09 August 2021: Clinical Research
Correlation Between Sleep, Life, Mood, and Diet and Severity of Inflammatory Bowel Disease in China: A Retrospective Study
Jiahao Xu1BCDEF, Xuejie Chen1AB, Kejia Ma1B, Kai Nie1B, Weiwei Luo1B, Xing Wu1B, Shiyu Pan1B, Xiaoyan Wang1A*DOI: 10.12659/MSM.930511
Med Sci Monit 2021; 27:e930511
Abstract
BACKGROUND: The aim of the present study was to assess the relationship between inflammatory bowel disease (IBD) and quality of sleep, quality of life (QoL), mental health, and dietary intake to identify potential risk factors for IBD.
MATERIAL AND METHODS: This was a retrospective analysis from September 2019 to August 2020. We enrolled 71 patients with IBD aged 14 to 69 years who completed the IBD-Life Habits Questionnaire, which included data on demographics, environmental factors, and dietary habits; the Pittsburgh Sleep Quality Index (PSQI); Patient Health Questionnaire (PHQ-9); Generalized Anxiety Disorder-7 (GAD-7); and the Inflammatory Bowel Disease Questionnaire (IBDQ). Of the patients, 46 had IBD that was in remission and in 25 the disease was active, based on scores used to assess clinical symptoms. The Crohn’s Disease Activity Index (CDAI) and the Partial Mayo Score were used for Crohn disease (CD) and ulcerative colitis (UC), respectively. The patients were divided into 2 groups, based on disease status: remission (CDAI <150 or Mayo Score=0) and active (CDAI ≥150 or Mayo Score >0). Because sleep and dietary habits in the patients with UC and CD were not significantly different, the 2 groups of patients were eventually combined into a single IBD group. The IBD-Life Habits Questionnaire, except for IBDQ, was completed by 68 age- and sex-matched healthy controls.
RESULTS: Scores for PSQI (P=.001), PHQ-9 (P=.003), GAD-7 (P=.007), and IBDQ (P=.001) were significantly higher in the patients with active IBD. An IBDQ score >168.0 (PSQI score >7.5) indicates a clinically active state of IBD with a sensitivity of 84.8% (72.0%) and a specificity of 88.0% (82.6%). Diet composition was not related to disease activity. An analysis of patients and controls showed that lack of siblings could be a protective factor for onset of IBD (OR 0.300, 95% CI 0.119-0.785), while not being breastfed (OR 2.753, 95% CI 1.025-7.396) and consuming spicy foods could be risk factors for onset of IBD (OR 2.186, 95% CI 1.370-3.488).
CONCLUSIONS: In patients with IBD, poor sleep quality, poor QoL, depression, and anxiety were related to having active disease, whereas diet was not. Attempting to control dietary composition in patients with IBD may not be effective in preventing disease flare, but attention should be paid to intake of spicy foods.
Keywords: Inflammatory Bowel Diseases, Mental Health, Sleep Disorders, Circadian Rhythm, Adolescent, Anxiety, Colitis, Ulcerative, Crohn Disease, Depression, Diet Surveys, Feeding Behavior, Quality of Life, Risk Factors, self report, Severity of Illness Index, sleep quality, young adult
Background
Inflammatory bowel disease (IBD) is a chronic disease with frequent relapses. The incidence is on the increase in China [1,2], a nation that has become more industrialized. IBD includes Crohn disease (CD), ulcerative colitis (UC), and IBD unclassified (IBD-U) [3]. More than 50% of patients with IBD present with rectal bleeding, crampy abdominal pain, fatigue, and body weakness at least once a week during disease relapses, which significantly affect their daily life, work, and intimate relationships [4]. Symptomatic relapse is defined by clinical symptoms, such as blood in the stool and abdominal pain, whereas relapse is identified on endoscopy or with other inflammatory markers and the focus is on mucosal conditions and biochemical examinations. We used the former definition in our study because we were inclined to investigate the relationship between sleep, life, mood, and diet and clinical disease status in patients with IBD.
In the present study, we focused on sleep disturbance, mental health, and dietary habits as the 3 lifestyle elements associated with IBD. A previous study found poor sleep quality in patients with IBD who had severe exacerbations [5]. In addition, specific changes in certain serum substances could account for abnormalities in circadian rhythm [6]. Sleep and IBD have important correlations [7]. Sleep quality can be assessed subjectively with questionnaires and objectively with polysomnography. Early in 2003, a study using the Multisystem Instrument Score for sleep quality found that patients with IBD had more sleep disturbances, on average, than healthy people [8]. Another study using the Pittsburgh Sleep Quality Index (PSQI) score also found poor sleep quality in individuals with IBD [9]. High levels of C-reactive protein and depression also were associated with poor sleep in a cohort study [10]. As for mental health, a systematic review study found that symptoms of depression were more common in patients with active IBD than in those whose disease was in remission [11]. One explanation for this is the gut-brain axis, that is, gut microbiota play a role in brain functions such as cognition, emotions, and physical activity, an effect that is bi-directional [12]. Another cohort study found that a higher level of stress was associated with increased relapse of IBD [13]. As for diet, dietary habits are key to keeping the intestinal environment in balance and they affect intestine microbial structure and function. Changing from a fiber-rich to a protein-rich diet, for example, could modify the intestinal microbiota, decreasing fiber-related degradation of bacteria such as
Material and Methods
PATIENTS:
We enrolled 71 patients with IBD who had visited The Third Xiangya Hospital from September 2019 to August 2020. All of them completed the IBD-Life Habits Questionnaire and their disease status was determined by physicians using the Crohn’s Disease Activity Index (CDAI) and Mayo scores for CD and UC, respectively. All of them were receiving biologic therapy, such as an anti-tumor necrosis factor drug like infliximab. We included participants based on their disease status and completion of the questionnaire; patients who did not complete the questionnaire were excluded.
HEALTHY CONTROLS:
A total of 68 healthy individuals were recruited from The Third Xiangya Hospital Physical Examination Center to act as controls in the present study. They also completed the IBD-Life Habits Questionnaire, except for the Inflammatory Bowel Disease Questionnaire (IBDQ) portion. Individuals with disease of the digestive system were excluded. The healthy controls and patients with IBD were mainly from the southern part of China.
DATA COLLECTION:
General information was collected about sex, age, residence, income, and level of education. The IBD-Life Habits Questionnaire was developed to help characterize the lifestyles of patients with IBD. It included an assessment of dietary habits before and after IBD diagnosis, such as the frequency of eating food that was spicy, salty, or sweet. In addition, daily intake of 21 kinds of foods, including vegetables, fruit, pork, and milk, was scored using a Likert scale.
The questionnaire also included the PSQI to evaluate sleep quality, the Patient Health Questionnaire (PHQ-9) and the Generalized Anxiety Disorder-7 (GAD-7) to assess the mental state of patients, and the IBDQ. The PSQI consists of 9 survey questions designed to score sleep quality during the past month. A score higher than 10 indicates poor sleep. The PHQ-9 questionnaire consists of 9 questions, each of which is scored on a scale from 0 to 3 points. Patients who score more than 4 points are considered to be depressed. The GAD-7 questionnaire consists of 7 questions, each of which is scored on a scale from 0 to 3 points. Patients who score more than 4 points are considered anxious. Finally, the IBDQ questionnaire consists of 28 questions designed to evaluate the QoL of patients with IBD over the previous 2 weeks.
The patients whose IBD was in remission and the healthy controls completed the questionnaire based on their current state. Those with active IBD were asked to answer the questions based on their recollection of conditions before their last relapse. Questions aimed at determining risk factors for onset of IBD were to be answered based on conditions before a patient’s diagnosis.
