12 October 2021: Review Articles
Silent Hypoxemia in Patients with COVID-19 Pneumonia: A Review
Lizhe Guo12E, Zhaosheng Jin3F, Tong J. Gan3E, E Wang12A*DOI: 10.12659/MSM.930776
Med Sci Monit 2021; 27:e930776
Abstract
ABSTRACT: During the coronavirus disease 2019 (COVID-19) pandemic due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, patients presented with COVID-19 pneumonia of varying severity. The phenomenon of severe hypoxemia without signs of respiratory distress is also known as silent or hidden hypoxemia. Although silent hypoxemia is not unique to pneumonia due to SARS-CoV-2 infection, this phenomenon is now recognized to be associated with severe COVID-19 pneumonia. Proper management of critically ill patients is the key to reducing mortality. Herein, we summarize the possible and rare factors contributing to silent hypoxemia in patients with COVID-19. Microvascular thrombosis causes dead space ventilation in the lungs, and the flow of pulmonary capillaries is reduced, which leads to an imbalance in the V/Q ratio. The dissociation curve of oxyhemoglobin shifts to the left and limits the release of oxygen to the tissue. SARS-CoV-2 interferes with the synthesis of hemoglobin and reduces the ability to carry oxygen. The accumulation of endogenous carbon monoxide and carboxyhemoglobin will reduce the total oxygen carrying capacity and interfere with pulse oxygen saturation readings. There are also some non-specific factors that cause the difference between pulse oximetry and oxygen partial pressure. We propose some potentially more effective clinical alternatives and recommendations for optimizing the clinical management processes of patients with COVID-19. This review aims to describe the prevalence of silent hypoxemia in COVID-19 pneumonia, to provide an update on what is known of the pathophysiology, and to highlight the importance of diagnosing silent hypoxemia in patients with COVID-19 pneumonia.
Keywords: COVID-19, Hypoxis, Intensive Care, Oximetry, Asymptomatic Diseases, COVID-19, Humans, Lung, Microvessels, Oxygen, Pneumonia, Viral, Prevalence, SARS-CoV-2, Thrombosis
Background
The clinical presentations of coronavirus disease-2019 (COVID-19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), include fever, cough, fatigue, shortness of breath, hypoxemia, and respiratory failure [1]. A small proportion of patients with COVID-19 require hospitalization, of which some will require invasive mechanical ventilation [2]. Radiologically, computed tomography of the chest typically demonstrates bilateral ground-glass infiltration, which may progress to widespread consolidation [3]. Respiratory failure and hypoxemia are common clinical manifestations of severe pneumonia secondary to COVID-19. In such cases, disease progression typically comprises a compensated hypoxic phase, often with non-specific signs and symptoms. This phase may be followed by decompensation and rapid deterioration to severe respiratory failure, requiring invasive ventilation or even extracorporeal membrane oxygenation (ECMO) rescue. Therefore, the correct timing of respiratory support therapy is critical. However, due to the special pathophysiological characteristics of a very small number of COVID-19 patients, coupled with the limitations of pulse oximetry, the clinician’s assessment of the patient’s progress may be delayed, resulting in suboptimal treatment timing, greatly prolonging the patient’s hospital stay and increasing the hospitalization cost and mortality.
This review aims to describe the prevalence of silent hypoxemia in COVID-19 pneumonia, to provide an update on what is known of the pathophysiology, and to highlight the importance of diagnosing silent hypoxemia in patients with COVID-19 pneumonia.
Microvascular Thrombosis-Related Hypoxemia in COVID-19 Patients
It is hypothesized that hypoxemia in COVID-19 is the result of V/Q mismatch due to vascular pathology, especially in the early stages. In this case, the loss of hypoxic pulmonary vascular constriction is considered to be a mechanism. Microvascular thrombosis has been fully demonstrated in the pathology of COVID-19. This event may be due to inflammation in the fine blood vessel network in the lungs, which triggers the activation of a series of proteins to promote blood clotting [4]. During this process, a dead space is formed in the lungs, and the flow of pulmonary capillaries is reduced without affecting ventilation, thus resulting in a higher V/Q ratio. However, because inflammation causes the production of inducible nitric oxide synthase (iNOS), the release of nitric oxide (NO), and local vasodilation, it is hypothesized that the obvious inflammatory process that occurs in COVID-19 will cause excessive capillary perfusion. In the same lung, the spread of units with different V/Q ratios can cause mismatch and hypoxemia [5].
