22 October 2021: Database Analysis
Comparison of Magnetic Resonance Imaging of the Lower Uterine Segment in Pregnant Women with Central Placenta Previa with and without Placenta Accreta Spectrum from a Single Center
Shunyu Hou1G, Ye Song1B, Jiahui Wu2D, Liping Zhou1B, Suya Kang1F, Xi Chen3C, Lei Zhang4F, Yanli Lu5C, Yongfei Yue1ACE*DOI: 10.12659/MSM.932759
Med Sci Monit 2021; 27:e932759
Abstract
BACKGROUND: Placenta accreta spectrum (PAS) includes placenta increta, placenta percreta, and placenta accreta. PAS is due to abnormal decidualization and can lead to severe maternal hemorrhage and occurs in up to 3% of women with central placental previa (CPP). This study from a single center aimed to compare the magnetic resonance imaging (MRI) changes in the lower uterine segment in pregnant women with CPP, with and without PAS.
MATERIAL AND METHODS: This retrospective study includes 90 pregnant women with PAS and 66 pregnant women without PAS. All participants were confirmed to have CPP by MRI. Eight MRI parameters were assessed and compared with perinatal outcomes for mothers and newborns.
RESULTS: The pregnancies in the non-PAS group had less operative time (P=0.001), less intrapartum hemorrhage (P<0.001), and less blood transfusion than the PAS group (P<0.001). The 8 MRI variables with different odds ratios were placenta thickness (4.20), cervical lengths (3.27), placental dark T2 bands area (5.10), cervical marginal sinus (3.04), bladder bulge (3.55), myometrial thinning (6.41), lower uterine segment bulge (4.61), and placental signals in the cervix (9.14). The sensitivity and specificity of MRI in the diagnosis of PAS were 82.22% and 91.09%, respectively, by the combined 8 MRI features, and the area under the curve (AUC) was 0.816.
CONCLUSIONS: The findings from this study showed that MRI of the lower uterine segment had high sensitivity and specificity for the diagnosis of PAS in pregnant women with CPP.
Keywords: Magnetic Resonance Imaging, Placenta Accreta, Placenta Previa, Female, Humans, Pregnancy, Prenatal Diagnosis, Sensitivity and Specificity
Background
Placenta accreta spectrum (PAS) includes placenta increta, placenta percreta, and placenta accreta, and was formerly known as morbidly adherent placenta. PAS is due to abnormal decidualization and can lead to severe maternal hemorrhage [1]. PAS has been reported to occur in up to 3% of women with central placental previa with no prior cesarean deliveries [2]. Placenta previa (PP) is classified into 4 categories based on the distance from placental edge to the internal cervix: low-lying placenta, marginal placenta, partial placenta, and complete placenta previa (CPP). PAS has a high incidence among patients with CPP, which is considered a severe life-threatening obstetric disease and may be associated with bladder injury and massive intraoperative and postpartum hemorrhage [3]. PAS is associated with a number of causes, including PP, miscarriage, cesarean section, smoking, and history of uterine surgery [4]. The incidence of PAS has risen from 0.8% in the 1980s to 3% in the 2010s [5]. During cesarean section, most PAS patients need blood transfusions or even hysterectomies.
Ultrasound is the first-line imaging modality. However, in recent years, magnetic resonance imaging (MRI) is increasingly widely used in obstetrics and benefits from the experience of functional MRI, which allows the assessment of PAS and abnormalities around the lower uterine segment. It has been found that MRI has high sensitivity and specificity in the diagnosis of PAS [6,7]. PP is mostly located in the lower uterine segment, where the muscle tissue is thinner than in the body of the uterus. Placental villi are prone to implant in the uterine wall in order to obtain enough nutrients for fetal growth. In the third trimester, the uterine wall in the lower uterine segment is thinner and there is a high probability of placental villi penetrating the uterine wall and implanting in the bladder. The bleeding spots are in the uterine body and lower uterine segment in partial and marginal PP women, while bleeding spots are often in the lower uterine segment and cervical canal in CPP patients. The uterine body is free and completely exposed to the field of vision, so we can use figure 8 suturing, uterine artery ligation, B-Lynch, and other methods to stop the hemorrhage in the uterine body. In front of the lower segment of the uterus is the bladder, behind is the rectum, and on both sides is the ureter. Moreover, the cervical canal space is narrow, which creates great difficulty for surgical hemostasis.
The antenatal diagnosis of PAS is helpful for obstetricians to make adequate preoperative preparation and reduce hemorrhagic morbidity [8]. There are abundant studies on evaluation of PAS by MRI, but there is no uniform standard for PAS and severity assessment. Therefore, this study from a single center aimed to compare the sensitivity and specificity of MRI changes in the lower uterine segment in pregnant women with CPP, with and without PAS.
Material and Methods
PATIENT SELECTION:
Informed consents were signed by pregnant women and the study was approved by the ethics review board of our hospital. From August 2014 to October 2020, a total of 156 pregnant women with CPP diagnosed by MRI examinations were included in our study at our hospital. All patients had a prenatal ultrasound before MRI evaluation. We included 90 pregnant women with PAS and 66 women without PAS. Inclusion criteria were CPP patients with gestational age between 32 and 37 weeks, and complete pelvic MRI images. All of the participants had at least 1 previous cesarean section. Patients with multiple pregnancies, marginal placenta previa, partial placenta previa, did not undergo MRI examination, or delivered in other hospitals were excluded. The PAS were diagnosed after laparotomy, which was based on Collins’s three-step diagnostic method [9]. The detailed exclusion criteria and the flow chart of the study design are shown in Figure 1.
PATIENT AND PUBLIC INVOLVEMENT:
Patients or public were not involved in this study.
MRI DATA ACQUISITION:
Before cesarean delivery, all patients underwent pelvic MRI using the 3.0 T MRI system (Siemens Medical Solutions, Erlangen, Germany). Sedation and intravenous gadolinium injection were not used for any patients. Patients were in the supine or left lateral decubitus position with moderately full bladder. The MRI protocol comprised T1WI and T2WI images in sagittal, coronal, and axial orientations. The scanning time was less than 1 s/image, and the entire MRI study was completed within 30 min. In this study, we used the following MRI signs in the lower uterine segment to evaluate PAS: placenta thickness, cervical lengths, placental dark T2 bands area, cervical marginal sinus, bladder bulge, myometrial thinning, lower uterine segment bulge, and placental signals in the cervix.
