diagnostic criteria of sepsis were based on SEPSIS 3.0 guidelines. SIMD was defined as LVEF <50% by echocardiography and global ejection fraction <25% by transpulmonary thermodilution during hospitalization. The lymphocyte markers and interleukins were detected by flow fluorescence immunomicrobead assay, and C3 and C4 were detected by enzyme-linked immunosorbent assay.
RESULTS: Plasma levels of IL-8 in the SIMD group were significantly higher than those in the NO-SIMD group, 133.90 (80.20, 402.79) vs 46.35 (16.80, 125.00) pg/mL (P<0.001). Logistic regression showed that N terminal pro B type natriuretic peptide (NT-proBNP; 95%CI 1.000-1.000, P<0.001) and IL-8 (95%CI 1.000-1.002, P=0.019) were independent risk factors for SIMD. Receiver operating characteristic curve analysis showed NT-proBNP, IL-8, and cardiac troponin T (cTnT) had different predictive values for SIMD: AUCNT-proBNP (0.810) >AUCIL-8 (0.748) >AUCcTnT (0.710). The cut-off value of IL-8 was 67.55 pg/mL; using this cut-off value, IL-8 predicted SIMD in sepsis with a sensitivity of 83.3% and specificity of 59.3%.
CONCLUSIONS: Increased plasma levels of IL-8 were significantly associated with cardiac dysfunction in patients with SIMD.
Keywords: Interleukin-8, inflammatory mediators, Sepsis, ejection fraction, Cardiomyopathies, Female, Humans, Male
Background
Sepsis and septic shock are medical emergencies, and SEPSIS 3.0 guidelines recommend immediate treatment and resuscitation, including initial resuscitation within the first 3 hours, norepinephrine as the first-choice vasopressor, and the use of echocardiography for further hemodynamic assessment (such as assessing cardiac function) to determine the type of shock [1]. Sepsis-induced myocardial dysfunction (SIMD) is cardiac dysfunction caused by sepsis, which is characterized by decreased left ventricular dilation and left ventricular ejection fraction (LVEF); however, this dysfunction can resolve over 7 to 10 days after sepsis [2,3]. SIMD was first reported by Parker et al in 1984, and has a development history of nearly 40 years [4]; however, SIMD has not been totally understood. Unfortunately, 18% to 65% of incidences of myocardial dysfunction occur in patients with sepsis [5], and up to 80% occur in patients with septic shock [6]. SIMD is the main risk factor for death by sepsis, and the mortality rate can rise to 70% once it occurs [3]. Therefore, early identification of SIMD in sepsis is important; however, the obstacle is that there are no clear diagnostic criteria for SIMD at present.
The most important examination method for SIMD is echocardiography, which is widely used because it is easy to implement, noninvasive, and repeatable [7]. SIMD has been defined as an LVEF <50% and a decrease ≥10% of LVEF in patient baseline values in some studies [5,8]. However, LVEF has been increasingly regarded as an inaccurate indicator because of it is heavily dependent on cardiac preload [9–11]. Therefore, some specific serum biomarkers that provide independent relevant information for myocardial injury or cardiac function can be used as a complement to the diagnosis of SIMD [7]. Troponin T (TnT) and B type natriuretic peptide (BNP), are considered as potential indicators of myocardial dysfunction [12], and these 2 cardiac biomarkers are generally elevated in sepsis [13]. Some studies have shown that cardiac biomarkers were significantly associated with SIMD on echocardiography, such as left ventricular systolic function, diastolic dysfunction, and even atrial arrhythmia [14,15]. However, other results are inconsistent or even contrary [13,16], supporting that increased plasma BNP or TnT levels seem to reflect the severity of illness but not specifically SIMD [17,18].
In sepsis, infection results in an imbalance of the host immune response, leading to organ dysfunction and even death [1]. The pathophysiological cascade begins with the host immune system’s response to invasive pathogens, then the initial immune response is activated, which promotes the release of inflammatory mediators and signaling molecules and activates positive and negative feedback in the immune system. Whether this process is beneficial or harmful has not yet been confirmed [19]. Endotoxins, cytokines, and nitric oxide (NO) have been considered the main mediators in the pathogenesis of SIMD [5,20]. An imbalance of inflammatory response is directly related to dysfunction of cardiomyocytes in SIMD. The interleukin (IL)-1β/tumor necrosis factor (TNF)-α/IL-6/P38 pathway, complement system, NO dysfunction, and PAMPs/DAMPs are considered to have important roles in the occurrence of SIMD [9,21,22]. In sepsis, the complement system is activated, leading to a cytokine storm and cardiomyopathy. In addition, the effect of complement components on intracellular calcium homeostasis is also part of the pathogenesis of SIMD [23,24]. The direct injury of inflammatory factors to myocardial cells is considered to be another mechanism of SIMD; (IL-1) can inhibit cardiac contractility by stimulating NO, and IL-6 has been shown to be involved in the pathogenesis of SIMD [25]. IL-8 may be, by its effects on neutrophils, part of an inflammatory cascade that contributes to injury of the reperfused myocardium [26]. The differences in IL-8 levels are probably more related to the acute myocardial infarction and the accompanying degree of heart failure [26]. Husebye et al suggested a possible role of IL-8 in the reperfusion-related injury and adverse left ventricular remodeling of post-ischemic myocardium [26]. However, the association between the complement, lymphocytes, and inflammatory cytokines has not been addressed in clinical trials to date.
Therefore, in this retrospective study from a single center, we aimed to evaluate the association between complement (C)3 and C4, lymphocytes markers CD4 and CD8, and the interleukins IL-1β, IL-2R, IL-6, IL-10, and IL-8 in patients with SIMD with a reduced LVEF of <50%.
