16 April 2021: Clinical Research
An Observational Cohort Study of the 2-Month Use of Regional Citrate Anticoagulation in Maintenance Hemodialysis Patients with Cerebral Hemorrhage
Xiao Bi1ABCDEF, Qi Zhang1ABE, Feng Zhuang1B, Wei Lu1B, Yingdeng Wang1AE, Feng Ding1ADE*DOI: 10.12659/MSM.930513
Med Sci Monit 2021; 27:e930513
Abstract
BACKGROUND: Regional citrate anticoagulation (RCA) is a recommended anticoagulation alternative for patients at high risk of bleeding while undergoing intermittent hemodialysis. Previous reports implied the risk of citrate application on bone metabolism. It is unclear whether long-term use of RCA is safe for maintenance hemodialysis patients in terms of bone metabolism.
MATERIAL AND METHODS: Seven patients with cerebral hemorrhage were included in the study. Blood samples were collected at baseline and 4 and 8 weeks after treatment. Spent dialysate samples were collected during each mid-week dialysis session, using the partial dialysate collection method. All patients were treated with RCA for 4 to 8 weeks, according to their clinical condition. We assessed bone metabolism-associated parameters, bone turnover markers, and magnesium loss at each dialysis session.
RESULTS: Serum magnesium levels were 1.24±0.13 mmol/L at baseline and significantly decreased to 1.16±0.14 mmol/L after 4 weeks of RCA treatment (P=0.025). Most patients had negative magnesium balance during citrate hemodialysis. Serum total calcium levels did not change significantly after treatment. One bone marker, N-terminal propeptide of type I procollagen (PINP), significantly decreased from 146.07±130.12 mmol/L to 92.42±79.01 mmol/L after citrate treatment (P=0.018). No significant changes were detected in other bone turnover markers.
CONCLUSIONS: Relatively long-term RCA treatment may decrease serum magnesium levels due to negative magnesium balance. Bone formation marker PINP seemed to decrease after treatment, while other bone turnover markers did not change significantly. Further investigation is needed to verify the effect of RCA on bone remodeling.
Keywords: Anticoagulants, Magnesium Deficiency, Renal Dialysis, Aged, 80 and over, Bone Remodeling, Cerebral Hemorrhage, Citric Acid, Cohort Studies, Osteogenesis, Peptide Fragments, Procollagen
Background
Patient undergoing hemodialysis have an increased risk of bleeding, such as intracranial and extradural hemorrhage, which is reported to be 10 to 20 times greater than that of the general population [1]. For the patients who have recently had intracranial hemorrhage, systemic anticoagulants should be avoided [2]. As such, regional citrate anticoagulation (RCA) is a preferred treatment because its anticoagulation effect is limited to the extracorporeal circuit. Citrate is a very effective regional anticoagulant. It decreases serum ionized calcium levels by forming a citrate-calcium complex. Therefore, calcium, which is essential for the coagulation process, is no longer available [3]. During RCA treatment, citrate is dripped into the arterial line to maintain the ionized calcium concentration of the extracorporeal circuit, within the range of 0.2 to 0.4 mmol/L. A calcium substitution is administered in the venous line to keep the normal systematic ionized calcium level [4]. Currently, RCA treatment is considered as an alternative anticoagulation treatment for patients who are actively bleeding, at increased risk of bleeding, or have heparin-induced thrombocytopenia [5].
Although many reports have reported the advantages of RCA, it is still unclear whether the long-term exposure to citrate would do harm to patients. This requires further investigation because some reports showed that plasma and thrombocyte donors exposed to citrate anticoagulation exhibited changes in mineral and bone metabolism [6,7]. Previous case reports also showed that bone loss and fracture occurred in 2 patients who received long-term RCA treatment [8,9]. Considering the importance of bone loss and fracture, which are associated with the prognostic maintenance of hemodialysis patients [10,11], it is of concern whether treatment with RCA for a period of time will affect the bone metabolism of these patients.
There are currently no reports about the effects of relatively long-term use of RCA on bone remodeling in patients undergoing intermittent hemodialysis. The objective of this study is to evaluate the impact of RCA on bone metabolism-associated parameters and bone turnover markers and to investigate whether the use of RCA for a longer period of time is safe for patients undergoing maintenance hemodialysis, in terms of bone metabolism.
Material and Methods
STATEMENT OF ETHICS:
This study was approved by the Ethics Committee of Shanghai Ninth People’s Hospital, School of Medicine, Shanghai Jiao tong University (approval No. SH9H-2018-T78-2). Informed consent was signed by all participants or their close relatives before they were enrolled in the study.
PATIENTS:
A total of 7 patients undergoing intermittent hemodialysis at the Kidney Intensive Care Unit of Shanghai Ninth People’s Hospital from May 2019 to March 2020 were included in this study. The patients began the treatment with RCA because of experiencing cerebral hemorrhage. The patients were included after returning to the dialysis center. All patients had uremia and anuria. Exclusion criteria were patients with known osteoporosis, severely impaired liver function, or malignant tumors; fracture/orthopedic surgery in the last 6 months; changes of the dose of phosphate binders, vitamin D, or calcimimetics within 4 weeks of study entry; and treatment with citrate anticoagulation, blood, blood products, or plasma exchange in the last 3 months.
HEMODIALYSIS:
Dialysis was performed using the Fresenius 4008 dialysis machine or Aquarius DBB27 dialysis machine. The dialysate flow rate was set at 500 mL/min. Dialysate was calcium-free, containing 140 mmol/L sodium, 108.5 mmol/L chlorine, 2.0 mmol/L potassium, 31 mmol/L bicarbonate, and 0.5 mmol/L magnesium. The blood flow rate and ultrafiltration rate were set and adjusted according to the patients’ therapeutic needs. Blood flow rates ranged from 200 mL/min to 250 mL/min. Dialysis access was via an arteriovenous fistula.