The measurement of placental thickness was the shortest distance from the interior cervix orifice to the fetal surface of placenta (Figure 2A). The length of cervical canal was measured from the interior cervix orifice to the external cervix orifice (Figure 2A). Three MRI features (placental dark T2 bands, myometrial thinning, and lower uterine segment bulge) were derived from the lower uterine segment, which we defined in our study as follows: The tangent line at the internal cervix (line A), parallel line 5 cm from line A (line B), the lower uterine segment (white arrow) between line A and line B is the area that we studied (Figure 2B). The cervix was thicker than normal with varicosities suggests that the cervix was rich in blood supply (Figure 2C (white circle)). We defined uterine wall partial defect or even placental tissue signals seen in myometrium as myometrial thinning by MRI (Figure 2C (red circle). When the placenta was located entirely in the lower uterine segment, the lower uterine segment was often enlarged and dilated (Figure 2D). MRI signs consistent with placental tissue were seen in the cervix, which means that the placenta might be invaded into the cervix (Figure 2D (white circle)). The MRI images were analyzed by 2 experienced radiologists on female pelvic MRI. Radiologists were blinded to all clinical information, ultrasonoscopy imaging, and the impressions of the other radiologists. Any disagreement in the process of interpretation was resolved by the senior radiologist.
STATISTICS ANALYSIS:
Continuous variables are expressed as mean (standard deviation) or median (interquartile range) depending on the underlying distribution of the data, and nominal data are expressed in the form of frequency (percentage). The sensitivity and specificity of MRI examination were calculated. Kappa statistics for different radiologist’s reliability were calculated for each sign. The chi-squared test, Fisher’s exact test, or Mann-Whitney U test were used to evaluate the significance of clinical factors between the 2 groups. Receiver operating characteristic curve (ROC) was used to evaluate the diagnostic value of different MRI features. A
Results
CLINICAL CHARACTERISTICS OF THE PATIENTS:
We included 156 women in the study, and the clinical characteristics of CPP are presented in Table 1. Ninety of 156 patients had evidence of PAS intraoperatively (accreta/increta, n=57; percreta, n=33). In all patients, cesarean section was performed. No statistically significant relation could be detected as regards age, BMI, previous placenta pervia, bladder injury, cesarean hysterectomy, or Apgar score in correlation to the type of PAS. No maternal deaths were recorded.
The gestational weeks of delivery (35.56±1.14 vs 36.38±1.06;
INTERRATER AGREEMENT:
The level of agreement between the 2 radiologists was basically satisfactory for the identification of each of the MRI signs, with kappa values ranging from 0.795 to 0.967. Interpretation of kappa scores was evaluated according to the following definitions: 1.0 perfect agreement, 0.91 to 0.99 almost perfect agreement, 0.81 to 0.90 substantial agreement, 0.71 to 0.80 moderate agreement, 0.61 to 0.70 fair agreement, and <0.6 slight agreement. The detailed results are shown in Table 2.
COMPARISON OF MRI FEATURES BETWEEN PA AND NON-PA PATIENTS:
Significant differences were found in the 8 depicted MRI features between PAS pregnancies and non-PAS pregnancies (P<0.05). The single MRI feature has lower sensitivity (from 14.44% to 55.56%) and higher specificity (from 80.30% to 96.97%), but the combination of 8 indicators has higher sensitivity (82.22%) and specificity (91.09%) at the same time (Table 3). According to our results, the predictive value of a single MRI feature on PA is limited (AUC from 0.542 to 0.639), but the presence of 8 MRI signs have a good predictive effect for PA, with an area under the curve (AUC)=0.816 (Table 3, Figure 3). Figure 4 shows MRI characteristics in central placenta previa patients with and without placenta accreta (sagittal T2-weighted). We found much thicker placenta, much shorter cervix, heterogeneous placenta, the dilated lower part of the uterus, and myometrium defect (Figure 4A). Figure 5 shows the ORs concerning the prediction of PAS in relation to 8 MRI features. The odds ratio using the 8 above MRI findings ranged from 3.04 to 9.14. CPP patients with MRI sign (placental signals in the cervix) had the highest odds ratio (9.14, 95% CI: 2.06–40.67, P=0.001) for PAS. The placental thickness in PAS patients were significantly thicker than those in non-PAS patients (5.28±1.23 cm vs 4.05±1.22 cm; P<0.001). Compared with patients in non-PAS group, the cervical lengths of patients with PAS were significantly shorter (2.74±0.53 cm vs 3.28±0.40 cm; P<0.001) (Figure 6).
Discussion
Ultrasound is widely used in the diagnosis of PP, but MRI can accurately assess placenta position and whether the placenta invades surrounding organs, and is not affected by maternal fat thickness. In recent years, MRI has played an increasingly important role in the diagnosis of PP and PAS [10]. MRI provides multiplane imaging and high soft-tissue resolution, and it can more accurately assess the presence of PAS and bladder invasion compared with ultrasound [11,12]. The danger of CPP is largely determined by PAS in the lower uterine segment and the cervical canal, so our study focused on MRI features in this part of the uterus.
Multiple studies have shown an association between PAS and more prior cesarean deliveries [13]. All pregnant women in our study had a history of at least 1 cesarean section. As many reports described, previous cesarean deliveries and/or PP were independent clinical risk factors of PAS [14,15]. In our study, the gravidity and parity in PAS group were significantly higher than that in the control group. CPP with PAS is a potential life-threatening obstetric disease that needs a multidisciplinary approach for appropriate management. CPP accompanied by PAS had a higher hysterectomy rate in order to save maternal life; however, the hysterectomy rate (3.33% in PAS group) was low in our study, mainly due to adequate preoperative evaluation and skilled surgical procedures (uterine artery ligation, anti-arcuate compression suturing, and B-lynch suturing) [16]. The uterus is thin in the third trimester, so PAS lacks specificity determined by direct signs of MRI. Sometimes, we can only judge PAS by some indirect signs of placenta in the lower uterine segment. Identification the site of PAS is very important because it requires different surgical procedures for therapy. We can easily remove the placenta and suture the local bleeding point if the site of PAS is in the body of the uterus. However, when the placenta is implanted in the lower uterine segment, we need to carefully remove the placenta and stop bleeding, otherwise the surrounding bladder and ureters may be damaged. It was very thin and lacked blood supply in the lower uterine segment of pregnant women with a history of cesarean section, the placental villi easily proliferates and implant, and even penetrates the uterine wall to get more blood nutrients.