ASSESSMENT OF DISEASE ACTIVITY:
Endoscopy is important for defining disease activity in IBD because remission depth for COD is measured based on endoscopic healing and for UC, is measured based on histologic healing [20]. Our IBD-Life Habits Questionnaire, however, was designed to elicit information about clinical symptoms. CDAI is a noninvasive, clinical, criterion standard scale for measuring disease activity [21] and the Partial Mayo Score is a scale based on several endoscopic conditions (erythema, vascular pattern, friability and erosions). The disease status of the enrolled patients was assessed using both scales. For patients with CD, a CDAI score <150 was considered remission; 150 to 220 consisted mild disease; 220 to 450 was regarded as CD; and a CDAI score >450 was considered severe CD [22]. For UC, a Partial Mayo Score of 0 was defined as clinical remission; 1 as mild UC; 2 as moderate; and 3 as severe UC. Based on the scores and the sample size, patients were divided into 2 groups, based on disease status: remission (CDAI <150 or Partial Mayo Score=0) and active (CDAI ≥150 or Partial Mayo Score >0). Because no significant differences were found between the patients with UC and CD in their scores for IBDQ, PHQ-9, GAD-7 or PSQI (Supplementary Table 1), they were eventually combined into a single IBD group.
STATISTICAL ANALYSIS:
All of the raw data were analyzed using SPSS. The demographic data and logistic regression were analyzed using SPSS, version 26.0. The comparison between groups was analyzed using Prism 8.0 and a
Results
PATIENT CHARACTERISTICS:
Approximately 100 IBD-Life Style questionnaires were hand-delivered to patients with IBD, of which 71 were completed at The Third Xiangya Hospital and The Hunan IBD Clinical Center. Table 1 lists the general and clinical information that was collected from participants. At the time of the study, the average age of the patients with IBD was 36 years (range, 14 to 69). Of them, 45 (63.4%) were men. Most of the patients (69.0%) had at least a high school education. Of the patients, 37.3% had an annual income of approximately ¥50 000; their residences were equally distributed between city and rural areas. Among the 71 patients, 25 (35.2%) had active IBD while 46 (64.8) had disease that was in remission at the time they completed the questionnaires. No significant difference was found between the patients with active IBD versus disease remission. As for the patients with IBD and the healthy controls, they shared most of the same features, except for education, income, and residence, which would not have affected the PSQI, IBDQ, PHQ-9, and GAD-7 scores, because there was no correlation between scores for the 4 scales and education, income, or place of residence (Supplementary Table 2 ).
PSQI, PHQ-9, AND GAD-7 SCORES:
As shown in Figure 1, no significant difference was found in the scores for the 3 indices between the group with IBD in remission and the healthy controls. However, the patients in the group with IBD had significantly higher scores on the 3 indices compared to the patients with disease in remission (P<0.05).
RECEIVER OPERATING CHARACTERISTIC CURVE FOR IBDQ, PHQ-9, AND PSQI:
To identify potential diagnostic markers for IBD relapse, we carried out a receiver operating characteristic analysis to determine values most predictive of clinically active disease. The area under the curve was 0.910 for the IBDQ score, 0.815 for the PHQ-9 score, 0.730 for the PSQI score, and 0.711 for the GAD-7 score. The analysis showed that a global IBDQ score <168.0 indicates active IBD with 84.8% sensitivity and 88.0% specificity, whereas a PSQI score >7.5 also indicates active IBD with 72.0% sensitivity and 82.6% specificity (Figure 2).
RELATIONSHIP BETWEEN IBD AND EMOTIONAL STATE, SLEEP, AND QUALITY OF LIFE:
In our study, 26.8% of patients with IBD (19/71) had poor sleeping habits (PSQI >10). We further divided the patients into groups who slept poorly and well and then compared their emotional states. We found that patients with poor sleep had higher PHQ-9 scores than the patients who slept well. This suggests that the patients with poor sleep were more depressed, and 45.5% of them were categorized as being depressed based on their PHQ-9 scores (Figure 3A). We then divided the patients into a group that was depressed group (PHQ-9 >4 points) and one that was not depressed (PHQ-9 ≤4 points) and compared their QoL. We observed a statistically significant difference in IBDQ scores in the depressed group versus the group that was not depressed (Figure 3B). QoL was better in patients with IBD who were not depressed than in those who were depressed.
Further correlation analysis (Figure 3C) revealed that IBDQ was significantly negatively correlated with PSQI, PHQ-9, and GAD-7 scores (P<0.05). PSQI was positively correlated with PHQ-9 and GAD-7 score (P<0.05) and PHQ-7 was positively correlated with GAD-7 scores (P<0.05). To visualize and investigate the relationship among the IBDQ, PHQ-9, and PSQI indices, we constructed a 3-dimensional (3D) dispersion plot, with PHQ-9 on the x-axis, IBDQ on the y-axis, and PSQI on the z-axis. Because GAD-7 was significantly related to PHQ-9, we did not include it in the 3D plot. Blue spots represent patients with active disease whereas red spots represent patients whose IBD was in remission. The groups with IBD in remission had worse QoL, poorer sleep quality, and were more depressed than the patients in the groups with active disease (Figure 3D).
DIET COMPOSITION:
The questionnaire also evaluated weekly patient of 22 different types of food, using a semiquantitative Likert scale, as previously described. The foods were divided into 7 groups (grains and beans; vegetables and fruit; proteins; eggs; milk and yogurt; energy food; and junk food) for easier analysis. Among the 22 foods, soybeans, potatoes, and grains are full of carbohydrates; therefore, we named them “grains and beans.” A diet high in carbohydrates is believed to have a positive effect on symptoms of IBD [23]. Vegetables and fruit were grouped because they are full of vitamins and fiber. While the association between different kinds of meat and IBD flare is unclear [24], we grouped pork, poultry, beef, lamb, seafood, and animal organs as proteins to assess the effect of high protein intake. Eggs were regarded as a single group because there is a positive association between eggs and cholesterol [25]. Milk and yogurt were grouped because research has shown that fermented milk can alleviate inflammatory responses [26]. Nuts contain 460 Kcal/100 g while chocolates contain 406 Kcal per 2 bars. In the scale, we used chocolate most often as an example to explain to patients what we meant by desserts. We regarded nuts and desserts as “energy food.” Finally, fried and fatty foods, fried fish, pickles, cured food, and barbecue were grouped as “junk foods.” The frequency of intake for each type of food was rated on a scale of 0 to 5 and was evaluated for the preceding several months or 1 year before disease relapse. We performed a clustering consistency analysis of the 7 food groups among the 71 patients. The consensus matrix showed that 2 kinds of clusters had a positive impact on IBD (Figure 4A). In the cumulative distribution function plot, the red line was almost horizontal between 0.2 and 0.8 (Figure 4B). Therefore, we divided the patients into 2 clusters and constructed a heat map, based on the frequency of intake of the 7 groups of food (Figure 4C). The heat map showed that the patients in cluster 2 mainly ate proteins, such as pork, poultry, and beef, and junk foods, such as fatty and fried food, whereas those in cluster 1 mainly ate energy foods (nuts and desserts). However, there was no difference in IBDQ scores between the 2 clusters. Because dietary intake is a stable variable, we evaluated whether it was associated with IBD relapse. We used a chi-square test to determine the relationship between diet and IBD disease status. The result was 0.164 chi-square points with P=0.688, which means that dietary intake in cluster 2 was not associated with IBD relapse.
DIETARY SPICE LEVELS:
The diet of the Chinese population, particularly in Hunan, the diet is mainly composed of spicy and salty food. We compared the frequency of consumption of spicy and salty food and the degree of spiciness of food eaten by the healthy controls and the patients with IBD before diagnosis (Table 2). Patients with IBD tended to favor spicier food than did the healthy controls (P<0.05). About 54.9% (39/71) of the patients with IBD had a diet that consisted largely of foods with a medium degree of spice (≥3 degrees vs 4 degrees for extremely spicy), whereas only 16.1% of the healthy controls (11/68) consistently ate food that spicy.
