26 April 2021: Clinical Research
Electron-Dense Deposition Patterns and the Outcomes of Nephrotic Idiopathic Membranous Nephropathy Treated with Tacrolimus in Chinese Adults
Xin Xiang1ABCDEF, Zhenwei Feng2ABCDEF, Qifeng Jiang3BDE, Diansheng Huang4BCDE, Zhandong Meng2BCE, Zuojie Luo1ABCEF*DOI: 10.12659/MSM.930500
Med Sci Monit 2021; 27:e930500
Abstract
BACKGROUND: Tacrolimus may be effective in the short-term treatment of idiopathic membranous nephropathy (IMN). However, it is not clear whether an electron microscopic classification of the homogeneous and heterogeneous types of nephrotic IMN is related to the efficacy of tacrolimus in patients with IMN. This study aimed to explore this question and to provide evidence for individualized patient treatment.
MATERIAL AND METHODS: This 6-month retrospective study included 61 Chinese patients previously diagnosed with IMN. Patients received treatment was tacrolimus plus glucocorticoid. The patients were divided into a homogeneous group and a heterogeneous group based on the evaluation of electron-dense deposits. The initial clinicopathologic factors in the 2 groups were analyzed, and the difference in efficacy of tacrolimus in the 2 groups was assessed. The factors predicting remission were also studied.
RESULTS: No significant alteration in the initial clinicopathologic status was found between the 2 groups, except for proteinuria, serum albumin levels, systolic blood pressure, and renal biopsy results (stages I/II/III/IV). After 3 months of treatment, the difference in remission was not significant between the 2 groups. However, after 6 months of treatment, a significant difference in remission rates was observed between the 2 groups. The binary logistic model showed that the homogeneous nephrotic IMN was independently associated with total remission (partial plus complete remission), and was also related to complete remission.
CONCLUSIONS: The results of our study revealed that the homogeneous type of nephrotic IMN had a higher short-term remission rate and a predictive value for partial or complete remission, and it might be a meaningful marker of the short-term response to tacrolimus.
Keywords: Glomerulonephritis, Membranous, Outcome Assessment (Health Care), Tacrolimus, Immunosuppressive Agents, Microscopy, Electron, Nephrons, Precision Medicine
Background
Idiopathic membranous nephropathy (IMN) is the most commonly prevailing cause of nephrotic syndrome in middle-aged and elderly people [1]. However, in recent years, the incidence of IMN has tended to occur in a younger population. Clinically, IMN is characterized by the thickening of the glomerular capillary basement membrane and the depositing of immune complexes in the subepithelial layer. Currently, its pathogenesis remains undiscovered, and its complete cure is difficult to achieve. An increased amount of attention has been paid to the role of M-type anti-PLA2R in the pathogenesis and patient response to therapy of IMN [2–6].
Previous studies have confirmed that glucocorticoid therapy alone is ineffective for treating IMN. Therefore, immunosuppressive agents in combination with glucocorticoid therapy are required. The development of IMN has been found to be diverse; therefore, the Kidney Disease Improving Global Outcomes guidelines [7] and Chinese Nephrotic Syndrome Work Group guidelines [8] currently recommend glucocorticoid and immunosuppressive therapy only for “high-risk” patients, which includes patients with severe nephrotic syndrome, serum creatinine significantly increased within 1 year of diagnosis, and hypotension and RAS inhibitor with urinary protein exceeding 4 g/d for more than 6 months. Several clinical studies [9–12] have shown that tacrolimus has a high level of safety and efficacy in the treatment of IMN and can be used as the first-choice alternative treatment with cyclophosphamide.
However, these guidelines lack renal pathology assessment indicators to aid in choosing treatment regimens. Many previous studies have highlighted the prognostic value of results of tubulointerstitial changes (fibrosis and cellular infiltration) [13–15], glomerular alterations with focal segmental sclerosis [16], and progressive histologic stage of electron-dense deposits [17], which are III to IV, according to the criteria of Ehrenreich et al [18]. Rosen et al [19] reported the division of subepithelial electron-dense deposits into 4 groups based on clinical and morphological configurations as short with 1 generation of deposits, short/repeated, long/rapid, and long/slow. Yokoyama et al [20] simplified this classification by combining the homogenous type with 1 generation of deposits, short/repeated, and long/rapid. They also designated the long/slow cases based on synchronicity and clinical course as the heterogeneous type. The authors’ further study indicated that the classification of electron microscope findings was a beneficial marker of the outcomes in membranous nephropathy among their Japanese patients.