RCA PROTOCOL:
We created the RCA protocol for intermittent hemodialysis based on our previous research [12,13]. This protocol has been performed as the standard protocol for RCA since 2016. The citrate anticoagulant (2.2%, 113 mmol/L) was dripped into the arterial line prior to the blood pump at a dose of 4 mmol/L per plasma flow, while the calcium chloride solution (5%, 340 mmol/L) was infused into the venous return as supplementation. The level of ionized calcium in the extracorporeal circuit was maintained within the range of 0.2 to 0.4 mmol/L to ensure sufficient anticoagulation, while the systematic ionized calcium level was kept within the standard range (1.0–1.2 mmol/L). Blood gas analyses were done to measure the ionized calcium concentration during the treatment.
MONITORING AND LABORATORY TESTS:
Blood samples were drawn at baseline, after 4 weeks of treatment, and after 8 weeks of treatment. Effluent samples were collected during each mid-week dialysis session using the partial dialysate collection method, based on a previous report [14]. Accordingly, a small pump was installed on the spent dialysate line to draw a small flow at about 8 to 10 mL/min for collection in a small vessel. A sample of the collected dialysate was well mixed and sent for calcium concentration measurement. Intact fibroblast growth factor (FGF)-23, bioactive parathyroid hormone (PTH) 1–84, N-terminal propeptide of type I procollagen (PINP), beta carboxy-terminal cross-linking telopeptide of type I collagen (CTX), osteocalcin, and bone alkaline phosphatase (b-ALP) were measured using commercially available ELISA kits, according to the manufacturer’s instructions (ELISA kits were from Immunodiagnostic Systems, Tyne and Wear, UK and Quidel Corporation, San Diego, CA, USA). The calcium, phosphate, and magnesium concentrations of the blood and effluent sample were measured according to the routine laboratory procedures of our hospital.
CALCULATIONS OF MAGNESIUM FLUXES FROM EXTRACORPOREAL CIRCUIT TO THE EFFLUENT:
The amount of magnesium removed by the filters was deducted from the amount of magnesium delivered in the dialysate. Effluent loss of magnesium was calculated from the effluent flow multiplied by the magnesium concentration in the effluent. Magnesium input was calculated as dialysate flow multiplied by the magnesium concentration in dialysate.
STATISTICAL ANALYSIS:
All continuous variables were normally distributed (assessed by the Shapiro-Wilk test) and expressed as mean±standard deviation. A paired
Results
BASELINE CHARACTERISTICS:
All patients were treated with hemodialysis RCA 3 times per week for 4 weeks. After 4 weeks, 3 patients continued treatment with RCA because of recurrent cerebral hemorrhage; 3 patients were anticoagulated by heparin during hemodialysis; and 1 patient died from intracranial hemorrhage. The patients’ baseline characteristics are shown in Table 1. Accordingly, the mean levels of serum blood urea nitrogen (BUN), creatine, phosphate, and total calcium were 25.2±7.6 mmol/L, 657.6±274.1 umol/L, 1.7±0.4 mmol/L, and 2.2±0.1 mmol/L, respectively. We evaluated the KT/V values of all patients before the study and every month during the study to ensure the dialysis adequacy. The KT/V values of the patients included in this study were all above 1.2.
CHANGES OF BONE METABOLISM-ASSOCIATED BIOCHEMICAL PARAMETERS:
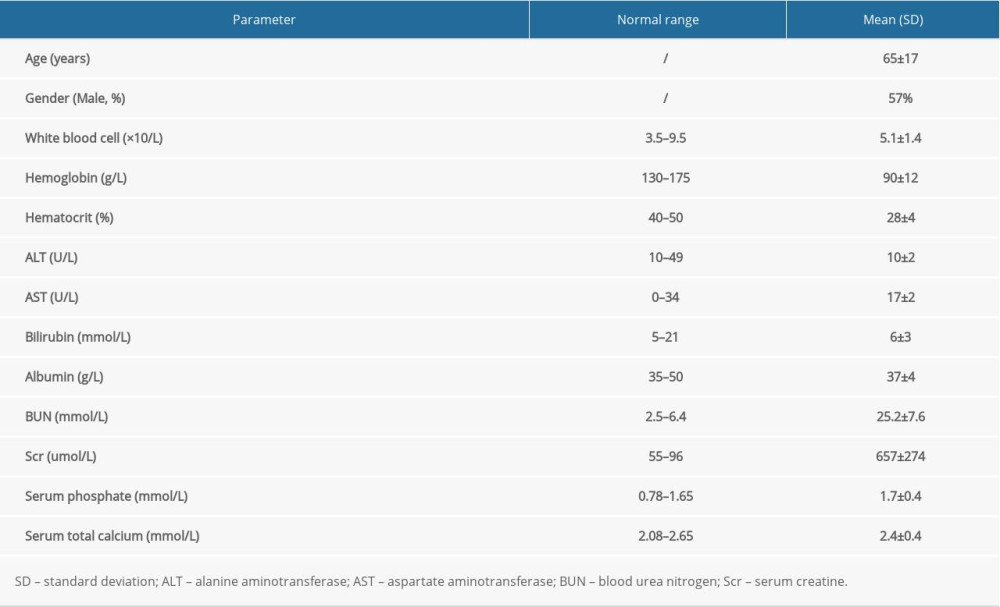
Serum calcium, serum phosphate, serum magnesium, PTH, and FGF-23 are associated with mineral and bone metabolism. Changes in these parameters are shown in Figure 1. Serum magnesium levels were 1.24±0.13 mmol/L at baseline, and they were significantly decreased to 1.16±0.14 mmol/L after 4 weeks of RCA treatment (P=0.025). Serum calcium and phosphate levels were 2.42±0.38 mmol/L and 1.72±0.39 mmol/L, respectively, at baseline. These levels were not significantly different after 4 weeks of RCA treatment. On the other hand, FGF-23 levels were significantly elevated after 4 weeks of RCA treatment compared with the levels at baseline (P=0.018), and bioactive intact PTH levels of the patients did not change significantly after exposure to 4 weeks of RCA treatment (P=0.59). Furthermore, 3 patients had an additional 4 weeks of RCA treatment. The trend of these relevant parameters is shown in Figure 2. During RCA treatment, 2 of the 3 patients showed continuously decreased levels of serum magnesium, and 1 of the 3 patients exhibited continuously increased levels of FGF-23. In addition, another 3 patients were treated with heparin anticoagulation in the 4 weeks following the 4 weeks of RCA treatment. Variations in the serum magnesium and FGF-23 concentrations of these patients are shown in Figure 3A–3C. Serum magnesium of the 3 patients seemed to increase slightly after stopping RCA treatment.