POTENTIAL RISK FACTORS FOR IBD:
In the questionnaire, we asked study participants about aspects of their childhoods before IBD diagnosis. Factors assessed included living with or sharing the same room with siblings; undergoing tonsillectomy, appendectomy, or cholecystectomy; being breastfed; having asthma, eczema, measles, whooping cough, rubella, chickenpox, mumps, or scarlet fever; exposure to antibiotics; home environment and sanitary conditions; exposure to sports; and preference for salty, spicy, or sweet foods. Logistic regression analysis (Table 3) showed that not having siblings was protective against IBD (OR 0.300, 95% CI 0.119–0.785), whereas not being breastfed (OR 2.753, 95% CI 1.025–7.396) and having a spicy diet were risk factors for IBD (OR 2.186, 95% CI 1.370–3.488).
Discussion
Based on the data collected using the IBD-Life Habits Questionnaire, we found that patients who were more depressed, anxious, or had poor sleep quality were more likely to have active IBD. An IBDQ score <168 and a PSQI score >7.5 were reliable tools for distinguishing patients with active IBD from those whose disease was in remission. However, there was no difference in PHQ-9, GAD-7, or PSQI scores between patients whose IBD was in remission and the healthy controls, which means the former group had lifestyles similar to those of the healthy controls. An analysis of participant diets revealed that patients with IBD preferred spicy food before they were diagnosed, whereas the healthy controls preferred milder food. We then divided the diet composition of the patients into 2 clusters, using clustering consistency analysis. The patients in cluster 2 consumed food that was mainly proteins and junk food, similar to a Western diet; however, there was no relationship between diet cluster and IBD activity. The results from our study also showed that not having siblings was protective against IBD, whereas eating spicy food and not being breastfed were risk factors for onset of IBD.
In our study, there was no difference in PHQ-9 and PSQI scores between the healthy controls and the patients whose IBD was in remission. Therefore, we compared the scores of patients with active IBD and whose disease was in remission. Although a previous study found that poor sleep quality was associated with active Crohn disease [27], the authors did not compare mental health, IBDQ, or PSQI scores between patients with IBD and those whose disease was in remission. In our study, we found that the degree of depression was associated with the quality of sleep; sleep quality, in turn, was associated with QoL in patients with IBD. Consequently, by using the 3 elements in the 3D plot, we were able to group patients with active IBD and whose disease was in remission. These data and the ROC analysis suggest that IBDQ and PSQI scores can be used as markers for disease relapse.
CDAI and endoscopic scores are widely used criterion standards for diagnosis of active IBD, but the former requires results of blood tests and endoscopy is invasive. Self-reported scales, in contrast, require no biochemical parameters to produce relatively reliable results.
Sleep disturbances among patients with active IBD may be due to diarrhea, abdominal pain, and other symptoms that interrupt their sleep patterns, and depression could lead to lack of sleep because it is associated with insomnia [28]. Regarding mental health, the cost of managing IBD in China may play a role in development of depression in patients with active IBD because only 39.4% of them earn more than ¥50 000 per year, making the disease financially burdensome. Loss of normal life and social functions or sleep disturbance caused by clinical symptoms such as nocturnal abdominal pain also could account for patient depression and anxiety. In the present study, patient QoL was influenced by both mental health and sleep conditions. The relationship between QoL, sleep, and mental health is complex. The data from our study provide new insights suggesting that patients with IBD might benefit from sleep aids and psychological support as part of a more comprehensive approach to therapy for IBD.
For our cluster analysis, we divided patients into 2 clusters, based on their intake of 7 groups of food. One cluster had a preference for a Western-like diet, which is mainly composed of proteins and food that contains saturated fats. The other cluster had a preference for a Mediterranean-like diet, which is mainly composed of fruits, vegetables, proteins, fish, and olives [29]. A Western diet is known to negatively affect the heart, kidneys, and immune system [30], whereas the immune system may be a key factor in IBD pathogenesis. In addition, high-carbohydrate meals have been found to induce expression of toll-like receptor-4 (TLR-4) [31], which is related to the onset of IBD [32]. Studies have shown that dietary patterns with potential to induce inflammation are associated with an increased risk of CD but not of UC. This is based on scores calculated according to the weighted sums of 18 food groups [33]. The impact of diet composition or patterns on relapse of IBD has not been studied in Chinese patients. Our analysis of retrospective data suggests certain dietary patterns in this population, but we did not observe any association between diet and IBD status in our study, and there was no difference in PHQ-9, GAD-7, or PSQI scores between the patients in the 2 diet clusters. Our results suggest that dietary patterns may not affect disease relapse but the stability of diet during certain periods may be important for IBD management.
Compared with the healthy controls, the patients with IBD preferred spicier foods before they were diagnosed with the diseases. Logistic regression analysis suggested that the spicy diet common in Hunan province may be a risk factor for IBD, which points to a need for vigilance in this geographic area regarding monitoring patient intake of food that is too spicy.
There are several limitations of the present study. First, responses to the questionnaire were based on participant recollection, which is subject to recall bias. Second, although all of the patients were receiving biologic therapy, duration and efficacy of the treatment differed among the participants. Those who had access to better biologic therapy would have better sleep, QoL, and emotional health. In a future study investigating the effect that sleep or diet have on IBD flare, clinical features of the cohort should be controlled. Third, the diet questionnaire was semiquantitative and focused on frequency of food intake rather than an accurate determination of the amount of each food that was eaten. As to the 7 groups of food, the food grouping here was based on literature reviews instead of exact energy or nutrition counts or groups. Fourth, data from endoscopic evaluations would be a better tool for determining disease remission. Fifth, measurement of sleep quality would have been better with use of electric sleep monitors; we are conducting a randomized controlled trial to obtain objective data on sleep. Sixth, the use of retrospective data to evaluate the relationship between sleep quality, QoL, emotional health, diet, and disease relapse is less reliable than following up on a cohort of patients with IBD.
Conclusions
In the present study, patients who had poor sleep quality, less QoL, and were more depressed and anxious were more likely to have relapsed IBD. As for dietary intake, neither a Mediterranean-like diet nor a Western diet was associated with IBD flare, whereas a spicy diet may be a risk factor for onset of IBD in China. In the future, management of patients with IBD should be multifaceted and include a focus on improving sleep and mood, but strict regulation and control of diet may not be necessary. Another cohort study is assessing whether a stable and nutritious diet is a better choice for patients in terms of managing their disease.