Material and Methods
PATIENTS:
This retrospective observational study was approved by the Medical Ethics Committee at the First Affiliated Hospital of Chongqing Medical University. All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments. All patients or their guardians provided written informed consent.
DEFINITION OF SEPSIS AND SIMD:
The diagnostic criteria of sepsis were based on the following SEPSIS 3.0 guideline [1]: “Sepsis is defined as a life-threatening organ dysfunction caused by a dysregulated host response to infection, which can be associated with a Sepsis-related Organ Failure Assessment (SOFA) score ≥2 points consequent to the infection”.
SIMD was defined as patients with sepsis with LVEF <50% by echocardiography during hospitalization (this diagnostic criterion referred to the study of Sato et al [5]), and the global ejection fraction (GEF) <25% by pulse index continuous cardiac output (PICCO). Echocardiography was performed by a cardiovascular physician or sonographer when the patient was not on cardiac stimulants on admission and was confirmed by an additional physician. PICCO involved advanced hemodynamic monitoring, integrating static and dynamic hemodynamic data by combining trans-cardiopulmonary thermodilution with pulse contour analysis, as reported by Litton [27].
INCLUSION AND EXCLUSION CRITERIA AND GROUPING:
In total, 329 patients diagnosed with sepsis in the Critical Care Medicine Department of our hospital from July 2017 to December 2020 were included in this study. The following patients were excluded: those with previous history of heart failure (29 patients), neuroendocrine tumors (7 patients), trauma with hemopneumothorax or pericardial tamponade (8 patients), age younger than 16 years (16 patients), history of pulmonary lobectomy surgery (4 patients), and lack of informed consent (9 patients). The included patients were divided into the SIMD group and NO-SIMD group according to the results of LVEF by transthoracic echocardiography on admission and GEF by PICCO (Figure 1). The patients in the SIMD group met the definition of SIMD in this study, including LVEF <50% by echocardiography and the GEF <25% by PICCO. Patients not meeting these criteria were included in the NO-SIMD group.
CLINICAL DATA COLLECTION AND LABORATORY EXAMINATION:
General clinical data including age, sex, hospitalization time, 30-day survival, vital signs at admission, and previous history were recorded. Blood routine examination, biochemical examination of liver/renal function and the cardiac biomarkers cardiac troponin T (cTnT) and N terminal pro B type natriuretic peptide (NT-proBNP) were detected in all patients within 24 h of admission. The Acute Physiology and Chronic Health Evaluation II (APACHE II) score and SOFA score were calculated.
The plasma concentrations of C3 and C4, lymphocytes markers CD4 and CD8, and interleukins IL-1β, IL-2R, IL-6, IL-10, and IL-8 were detected by the clinical Molecular Test Center of our hospital at admission. The lymphocytes markers CD4 and CD8 were detected by flow cytometry (CD3-FITC/CD16+56-PE/CD45-PerCP-Cy5.5/CD4-PC7/CD19-APC/CD8-APC-Cy7; fluorescent monoclonal antibody kit, Beijing Tongsheng Shidai Biotech Co, Ltd; detecting instrument: BD FACSCanto II). The interleukins IL-1β, IL-2R, IL-6, IL-10, and IL-8 were detected by flow fluorescence immunomicrobead assay (12 Cytokine Detection Kits, Product code R701002, Qingdao Raisecare Biotech Co, Ltd; detecting instrument: Navios Beckman Coulter). Plasma IL-8 concentration was measured in strict accordance with the kit instructions, including extraction of plasma (venous blood samples were collected with an EDTA anticoagulant tube and centrifuged at 1000 g for 30 min), preparation of matrix, calibration reagents, and flow cytometry.
STATISTICAL ANALYSIS:
SPSS version 17.0 was used for statistical analysis and PASS was used for power analysis. Continuous variables were expressed as mean±standard deviation or median (quartile) depending on whether the data conformed to a normal distribution or not. Groups were compared using the
Results
COMPARISON OF CLINICAL DATA AND LABORATORY EXAMINATIONS BETWEEN SIMD AND NO-SIMD GROUPS:
A total of 256 patients with sepsis were included in this study. The average age of the patients was 66 years (53–74 years), and 61.72% were men. A total of 48 patients required vasoactive agents to maintain blood pressure, 86 patients underwent mechanical ventilation, 107 patients developed a new arrhythmia, mainly atrial arrhythmia. According to the results of transthoracic echocardiography and PICCO, patients were divided into either the SIMD group (42 patients) or NO-SIMD group (214 patients). More patients in the SIMD group than in the NO-SIMD group needed vasoactive drugs (P<0.001). The plasma concentrations in the SIMD and NO-SIMD groups were cTnT 0.0945 (0.0405, 0.2133) vs 0.0360 (0.0155, 0.0795) ug/L; NT-proBNP 7782.00 (2601.50, 28415.50) vs 1553.50 (422.00, 4228.00) ng/L; lactate 2.45 (1.96, 4.78) vs 1.58 (1.05, 2.75) mmol/L; blood urea nitrogen (BUN) 15.00 (11.10, 19.18) vs 9.20 (5.98, 14.45) mmol/L; creatinine 134.50 (87.50, 236.75) vs 91.00 (60.00, 135.00) μmol/L; and procalcitonin 4.40 (2.50, 23.67) vs 0.84 (0.15, 5.66) mmol/L, respectively. The differences between groups were statistically significant (P<0.05). No significant differences were found in general clinical data, mortality, blood gas analysis, and routine blood and other laboratory examinations (Table 1).