Over the past few years, many studies have been reported that some of MRI signs (eg, focal myometrial interruption, dark placental bands, uterine bulge, and placental heterogeneity) were associated with PAS [17,18]. The placenta thickness in PAS group was much thicker than non-PAS group (OR, 4.20; 95% CI, 1.79–9.87), which indicates proliferation of placental villi, and even PAS. Shortening of the cervical was an important sign of PAS and can make the cesarean section more difficult [19]. Our results showed that patients with the PAS had shorter cervical lengths than the patients without PAS. Abnormal vascularity and percretism signs were the 2 most predictive MRI features of PAS [20]. Our study found that placental signal in the cervix was the highest risk sign (OR, 9.14; 95% CI, 2.06–40.67) for placental implantation and it is difficult to stop bleeding in the cervix. Many researches have demonstrated that the dark intraplacental band on T2WI was a useful MRI feature for predicting PAS [21,22]. The signs of dark intraplacental bands, focal outward bulging of the placenta, and abnormal placental vascularity are reliable signs of PAS [23]. The dark intraplacental band on T2WI usually represents fibrin deposition or placental infarction, which was associated with placental invasion [24]. Uterine bulging was another useful MRI feature, which indicated that extensive growth of placental villus leads to PAS [25]. Our study results suggested that PAS had a higher risk in women with lower uterine segment bulge (OR, 4.61; 95%CI, 1.97–10.82). The value of a single MRI feature for PAS prediction was limited; however, the sensitivity and specificity of predicting PAS were 82.22% and 91.09%, respectively, by using the combination of these 8 MRI features.
However, there were some potential limitations in our study. The scale of the study, which included 156 pregnant women with CPP, was not large enough. Therefore, we need a larger-sample and multi-center study to further verify our conclusions.
Conclusions
MRI features of the lower uterine segment have high sensitivity and specificity for the diagnosis of PAS in pregnant women with CPP. The placental signals in the cervix, myometrial thinning and placental dark T2 bands area, are the most useful signs for the diagnosis of PAS. We found they may play an important role in assessing the risk of disease in pregnant women and making adequate preoperative preparation for obstetricians.
Figures
 Figure 1. Flow chart of the study design. (Microsoft office word 2007, Microsoft, USA).
Figure 1. Flow chart of the study design. (Microsoft office word 2007, Microsoft, USA). 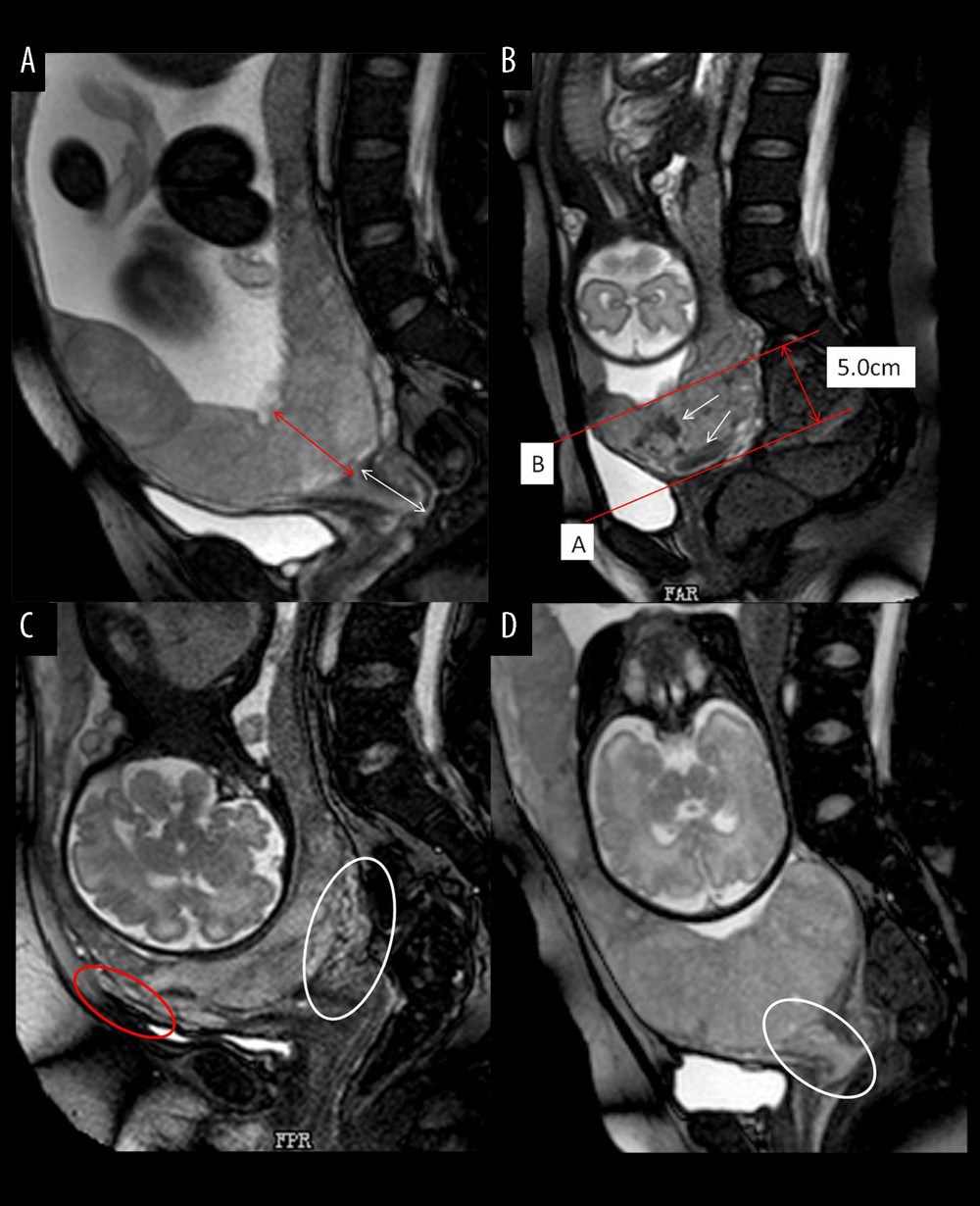 Figure 2. Different imaging characteristics of magnetic resonance imaging (MRI) in central placenta previa patients (Sagittal T2-weighted). (Microsoft office PowerPoint 2007, Microsoft, USA). (A) Placenta thickness: Placental thickness was measured from the internal cervix (red arrow); Cervical length: White arrow indicate the measurement of cervical length. (B) Placental dark T2 bands: The tangent line at the internal cervix (line A), parallel line 5 cm from line A (line B), the dark band (white arrow) between line A and line B is the area that we studied. (C). Cervical marginal sinus: Assessment of cervical marginal sinus signs using MRI (white circle); Myometrial thinning: The myometrium was partially defective, and placental tissue signals were seen in myometrium (red circle). (D). Placental signals in the cervix: Signs consistent with placental tissue were seen in the cervix, which means that the placenta may be inserted into the cervix (white circle); Lower uterine segment bulge: The placenta located entirely in the lower uterine segment, the lower uterine segment was enlarged and dilated.