Figures
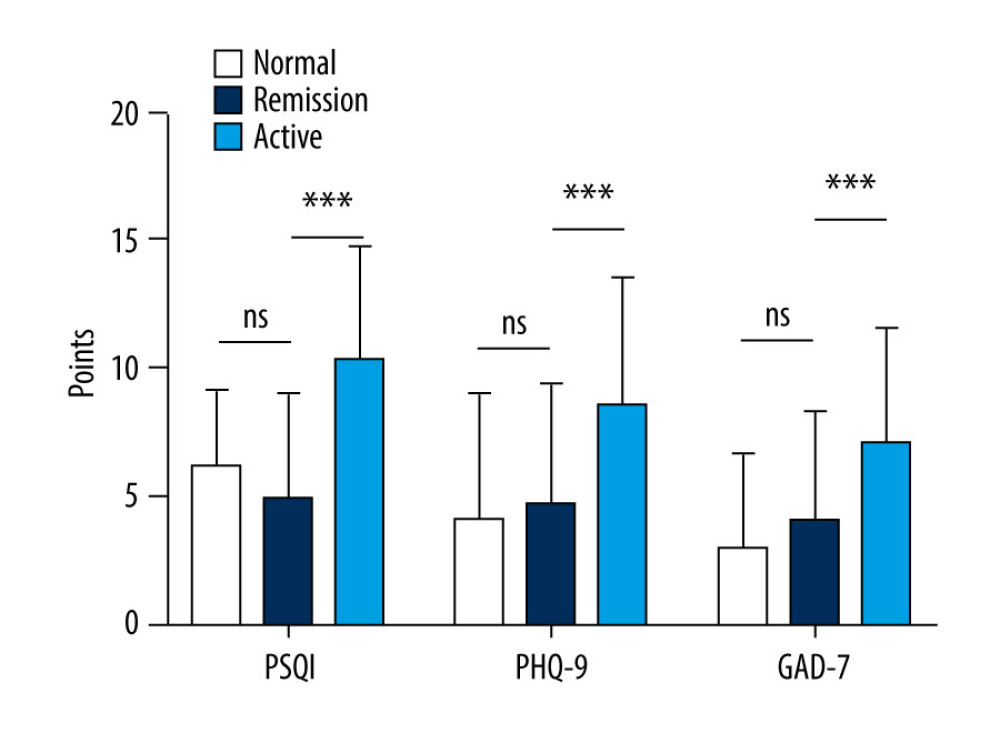 Figure 1. Comparison of Pittsburgh Sleep Quality Index (PSQI), Patient Health Questionnaire (PHQ-9), and Generalized Anxiety Disorder (GAD-7) scores in patients versus healthy controls. (PSQI: normal 6.20±2.95, remission 4.97±4.04, active 10.36±4.40; PHQ-9: normal 4.16±4.80, remission 4.72±4.71, active 8.56±5.03; GAD-7: normal 3.02±3.65, remission 4.07±4.22, active 7.12±4.42).
Figure 1. Comparison of Pittsburgh Sleep Quality Index (PSQI), Patient Health Questionnaire (PHQ-9), and Generalized Anxiety Disorder (GAD-7) scores in patients versus healthy controls. (PSQI: normal 6.20±2.95, remission 4.97±4.04, active 10.36±4.40; PHQ-9: normal 4.16±4.80, remission 4.72±4.71, active 8.56±5.03; GAD-7: normal 3.02±3.65, remission 4.07±4.22, active 7.12±4.42). ![Status of inflammatory bowel disease in patients determined based on receiver operating characteristic curves for their Inflammatory Bowel Disease Questionnaire (IBDQ), Pittsburgh Sleep Quality Index (PSQI), Patient Health Questionnaire (PHQ-9), and Generalized Anxiety Disorder scores. (IBDQ: area under the curve [AUC] 0.91; 84.8% sensitivity and 88.0% specificity; PSQI: AUC 0.815; 72.0% sensitivity and 82.6% specificity).](https://jours.isi-science.com/imageXml.php?i=medscimonit-27-e930511-g002.jpg&idArt=930511&w=1000) Figure 2. Status of inflammatory bowel disease in patients determined based on receiver operating characteristic curves for their Inflammatory Bowel Disease Questionnaire (IBDQ), Pittsburgh Sleep Quality Index (PSQI), Patient Health Questionnaire (PHQ-9), and Generalized Anxiety Disorder scores. (IBDQ: area under the curve [AUC] 0.91; 84.8% sensitivity and 88.0% specificity; PSQI: AUC 0.815; 72.0% sensitivity and 82.6% specificity).
Figure 2. Status of inflammatory bowel disease in patients determined based on receiver operating characteristic curves for their Inflammatory Bowel Disease Questionnaire (IBDQ), Pittsburgh Sleep Quality Index (PSQI), Patient Health Questionnaire (PHQ-9), and Generalized Anxiety Disorder scores. (IBDQ: area under the curve [AUC] 0.91; 84.8% sensitivity and 88.0% specificity; PSQI: AUC 0.815; 72.0% sensitivity and 82.6% specificity). 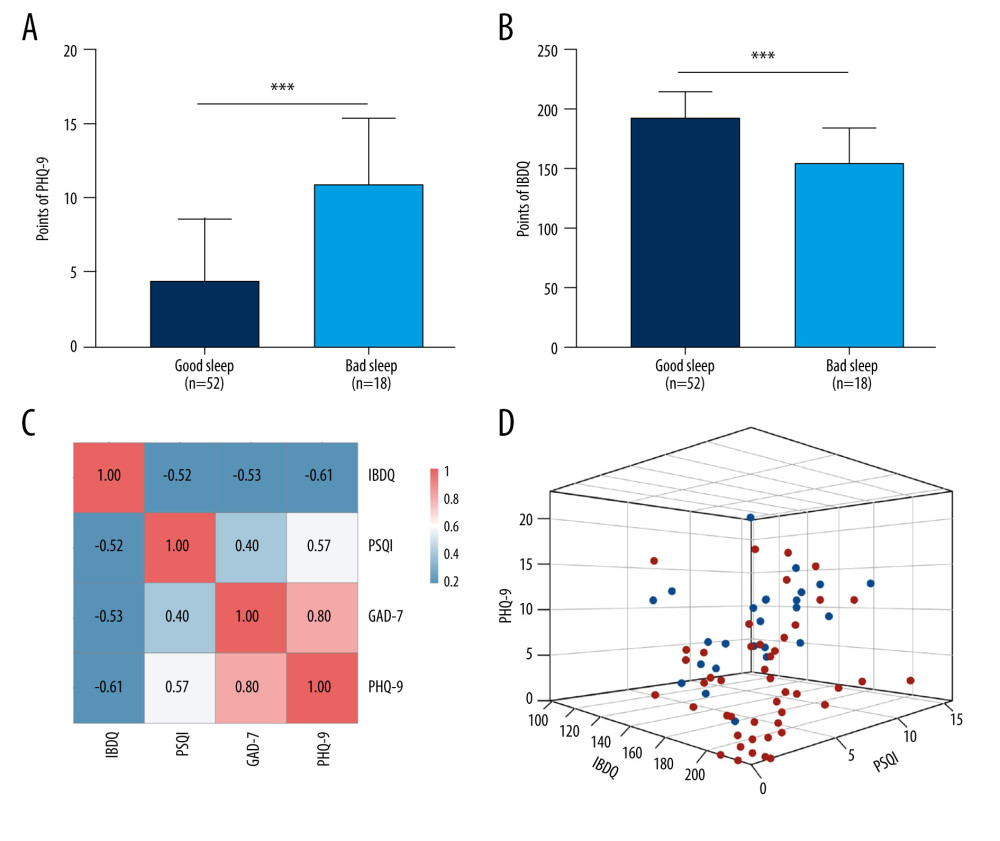 Figure 3. Relationship between sleep, emotional state, and quality of life and inflammatory bowel disease. (A) Comparison of Patient Health Questionnaire scores between patients with good and poor sleep quality (4.33±4.19 versus 10.84±4.50; P<0.0001). (B) Comparison of Inflammatory Bowel Disease Questionnaire scores between patients with and without depression (153.94±28.95 versus 184.53±24.74). (C) Heat map of the correlation coefficients between Inflammatory Bowel Disease Questionnaire (IBDQ), Pittsburgh Sleep Quality Index (PSQI), Patient Health Questionnaire (PHQ-9) and Generalized Anxiety Disorder-7 (for each one coefficient: P<0.05). (D) Three-dimensional dispersion plot of patients with active disease (blue spots) and those in remission (red spots), based on PSQI, IBDQ, and PHQ-9 scores.