CHANGES OF BONE TURNOVER MARKERS:
We measured bone formation markers including PINP, osteocalcin, b-ALP, as well as bone absorption markers, such as CTX. As shown in Figure 4, the serum PINP concentration was significantly decreased after 4 weeks of RCA treatment (P=0.018). Other bone turnover markers, including osteocalcin, b-ALP, and CTX, did not differ significantly from their baseline levels. Moreover, serum PINP concentrations seemed to decrease continuously in 2 of the 3 patients after receiving an additional 4 weeks of RCA treatment (Figure 5).
MAGNESIUM BALANCE DURING RCA TREATMENT:
Since serum magnesium levels were significantly decreased after 4 weeks of citrate treatment, we assessed the amount of magnesium input and the amount of magnesium lost in the extracorporeal circuit. The magnesium input was about 60 mmol for every 4-hour hemodialysis session. The amounts magnesium lost in every session of week 1, week 2, week 3, and week 4 were 63.7±3.9 mmol, 62.2±2.9 mmol, 64.2±3.5 mmol, and 63.2±2.1 mmol, respectively (Figure 6). For most patients, the amount of magnesium lost was more than the magnesium delivered in the dialysate, and negative magnesium balance was likely inevitable.
Discussion
Compared with the general population, patients undergoing maintenance hemodialysis are at a higher risk of cerebral hemorrhage [15], which is associated with the high prevalence of hypertension, vascular comorbidities linked to kidney impairment, and complications resulting from uremia, such as vascular calcification and malnutrition-inflammation-atherosclerosis syndrome [16]. The use of heparin is essential for dialysis therapy, but is hazardous in the case of cerebral hemorrhage.
The anticoagulation effect of RCA treatment is limited in the extracorporeal circuit. To date, it has been recognized that RCA has some advantages compared with heparin, including prolonged filter life, reduction of bleeding risk, and better membrane biocompatibility [17]. The removal of calcium in the extracorporeal circuit during RCA has also been shown to attenuate neutrophil degranulation, and thus reduce oxidative stress [18]. For patients undergoing maintenance hemodialysis and who have cerebral hemorrhage, RCA may be used for long-term treatment to reduce the bleeding risk. However, it is still unclear whether long-term exposure to RCA is safe for patients undergoing maintenance hemodialysis. Previous reports demonstrated that blood donors who were exposed to citrate during apheresis exhibited compromised bone mass density at the lumbar spine [7]. Moreover, some cases showed that marked bone loss and spontaneous fracture occurred in several patients who were sustained with prolonged continuous renal replacement therapy using RCA. Therefore, the long-term application of RCA may induce harmful effects on patients undergoing hemodialysis, and this deserves further investigation.
In this study, we found that, after citrate treatment, there were no significant changes in serum total calcium and serum phosphate levels; however, serum magnesium levels were significantly decreased after 4 weeks of RCA treatment, which may be associated with the negative balance of magnesium in each hemodialysis session. Magnesium is essential for bone health. During RCA, magnesium is chelated by citrate, similarly to calcium. The magnesium concentration of 0.5 mmol/L is common in commercially available dialysate. Kozik et al found a slight decrease of serum magnesium in patients treated with high-flux hemodialysis using 0.5 mmol/L dialysate magnesium [19]. Brain et al also found a significantly negative magnesium balance using dialysate containing 0.5 mmol/L of magnesium [20]. Our results were consistent with previous reports and suggest that the spontaneous decrease of serum magnesium may occur during the long-term use of RCA treatment. Moreover, serum magnesium levels were reported to be related with both cardiovascular and non-cardiovascular mortality, as well as the risk of hip fracture [21,22]. Therefore, more attention needs to be given to the magnesium concentration of dialysis/substitution fluids or additional magnesium supplementation. In this study, we did not strictly restrict magnesium intake from the patients’ diet. Our patients took an oral phosphate binder that was free of magnesium. Further studies taking daily magnesium intake into consideration should be performed to confirm the effect of RCA treatment on the serum magnesium level.
In addition, serum FGF-23 levels were increased in the patients treated with 4 weeks of citrate anticoagulation in this study. FGF-23 is an endocrine hormone, which is secreted by osteocytes and osteoblasts. Increased levels of serum FGF-23 is a risk factor for cardiovascular events and mortality in patients undergoing hemodialysis [23]. There are several reports on the correlation between serum magnesium and FGF-23, although the precise mechanism remains unknown. The magnesium-deficiency diet was reported to increase serum FGF-23 levels in rats with normal renal function [24]. Another report demonstrated that serum magnesium concentration was inversely associated with FGF-23 in patients undergoing hemodialysis [25]. This agrees with the results of our present study. Some researchers have suggested that magnesium may suppress FGF-23 secretion through a calcium-sensing receptor expressed in osteoblasts [26,27]. Other researchers suggested that magnesium may suppress FGF-23 production via the L-type voltage-sensitive calcium channel or transient receptor potential melastatin 7 channel [28,29]. These speculations need further experimental validation. Also, serum FGF-23 levels are related with serum calcium, phosphate, and PTH levels. Elevated PTH levels were detected in patients using RCA with a relatively low target of serum ionized calcium [30]. Although serum calcium and PTH levels did not change significantly in our present study, possibly due to the relative high target of serum ionized calcium in the study, the potential effect of RCA on the metabolism of calcium, PTH, and FGF-23 increases our concern of the use of long-term RCA treatment.