The results of studies in Chinese patients with IMN showed that tacrolimus was effective in short-term treatment [9–11]. In addition to clinical factors, it is still unclear whether an electron microscopic classification under the electron microscope is related to the efficacy of tacrolimus combined with glucocorticoid therapy. Therefore, we retrospectively analyzed 61 patients with nephrotic IMN, confirmed by renal biopsy. We analyzed the difference in remission rates between patients with homogenous and heterogeneous types of IMN within 6 months of treatment to provide evidence for individualized IMN treatment, according to electron microscopic classification.
Material and Methods
STUDY POPULATION:
This retrospective study included patient data from January 2018 to October 2020. All eligible patients from the Guangxi Minzu Hospital, the Affiliated Minzu Hospital of Guangxi Medical University were screened according to the inclusion and exclusion criteria. Inclusion criteria were (1) patients over 18 years old; (2) symptoms of nephrotic syndrome: 24-h urine protein >3.5 g/d, serum albumin <30 g/L, edema, and/or hyperlipidemia; (3) IMN confirmed by renal biopsy; (4) initial serum creatinine levels <133 μmol/L; (5) antiproteinuric therapy with angiotensin-converting enzyme inhibitor (ACEI)/angiotensin receptor blocker (ARB) at least 3 months before the study, with no spontaneous remission or no response to treatment, and no previous treatment with tacrolimus and glucocorticoid; and (6) high-risk patients treated directly with tacrolimus and glucocorticoid during the study period. Exclusion criteria were (1) current active infection or autoimmune disorder; (2) serum creatinine >133 μmol/L; (3) diabetes mellitus, tumors, hepatic function test irregularities, or active peptic ulcer illness; and (4) secondary membranous nephropathy, such as lupus nephritis or membranous nephropathy related to malignancy or hepatitis B virus.
Because of limited capabilities at our hospital, our renal biopsy pathological specimens have been sent to the Guangzhou Huayin Medical Laboratory Center for electron microscope examination of renal pathology since January 2018. In total, we retrospectively enrolled 61 local Chinese patients, including 23 women and 38 men, with an age range of 18 to 74 years. Written informed consent was obtained from all recruited patients before their renal biopsies were conducted and tacrolimus with glucocorticoid therapy began. The study was approved by the Medical Ethics Committee of Guangxi Minzu Hospital (IRB approval No. [2020]18).
TREATMENT REGIMENS:
During the study, ACEIs or ARBs were administered to a few patients for blood pressure control. All of the patients received anticoagulant drugs and statins.
An initial dosage of tacrolimus of 2 mg daily (body weight ≤60 kg) or 3 mg daily (body weight >60 kg) was administered to the patients. The given dosage of tacrolimus was adjusted based on measured serum values. During the first month of therapy, the patients visited the doctor every 2 weeks, and their tacrolimus dosage was adjusted based on serum concentration (5–10 ng/mL). A 0.5 mg/kg per day dosage of prednisone was also administered to the patients. The dosage of tacrolimus was reduced gradually but was administered longer than prednisone.