We compared the differences of C3 and C4, lymphocytes markers CD4 and CD8, and interleukins IL-1β, IL-2R, IL-6, IL-10, and IL-8 between the SIMD and NO-SIMD groups in this study (Table 2). The results showed that the concentration of plasma C4 in the SIMD group (0.18±0.04 g/L) was significantly lower than that in the NO-SIMD group (0.21±0.07 g/L), P=0.028. The concentrations of plasma IL-1β, IL-2R, IL-10, and IL-8 in the SIMD group were significantly higher than those in the NO-SIMD group: IL-1β 5.00 (5.00, 5.86) vs 4.90 (4.90, 5.00) pg/mL, P<0.001; IL-2R 1673.00 (1027.00, 4024.25) vs 1136.50 (584.00, 2744.00) IU/mL, P=0.015; IL-10 19.40 (8.78, 55.86) vs 7.10 (4.90, 26.05) pg/mL, P=0.002; and IL-8 133.90 (80.20, 402.79) vs 46.35 (16.80, 125.00) pg/mL, P<0.001, respectively. There were no differences in other immune parameters (C3, CD4, CD8) and IL-6 between the SIMD and NO-SIMD groups.
RISK FACTORS OF SIMD IN SEPSIS:
Binary logistic regression was used to analyze the independent risk factors of SIMD. The results showed that only NT-proBNP (P<0.001) and IL-8 (P=0.019) were independent risk factors for SIMD in this study (Table 3). The logistic regression model also revealed that cTnT (P=0.083), BUN (P=0.072), creatinine (P=0.077), procalcitonin (P=0.154), lactate (P=0.399), C4 (P=0.179), IL-1β (P=0.174), IL-2R (P=0.890), and IL-10 (P=0.485) were not associated with SIMD in our study.
ANALYSIS OF CORRELATION BETWEEN IL-8 AND OTHER VARIABLES IN SEPSIS:
Pearson correlation analysis showed that plasma IL-8 concentration levels were significantly correlated with the concentration of cTnT (r=0.490, P<0.001), NT-proBNP (r=0.328, P<0.001), creatinine (r=0.458, P<0.001), BUN (r=0.285, P<0.001), IL-2R (r=0.160, P=0.010), and IL-10 (r=0.184, P=0.003) in patients with sepsis (Table 4).
PREDICTIVE VALUE OF IL-8, CTNT AND NT-PROBNP FOR SIMD IN SEPSIS:
ROC curve analysis was used to evaluate the predictive value of IL-8, cTnT, and NT-proBNP for SIMD in sepsis. The results showed that NT-proBNP, IL-8, and cTnT had different predictive values for SIMD (Figure 2), with an area under the ROC curve (AUC) of AUCNT-proBNP (0.810) >AUCIL-8 (0.748) >AUCcTnT (0.710) (Figure 2, Table 5). The cut-off value of IL-8 was 67.55 pg/mL, and with this cut-off value IL-8 could predict SIMD in sepsis with a sensitivity of 83.3% and specificity of 59.3%.
POWER ANALYSIS:
Using the cut-off value of IL-8 to define exposed patients, the exposure percentage was P1=35/42=83% in the SIMD group and P2=87/214=41% in the NO-SIMD group. The odds ratio (OR) between the SIMD group and the NO-SIMD group=[P1/(1-P1)]/[P2/(1-P2)]=7.3. Power analysis was based on the comparison of 2 independent sample rates. The null hypothesis was H0: OR=1, alternative hypothesis was H1: OR≠1, and the overall class I error level was α=0.05. PASS software was used to calculate the power=100% under the current sample size.
Discussion
LIMITATIONS:
There were some limitations in our study. First, it was a single-center retrospective study with a small sample size, in which we attempted to explore the correlation between some cytokines and SIMD but not the specific mechanism of IL-8 in SIMD. The small cohort population and unblinded design are major confounders for this study. Second, sepsis is an acute syndrome caused by infection, and the inclusion of patients was heterogeneous owing to differences in primary disease. Third, this study did not completely exclude patients with preexisting cardiovascular disease.
Conclusions
In this study, increased plasma levels of IL-8 were most significantly associated with cardiac dysfunction in patients with SIMD. Plasma IL-8-concentration was a potential biomarker for predicting SIMD in sepsis.
Figures
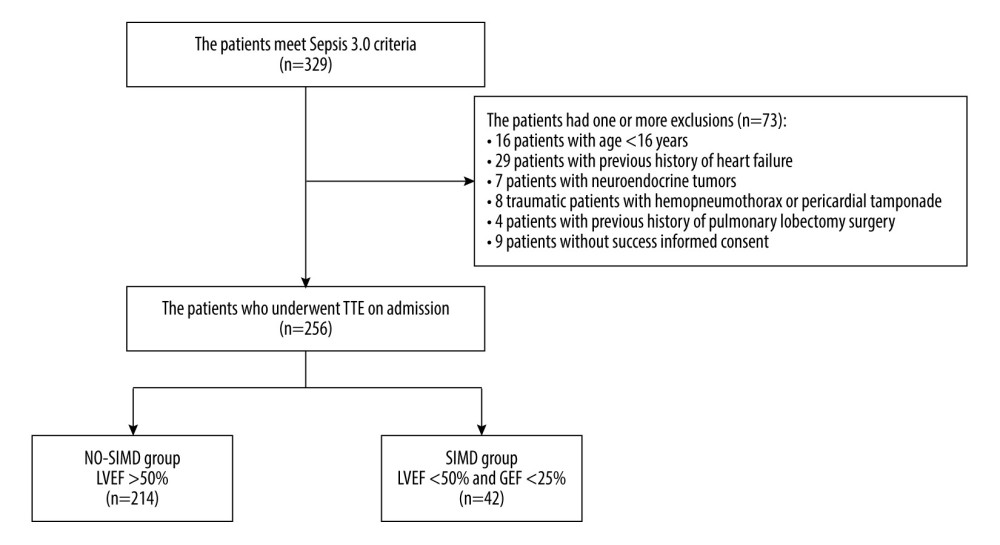 Figure 1. Study flow diagram. In total, 329 patients had sepsis or septic shock. The study included 256 patients with sepsis. Patients were divided into a sepsis-induced myocardial dysfunction group (SIMD; n=42) and a without sepsis-induced myocardial dysfunction group (NO-SIMD; n=214) by transthoracic echocardiography (TTE). LVEF – left ventricular ejection fraction; GEF – global ejection fraction.