Bone formation and resorption markers are proteins produced by active bone cells or fragments of collagen released into the circulation during bone remodeling. Some studies have indicated that high levels of bone remodeling markers were correlated with abnormal cortical and trabecular density and microarchitecture [31,32]. In the present study, we found that serum PINP levels were decreased after 4 weeks of RCA treatment, and other markers including osteocalcin, b-ALP, and CTX did not change significantly. PINP is a sensitive marker of bone formation, which is cleared by the kidneys. We think its decline may be related to magnesium deficiency because magnesium has been regarded as promoting bone formation. A recent study that was focused on the dialysate magnesium concentration showed that increasing dialysate magnesium levels resulted in higher levels of b-ALP, another marker of bone formation [33]. Although there were no significant changes in b-ALP in our study, the potential effect of citrate treatment on bone formation needs further research. Therefore, it was difficult to draw any conclusions on the effect of RCA on bone remodeling, but we need to stay focused on this issue. We did not perform bone density scans because changes of bone density reflect 3 to 6 months of bone remodeling. Larger and longer cohort studies evaluating bone loss by imaging examination need to be conducted to verify the effect of RCA on bone remodeling.
There were several limitations in this study. First, the number of the patients included in the study was relatively small. Second, we did not strictly restrict the regular diet and consider patients’ magnesium intake. The effect of RCA on serum magnesium levels needs to be further validated. Third, no significant changes were detected in most of the bone markers measured in this study. It was uncertain whether prolonged RCA treatment would affect bone mass density or other prognostic markers. Fourth, we sampled only once at every time point. Multiple sampling at every time point would increase the value of the study.
Conclusions
Our study demonstrated that long-term RCA treatment may decrease serum magnesium levels, possibly due to negative magnesium balance. PINP levels were also decreased after the treatment, while other bone turnover markers did not change significantly. Further investigations are needed to determine whether dialysate magnesium concentration needs to be elevated during the long-term use of RCA. In the future, we will perform larger and longer cohort studies to verify the effect of RCA on patients undergoing hemodialysis.
Figures
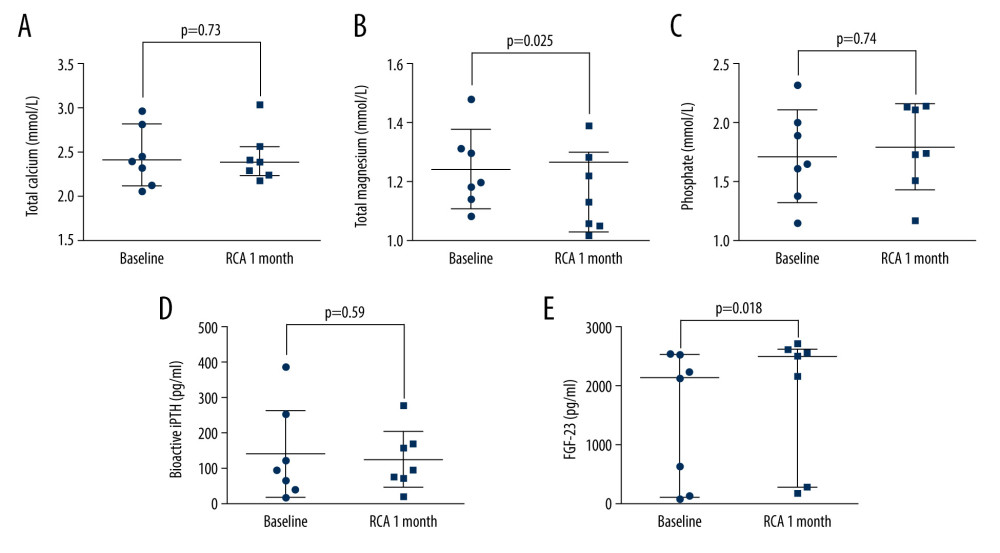 Figure 1. The changes in bone metabolism-associated biochemical parameters before and after 4 weeks of regional citrate anticoagulation (RCA) treatment: (A) total calcium; (B) total magnesium; (C) phosphate; (D) bioactive intact parathyroid hormone (PTH); (E) fibroblast growth factor (FGF)-23.