Figure 3. Relationship between sleep, emotional state, and quality of life and inflammatory bowel disease. (A) Comparison of Patient Health Questionnaire scores between patients with good and poor sleep quality (4.33±4.19 versus 10.84±4.50; P<0.0001). (B) Comparison of Inflammatory Bowel Disease Questionnaire scores between patients with and without depression (153.94±28.95 versus 184.53±24.74). (C) Heat map of the correlation coefficients between Inflammatory Bowel Disease Questionnaire (IBDQ), Pittsburgh Sleep Quality Index (PSQI), Patient Health Questionnaire (PHQ-9) and Generalized Anxiety Disorder-7 (for each one coefficient: P<0.05). (D) Three-dimensional dispersion plot of patients with active disease (blue spots) and those in remission (red spots), based on PSQI, IBDQ, and PHQ-9 scores. 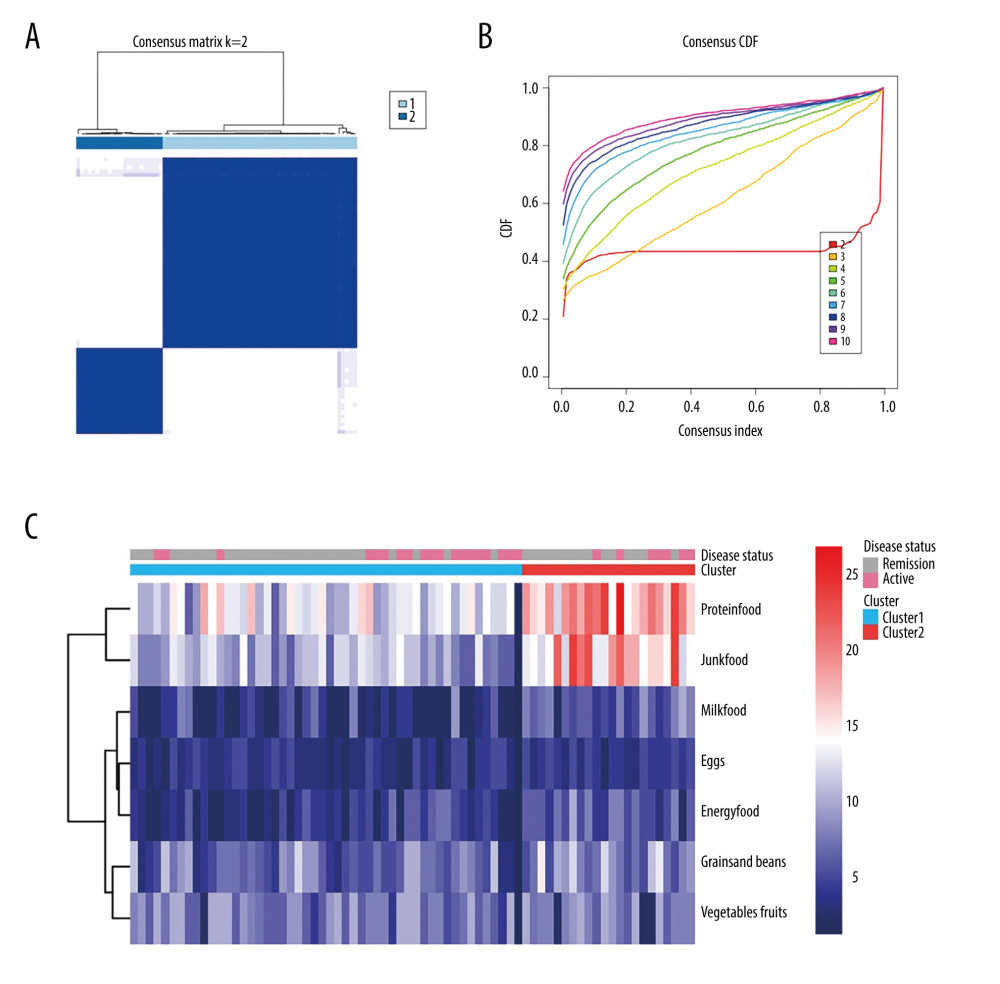 Figure 4. Consensus cluster based on patient preference for 7 groups of food. (A) Consensus matrix of clustering. The blue square indicates that the result was best when the cluster equaled 2. (B) Cumulative distribution function curve. The red line between 0.2 and 0.8 that is the closest to being horizontal is the consensus index and indicates the best cluster design. (C) Heat map of preferences for 7 groups of food among 71 patients, with individual disease status listed at the top. The cluster results showed that diet composition was similar in clusters 1 and 2.
Figure 4. Consensus cluster based on patient preference for 7 groups of food. (A) Consensus matrix of clustering. The blue square indicates that the result was best when the cluster equaled 2. (B) Cumulative distribution function curve. The red line between 0.2 and 0.8 that is the closest to being horizontal is the consensus index and indicates the best cluster design. (C) Heat map of preferences for 7 groups of food among 71 patients, with individual disease status listed at the top. The cluster results showed that diet composition was similar in clusters 1 and 2. Tables
Table 1. Baseline characteristics of the study population.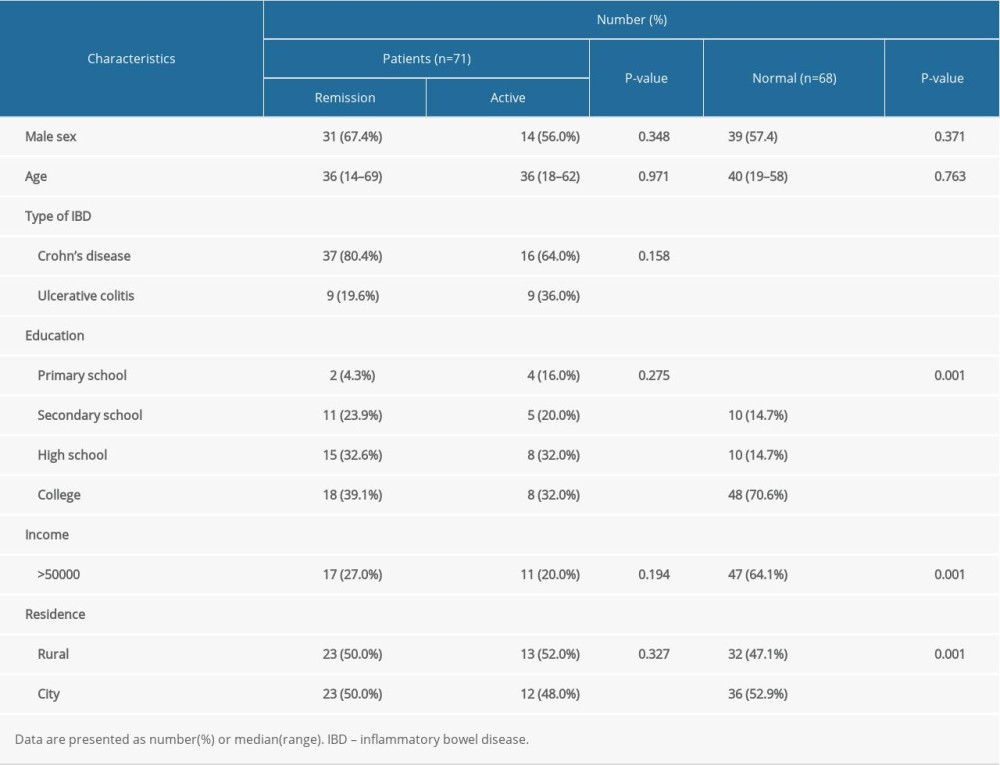 Table 2. Comparison of intake of spicy foods in healthy subjects versus patients with inflammatory bowel disease (before diagnosis).