Figure 1. Study flow diagram. In total, 329 patients had sepsis or septic shock. The study included 256 patients with sepsis. Patients were divided into a sepsis-induced myocardial dysfunction group (SIMD; n=42) and a without sepsis-induced myocardial dysfunction group (NO-SIMD; n=214) by transthoracic echocardiography (TTE). LVEF – left ventricular ejection fraction; GEF – global ejection fraction. 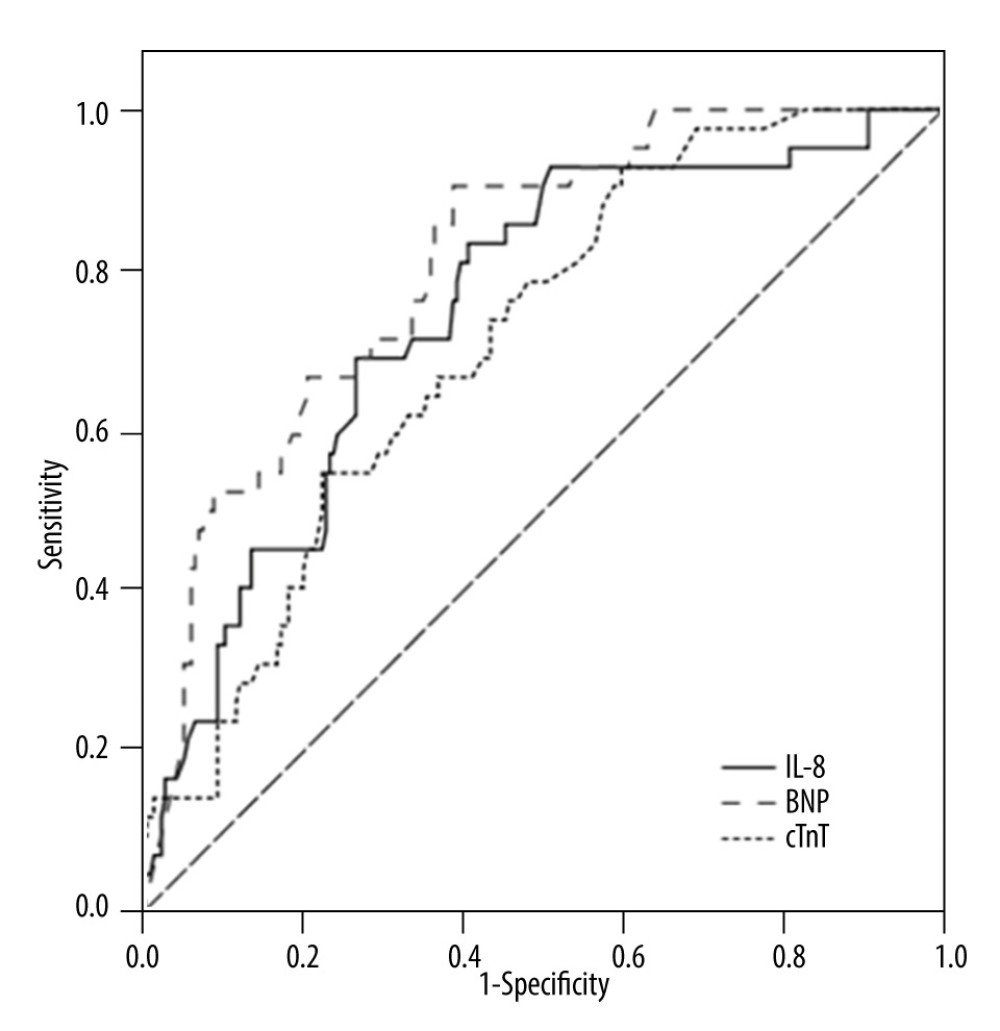 Figure 2. Receiver operating characteristic curve of cardiac troponin T (cTnT), N terminal pro B type natriuretic peptide (NT-proBNP), and interleukin (IL)-8 to predict sepsis-induced myocardial dysfunction (SIMD) in sepsis.