Figure 1. The changes in bone metabolism-associated biochemical parameters before and after 4 weeks of regional citrate anticoagulation (RCA) treatment: (A) total calcium; (B) total magnesium; (C) phosphate; (D) bioactive intact parathyroid hormone (PTH); (E) fibroblast growth factor (FGF)-23. 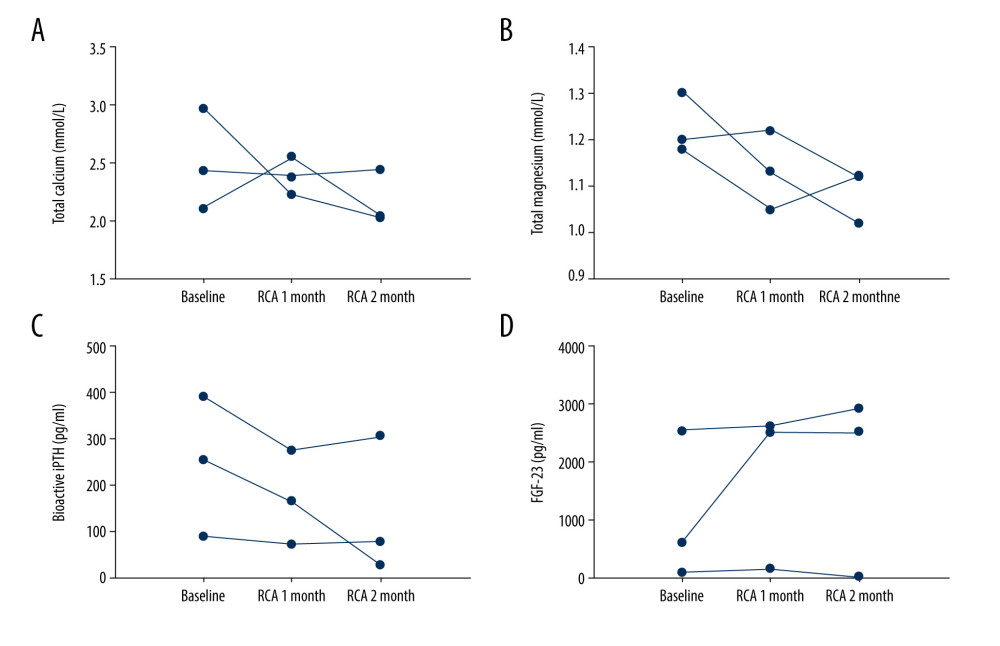 Figure 2. The trend of total calcium, total magnesium, bioactive intact parathyroid hormone (PTH), and FGF-23 in patients receiving 8 weeks of continuous regional citrate anticoagulation (RCA) treatment: (A) total calcium; (B) total magnesium; (C) bioactive PTH; (D) fibroblast growth factor (FGF)-23.
Figure 2. The trend of total calcium, total magnesium, bioactive intact parathyroid hormone (PTH), and FGF-23 in patients receiving 8 weeks of continuous regional citrate anticoagulation (RCA) treatment: (A) total calcium; (B) total magnesium; (C) bioactive PTH; (D) fibroblast growth factor (FGF)-23. 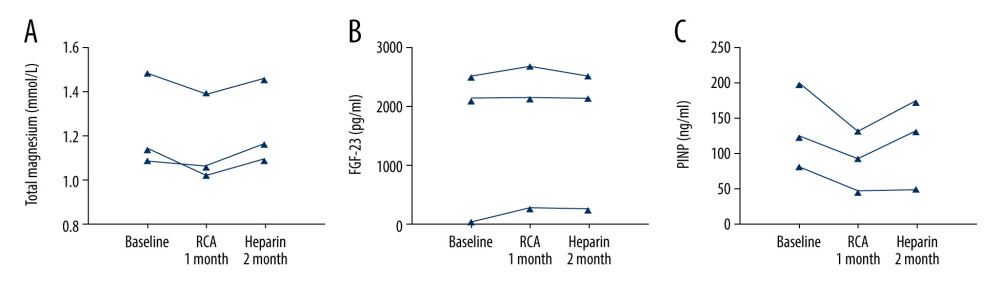 Figure 3. The trend of total magnesium, fibroblast growth factor (FGF)-23 in patients treated with 4 weeks of regional citrate anticoagulation (RCA) treatment and subsequent 4 weeks of heparin anticoagulation: (A) total magnesium; (B) FGF-23; (C) PINP.
Figure 3. The trend of total magnesium, fibroblast growth factor (FGF)-23 in patients treated with 4 weeks of regional citrate anticoagulation (RCA) treatment and subsequent 4 weeks of heparin anticoagulation: (A) total magnesium; (B) FGF-23; (C) PINP. 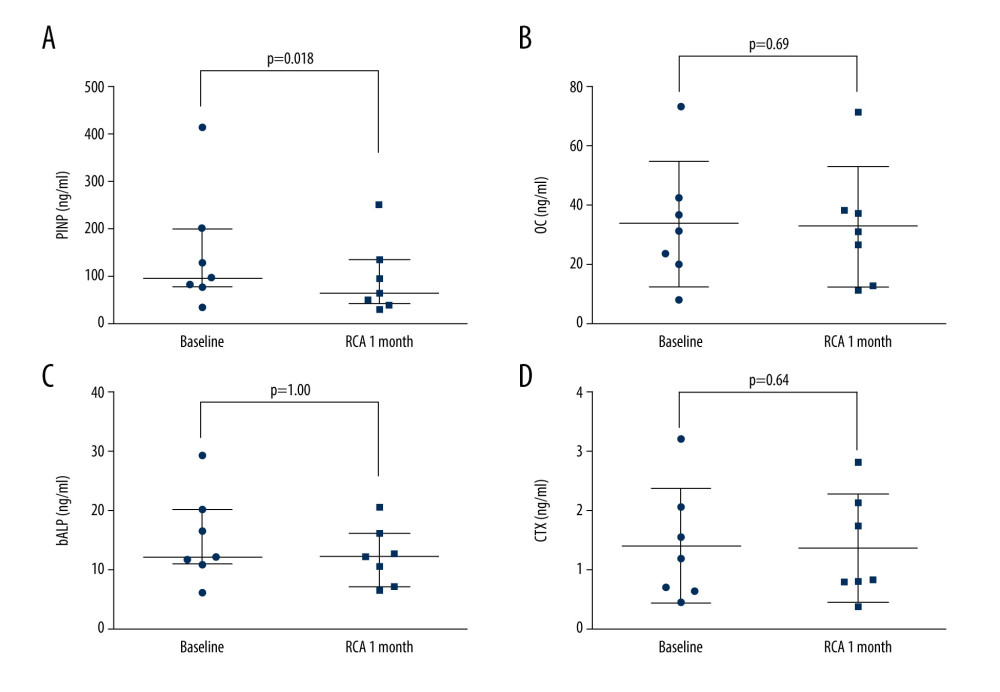 Figure 4. The changes in bone formation and resorption markers before and after 4 weeks of regional citrate anticoagulation (RCA) treatment: (A) N-terminal propeptide of type I procollagen (PINP); (B) osteocalcin; (C) bone alkaline phosphatase (b-ALP); (D) beta carboxy-terminal cross-linking telopeptide of type I collagen (CTX).