ELECTRON-DENSE DEPOSITION TYPES:
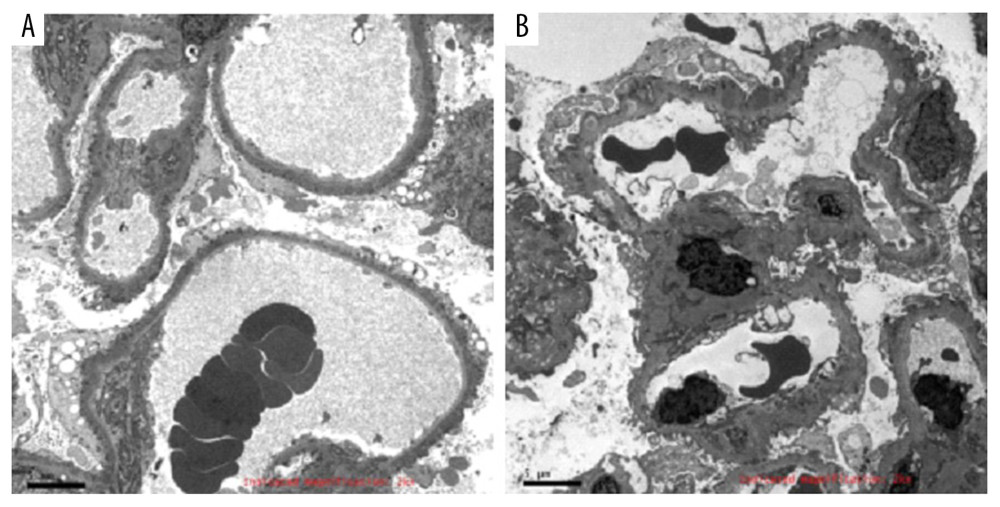
During the study, 2 pathologists examined the renal tissue samples to make a diagnosis using standard pathological procedures. Patient identity and disease status were not disclosed to the pathologists to ensure an independent diagnosis. The classification of patients into homogenous and heterogeneous groups was based on the criteria established by Yokoyama, et al [20]. The samples with synchronous electron-dense deposits with a single-stage were classified as the homogeneous type (44 patients) and samples with numerous stages of electron-dense deposits were classified as the heterogeneous type (17 patients) (Figure 1).
CLINICAL DATA:
The clinical data were collected before tacrolimus treatment and after 3 and 6 months of tacrolimus treatment. Levels of the 24-h urinary protein, serum creatinine, serum albumin, and serum cholesterol levels, and systolic and diastolic blood pressure were collected before tacrolimus treatment. Similarly, the 24-h urinary protein, serum creatinine, and serum albumin levels were collected after 3 and 6 months of tacrolimus treatment.
OUTCOME PARAMETERS:
The end of the study was divided into 3 categories based on curative effects: complete remission, partial remission, or no remission. A daily proteinuria level of less than 0.3 g and a normal serum creatinine concentration was classified as complete remission. Partial remission was defined as a decrease of at least 50% in daily proteinuria, with a serum albumin concentration of at least 30 g/L and stable renal function. No remission was defined as a decrease in daily proteinuria of less than 50% and/or greater than 3.5 g per day of urinary protein. The efficacy of tacrolimus was evaluated at 3 months and 6 months of follow-up.
STATISTICAL ANALYSIS:
The acquired data were evaluated using SPSS 16.0 software. The data were presented as mean±standard deviation, and an independent
Results
PATIENTS AND BASELINE FEATURES AT THE START OF TACROLIMUS TREATMENT:
Table 1 shows the baseline clinicopathological features of the 61 patients included in the study. Nephrotic syndrome was exhibited by all patients at the start of their tacrolimus therapy. The early clinicopathological states were similar in the homogeneous and heterogeneous groups: age (52.20 years vs 51.59 years); male/female ratio (30/14 vs 8/9); serum creatinine (81.82 μmol/L vs 85.88 μmol/L); diastolic blood pressure (83.98 mmHg vs 90.12 mmHg); cholesterol (8.38 mmol/L vs 9.67 mmol/L); and ACEI/ARB used (26 cases vs 10 cases). The following variables differed between the homogeneous and heterogeneous groups: proteinuria (4.56 g/24-h vs 5.06 g/24-h; P=0.042); serum albumin (24.71 g/L vs 22.01 g/L; P=0.014); systolic blood pressure (137.16 mmHg vs 147.94 mmHg; P=0.037); basement membrane thickness (986.36 nm vs 1198.8 nm; P=0.005); and renal biopsy (I/II/III/IV; P =0.000).
EFFECT OF TACROLIMUS ON NEPHROTIC IMN:
The 61 patients diagnosed with nephrotic IMN were observed for 6 months after the start of therapy (Table 2). At the 3-month follow-up in the homogeneous group, 1 patient achieved clinical complete remission, 17 patients showed partial remission, and 26 patients showed no remission. At the 3-month follow-up in the heterogeneous group, no patients exhibited complete remission, 4 patients showed partial remission, and 13 patients showed no remission. The differences between the 2 groups were not statistically significant (P=0.545). After 6 months of treatment, in the homogeneous group, 12 patients achieved clinical complete remission, 27 patients showed partial remission, and 5 patients showed no remission. After 6 months of treatment, in the heterogeneous group, 2 patients achieved complete remission, 8 patients showed partial remission, and 7 patients showed no remission. The differences between the 2 groups were statistically significant (P=0.026). These findings indicate that a higher remission rate was observed in the patients in the homogeneous group.