Figure 2. Receiver operating characteristic curve of cardiac troponin T (cTnT), N terminal pro B type natriuretic peptide (NT-proBNP), and interleukin (IL)-8 to predict sepsis-induced myocardial dysfunction (SIMD) in sepsis. Tables
Table 1. Comparison of basic information and clinical characteristics between the 2 groups.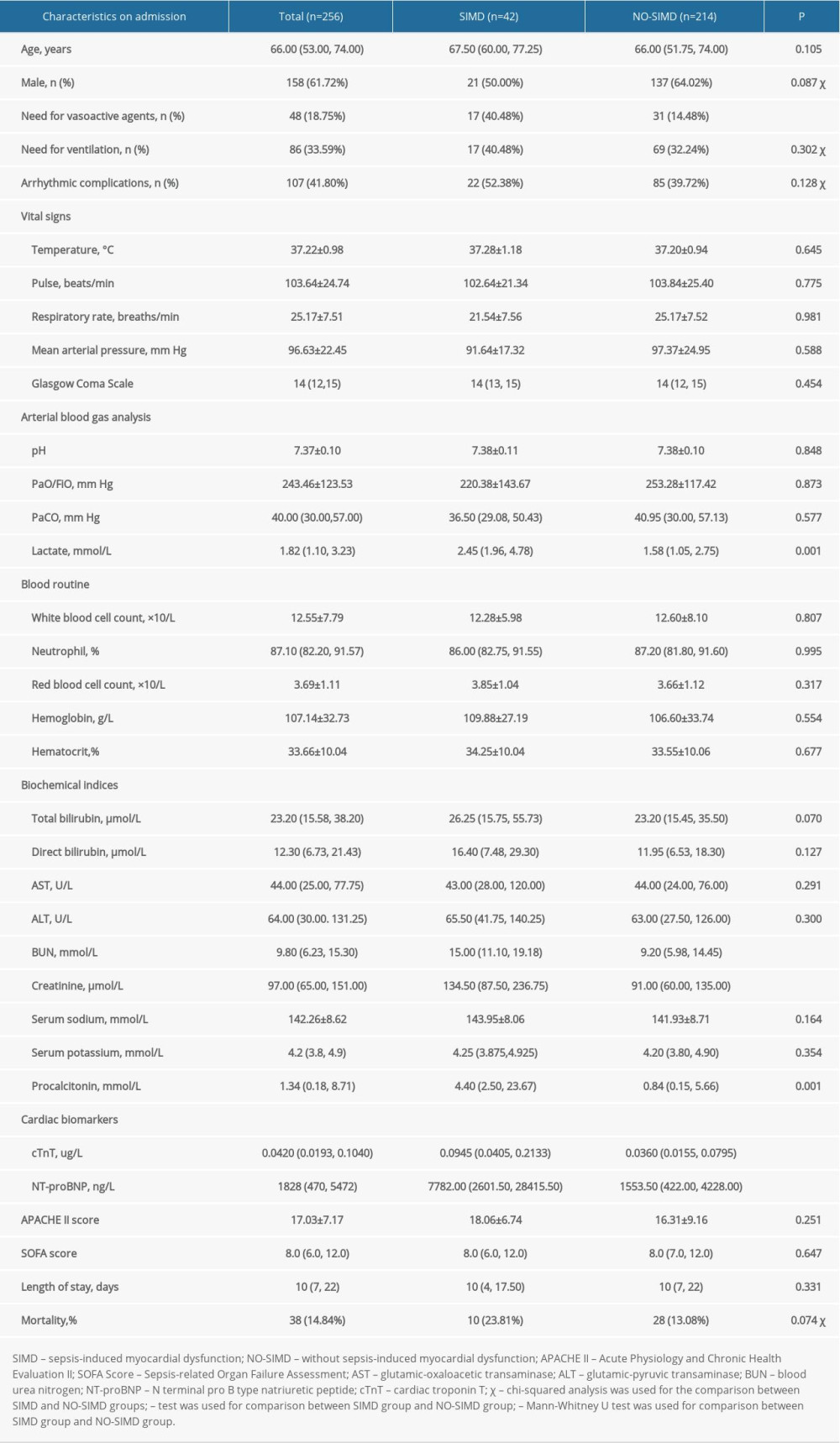 Table 2. Comparison of immune parameters and inflammatory mediators between the 2 groups.
Table 2. Comparison of immune parameters and inflammatory mediators between the 2 groups. Table 3. Risk factors of sepsis-induced myocardial dysfunction using a binary logistic regression model.
Table 3. Risk factors of sepsis-induced myocardial dysfunction using a binary logistic regression model.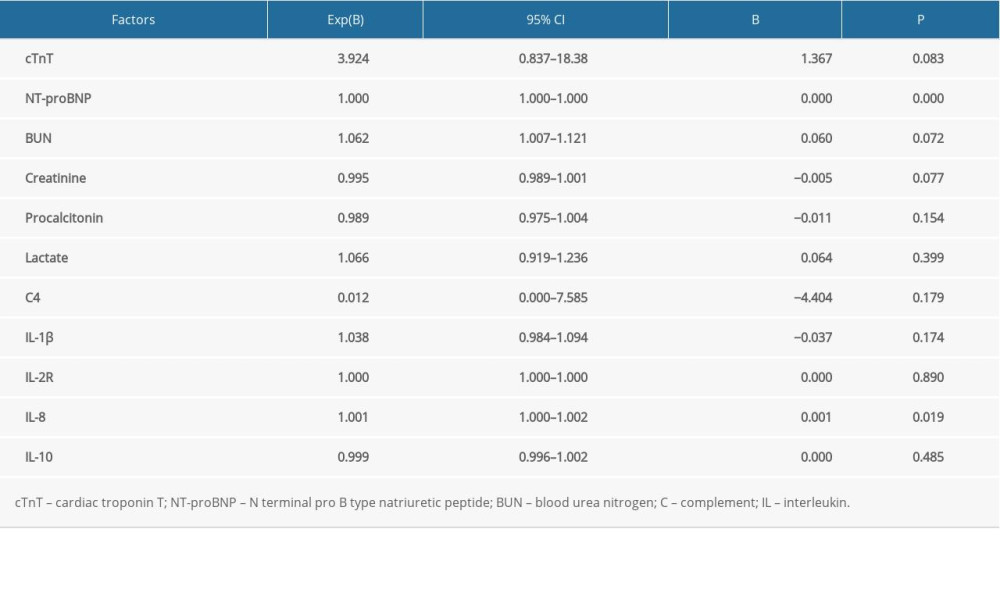 Table 4. Pearson correlation between interleukin 8 and other variables.