Figure 4. The changes in bone formation and resorption markers before and after 4 weeks of regional citrate anticoagulation (RCA) treatment: (A) N-terminal propeptide of type I procollagen (PINP); (B) osteocalcin; (C) bone alkaline phosphatase (b-ALP); (D) beta carboxy-terminal cross-linking telopeptide of type I collagen (CTX). 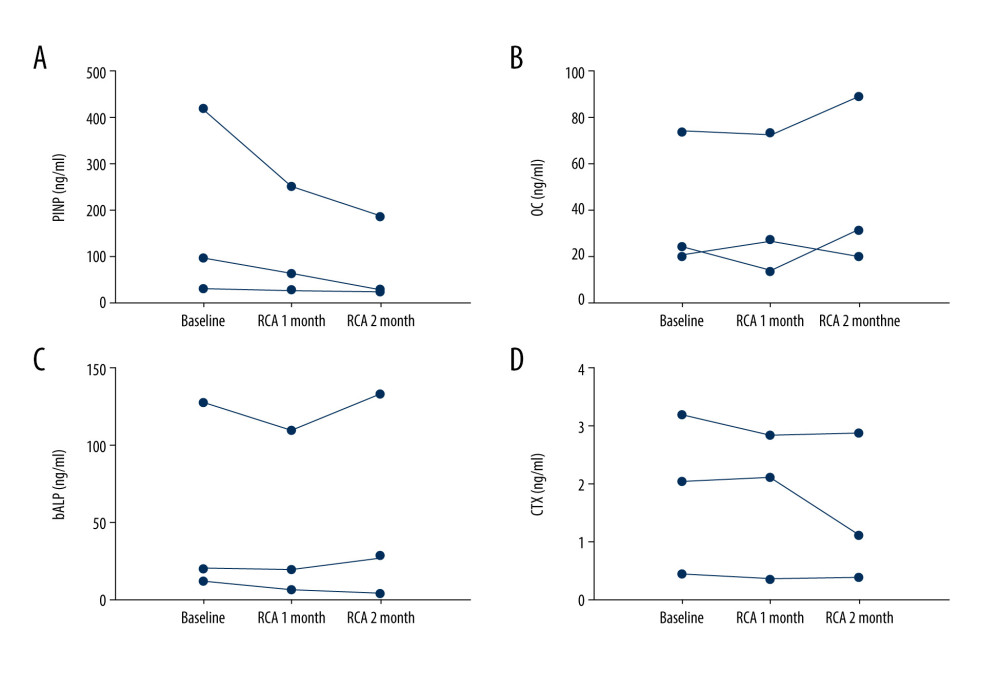 Figure 5. The trend of N-terminal propeptide of type I procollagen (PINP), osteocalcin, bone alkaline phosphatase (b-ALP), and beta carboxy-terminal cross-linking telopeptide of type I collagen (CTX) in patients treated with 8 weeks of continuous regional citrate anticoagulation (RCA): (A) PINP; (B) osteocalcin; (C) b-ALP; (D) CTX.
Figure 5. The trend of N-terminal propeptide of type I procollagen (PINP), osteocalcin, bone alkaline phosphatase (b-ALP), and beta carboxy-terminal cross-linking telopeptide of type I collagen (CTX) in patients treated with 8 weeks of continuous regional citrate anticoagulation (RCA): (A) PINP; (B) osteocalcin; (C) b-ALP; (D) CTX. 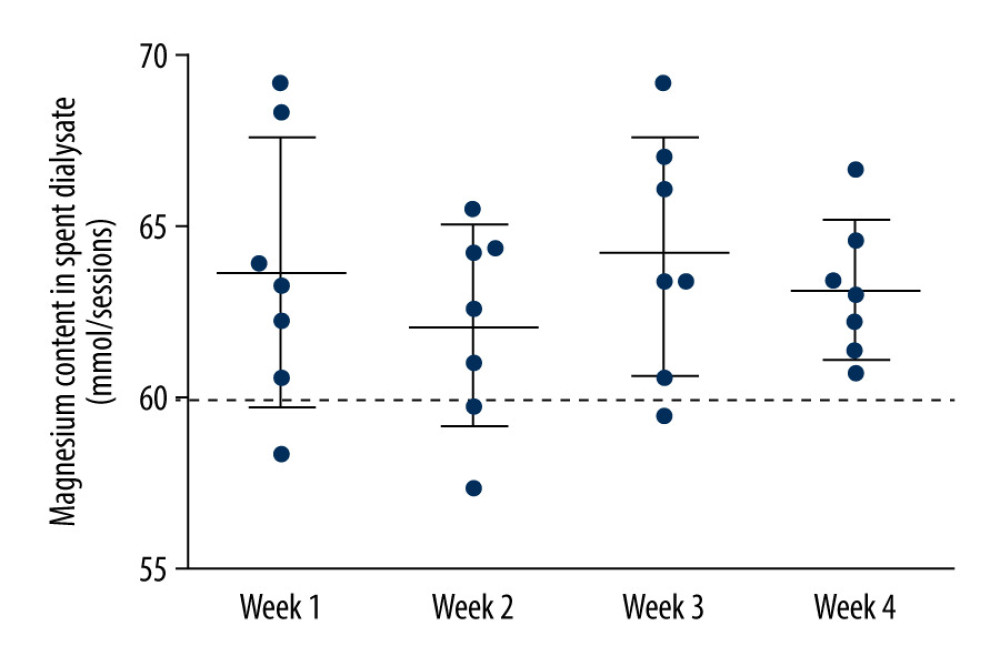 Figure 6. Magnesium loss of each hemodialysis session in the dialysate during week 1, week 2, week 3, and week 4. The dialysate was sampled during each mid-week dialysis session. The dotted line means the amount of magnesium delivered in the dialysate, which was approximately 60 mmol in each session.