FACTORS PREDICTING REMISSION AFTER TACROLIMUS TREATMENT:
The clinicopathological characteristics of patients with various responses (complete, partial, and no remission) to tacrolimus after 6 months of treatment are shown in Table 3. There were older patients, thicker basement membranes, increased levels of creatinine, and a higher number of heterogeneous types of IMN in the no remission group than in the other remission groups. The male/female ratio, proteinuria, cholesterol, serum albumin, systolic blood pressure, and diastolic blood pressure at baseline were the same among the 3 remission groups. The percentage of patients receiving ACEI or ARB parallel with tacrolimus therapy was not different in the 3 groups of patients. Remission-associated factors were assessed in detail in the logistic models (Table 4). In binary logistic analyses, the binary logistic model showed that the proportion of homogeneous type of INM was independently associated with total remission (partial plus complete remission) after tacrolimus treatment (odds ratio [OR] 4.795, 95% confidence interval [CI] 1.145–20.075) and was independently associated with complete remission after tacrolimus therapy (OR 12.134, 95% CI 1.014–145.205), whereas age, serum creatinine, and basement membrane thickness were not significant.
Discussion
In the pathogenesis and pathology of IMN, B and T lymphocytes play a significant role [21]. Tacrolimus has been used as therapy in nephrotic syndrome since 1990 to prevent graft rejection following organ transplantation. Tacrolimus forms a complex with the specific immunophilin, known as FK-506-binding protein 12, to impede calcineurin phosphatase and, thus, exerts its immunosuppressive effect by activating T-cell nuclear factor for the consecutive transcription of cytokines such as IL-2.
In China, in recent years, the incidence of IMN in renal biopsies of primary glomerular disease has increased. Hou et al [22] reported that the incidence of IMN increased from 10.4% in 2003 to 2006 to 24.1% in 2011 to 2014. A study found that the increased risk of IMN was related to air pollution [23]. China is a populous country with many patients with IMN, and the clinical treatment schemes used in various medical units vary greatly. Around 30% to 50% of patients with IMN develop chronic renal function or end-stage renal disease after 10 to 15 years. Therefore, standardized guidelines are needed to guide clinical treatment, improve prognosis, and achieve a satisfactory curative effect. It is also necessary to further evaluate the relationship between clinicopathologic features and the efficacy of IMN therapies to provide individualized treatment for patients.
Yoshimoto et al [24] and Yokoyama et al [20] conducted a retrospective study in Japanese patients previously diagnosed with IMN and assessed the clinicopathologic factors. The patients were observed closely for 5 years, following initial renal biopsy. The results indicated that the category of electron microscope findings was a beneficial marker of patient outcomes; an electron microscopic classification as heterogeneous type IMN or the deep subgroup type were independent indicators of poor prognosis at initial biopsy. In another study, Yokoyama et al [25] revealed that a relatively low dose of intravenous immune globulin therapy for a short duration could be beneficial in the early onset of remission in homogeneous type IMN with electron microscope results of synchronous electron-dense deposits.
Unfortunately, there are few studies on electron microscopic classification and its relationship to the therapeutic efficacy for IMN. Also, the relationship between the efficacy of tacrolimus and electron microscopic classification has not been analyzed, and no subsequent studies have been published.
In the present study, we found that the patients with the heterogeneous type of IMN had more proteinuria, lower serum albumin levels, higher systolic blood pressure, a thicker basement membrane, higher stages of pathology, and lower short-term remission rates after tacrolimus treatment. However, the short-term remission rate was significant in the patients with homogenous type IMN. Our study showed the patients with homogeneous type IMN had a greater possibility of complete remission and total remission resulting from tacrolimus treatment. However, female sex and proteinuria at baseline were not associated with the possibility of remission in the present study, which is not consistent with the results of previous studies [26,27]. The difference in research conclusions may be due to differences in factors such as region, sample sizes, genetic background, and treatment regimen. Therefore, the classification of electron microscope findings was a meaningful marker of the short-term efficacy of tacrolimus in nephrotic IMN and could assist in making therapeutic decisions. In the future, whether tacrolimus with glucocorticoid should be recommended as the preferred treatment for patients with the homogenous type of IMN needs to be confirmed by additional randomized controlled studies.