Table 4. Pearson correlation between interleukin 8 and other variables.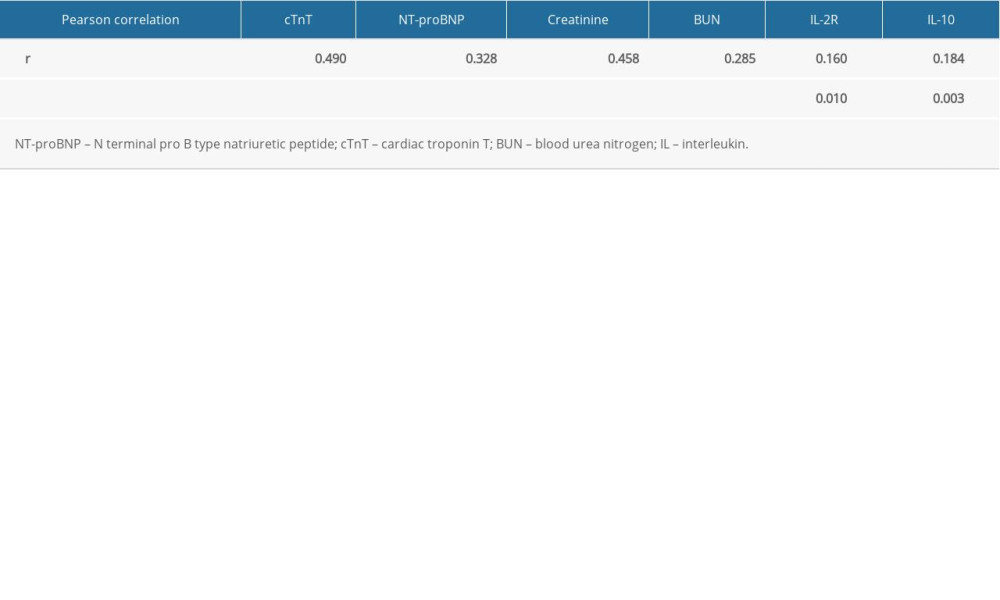 Table 5. Receiver operating characteristic curve analysis of cardiac troponin T, N terminal pro B type natriuretic peptide, interleukin 8 in predicting sepsis-induced myocardial dysfunction.
Table 5. Receiver operating characteristic curve analysis of cardiac troponin T, N terminal pro B type natriuretic peptide, interleukin 8 in predicting sepsis-induced myocardial dysfunction.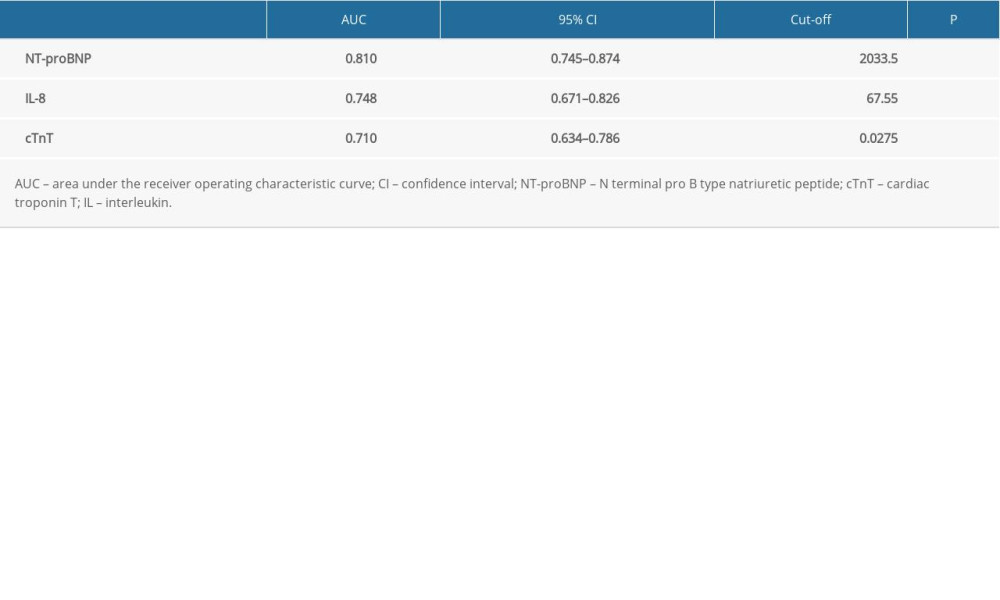
References
1. Rhodes A, Evans LE, Alhazzani W, Surviving sepsis campaign: International guidelines for management of sepsis and septic shock: Crit Care Med, 2017; 45(3); 1
2. Jeong HS, Lee TH, Bang CH, Risk factors and outcomes of sepsis-induced myocardial dysfunction and stress-induced cardiomyopathy in sepsis or septic shock: Medicine, 2018; 97(13); e0263
3. Romero-Bermejo FJ, Ruiz-Bailen M, Gil-Cebrian J, Huertos-Ranchal MJ, Sepsis-induced Cardiomyopathy: Curr Cardiol Rev, 2011; 7(3); 163-83
4. Parker MM, Shelhamer JH, Bacharach SL, Profound but reversible myocardial depression in patients with septic shock: Ann Intern Med, 1984; 100(4); 483
5. Sato R, Kuriyama A, Takada T, Nasu M, Luthe SK, Prevalence and risk factors of sepsis-induced cardiomyopathy: A retrospective cohort study: Medicine, 2016; 95(39); e5031
6. Beraud AS, Guillamet CV, Hammes JL, Efficacy of transthoracic echocardiography for diagnosing heart failure in septic shock: Am J Med Sci, 2014; 347(4); 295-98
7. Ehrman RR, Sullivan AN, Favot MJ, Pathophysiology, echocardiographic evaluation, biomarker findings, and prognostic implications of septic cardiomyopathy: A review of the literature: Crit Care, 2018; 22(1); 112
8. Sato R, Nasu M, A review of sepsis-induced cardiomyopathy: J Intensive Care, 2015; 3(1); 48
9. Beesley SJ, Weber G, Sarge T, Septic cardiomyopathy: Crit Care Med, 2018; 46(4); 625-34
10. Lanspa MJ, Pittman JE, Hirshberg EL, Association of left ventricular longitudinal strain with central venous oxygen saturation and serum lactate in patients with early severe sepsis and septic shock: Crit Care, 2015; 19(1); 304
11. Dalla K, Hallman C, Bech-Hanssen O, Strain echocardiography identifies impaired longitudinal systolic function in patients with septic shock and preserved ejection fraction: Cardiovasc Ultrasound, 2015; 13; 30
12. Maeder M, Fehr T, Rickli H, Ammann P, Sepsis-associated myocardial dysfunction: Diagnostic and prognostic impact of cardiac troponins and natriuretic peptides: Chest, 2006; 129(5); 1349-66
13. Landesberg G, Jaffe AS, Gilon D, Troponin elevation in severe sepsis and septic shock: The role of left ventricular diastolic dysfunction and right ventricular dilatation: Crit Care Med, 2013; 42(4); 790-800
14. Sheyin O, Davies O, Duan W, Perez X, The prognostic significance of troponin elevation in patients with sepsis: A meta-analysis: Heart Lung, 2015; 44(1); 75-81
15. Lee YJ, Lee H, Park JS, Cardiac troponin I as a prognostic factor in critically ill pneumonia patients in the absence of acute coronary syndrome: J Crit Care, 2014; 30(2); 390-94
16. Zaky A, Gill EA, Lin CP, Characteristics of sepsis-induced cardiac dysfunction using speckle-tracking echocardiography: A feasibility study: Anaesth Intensive Care, 2016; 44(1); 65-76
17. Papanikolaou J, Makris D, Mpaka M, New insights into the mechanisms involved in B-type natriuretic peptide elevation and its prognostic value in septic patients: Crit Care, 2014; 18(3); R94
18. Ostermann M, Ayis S, Tuddenham E, Cardiac troponin release is associated with biomarkers of inflammation and ventricular dilatation during critical illness: Shock, 2017; 47(6); 702-8
19. Conway-Morris A, Wilson J, Shankar-Hari M, Immune activation in sepsis: Crit Care Clin, 2017; 34(1); 29-42