Figure 6. Magnesium loss of each hemodialysis session in the dialysate during week 1, week 2, week 3, and week 4. The dialysate was sampled during each mid-week dialysis session. The dotted line means the amount of magnesium delivered in the dialysate, which was approximately 60 mmol in each session. References
1. Leonard A, Shapiro FL, Subdural hematoma in regularly hemodialyzed patients: Ann Intern Med, 1975; 82; 650-58
2. Davenport A, Changing the hemodialysis prescription for hemodialysis patients with subdural and intracranial hemorrhage: Hemodial Int, 2013; 17(Suppl 1); S22-27
3. Buturovic-Ponikvar J, Is regional citrate anticoagulation the future of hemodialysis?: Ther Apher Dial, 2016; 20; 234-39
4. Morgera S, Schneider M, Slowinski T, A safe citrate anticoagulation protocol with variable treatment efficacy and excellent control of the acid-base status: Crit Care Med, 2009; 37; 2018-24
5. Khwaja A, Kdigo clinical practice guidelines for acute kidney injury: Nephron Clin Pract, 2012; 120; c179-84
6. Bialkowski W, Bruhn R, Edgren G, Papanek P, Citrate anticoagulation: Are blood donors donating bone?: J Clin Apher, 2016; 31; 459-63
7. Amrein K, Katschnig C, Sipurzynski S, Apheresis affects bone and mineral metabolism: Bone, 2010; 46; 789-95
8. Wang PL, Meyer MM, Orloff SL, Anderson S, Bone resorption and “relative” immobilization hypercalcemia with prolonged continuous renal replacement therapy and citrate anticoagulation: Am J Kidney Dis, 2004; 44; 1110-14
9. Klingele M, Seiler S, Poppleton A, The gap between calculated and actual calcium substitution during citrate anticoagulation in an immobilised patient on renal replacement therapy reflects the extent of bone loss – a case report: BMC Nephrol, 2014; 15; 163
10. Orabona N, Bove A, Smeraglia F, The impact of hemodialysis on mortality and personal independence after hip fracture. A prospective matched cohort study: J Orthop Trauma, 2019; 33; 577-82
11. Kaneko TM, Foley RN, Gilbertson DT, Collins AJ, Clinical epidemiology of long-bone fractures in patients receiving hemodialysis: Clin Orthop Relat Res, 2007; 457; 188-93
12. Yu W, Zhuang F, Ma S, Optimized calcium supplementation approach for regional citrate anticoagulation: Nephron, 2019; 141; 119-27
13. Zheng Y, Xu Z, Zhu Q, Citrate pharmacokinetics in critically ill patients with acute kidney injury: PLoS One, 2013; 8; e65992
14. Argilés A, Ficheux A, Thomas M, Precise quantification of dialysis using continuous sampling of spent dialysate and total dialysate volume measurement: Kidney Int, 1997; 52; 530-37
15. Iseki K, Kinjo K, Kimura Y, Evidence for high risk of cerebral hemorrhage in chronic dialysis patients: Kidney Int, 1993; 44; 1086-90
16. Ozelsancak R, Micozkadioglu H, Torun D, Tekkarismaz N, Cerebrovascular events in hemodialysis patients; A retrospective observational study: BMC Nephrol, 2019; 20; 466
17. Liu C, Mao Z, Kang H, Regional citrate versus heparin anticoagulation for continuous renal replacement therapy in critically ill patients: A meta-analysis with trial sequential analysis of randomized controlled trials: Crit Care, 2016; 20; 144
18. Schilder L, Nurmohamed SA, ter Wee PM, Citrate confers less filter-induced complement activation and neutrophil degranulation than heparin when used for anticoagulation during continuous venovenous haemofiltration in critically ill patients: BMC Nephrol, 2014; 15; 19
19. Kozik-Jaromin J, Nier V, Heemann U, Citrate pharmacokinetics and calcium levels during high-flux dialysis with regional citrate anticoagulation: Nephrol Dial Transplant, 2009; 24; 2244-51
20. Brain M, Anderson M, Parkes S, Fowler P, Magnesium flux during continuous venovenous haemodiafiltration with heparin and citrate anticoagulation: Crit Care Resusc, 2012; 14; 274-82
21. Sakaguchi Y, Fujii N, Shoji T, Hypomagnesemia is a significant predictor of cardiovascular and non-cardiovascular mortality in patients undergoing hemodialysis: Kidney Int, 2014; 85; 174-81
22. Sakaguchi Y, Hamano T, Wada A, Magnesium and risk of hip fracture among patients undergoing hemodialysis: J Am Soc Nephrol, 2018; 29; 991-99
23. Jean G, Terrat JC, Vanel T, High levels of serum fibroblast growth factor (fgf)-23 are associated with increased mortality in long haemodialysis patients: Nephrol Dial Transplant, 2009; 24; 2792-96
24. Matsuzaki H, Kajita Y, Miwa M, Magnesium deficiency increases serum fibroblast growth factor-23 levels in rats: Magnes Res, 2013; 26; 18-23
25. Iguchi A, Watanabe Y, Iino N, Serum magnesium concentration is inversely associated with fibroblast growth factor 23 in haemodialysis patients: Nephrology (Carlton), 2014; 19; 667-71
26. Koizumi M, Komaba H, Nakanishi S, Cinacalcet treatment and serum fgf23 levels in haemodialysis patients with secondary hyperparathyroidism: Nephrol Dial Transplant, 2012; 27; 784-90
27. Zhang C, Zhang T, Zou J, Structural basis for regulation of human calcium-sensing receptor by magnesium ions and an unexpected tryptophan derivative co-agonist: Sci Adv, 2016; 2; e1600241