IMN can be successfully diagnosed by anti-PLA2R antibody levels, which play an important role as a biomarker [2,3]. Several studies have suggested that the anti-PLA2R antibody titers of patients with IMN were associated with proteinuria and response to therapy [4–6]. Lambeau, et al [28] found that there were multiple antigenic epitopes in the cysteine-rich, C-type lectin domain 1 and C-type lectin domain 7 domains against PLA2R, and the expression of epitopes was correlated with clinical features and disease outcome. The detection of autoantibodies to different antigenic epitopes is helpful to better evaluate the clinical severity of the disease and predict its prognosis, and it might be a significant indicator to guide the type of therapy for IMN. But it is not clear whether the difference of electron-dense deposition patterns is related to the epitope spreading of an autoantibody response to PLA2R. Since the anti-PLA2R antibody was not involved in our study, we cannot reach a conclusion regarding its value, but it is necessary to address this question in future research.
There are several limitations to this study. Our study was a retrospective and observational study, with a small number of patients and a short follow-up time. Furthermore, no research on the mechanism of IMN was conducted. In the future, it will be necessary to include more cases, extend the follow-up period, carry out prospective randomized controlled studies, and conduct research on the relationship between epitope spreading of autoantibody response to PLA2R and electron-dense deposition patterns.
Conclusions
In conclusion, our results indicated that patients with the homogeneous type of nephrotic IMN, with a synchronous phase of electron-dense deposits, had a higher short-term remission rate. The classification of homogeneous IMN had a predictive value for partial or complete remission. Thus, homogeneous status may be a meaningful marker of the short-term response to tacrolimus treatment.
Tables
Table 1. Baseline clinicopathological features of 61 patients with idiopathic membranous nephropathy.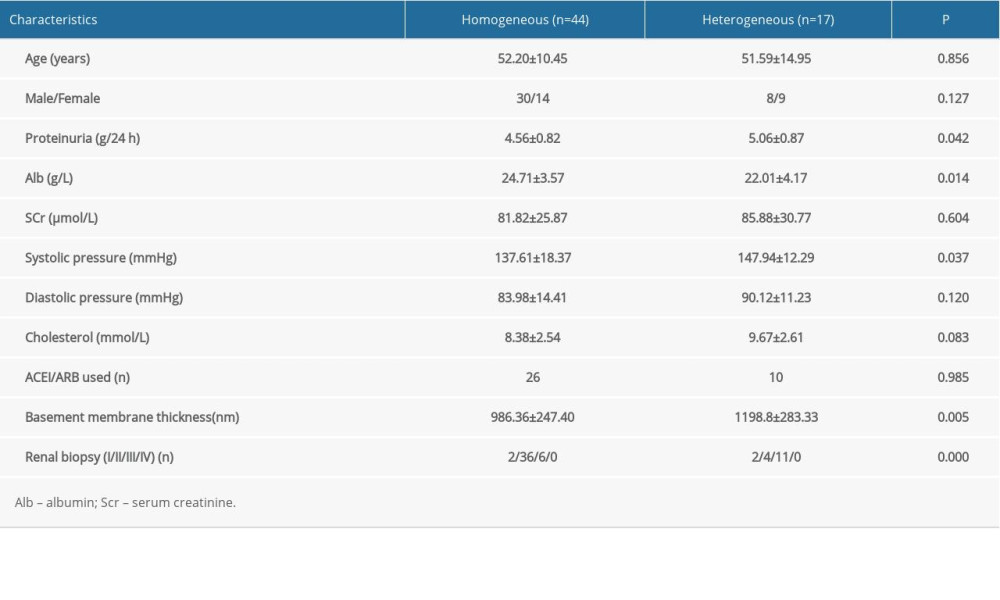 Table 2. Response to tacrolimus treatment in the homogeneous and heterogeneous groups.