Figure 2. Different imaging characteristics of magnetic resonance imaging (MRI) in central placenta previa patients (Sagittal T2-weighted). (Microsoft office PowerPoint 2007, Microsoft, USA). (A) Placenta thickness: Placental thickness was measured from the internal cervix (red arrow); Cervical length: White arrow indicate the measurement of cervical length. (B) Placental dark T2 bands: The tangent line at the internal cervix (line A), parallel line 5 cm from line A (line B), the dark band (white arrow) between line A and line B is the area that we studied. (C). Cervical marginal sinus: Assessment of cervical marginal sinus signs using MRI (white circle); Myometrial thinning: The myometrium was partially defective, and placental tissue signals were seen in myometrium (red circle). (D). Placental signals in the cervix: Signs consistent with placental tissue were seen in the cervix, which means that the placenta may be inserted into the cervix (white circle); Lower uterine segment bulge: The placenta located entirely in the lower uterine segment, the lower uterine segment was enlarged and dilated. 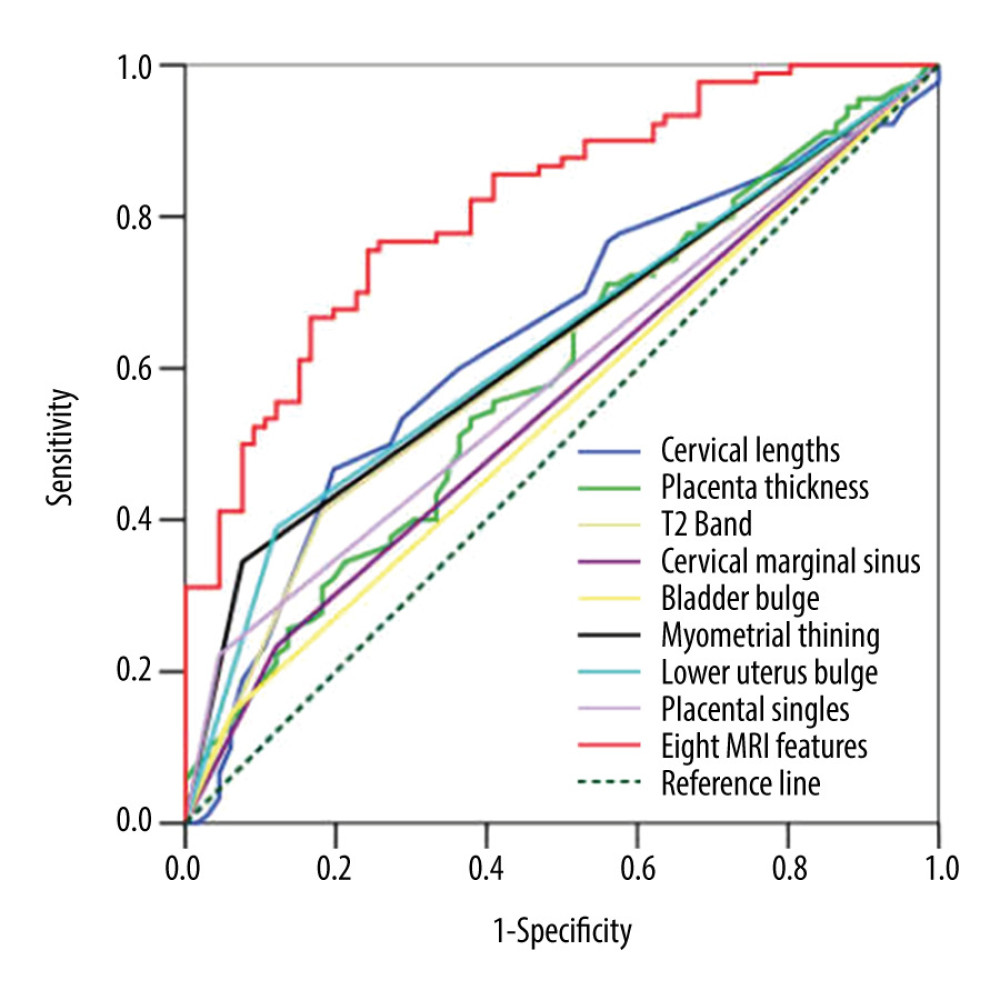 Figure 3. Receiver operating characteristic curve (ROC) of different magnetic resonance imaging (MRI) features for all patients. (SPSS 23.0 statistics software, IBM America).
Figure 3. Receiver operating characteristic curve (ROC) of different magnetic resonance imaging (MRI) features for all patients. (SPSS 23.0 statistics software, IBM America). 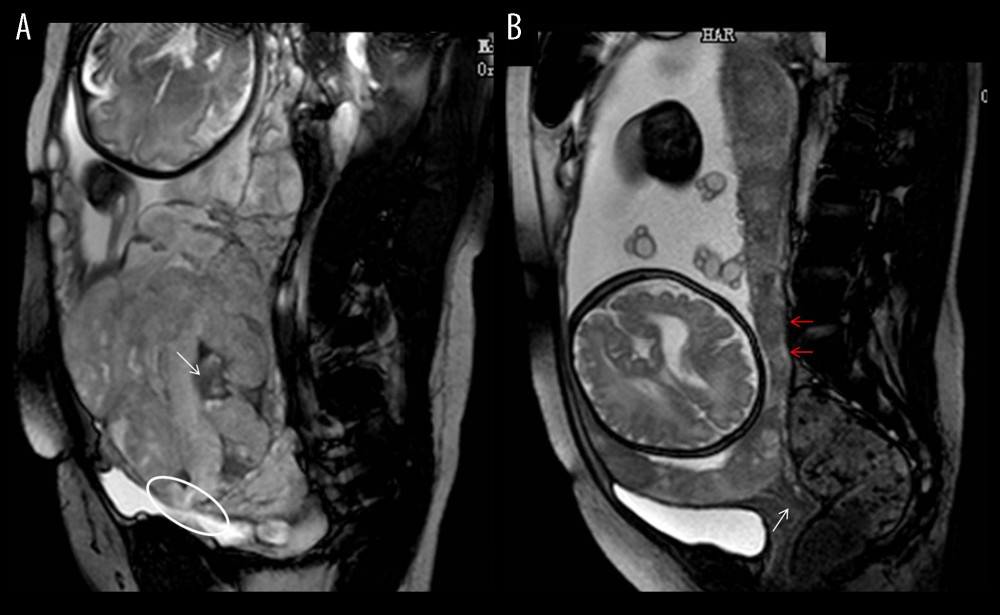 Figure 4. Magnetic resonance imaging (MRI) characteristics in central placenta previa patients with and without placenta accreta (Sagittal T2-weighted). (Microsoft Office PowerPoint 2007, Microsoft, USA). (A) Central placenta previa with placenta accrete: Placenta thickness is 12.3 centimeters; The cervical canal is almost completely eroded by the placenta and we can hardly see a clear cervix; The placental signal is heterogeneous and many dark T2 bands are seen in the placenta (white arrow); The whole placenta is located in the lower part of the uterus, which is dilated; A partial myometrium defect can be seen at the uterine and placental interface (white circle); (B) Central placenta previa without placenta accrete: Placenta thickness is 3.2 centimeters; We can see a clear cervix (white arrow); Placenta signals are homogeneous; The boundary between the uterine myometrium and placenta is clear (red arrow).