Table 2. Comparison of intake of spicy foods in healthy subjects versus patients with inflammatory bowel disease (before diagnosis).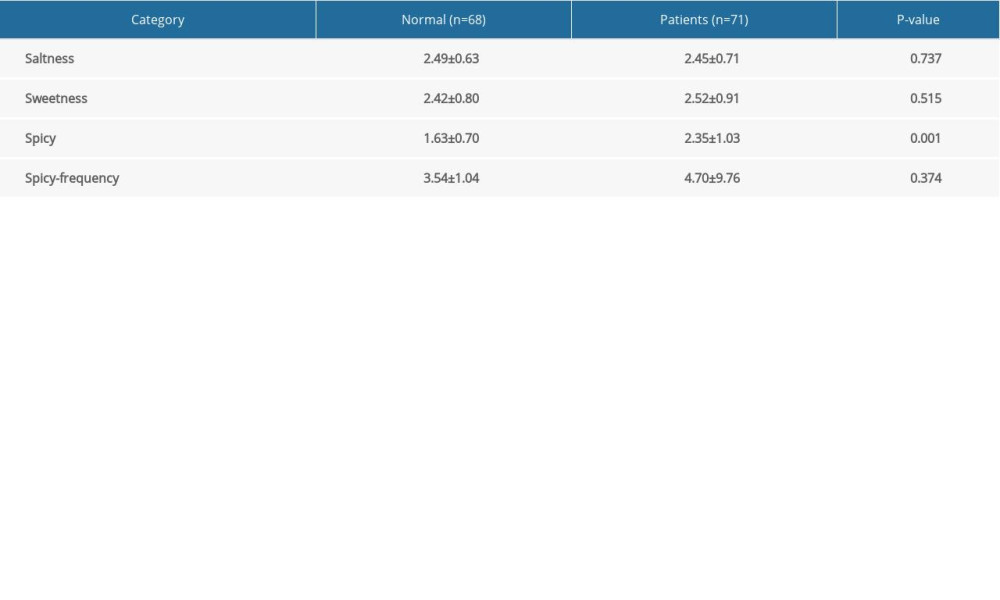 Table 3. Logistic regression analysis of factors related to the onset of inflammatory bowel disease.
Table 3. Logistic regression analysis of factors related to the onset of inflammatory bowel disease.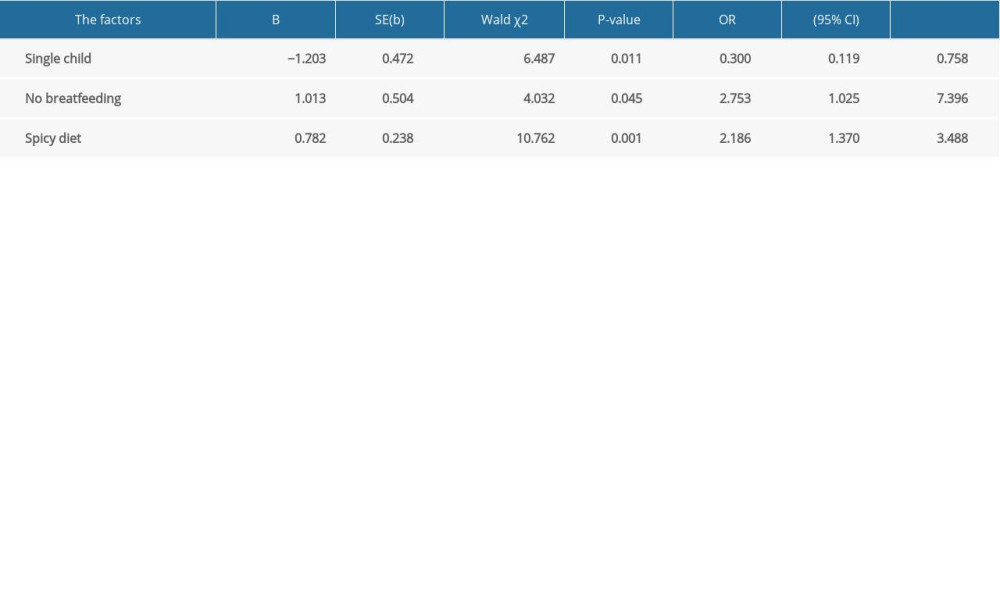 Supplementary Table 1. Comparison between Crohn disease and ulcerative colitis.
Supplementary Table 1. Comparison between Crohn disease and ulcerative colitis.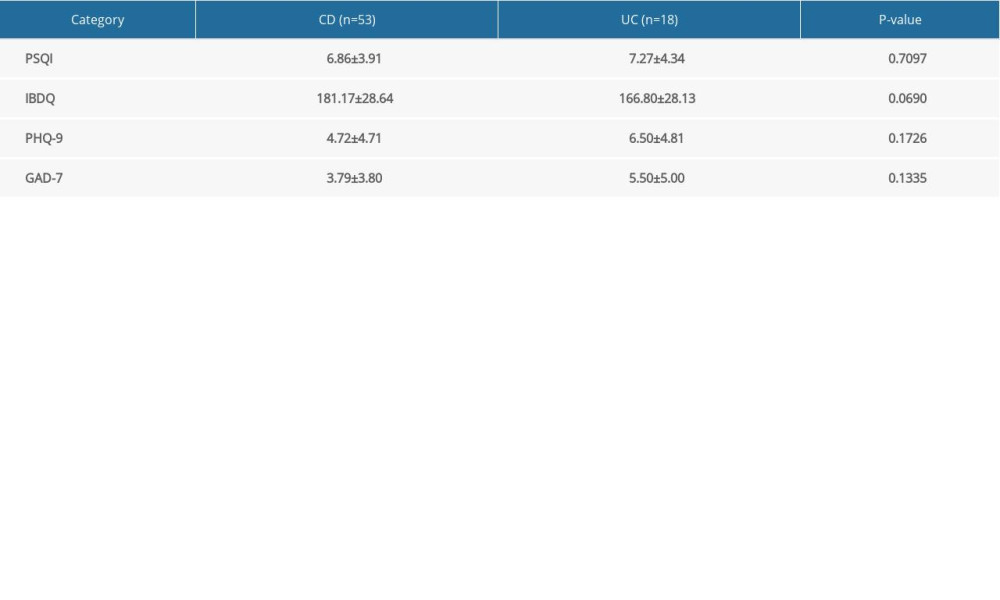 Supplementary Table 2. Spearman rank correlation of clinical and demographic data.