20. Kumar A, Thota V, Dee L, Tumor necrosis factor alpha and interleukin 1beta are responsible for in vitro myocardial cell depression induced by human septic shock serum: Resuscitation, 1996; 32(2); 949-58
21. Tzeng HP, Fan J, Vallejo JG, Negative inotropic effects of high mobility box group 1 protein in isolated contracting cardiac myocytes: Am J Physiol, 2008; 294(2); 1490-96
22. Singer M, Rudiger A, The heart in sepsis: From basic mechanisms to clinical management: Curr Vasc Pharmacol, 2013; 11(2); 187-95
23. Fattahi F, Frydrych LM, Bian G, Role of complement C5a and histones in septic cardiomyopathy: Mol Immunol, 2018; 102; 32-41
24. Ward PA, Fattahi F, New strategies for treatment of infectious sepsis: J Leukoc Biol, 2019; 106(1); 187-92
25. Ravikumar N, Sayed MA, Poonsuph CJ, Septic cardiomyopathy: From basics to management choices: Curr Probl Cardiol, 2021; 46(4); 100767
26. Husebye T, Eritsland J, Arnesen H, Association of interleukin 8 and myocardial recovery in patients with ST-elevation myocardial infarction complicated by acute heart failure: PLoS One, 2014; 9(11); e112359
27. Litton E, Morgan M, The PiCCO monitor: A review: Anaesth Intensive Care, 2012; 40(3); 393-409
28. Chen FC, Xu YC, Zhang ZC, Multi-biomarker strategy for prediction of myocardial dysfunction and mortality in sepsis: J Zhejiang Univ Sci B, 2020; 21(7); 537-48
29. Zaky A, Deem S, Bendjelid K, Treggiari MM, Characterization of cardiac dysfunction in sepsis: Shock, 2014; 41(1); 12-24
30. Abraham E, Singer M, Mechanisms of sepsis-induced organ dysfunction: Crit Care Med, 2007; 35(10); 2408-16
31. Sevilla Berrios RA, O’Horo JC, Velagapudi V, Pulido JN, Correlation of left ventricular systolic dysfunction determined by low ejection fraction and 30-day mortality in patients with severe sepsis and septic shock: A systematic review and meta-analysis: J Crit Care, 2014; 29(4); 495-99
32. Huang SJ, Nalos M, Mclean AS, Is early ventricular dysfunction or dilatation associated with lower mortality rate in adult severe sepsis and septic shock? A meta-analysis: Crit Care, 2013; 17(3); R96
33. Arthur W, Edgar D, Kaye GC, The measurement of impedance to assess myocardial contractility and rhythm stability: Physiol Meas, 2000; 21(4); R43-54
34. Masson S, Caironi P, Fanizza CAlbumin Italian outcome sepsis study investigators, sequential n-terminal pro-b-type natriuretic peptide and high-sensitivity cardiac troponin measurements during albumin replacement in patients with severe sepsis or septic shock: Crit Care Med, 2015; 44(4); 707-16
35. Kim JS, Kim M, Kim YJ, Troponin testing for assessing sepsis-induced myocardial dysfunction in patients with septic shock: Clin Med, 2019; 8; 239
36. Røsjø H, Varpula M, Hagve TAFINNSEPSIS Study Group, Circulating high sensitivity troponin T in severe sepsis and septic shock: Distribution, associated factors, and relation to outcome: Intensive Care Med, 2011; 37(1); 77-85
37. Jozwiak M, Persichini R, Monnet X, Teboul JL, Management of myocardial dysfunction in severe sepsis: Semin Respir Crit Care Med, 2011; 32(2); 206-14
38. Pathan N, Franklin JL, Eleftherohorinou H, Myocardial depressant effects of interleukin 6 in meningococcal sepsis are regulated by p38 mitogen-activated protein kinase: Crit Care Med, 2011; 39(7); 1692
39. Kumar A, Brar R, Wang P, Role of nitric oxide and cGMP in human septic serum-induced depression of cardiac myocyte contractility: Am J Physiol, 1999; 276(67); R265-76
40. Fernandes CJ, De Assuncao MSC, Myocardial dysfunction in sepsis: A large, unsolved puzzle: Crit Care Res Pract, 2012; 2012; 896430
41. Christian S, Shanmuganathan L, Pavel , Association of IL-8 with infarct size and clinical outcomes in patients with STEMI: J Am Coll Cardiol, 2018; 72(2); 187-98