28. Wang M, Berlin JR, Voltage-dependent modulation of l-type calcium currents by intracellular magnesium in rat ventricular myocytes: Arch Biochem Biophys, 2007; 458; 65-72
29. Elizondo MR, Budi EH, Parichy DM, Trpm7 regulation of in vivo cation homeostasis and kidney function involves stanniocalcin 1 and fgf23: Endocrinology, 2010; 151; 5700-9
30. van der Voort PH, Postma SR, Kingma WP, An observational study on the effects of nadroparin-based and citrate-based continuous venovenous hemofiltration on calcium metabolism: Blood Purif, 2007; 25; 267-73
31. Jorgensen HS, Winther S, Bottcher M, Bone turnover markers are associated with bone density, but not with fracture in end stage kidney disease: a cross-sectional study: BMC Nephrol, 2017; 18; 284
32. Nickolas TL, Stein EM, Dworakowski E, Rapid cortical bone loss in patients with chronic kidney disease: J Bone Miner Res, 2013; 28; 1811-20
33. Bressendorff I, Hansen D, Pasch A, The effect of increasing dialysate magnesium on calciprotein particles, inflammation and bone markers: Post hoc analysis from a randomized controlled clinical trial: Nephrol Dial Transplant, 2019 [Online ahead of print]
Figures
 Figure 1. The changes in bone metabolism-associated biochemical parameters before and after 4 weeks of regional citrate anticoagulation (RCA) treatment: (A) total calcium; (B) total magnesium; (C) phosphate; (D) bioactive intact parathyroid hormone (PTH); (E) fibroblast growth factor (FGF)-23.
Figure 1. The changes in bone metabolism-associated biochemical parameters before and after 4 weeks of regional citrate anticoagulation (RCA) treatment: (A) total calcium; (B) total magnesium; (C) phosphate; (D) bioactive intact parathyroid hormone (PTH); (E) fibroblast growth factor (FGF)-23. Figure 2. The trend of total calcium, total magnesium, bioactive intact parathyroid hormone (PTH), and FGF-23 in patients receiving 8 weeks of continuous regional citrate anticoagulation (RCA) treatment: (A) total calcium; (B) total magnesium; (C) bioactive PTH; (D) fibroblast growth factor (FGF)-23.
Figure 2. The trend of total calcium, total magnesium, bioactive intact parathyroid hormone (PTH), and FGF-23 in patients receiving 8 weeks of continuous regional citrate anticoagulation (RCA) treatment: (A) total calcium; (B) total magnesium; (C) bioactive PTH; (D) fibroblast growth factor (FGF)-23. Figure 3. The trend of total magnesium, fibroblast growth factor (FGF)-23 in patients treated with 4 weeks of regional citrate anticoagulation (RCA) treatment and subsequent 4 weeks of heparin anticoagulation: (A) total magnesium; (B) FGF-23; (C) PINP.
Figure 3. The trend of total magnesium, fibroblast growth factor (FGF)-23 in patients treated with 4 weeks of regional citrate anticoagulation (RCA) treatment and subsequent 4 weeks of heparin anticoagulation: (A) total magnesium; (B) FGF-23; (C) PINP. Figure 4. The changes in bone formation and resorption markers before and after 4 weeks of regional citrate anticoagulation (RCA) treatment: (A) N-terminal propeptide of type I procollagen (PINP); (B) osteocalcin; (C) bone alkaline phosphatase (b-ALP); (D) beta carboxy-terminal cross-linking telopeptide of type I collagen (CTX).
Figure 4. The changes in bone formation and resorption markers before and after 4 weeks of regional citrate anticoagulation (RCA) treatment: (A) N-terminal propeptide of type I procollagen (PINP); (B) osteocalcin; (C) bone alkaline phosphatase (b-ALP); (D) beta carboxy-terminal cross-linking telopeptide of type I collagen (CTX). Figure 5. The trend of N-terminal propeptide of type I procollagen (PINP), osteocalcin, bone alkaline phosphatase (b-ALP), and beta carboxy-terminal cross-linking telopeptide of type I collagen (CTX) in patients treated with 8 weeks of continuous regional citrate anticoagulation (RCA): (A) PINP; (B) osteocalcin; (C) b-ALP; (D) CTX.
Figure 5. The trend of N-terminal propeptide of type I procollagen (PINP), osteocalcin, bone alkaline phosphatase (b-ALP), and beta carboxy-terminal cross-linking telopeptide of type I collagen (CTX) in patients treated with 8 weeks of continuous regional citrate anticoagulation (RCA): (A) PINP; (B) osteocalcin; (C) b-ALP; (D) CTX. Figure 6. Magnesium loss of each hemodialysis session in the dialysate during week 1, week 2, week 3, and week 4. The dialysate was sampled during each mid-week dialysis session. The dotted line means the amount of magnesium delivered in the dialysate, which was approximately 60 mmol in each session.
Figure 6. Magnesium loss of each hemodialysis session in the dialysate during week 1, week 2, week 3, and week 4. The dialysate was sampled during each mid-week dialysis session. The dotted line means the amount of magnesium delivered in the dialysate, which was approximately 60 mmol in each session. In Press
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952