Table 2. Response to tacrolimus treatment in the homogeneous and heterogeneous groups.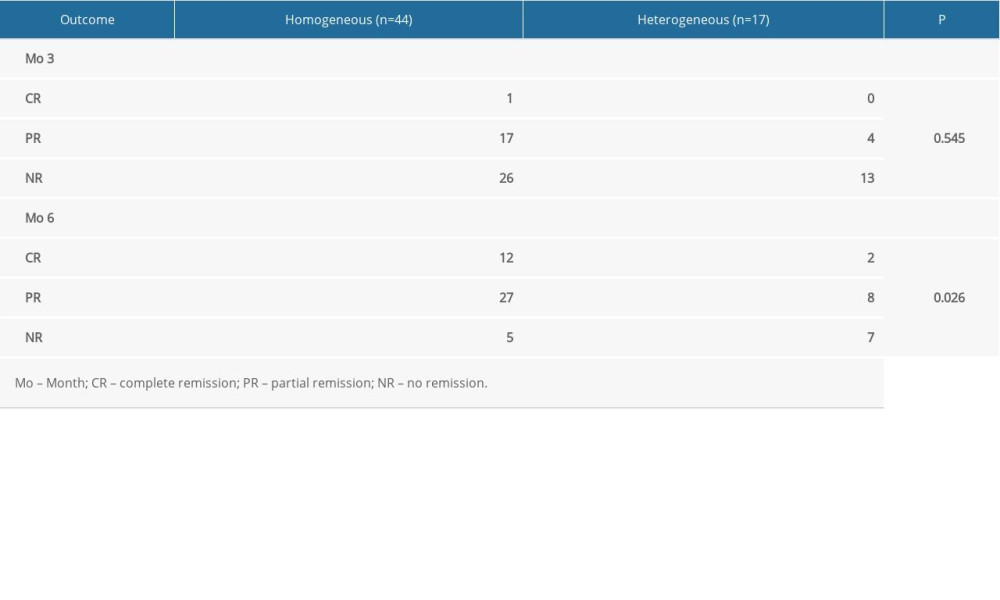 Table 3. Comparison of baseline clinicopathological features in patients with partial remission, complete remission, and no remission.
Table 3. Comparison of baseline clinicopathological features in patients with partial remission, complete remission, and no remission.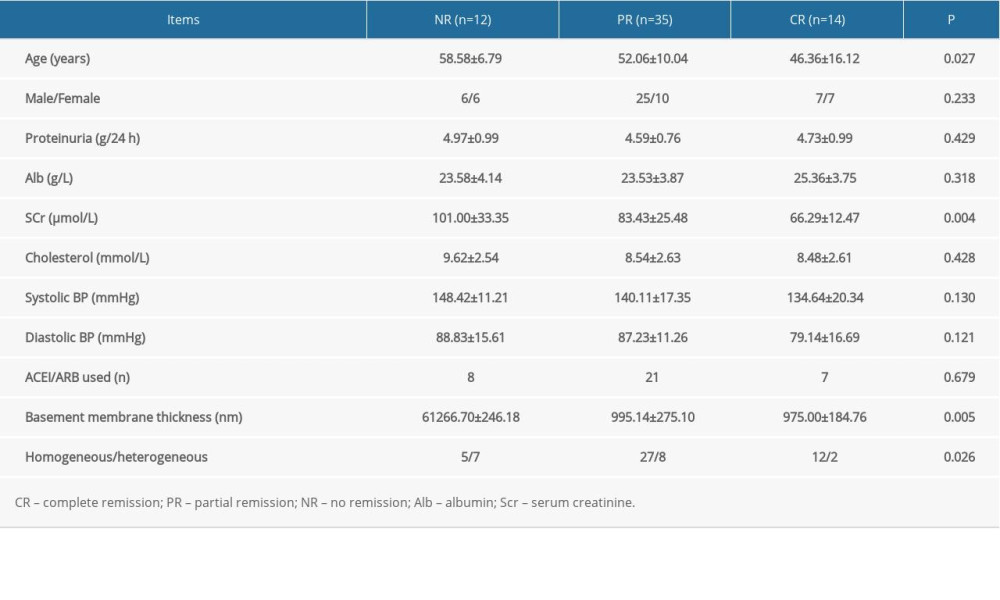 Table 4. Factors predicting remission after tacrolimus treatment in a binary logistic model.