Supplementary Table 2. Spearman rank correlation of clinical and demographic data.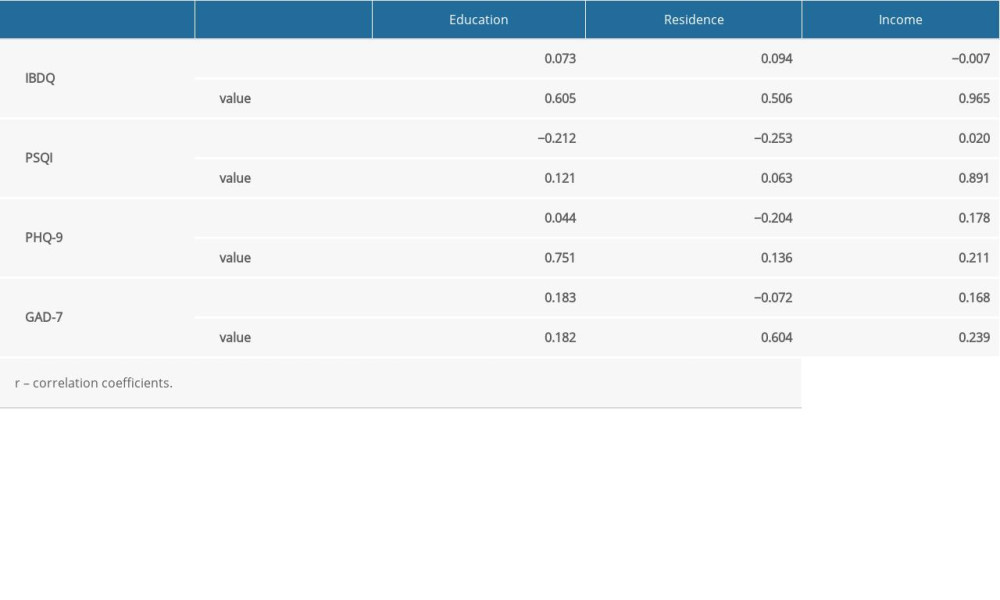
References
1. Ng SC, Shi HY, Hamidi N: Lancet, 2017; 390(10114); 2769-78
2. Cui G, Yuan A, Systematic review of epidemiology and risk factors associated with chinese inflammatory bowel disease: Front Med (Lausanne), 2018; 5; 183
3. Satsangi J, Silverberg MS, Vermeire S, Colombel J-F, The Montreal classification of inflammatory bowel disease: Controversies, consensus, and implications: Gut, 2006; 55(6); 749-53
4. Alrubaiy L, Rikaby I, Dodds P, Systematic review of health-related quality of life measures for inflammatory bowel disease: J Crohns Colitis, 2015; 9(3); 284-92
5. Sobolewska-Włodarczyk A, Wlodarczyk M, Sleep disturbance and disease activity in adult patients with inflammatory bowel diseases: J Physiol Pharmacol, 2018; 69(3); jpp.2018.3.09
6. Sobolewska-Włodarczyk A, Wlodarczyk M, Banasik J, Circadian rhythm abnormalities in patients with inflammatory bowel disease – association with adipokine profile: Scand J Gastroenterol, 2020; 55(3); 294-300
7. Qazi T, Farraye FA, Sleep and inflammatory bowel disease: An important bi-directional relationship: Inflamm Bowel Dis, 2019; 25(5); 843-52
8. Zimmerman J, Extraintestinal symptoms in irritable bowel syndrome and inflammatory bowel diseases: Nature, severity, and relationship to gastrointestinal symptoms: Dig Dis Sci, 2003; 48(4); 743-49
9. Ranjbaran Z, Keefer L, Farhadi A, Impact of sleep disturbances in inflammatory bowel disease: J Gastroenterol Hepatol, 2007; 22(11); 1748-53
10. Wilson RG, Stevens BW, Guo AY, High C-reactive protein is associated with poor sleep quality independent of nocturnal symptoms in patients with inflammatory bowel disease: Dig Dis Sci, 2015; 60(7); 2136-43
11. Neuendorf R, Harding A, Stello N, Depression and anxiety in patients with inflammatory bowel disease: A systematic review: J Psychosom Res, 2016; 87; 70-80
12. Skonieczna-Żydecka K, Marlicz W, Misera A, Microbiome – the missing link in the gut-brain axis: Focus on its role in gastrointestinal and mental health: J Clin Med, 2018; 7(12); 521
13. Bernstein CN, Sngh S, Graff LA, A prospective population-based study of triggers of symptomatic flares in IBD: Am J Gastroenterol, 2010; 105(9); 1994-2002
14. David LA, Maurice CF, Carmody RN, Diet rapidly and reproducibly alters the human gut microbiome: Nature, 2014; 505(7484); 559-63
15. Glassner KL, Abraham BP, Quigley EMM, The microbiome and inflammatory bowel disease: J Allergy Clin Immunol, 2020; 145(1); 16-27
16. Murray K, Wilkinson-Smith V, Hoad C, Differential effects of FODMAPs (fermentable oligo-, di-, mono-saccharides and polyols) on small and large intestinal contents in healthy subjects shown by MRI: Am J Gastroenterol, 2014; 109(1); 110-19
17. Rai SK, Fung TT, Lu N, The Dietary Approaches to Stop Hypertension (DASH) diet, Western diet, and risk of gout in men: prospective cohort study: BMJ, 2017; 357; j1794
18. De Filippo C, Di Paola M, Ramazzotti M, Diet, environments, and gut microbiota. A preliminary investigation in children living in rural and urban Burkina Faso and Italy: Front Microbiol, 2017; 8; 1979
19. Levine A, Rhodes JM, Lindsay JO, Dietary guidance from the International Organization for the Study of Inflammatory Bowel Diseases: Clin Gastroenterol Hepatol, 2020; 18(6); 1381-92
20. Turner D, Ricciuto A, Lewis A, STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining therapeutic goals for treat-to-target strategies in IBD: Gastroenterology, 2021; 160(5); 1570-83
21. Sandborn WJ, Feagan BG, Hanauer ST, A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn’s disease: Gastroenterology, 2002; 122(2); 512-30
22. Sturm A, Maaser C, Calabrese E, ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 2: IBD scores and general principles and technical aspects: J Crohns Colitis, 2019; 13(3); 273-84
23. Suskind DL, Lee D, Kim Y-M, The Specific Carbohydrate Diet and diet modification as induction therapy for pediatric Crohn’s Disease: A randomized diet controlled trial: Nutrients, 2020; 12(12); 3749
24. Albenberg L, Brensinger CM, Wu Q, A diet low in red and processed meat does not reduce rate of Crohn’s disease flares: Gastroenterology, 2019; 157(1); 128-136.e5
25. Sugano M, Matsuoka R, Nutritional viewpoints on eggs and cholesterol: Foods, 2021; 10(3); 494
26. Zhang X, Ton Y, Lyu X, Prevention and alleviation of dextran sulfate sodium salt-induced inflammatory bowel disease in mice with bacillus subtilis-fermented milk via inhibition of the inflammatory responses and regulation of the intestinal flora: Front Microbiol, 2020; 11; 622354
27. Gingold-Belfer R, Peled N, Levy S, Impaired sleep quality in Crohn’s disease depends on disease activity: Dig Dis Sci, 2014; 59(1); 146-51
28. Tsuno N, Besset A, Ritchie K, Sleep and depression: J Clin Psychiatry, 2005; 66(10); 1254-69
29. Voelker R, The Mediterranean diet’s fight against frailty: JAMA, 2018; 319(19); 1971-72
30. Myles IA, Fast food fever: Reviewing the impacts of the Western diet on immunity: Nutr J, 2014; 13; 61
31. Deopurkar R, Ghanim H, Friedman J, Differential effects of cream, glucose, and orange juice on inflammation, endotoxin, and the expression of Toll-like receptor-4 and suppressor of cytokine signaling-3: Diabetes Care, 2010; 33(5); 991-97
32. Lu Y, Li X, Liu S, Toll-like receptors and inflammatory bowel disease: Front Immunol, 2018; 9; 72
33. Lo CH, Lochhead P, Khalili H, Dietary inflammatory potential and risk of Crohn’s disease and ulcerative colitis: Gastroenterology, 2020; 159(3); 873-883.e1
Figures
 Figure 1. Comparison of Pittsburgh Sleep Quality Index (PSQI), Patient Health Questionnaire (PHQ-9), and Generalized Anxiety Disorder (GAD-7) scores in patients versus healthy controls. (PSQI: normal 6.20±2.95, remission 4.97±4.04, active 10.36±4.40; PHQ-9: normal 4.16±4.80, remission 4.72±4.71, active 8.56±5.03; GAD-7: normal 3.02±3.65, remission 4.07±4.22, active 7.12±4.42).