Figure 4. Magnetic resonance imaging (MRI) characteristics in central placenta previa patients with and without placenta accreta (Sagittal T2-weighted). (Microsoft Office PowerPoint 2007, Microsoft, USA). (A) Central placenta previa with placenta accrete: Placenta thickness is 12.3 centimeters; The cervical canal is almost completely eroded by the placenta and we can hardly see a clear cervix; The placental signal is heterogeneous and many dark T2 bands are seen in the placenta (white arrow); The whole placenta is located in the lower part of the uterus, which is dilated; A partial myometrium defect can be seen at the uterine and placental interface (white circle); (B) Central placenta previa without placenta accrete: Placenta thickness is 3.2 centimeters; We can see a clear cervix (white arrow); Placenta signals are homogeneous; The boundary between the uterine myometrium and placenta is clear (red arrow). 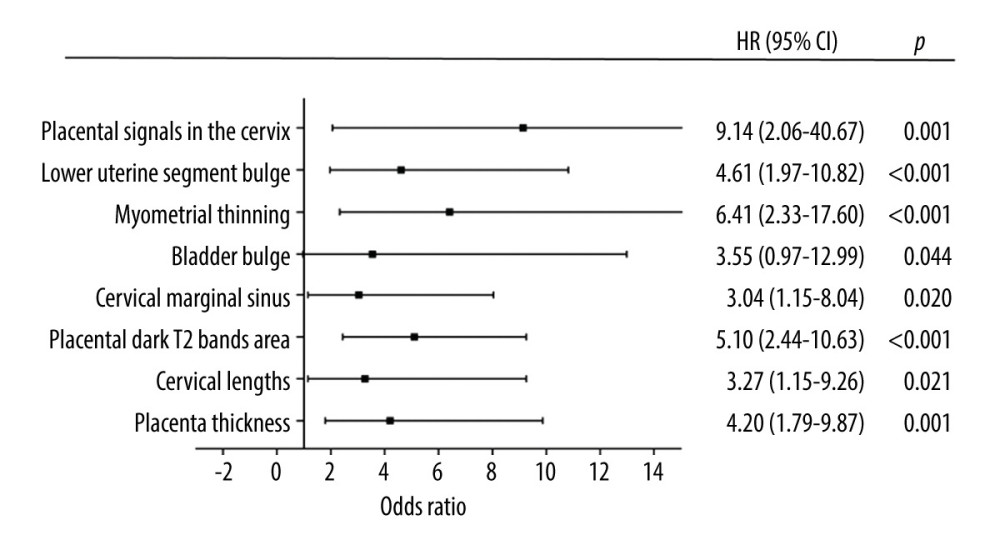 Figure 5. Graph of odds ratios (black square) and 95% CI for 8 statistically significant variables associated with placental accrete spectrum. (GraphPad Prism 5.0, GraphPad Software, USA).
Figure 5. Graph of odds ratios (black square) and 95% CI for 8 statistically significant variables associated with placental accrete spectrum. (GraphPad Prism 5.0, GraphPad Software, USA). 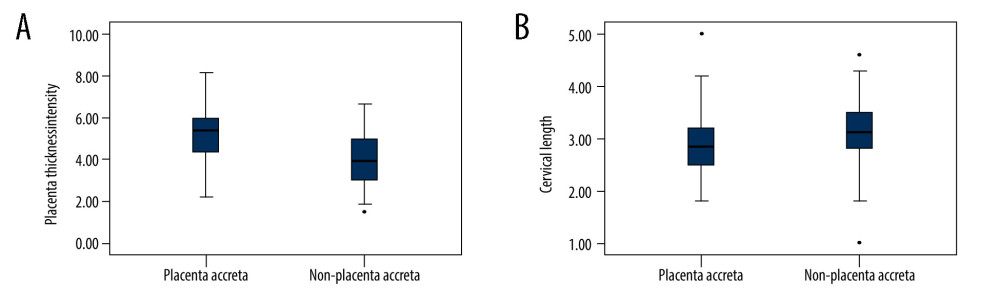 Figure 6. (A) The mean placenta thickness in cases with and without placenta accreta spectrum. (B) The mean cervical length in cases with and without placenta accreta spectrum. (SPSS 23.0 statistics software, IBM, USA).