Table 4. Factors predicting remission after tacrolimus treatment in a binary logistic model.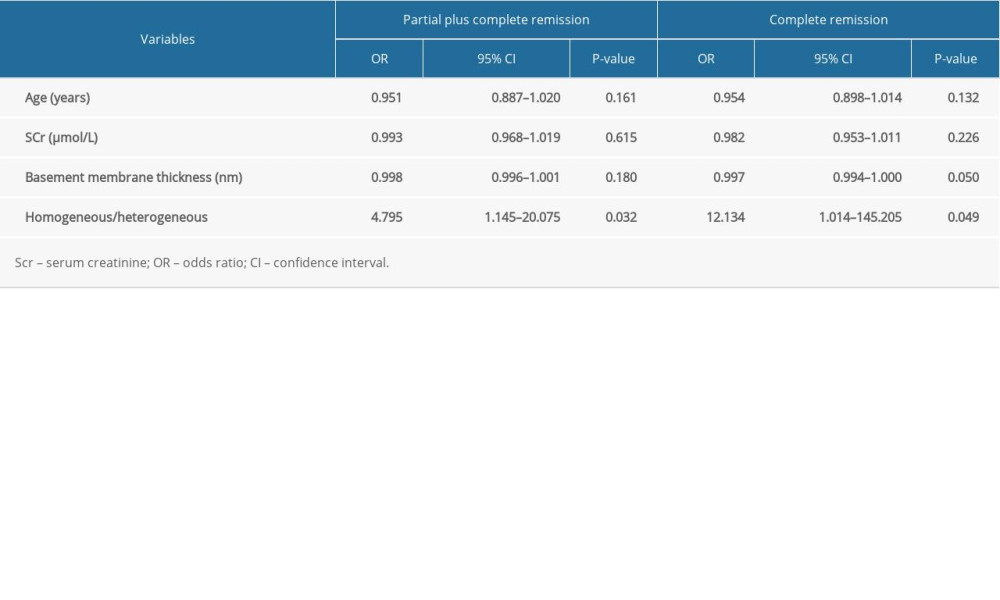
References
1. Ponticelli C, Glassock RJ, Glomerular diseases: Membranous nephropathy – a modern view: Clin J Am Soc Nephrol, 2014; 9(3); 609-16
2. Beck LH, Bonegio RG, Lambeau G, M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy: N Engl J Med, 2009; 363(1); 11-21
3. Qin W, Beck LH, Zeng C, Anti-phospholipase A2 receptor antibody in membranous nephropathy: J Am Soc Nephrol, 2011; 22(6); 1137-43
4. Hofstra JM, Beck LH, Beck DM: Clin J Am Soc Nephrol, 2011; 6(6); 1286-91
5. Wei SY, Wang YX, Li JS, Serum anti-PLA2R antibody predicts treatment outcome in idiopathic membranous nephropathy: Am J Nephrol, 2016; 43(2); 129-40
6. Zhang D, Zou J, Zhang C, Clinical and histological features of phospholipase A2 receptor-associated and thrombospondin type-I domain-containing 7A-associated idiopathic membranous nephropathy: A single center retrospective study from China: Med Sci Monit, 2018; 24; 5076-83
7. Kidney Disease Improving Global Outcomes Glomerulonephritis Work Group, KDIGO clinical practice guideline for glomerulonephritis: Kidney Int, 2012; 2; 139-274
8. Immunosuppressive Treatment for Chinese Adults with Nephrotic Syndrome Work Group, 2014 expert consensus on immunosuppressive treatment for Chinese adults with nephrotic syndrome: Chin J Nephrol, 2014; 30; 467-74
9. Zou HH, Jiang F, Xu GS, Effectiveness and safety of cyclophosphamide or tacrolimus therapy for idiopathic membranous nephropathy: Intern Med J, 2020; 50(5); 612-19
10. Zou H, Jiang F, Xu G, Effectiveness and safety of cyclophosphamide or tacrolimus therapy for idiopathic membranous nephropathy: Ren Fail, 2019; 41(1); 673-81
11. Cui W, Lu X, Min X, Therapy of tacrolimus combined with corticosteroids in idiopathic membranous nephropathy: Braz J Med Biol Res, 2017; 50(4); e5976
12. Ramachandran R, Hn HK, Kumar V, Tacrolimus combined with corticosteroids versus Modified Ponticelli regimen in treatment of idiopathic membranous nephropathy: Randomized control trial: Nephrology(Carlton), 2016; 21(2); 139-46
13. Zuo K, Wu Y, Li SJ, Long-term outcome and prognostic factors of idiopathic membranous nephropathy in the Chinese population: Clin Nephrol, 2013; 79(6); 445-53
14. Shiiki H, Saito T, Nishitani Y, Prognosis and risk factors for idiopathic membranous nephropathy with nephrotic syndrome in Japan: Kidney Int, 2004; 65(4); 1400-7
15. Lu WJ, Gong SH, Li J, Clinicopathological features and prognosis in patients with idiopathic membranous nephropathy with hypertension: Exp Ther Med, 2020; 19(4); 2615-21
16. Wakai S, Magil AB, Focal glomerulosclerosis in idiopathic membranous glomerulonephritis: Kidney Int, 1992; 41(2); 428-34
17. Marx BE, Marx M, Prediction of idiopathic membranous nephropathy: Kidney Int, 1999; 56(2); 666-73
18. Ehrenreich T, Porush JG, Churg J, Treatment of idiopathic membranous nephropathy: N Engl J Med, 1976; 295(14); 741-46
19. Rosen S, Tornroth T, Bernard DB, Membranous glomerulonephritis: Renal pathology with clinical and functional correlations, 1989; 196-227, Philadelphia, JB, Lippincott Company