Patients with COVID-19 are Prone to Alkalosis
Patients with COVID-19 may experience tissue hypoxia before pulse oximetry drops. In patients with early stages of COVID-19 pneumonia, PaCO2 is often reduced due to hyperventilation, resulting in respiratory alkalosis, which shifts the oxyhaemoglobin dissociation curve to the left, and limits oxygen release to the tissue. This was confirmed in a retrospective study of patients with COVID-19, comprising a centralized analysis of the medical records of 113 deceased patients; among the 35 deceased patients with arterial blood gas (ABG) values, type I respiratory failure was present in 51% of patients, and respiratory alkalosis was present in 40% of patients [6]. In patients with severe acid-base imbalance, oxygen saturation should be corrected for pH, temperature, and base excess, otherwise tissue hypoxia may occur before an appreciable reduction in the pulse oximetry reading [7].
SARS-CoV-2 is Dependent on Porphyrins and Attacks the 1-beta Chain of Hemoglobin
SARS-CoV-2 may affect the oxygen carrying capacity of hemoglobin due to its unique structure and attack mode. By means of conservative domain analysis, homologous modeling, and molecular docking, it has been shown that viral proteins, such as the open read frame (ORF) 8 protein and surface glycoproteins, can bind to porphyrin, whereas ORF1ab, ORF10, and ORF3a proteins can cooperate to attack the 1-beta chain of hemoglobin and dissociate the iron ion from the heme molecule. [6] These findings suggest that SARS-CoV-2 may interfere with hemoglobin synthesis, as well as hemoglobin function.
Several studies have reported that hemoglobin levels in blood samples from patients with COVID-19 are often decreased [6,9]. Another significant finding is that serum ferritin is elevated in conjunction with mild anemia in patients with COVID-19 [9], which could be explained by the fact that SARS-CoV-2 promotes iron dissociation and inhibits hemoglobin synthesis, resulting in serum iron accumulation. However, ferritin is also an acute-phase protein representing a systemic non-specific inflammatory response. More experimental data are needed to demonstrate the underlying cause of the increase in serum ferritin levels in COVID-19 patients.
Although not all studies support the theory that viral attacks cause hemoglobin levels to drop [10], it is possible that SARS-CoV-2 directly interferes with hemoglobin function and reduces the oxygen carrying capacity in some patients. This phenomenon might not directly affect the accuracy of blood oxygen saturation readings, but a drop in the total amount of hemoglobin may cause healthcare workers to become overconfident in readings of oxygen saturation within the safe range, which can lead to a reduction in vigilance against the severity of tissue hypoxia. In contrast to that noted in patients with long-term chronic anemia, acute hemoglobin reduction due to a viral attack decreases the reserve capacity of oxygen delivery. Within the same safe reading range, patients with COVID-19 could be less tolerant of hypoxia than patients with long-term chronic anemia. It has also been proposed that the higher average hemoglobin level in men than in women may contribute to the higher risk of symptomatic disease in me. [6, 9–11].
Endogenous Carbon Monoxide and Carboxyhaemoglobin Accumulation in Patients with Severe COVID-19
Endogenous carbon monoxide (CO) production and carboxyhemoglobin levels are increased in patients with severe COVID-19. CO binds to hemoglobin with an affinity 210 times greater than that of oxygen, and significant carboxyhemoglobinemia can therefore reduce the total oxygen carrying capacity, interfering with oxygen saturation readings. Unlike CO poisoning, most CO in patients with COVID-19 is produced by endogenous pathways. The heme oxygenase (HO)/CO system in the human body is the most important pathway for the production of endogenous CO [12]. HO-1 degrades heme, produces CO and biliverdin, and releases iron [13]. Oxidative stress, hypoxia, heavy metals, sodium arsenite, haem and haem derivatives, various cytokines, and exogenous CO can all induce the production of endogenous CO by increasing HO-1 expression and activity [14]. Increases in endogenous CO levels have been observed in the ABG results from patients with COVID-19 pneumonia, and more attention and clinical data are needed to confirm the relevance of this indicator to the progression of COVID-19.