Figure 1. Comparison of Pittsburgh Sleep Quality Index (PSQI), Patient Health Questionnaire (PHQ-9), and Generalized Anxiety Disorder (GAD-7) scores in patients versus healthy controls. (PSQI: normal 6.20±2.95, remission 4.97±4.04, active 10.36±4.40; PHQ-9: normal 4.16±4.80, remission 4.72±4.71, active 8.56±5.03; GAD-7: normal 3.02±3.65, remission 4.07±4.22, active 7.12±4.42).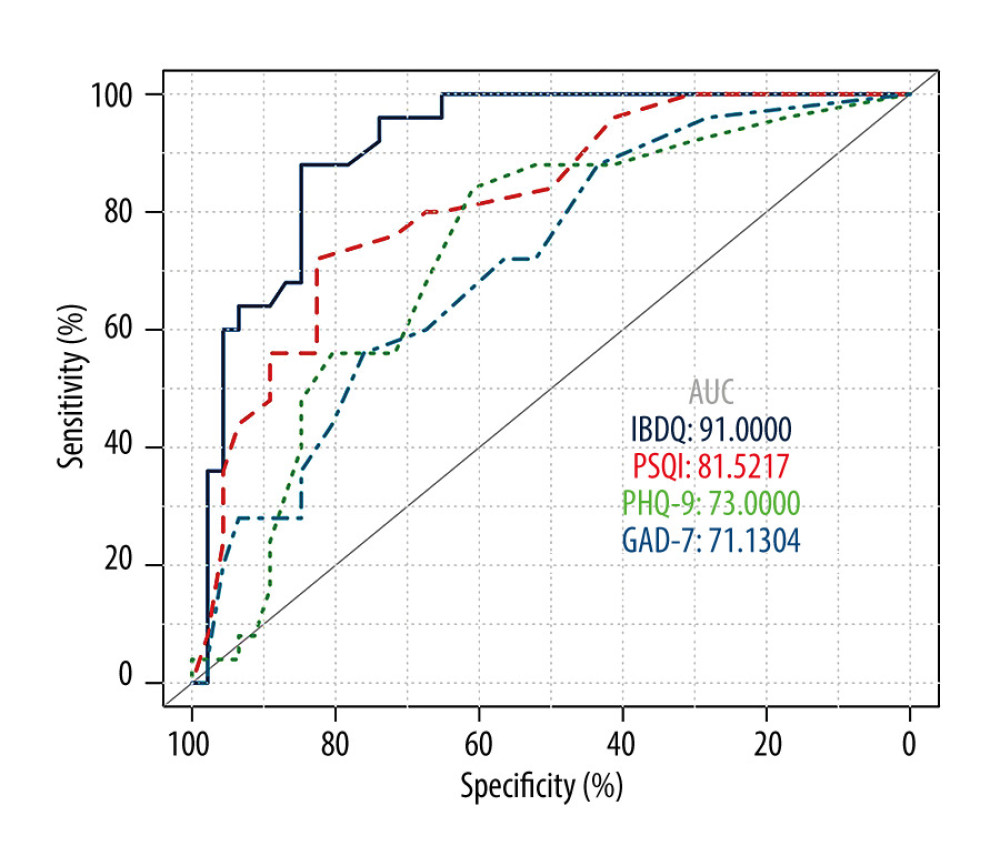 Figure 2. Status of inflammatory bowel disease in patients determined based on receiver operating characteristic curves for their Inflammatory Bowel Disease Questionnaire (IBDQ), Pittsburgh Sleep Quality Index (PSQI), Patient Health Questionnaire (PHQ-9), and Generalized Anxiety Disorder scores. (IBDQ: area under the curve [AUC] 0.91; 84.8% sensitivity and 88.0% specificity; PSQI: AUC 0.815; 72.0% sensitivity and 82.6% specificity).
Figure 2. Status of inflammatory bowel disease in patients determined based on receiver operating characteristic curves for their Inflammatory Bowel Disease Questionnaire (IBDQ), Pittsburgh Sleep Quality Index (PSQI), Patient Health Questionnaire (PHQ-9), and Generalized Anxiety Disorder scores. (IBDQ: area under the curve [AUC] 0.91; 84.8% sensitivity and 88.0% specificity; PSQI: AUC 0.815; 72.0% sensitivity and 82.6% specificity). Figure 3. Relationship between sleep, emotional state, and quality of life and inflammatory bowel disease. (A) Comparison of Patient Health Questionnaire scores between patients with good and poor sleep quality (4.33±4.19 versus 10.84±4.50; P<0.0001). (B) Comparison of Inflammatory Bowel Disease Questionnaire scores between patients with and without depression (153.94±28.95 versus 184.53±24.74). (C) Heat map of the correlation coefficients between Inflammatory Bowel Disease Questionnaire (IBDQ), Pittsburgh Sleep Quality Index (PSQI), Patient Health Questionnaire (PHQ-9) and Generalized Anxiety Disorder-7 (for each one coefficient: P<0.05). (D) Three-dimensional dispersion plot of patients with active disease (blue spots) and those in remission (red spots), based on PSQI, IBDQ, and PHQ-9 scores.
Figure 3. Relationship between sleep, emotional state, and quality of life and inflammatory bowel disease. (A) Comparison of Patient Health Questionnaire scores between patients with good and poor sleep quality (4.33±4.19 versus 10.84±4.50; P<0.0001). (B) Comparison of Inflammatory Bowel Disease Questionnaire scores between patients with and without depression (153.94±28.95 versus 184.53±24.74). (C) Heat map of the correlation coefficients between Inflammatory Bowel Disease Questionnaire (IBDQ), Pittsburgh Sleep Quality Index (PSQI), Patient Health Questionnaire (PHQ-9) and Generalized Anxiety Disorder-7 (for each one coefficient: P<0.05). (D) Three-dimensional dispersion plot of patients with active disease (blue spots) and those in remission (red spots), based on PSQI, IBDQ, and PHQ-9 scores. Figure 4. Consensus cluster based on patient preference for 7 groups of food. (A) Consensus matrix of clustering. The blue square indicates that the result was best when the cluster equaled 2. (B) Cumulative distribution function curve. The red line between 0.2 and 0.8 that is the closest to being horizontal is the consensus index and indicates the best cluster design. (C) Heat map of preferences for 7 groups of food among 71 patients, with individual disease status listed at the top. The cluster results showed that diet composition was similar in clusters 1 and 2.
Figure 4. Consensus cluster based on patient preference for 7 groups of food. (A) Consensus matrix of clustering. The blue square indicates that the result was best when the cluster equaled 2. (B) Cumulative distribution function curve. The red line between 0.2 and 0.8 that is the closest to being horizontal is the consensus index and indicates the best cluster design. (C) Heat map of preferences for 7 groups of food among 71 patients, with individual disease status listed at the top. The cluster results showed that diet composition was similar in clusters 1 and 2. Tables
 Table 1. Baseline characteristics of the study population.
Table 1. Baseline characteristics of the study population. Table 2. Comparison of intake of spicy foods in healthy subjects versus patients with inflammatory bowel disease (before diagnosis).
Table 2. Comparison of intake of spicy foods in healthy subjects versus patients with inflammatory bowel disease (before diagnosis). Table 3. Logistic regression analysis of factors related to the onset of inflammatory bowel disease.
Table 3. Logistic regression analysis of factors related to the onset of inflammatory bowel disease. Table 1. Baseline characteristics of the study population.
Table 1. Baseline characteristics of the study population. Table 2. Comparison of intake of spicy foods in healthy subjects versus patients with inflammatory bowel disease (before diagnosis).
Table 2. Comparison of intake of spicy foods in healthy subjects versus patients with inflammatory bowel disease (before diagnosis). Table 3. Logistic regression analysis of factors related to the onset of inflammatory bowel disease.
Table 3. Logistic regression analysis of factors related to the onset of inflammatory bowel disease. Supplementary Table 1. Comparison between Crohn disease and ulcerative colitis.
Supplementary Table 1. Comparison between Crohn disease and ulcerative colitis. Supplementary Table 2. Spearman rank correlation of clinical and demographic data.
Supplementary Table 2. Spearman rank correlation of clinical and demographic data. In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