Figure 6. (A) The mean placenta thickness in cases with and without placenta accreta spectrum. (B) The mean cervical length in cases with and without placenta accreta spectrum. (SPSS 23.0 statistics software, IBM, USA). Tables
Table 1. Clinical characteristics of pregnant women with placenta previa (PP).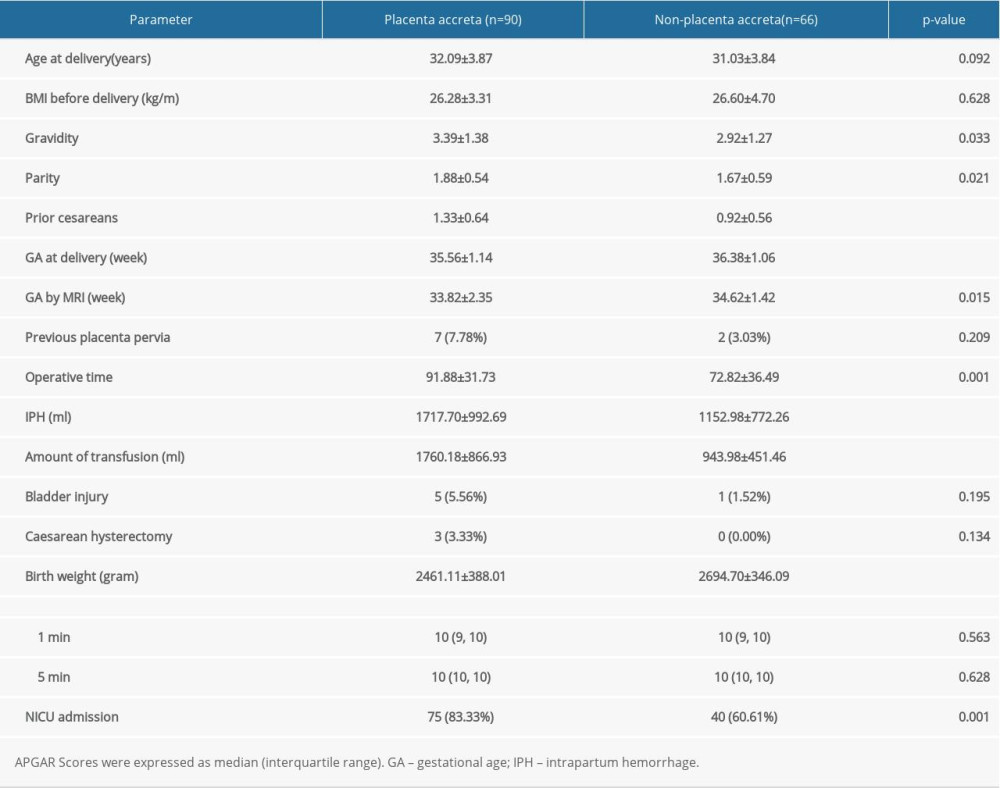 Table 2. Interobserver reliability of magnetic resonance imaging (MRI) in the diagnosis of placenta accreta spectrum (PAS).
Table 2. Interobserver reliability of magnetic resonance imaging (MRI) in the diagnosis of placenta accreta spectrum (PAS).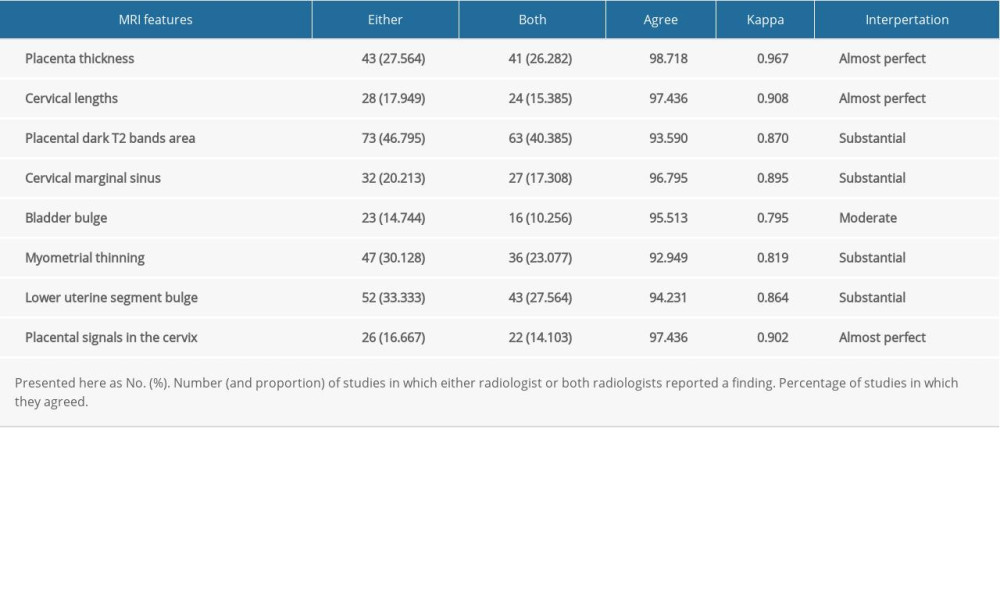 Table 3. Diagnostic indexes for the association of magnetic resonance imaging (MRI) features with placenta accreta spectrum (PAS).
Table 3. Diagnostic indexes for the association of magnetic resonance imaging (MRI) features with placenta accreta spectrum (PAS).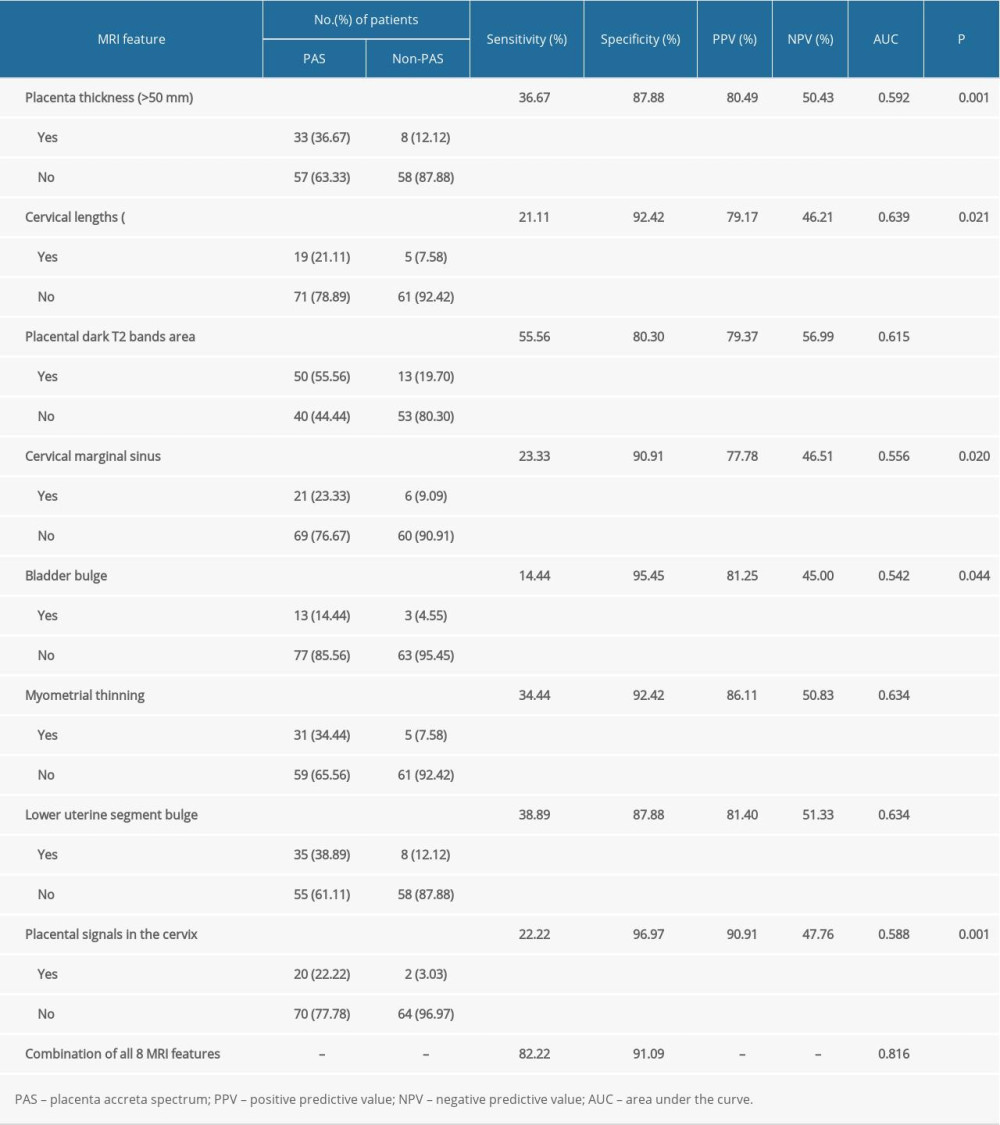
References
1. Society of Gynecologic Oncology; American College of Obstetricians and Gynecologists and the Society for Maternal – Fetal Medicine, Placenta accreta spectrum: Am J Obstet Gynecol, 2018; 219(6); B2-16
2. , Obstetric Care Consensus No. 7 Summary: Placenta Accreta Spectrum: Obstet Gynecol, 2018; 132(6); 1519-21
3. Faranesh R, Romanov S, Shalev E, Suggested approach for management of placenta percreta invading the urinary bladder: Obstet Gynecol, 2007; 110(2); 512-15
4. Miller DA, Chollet JA, Goodwin TM, Clinical risk factors for placenta previa – placenta accrete: Am J Obstet Gynecol, 1997; 177(1); 210-14
5. Publications Committee, Society for Maternal-Fetal Medicine, Placenta accreta: Am J Obstet Gynecol, 2010; 203(5); 430-39
6. D’Antonio F, Iacovella C, Palacios-Jaraquemada J, Prenatal identification of invasive placentation using magnetic resonance imaging: Aystematic review and meta-analysis: Ultrasound Obstet Gynecol, 2014; 44(1); 8-16
7. Delli Pizzi A, Tavoletta A, Narciso R, Prenatal planning of placenta previa: diagnostic accuracy of a novel MRI-based prediction model for placenta accreta spectrum (PAS) and clinical outcome: Abdom Radiol (NY), 2019; 44(5); 1873-82
8. Tikkanen M, Paavonen J, Loukovaara M, Antenatal diagnosis of placenta accreta leads to reduced blood loss: Acta Obstet Gynecol Scand, 2011; 90(10); 1140-46
9. Collins SL, Alemdar B, van Beekhuizen HJ, Evidence-based guidelines for the management of abnormally invasive placenta: Recommendations from the International Society for Abnormally Invasive Placenta: Am J Obstet Gynecol, 2019; 220(6); 511-16
10. Eshkoli T, Weintraub AY, Sergienko R, Placenta accreta: Risk factors, perinatal outcomes, and consequences for subsequent births: Am J Obstet Gynecol, 2013; 208(3); 1-7