An increase in endogenous CO levels is often regarded as a biological marker of oxidation and inflammation; increases in carboxyhaemoglobin levels have been observed in many diseases that cause increased levels of bacterial or aseptic inflammation and tissue damage, such as bronchial asthma, pneumonia, idiopathic pulmonary fibrosis, pyelonephritis, active rheumatoid arthritis, diabetes, and coronary heart disease [15]. As mentioned above, SARS-CoV-2 may increase carboxyhemoglobin levels by increasing the destruction of red blood cells and hemoglobin metabolism, and the consequent storm of inflammation further facilitates this process.
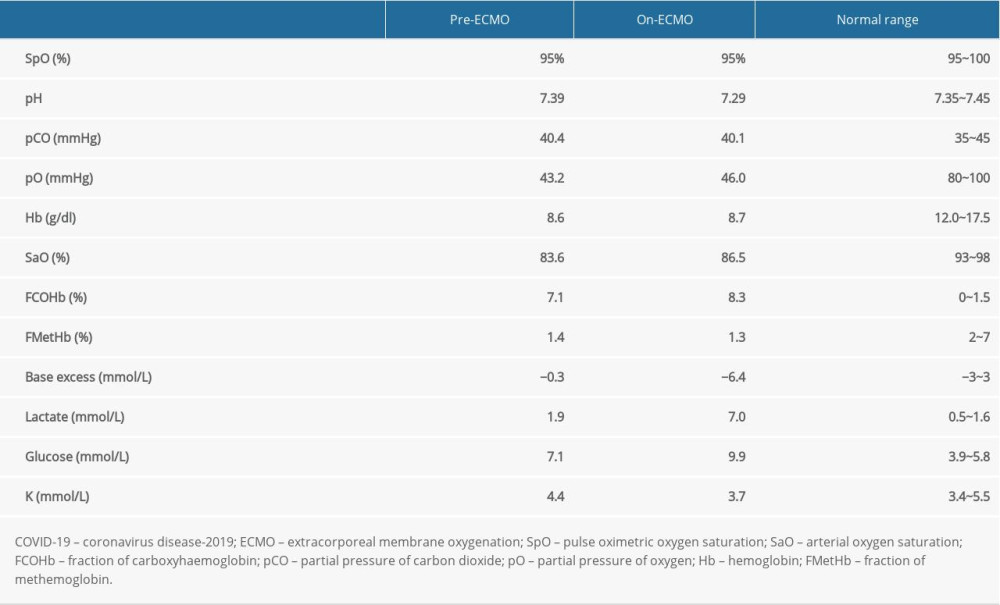
Venovenous ECMO (VV-ECMO) is used as a rescue treatment for critical patients with COVID-19 when acute respiratory failure does not respond to invasive ventilation. Hemolysis during VV-ECMO is common due to the high velocity blood flow and formation of blood clots in the circuit. Heme molecules from damaged red blood cells are broken down into free iron, biliverdin, and CO by hemoxygenase. In patients on VV-ECMO, CO and carboxyhaemoglobin accumulate. As noted in a comparison of ABG results in a patient with COVID-19 before and after using VV-ECMO (Table 1), the fraction of carboxyhaemoglobin (FCOHb) increased from 7.1% to 8.3% over a 40-hour period. The reliability of pulse oximetry would decrease due to carboxyhemoglobin interference [16].
In addition, increased CO production and carboxyhemoglobinemia are often observed in patients with pneumonia [17]. CO is mainly eliminated from the body through alveolar gas exchange and oxidation to CO2, which are both impaired in patients with COVID-19 due to respiratory function decline [18].