20. Yokoyama H, Yoshimoto K, Wada T, Electron-dense deposition patterns and the outcomes of idiopathic membranous nephropathy in Japanese: Med Electron Microsc, 2002; 35(2); 81-86
21. Beck LH, Bonegio RG, Lambeau G, M-type phospholipasea2 receptor as target antigen in idiopathic membranous nephropathy: N Engl J Med, 2009; 361(1); 11-21
22. Hou JH, Zhu HX, Zhou ML, Changes in the spectrum of kidney diseases: An analysis of 40,759 biopsy proven cases from 2003 to 2014 in China: Kidney Dis (Basel), 2018; 4(1); 10-19
23. Xu X, Wang G, Chen N, Long-term exposure to air pollution and increased risk of membranous nephropathy in China: J Am Soc Nephrol, 2016; 27(12); 3739-46
24. Yoshimoto K, Yokoyama H, Wada T, Pathologic findings of initial biopsies reflect the outcomes of membranous nephropathy: Kidney Int, 2004; 65(1); 148-53
25. Yokoyama H, Goshima S, Wada T, The short- and long-term outcomes of membranous nephropathy treated with intravenous immune globulin therapy: Nephrol Dial Transplant, 1999; 14(10); 2379-86
26. Qin HZ, Liu L, Liang SS, Evaluating tacrolimus treatment in idiopathic membranous nephropathy in a cohort of 408 patients: BMC Nephrol, 2017; 18(1)
27. Caro J, Gutiérrez-Solís E, Rojas-Rivera J, Predictors of response and relapse in patients with idiopathic membranous nephropathy treated with tacrolimus: Nephrol Dial Transplant, 2015; 30(3); 467-74
28. Seitz-Polski B, Dolla G, Payré C, Epitope spreading of autoantibody response to PLA2R associates with poor prognosis in membranous nephropathy: J Am Soc Nephrol, 2016; 27(5); 1517-33
Tables
 Table 1. Baseline clinicopathological features of 61 patients with idiopathic membranous nephropathy.
Table 1. Baseline clinicopathological features of 61 patients with idiopathic membranous nephropathy. Table 2. Response to tacrolimus treatment in the homogeneous and heterogeneous groups.
Table 2. Response to tacrolimus treatment in the homogeneous and heterogeneous groups. Table 3. Comparison of baseline clinicopathological features in patients with partial remission, complete remission, and no remission.
Table 3. Comparison of baseline clinicopathological features in patients with partial remission, complete remission, and no remission. Table 4. Factors predicting remission after tacrolimus treatment in a binary logistic model.
Table 4. Factors predicting remission after tacrolimus treatment in a binary logistic model. Table 1. Baseline clinicopathological features of 61 patients with idiopathic membranous nephropathy.
Table 1. Baseline clinicopathological features of 61 patients with idiopathic membranous nephropathy. Table 2. Response to tacrolimus treatment in the homogeneous and heterogeneous groups.
Table 2. Response to tacrolimus treatment in the homogeneous and heterogeneous groups. Table 3. Comparison of baseline clinicopathological features in patients with partial remission, complete remission, and no remission.
Table 3. Comparison of baseline clinicopathological features in patients with partial remission, complete remission, and no remission. Table 4. Factors predicting remission after tacrolimus treatment in a binary logistic model.
Table 4. Factors predicting remission after tacrolimus treatment in a binary logistic model. In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952