42. Zhao X, Zhang W, Xing D, Endothelial cells overexpressing IL-8 receptor reduce cardiac remodeling and dysfunction following myocardial infarction: Am J Physiol Heart Circ Physiol, 2013; 305(4); H590-98
Figures
 Figure 1. Study flow diagram. In total, 329 patients had sepsis or septic shock. The study included 256 patients with sepsis. Patients were divided into a sepsis-induced myocardial dysfunction group (SIMD; n=42) and a without sepsis-induced myocardial dysfunction group (NO-SIMD; n=214) by transthoracic echocardiography (TTE). LVEF – left ventricular ejection fraction; GEF – global ejection fraction.
Figure 1. Study flow diagram. In total, 329 patients had sepsis or septic shock. The study included 256 patients with sepsis. Patients were divided into a sepsis-induced myocardial dysfunction group (SIMD; n=42) and a without sepsis-induced myocardial dysfunction group (NO-SIMD; n=214) by transthoracic echocardiography (TTE). LVEF – left ventricular ejection fraction; GEF – global ejection fraction. Figure 2. Receiver operating characteristic curve of cardiac troponin T (cTnT), N terminal pro B type natriuretic peptide (NT-proBNP), and interleukin (IL)-8 to predict sepsis-induced myocardial dysfunction (SIMD) in sepsis.
Figure 2. Receiver operating characteristic curve of cardiac troponin T (cTnT), N terminal pro B type natriuretic peptide (NT-proBNP), and interleukin (IL)-8 to predict sepsis-induced myocardial dysfunction (SIMD) in sepsis. Tables
 Table 1. Comparison of basic information and clinical characteristics between the 2 groups.
Table 1. Comparison of basic information and clinical characteristics between the 2 groups. Table 2. Comparison of immune parameters and inflammatory mediators between the 2 groups.
Table 2. Comparison of immune parameters and inflammatory mediators between the 2 groups. Table 3. Risk factors of sepsis-induced myocardial dysfunction using a binary logistic regression model.
Table 3. Risk factors of sepsis-induced myocardial dysfunction using a binary logistic regression model. Table 4. Pearson correlation between interleukin 8 and other variables.
Table 4. Pearson correlation between interleukin 8 and other variables. Table 5. Receiver operating characteristic curve analysis of cardiac troponin T, N terminal pro B type natriuretic peptide, interleukin 8 in predicting sepsis-induced myocardial dysfunction.
Table 5. Receiver operating characteristic curve analysis of cardiac troponin T, N terminal pro B type natriuretic peptide, interleukin 8 in predicting sepsis-induced myocardial dysfunction. Table 1. Comparison of basic information and clinical characteristics between the 2 groups.
Table 1. Comparison of basic information and clinical characteristics between the 2 groups. Table 2. Comparison of immune parameters and inflammatory mediators between the 2 groups.
Table 2. Comparison of immune parameters and inflammatory mediators between the 2 groups. Table 3. Risk factors of sepsis-induced myocardial dysfunction using a binary logistic regression model.
Table 3. Risk factors of sepsis-induced myocardial dysfunction using a binary logistic regression model. Table 4. Pearson correlation between interleukin 8 and other variables.
Table 4. Pearson correlation between interleukin 8 and other variables. Table 5. Receiver operating characteristic curve analysis of cardiac troponin T, N terminal pro B type natriuretic peptide, interleukin 8 in predicting sepsis-induced myocardial dysfunction.
Table 5. Receiver operating characteristic curve analysis of cardiac troponin T, N terminal pro B type natriuretic peptide, interleukin 8 in predicting sepsis-induced myocardial dysfunction. In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