Other Non-Specific Factors Causing the Discrepancy Between Pulse Oximetric Oxygen Saturation and the Partial Pressure of Oxygen
Pulse oximetry is a part of standard non-invasive ward-based observations. It measures the tissue absorption of 660 nm and 940 nm light, and detects the variation in absorbance caused by pulsatile arterial flow. The variation is then used to estimate the arterial oxygen saturation based on the Beer-Lambert law [19]. Although it is easy to administer and many studies support a high correlation between pulse oximetric oxygen saturation (SpO2) and partial pressure of oxygen (PaO2), SpO2 occasionally does not reflect the findings from ABG analyses and the clinical condition in COVID-19 pneumonia [20–22]. A single-center retrospective study found that the ratio of SpO2 to the fraction of inspiratory O2 (FiO2) more effectively indicated an increased risk of death in mechanically ventilated children compared with the PaO2/FiO2 ratio [23]. Additionally, both animal and human experiments suggest that SpO2 is not a good indicator of the oxygenation of the detected object. SpO2 overestimates arterial oxygen saturation (SaO2) readings, especially in cases of hypoxemia. Thus, SpO2 might be too muted to allow the degree of impairment in oxygenation status to be judged by the decline in SpO2 [24,25]. In addition, pulse oximetry recordings are typically performed on the extremities, and poor peripheral perfusion can interfere with SpO2 monitoring accuracy. Therefore, this rare situation can occur in individual patients with signs of arterial blood hypoxemia and signs of respiratory distress, but the peripheral pulse oximeter may remain within the normal range. Relying on bedside pulse oximetry monitoring may delay the recognition of impending respiratory failure in these patients until it is too late for the best rescue treatment.
COVID-19 Monitoring and Treatment Optimization
The purpose of this review is to analyze the causes of silent hypoxemia in a small number of COVID-19 patients. Therefore, we analyzed each possible factor separately without emphasizing that any factor is the dominant factor. Due to the reasons outlined above, a reduction in the pulse oximetry reading is often a late sign of clinical deterioration and is often associated with significant mortality and morbidity [26]. The possible limitations of pulse oximetry in critical monitoring of COVID-19 patients should be considered, and clinical evaluations should focus on assessing the progression of signs and symptoms, such as shortness of breath, fatigue, and an inability to move and eat. The respiratory rate may be a more sensitive observation parameter in detecting deterioration.
ABG remains the most reliable method to evaluate oxygenation. Personal protective equipment used by health care professionals, such as heavy gowns, face shields, and gloves, reduces the manual dexterity necessary for bedside procedures. This increases the difficulty of arterial blood sample collection and creates a barrier to regular ABG monitoring. This information should be taken into account, and extra resources may need to be allocated to ensure that samples are taken promptly.
In the emergency department, signs of respiratory distress are sensitive and reliable criteria for patient triage; for patients with suspected severe disease, prompt ABG analysis is indicated. When making decisions on the level of care required, physicians should not rely exclusively on pulse oximetry. Ward-based early warning systems also need to be adapted to take this information into account. The respiratory rate should be monitored regularly, and tachypnea may warrant prompt escalation. In patients who are at risk of deterioration, frequent ABG analysis may be required to reliably assess oxygenation. Other early warning indicators for severe and critical disease include rapidly declining lymphocyte count, increasing serum lactate levels, and progression of radiological changes. The decision to start oxygen therapy should be made based on the clinical impression, ABG analysis, and lung imaging, and oxygen therapy should be titrated based on regular ABG analysis.
Patients who do not respond adequately to oxygen therapy should be promptly assessed, and decisions regarding an escalation of respiratory support should be made early. The choice of treatment is based on FiO2, PaO2, current respiratory support, and the patient’s overall clinical state. A normal pulse oximetry reading does not exclude the need for escalating respiratory support and critical care admission. For patients receiving respiratory support or ECMO in the critical care setting, regular arterial samples should be obtained (preferably using an arterial catheter) to accurately assess the response to treatments.
Conclusions
Severe hypoxemia without signs of respiratory distress is also known as silent or hidden hypoxemia. Silent hypoxemia is now recognized to be associated with severe COVID-19 pneumonia. This review has highlighted that clinicians who are managing patients with COVID-19 pneumonia need to be vigilant for the presence of silent hypoxemia. Therefore, signs and symptoms of shortness of breath in patients with severe COVID-19 pneumonia should alert the clinician to the possibility of silent hypoxemia and tissue hypoxia. Clinical evaluation should be modified such that the diagnosis of silent hypoxemia is not delayed or missed.