11. Seet EL, Kay HH, Wu S, Placenta accreta: Depth of invasion and neonatal outcomes: J Matern Fetal Med, 2012; 25(10); 2042-45
12. D’Antonio F, Iacovella C, Palacios-Jaraquemada J, Prenatal identification of invasive placentation using magnetic resonance imaging: Systematic review and meta-analysis: Ultrasound Obstet Gynecol, 2014; 44(1); 8-16
13. Silver RM, Landon MB, Rouse DJ, Maternal morbidity associated with multiple repeat cesarean deliveries: Obstet Gynecol, 2006; 107(6); 1226-32
14. Eshkoli T, Weintraub AY, Sergienko R, Placenta accreta: Risk factors, perinatal outcomes, and consequences for subsequent births: Am J Obstet Gynecol, 2013; 208(3); 219.e1-7
15. Bowman ZS, Eller AG, Bardsley TR, Risk factors for placenta accreta: A large prospective cohort: Am J Perinatol, 2014; 31(9); 799-804
16. Li Y, Luan X, Chen D, Use of anti-arcuate compression suturing in pernicious placenta previa with accrete spectrum disorders: A surgical technique: Med Sci Monit, 2020; 26; e922958
17. Baughman WC, Corteville JE, Shah RR, Placenta accreta: Spectrum of US and MR imaging findings: Radiographics, 2008; 28(7); 1905-16
18. Lax A, Prince MR, Mennitt KW, The value of specific MRI features in the evaluation of suspected placental invasion: Magn Reson Imag, 2007; 25(1); 87-93
19. Polat M, Kahramanoglu I, Senol T, Shorter the cervix, more difficult the placenta percreta operations: J Matern Fetal Neonatal Med, 2016; 29(14); 2327-31
20. Delli Pizzi A, Tavoletta A, Narciso R, Prenatal planning of placenta previa: Diagnostic accuracy of a novel MRI-based prediction model for placenta accreta spectrum (PAS) and clinical outcome: Abdom Radiol (NY), 2019; 44(5); 1873-82
21. Rahaim NS, Whitby EH, The MRI features of placental adhesion disorder and their diagnostic significance: Systematic review: Clin Radiol, 2015; 70(9); 917-25
22. Ueno Y, Kitajima K, Kawakami F, Novel MRI finding for diagnosis of invasive placenta praevia: Evaluation of findings for 65 patients using clinical and histopathological correlations: Eur Radiol, 2014; 24(4); 881-88
23. Huang F, Lai QQ, Wu H, Application of indirect signs of magnetic resonance imaging (MRI) in prenatal diagnosis of abnormally invasive placenta: Med Sci Monit, 2020; 26; e923272
24. Baughman CW, Corteville JE, Shah RR, Placenta accreta: Spectrum of US and MR imaging findings: Radiographics, 2008; 28(7); 1905-16
25. Kilcoyne A, Shenoy-Bhangle AS, Roberts DJ, MRI of placenta accreta, placenta increta, and placenta percreta: Pearls and pitfalls: Am J Roentgenol, 2017; 208(1); 214-21
Figures
 Figure 1. Flow chart of the study design. (Microsoft office word 2007, Microsoft, USA).