References
1. Chen Q, Quan B, Li X, A report of clinical diagnosis and treatment of nine cases of coronavirus disease 2019: J Med Virol, 2020; 92; 683-87
2. Yang X, Yu Y, Xu J, Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study: Lancet Respir Med, 2020; 8; 475-81
3. Bai HX, Hsieh B, Xiong Z, Performance of radiologists in differentiating COVID-19 from non-COVID-19 Viral pneumonia at chest CT: Radiology, 2020; 296; E46-54
4. Nitsure M, Sarangi B, Shankar GH, Mechanisms of hypoxia in COVID-19 patients: A pathophysiologic reflection: Indian J Crit Care Med, 2020; 24(10); 967-70
5. Couzin-Frankel J, The mystery of the pandemic’s ‘happy hypoxia’: Science, 2020; 368(6490); 455-56
6. Chen T, Wu D, Chen H, Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study: BMJ, 2020; 368; m1091
7. Bellingham AJ, Detter JC, Lenfant C, Regulatory mechanisms of hemoglobin oxygen affinity in acidosis and alkalosis: J Clin Invest, 1971; 50; 700-6
8. Liu W, Li H, COVID-19: Attacks the 1-beta chain of hemoglobin and captures the porphyrin to inhibit human heme metabolism: ChemRxiv, 2020 11938173.v9 [Preprint]
9. Chen N, Zhou M, Dong X, Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study: Lancet, 2020; 395; 507013
10. Huang C, Wang Y, Li X, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China: Lancet, 2020; 395; 497-506
11. Grasselli G, Zangrillo A, Zanella A, Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy: JAMA, 2020; 323; 1574-81
12. Otterbein LE, Foresti R, Motterlini R, Heme oxygenase-1 and carbon monoxide in the heart: the balancing act between danger signaling and pro-survival: Circ Res, 2016; 118; 1940-59
13. Tenhunen R, Marver HS, Schmid R, The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase: Proc Natl Acad Sci USA, 1968; 61; 748-55
14. Wu L, Wang R, Carbon monoxide: Endogenous production, physiological functions, and pharmacological applications: Pharmacol Rev, 2005; 57; 585-630
15. Owens EO, Endogenous carbon monoxide production in disease: Clin Biochem, 2010; 43; 1183-88
16. Nisar S, Gibson CD, Sokolovic M, Shah NS, Pulse oximetry is unreliable in patients on veno-venous extracorporeal membrane oxygenation caused by unrecognized carboxyhemoglobinemia: ASAIO J, 2020; 66(10); 1105-9
17. Corbacioglu SK, Kilicaslan I, Bildik F, Endogenous carboxyhemoglobin concentrations in the assessment of severity in patients with community-acquired pneumonia: Am J Emerg Med, 2013; 31; 520-23
18. Stevenson DK, Ostrander CE, Johnson JD, Effect of erythrocyte destruction on the pulmonary excretion rate of carbon monoxide in adult male Wistar rats: J Lab Clin Med, 1979; 94; 649-54
19. Barker SJ, Tremper KK, Pulse oximetry: Applications and limitations: Int Anesthesiol Clin, 1987; 25; 155-75
20. Zeserson E, Goodgame B, Hess JD, Correlation of venous blood gas and pulse oximetry with arterial blood gas in the undifferentiated critically ill patient: J Intensive Care Med, 2018; 33; 176-81
21. Khemani RG, Thomas NJ, Venkatachalam V: Crit Care Med, 2012; 40; 1309-16
22. Hay WW, Brockway JM, Eyzaguirre M, Neonatal pulse oximetry: Accuracy and reliability: Pediatrics, 1989; 83; 717-22
23. Khemani RG, Rubin S, Belani S: Intensive Care Med, 2015; 41; 94-102
24. Farrell KS, Hopper K, Cagle LA, Epstein SE: J Vet Emerg Crit Care (San Antonio), 2019; 29; 622-29
25. Wackernagel D, Blennow M, Hellström A, Accuracy of pulse oximetry in preterm and term infants is insufficient to determine arterial oxygen saturation and tension: Acta Paediatr, 2020; 109(11); 2251-57
26. Xu Z, Shi L, Wang Y, Pathological findings of COVID-19 associated with acute respiratory distress syndrome: Lancet Respir Med, 2020; 8; 420-22
In Press
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952