Figure 1. Flow chart of the study design. (Microsoft office word 2007, Microsoft, USA). Figure 2. Different imaging characteristics of magnetic resonance imaging (MRI) in central placenta previa patients (Sagittal T2-weighted). (Microsoft office PowerPoint 2007, Microsoft, USA). (A) Placenta thickness: Placental thickness was measured from the internal cervix (red arrow); Cervical length: White arrow indicate the measurement of cervical length. (B) Placental dark T2 bands: The tangent line at the internal cervix (line A), parallel line 5 cm from line A (line B), the dark band (white arrow) between line A and line B is the area that we studied. (C). Cervical marginal sinus: Assessment of cervical marginal sinus signs using MRI (white circle); Myometrial thinning: The myometrium was partially defective, and placental tissue signals were seen in myometrium (red circle). (D). Placental signals in the cervix: Signs consistent with placental tissue were seen in the cervix, which means that the placenta may be inserted into the cervix (white circle); Lower uterine segment bulge: The placenta located entirely in the lower uterine segment, the lower uterine segment was enlarged and dilated.
Figure 2. Different imaging characteristics of magnetic resonance imaging (MRI) in central placenta previa patients (Sagittal T2-weighted). (Microsoft office PowerPoint 2007, Microsoft, USA). (A) Placenta thickness: Placental thickness was measured from the internal cervix (red arrow); Cervical length: White arrow indicate the measurement of cervical length. (B) Placental dark T2 bands: The tangent line at the internal cervix (line A), parallel line 5 cm from line A (line B), the dark band (white arrow) between line A and line B is the area that we studied. (C). Cervical marginal sinus: Assessment of cervical marginal sinus signs using MRI (white circle); Myometrial thinning: The myometrium was partially defective, and placental tissue signals were seen in myometrium (red circle). (D). Placental signals in the cervix: Signs consistent with placental tissue were seen in the cervix, which means that the placenta may be inserted into the cervix (white circle); Lower uterine segment bulge: The placenta located entirely in the lower uterine segment, the lower uterine segment was enlarged and dilated. Figure 3. Receiver operating characteristic curve (ROC) of different magnetic resonance imaging (MRI) features for all patients. (SPSS 23.0 statistics software, IBM America).
Figure 3. Receiver operating characteristic curve (ROC) of different magnetic resonance imaging (MRI) features for all patients. (SPSS 23.0 statistics software, IBM America). Figure 4. Magnetic resonance imaging (MRI) characteristics in central placenta previa patients with and without placenta accreta (Sagittal T2-weighted). (Microsoft Office PowerPoint 2007, Microsoft, USA). (A) Central placenta previa with placenta accrete: Placenta thickness is 12.3 centimeters; The cervical canal is almost completely eroded by the placenta and we can hardly see a clear cervix; The placental signal is heterogeneous and many dark T2 bands are seen in the placenta (white arrow); The whole placenta is located in the lower part of the uterus, which is dilated; A partial myometrium defect can be seen at the uterine and placental interface (white circle); (B) Central placenta previa without placenta accrete: Placenta thickness is 3.2 centimeters; We can see a clear cervix (white arrow); Placenta signals are homogeneous; The boundary between the uterine myometrium and placenta is clear (red arrow).
Figure 4. Magnetic resonance imaging (MRI) characteristics in central placenta previa patients with and without placenta accreta (Sagittal T2-weighted). (Microsoft Office PowerPoint 2007, Microsoft, USA). (A) Central placenta previa with placenta accrete: Placenta thickness is 12.3 centimeters; The cervical canal is almost completely eroded by the placenta and we can hardly see a clear cervix; The placental signal is heterogeneous and many dark T2 bands are seen in the placenta (white arrow); The whole placenta is located in the lower part of the uterus, which is dilated; A partial myometrium defect can be seen at the uterine and placental interface (white circle); (B) Central placenta previa without placenta accrete: Placenta thickness is 3.2 centimeters; We can see a clear cervix (white arrow); Placenta signals are homogeneous; The boundary between the uterine myometrium and placenta is clear (red arrow). Figure 5. Graph of odds ratios (black square) and 95% CI for 8 statistically significant variables associated with placental accrete spectrum. (GraphPad Prism 5.0, GraphPad Software, USA).
Figure 5. Graph of odds ratios (black square) and 95% CI for 8 statistically significant variables associated with placental accrete spectrum. (GraphPad Prism 5.0, GraphPad Software, USA). Figure 6. (A) The mean placenta thickness in cases with and without placenta accreta spectrum. (B) The mean cervical length in cases with and without placenta accreta spectrum. (SPSS 23.0 statistics software, IBM, USA).
Figure 6. (A) The mean placenta thickness in cases with and without placenta accreta spectrum. (B) The mean cervical length in cases with and without placenta accreta spectrum. (SPSS 23.0 statistics software, IBM, USA). Tables
 Table 1. Clinical characteristics of pregnant women with placenta previa (PP).
Table 1. Clinical characteristics of pregnant women with placenta previa (PP). Table 2. Interobserver reliability of magnetic resonance imaging (MRI) in the diagnosis of placenta accreta spectrum (PAS).
Table 2. Interobserver reliability of magnetic resonance imaging (MRI) in the diagnosis of placenta accreta spectrum (PAS). Table 3. Diagnostic indexes for the association of magnetic resonance imaging (MRI) features with placenta accreta spectrum (PAS).
Table 3. Diagnostic indexes for the association of magnetic resonance imaging (MRI) features with placenta accreta spectrum (PAS). Table 1. Clinical characteristics of pregnant women with placenta previa (PP).
Table 1. Clinical characteristics of pregnant women with placenta previa (PP). Table 2. Interobserver reliability of magnetic resonance imaging (MRI) in the diagnosis of placenta accreta spectrum (PAS).
Table 2. Interobserver reliability of magnetic resonance imaging (MRI) in the diagnosis of placenta accreta spectrum (PAS). Table 3. Diagnostic indexes for the association of magnetic resonance imaging (MRI) features with placenta accreta spectrum (PAS).
Table 3. Diagnostic indexes for the association of magnetic resonance imaging (MRI) features with placenta accreta spectrum (PAS). In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








