21 June 2021: Lab/In Vitro Research
Salvianolic Acid A Protects H9C2 Cardiomyocytes from Doxorubicin-Induced Damage by Inhibiting NFKB1 Expression Thereby Downregulating Long-Noncoding RNA (lncRNA) Plasmacytoma Variant Translocation 1 (PVT1)
Yumeng Wu1ABF, Wei Xiu2CD, Yubo Wu1AEF*DOI: 10.12659/MSM.929824
Med Sci Monit 2021; 27:e929824
Abstract
BACKGROUND: A cardioprotective effect of salvianolic acid A (SalA) has been described, but it is unknown whether SalA can protect cardiomyocytes against doxorubicin (Dox)-induced cardiotoxicity. This study aimed to investigate whether SalA could inhibit Dox-induced apoptosis in H9C2 cells and to uncover the potential mechanism.
MATERIAL AND METHODS: H9C2 cardiomyocytes exposed to Dox were treated with SalA or not, and then cell viability, apoptosis, and the expression of nuclear factor-κB (NF-κB) signaling were detected by Cell Counting Kit-8, TUNEL staining, and western blot assays, respectively. Nuclear factor kappa B subunit 1 (NFKB1) was overexpressed in H9C2 cells, and then alterations in cell viability and apoptosis in H9C2 cells co-treated with Dox and SalA were investigated.
RESULTS: SalA (2, 10, and 50 μM) had no effect on H9C2 cell viability, while Dox reduced cell viability in a concentration-dependent manner. In addition, SalA rescued Dox-decreased cell viability. Dox also triggered apoptosis as evidenced by an increased ratio of TUNEL-positive cells, enhanced expression of pro-apoptotic proteins, and reduced expression of anti-apoptotic protein BCL-2, which were all partially blocked by SalA co-treatment. The proteins involved in NF-κB signaling including IκBα, IKKα, IKKβ, and p65 were activated by Dox but inactivated by SalA co-treatment. Moreover, Dox increased NFKB1 mRNA and nuclear expression, which was blocked by SalA. NFKB1 could bind to plasmacytoma variant translocation 1 (PVT1) and upregulate PVT1 expression. Mechanistically, the overexpression of NFKB1 blocked the inhibitory effect of SalA on Dox-induced cell viability impairment and apoptosis.
CONCLUSIONS: We demonstrated that SalA may exert a protective effect against Dox-induced H9C2 injury and apoptosis via inhibition of NFKB1 expression, thereby downregulating lncRNA PVT1.
Keywords: Salvia miltiorrhiza, Caffeic Acids, cardiotoxicity, Down-Regulation, Lactates, Plasmacytoma
Background
While anticancer drugs bring significant therapeutic effects to patients, they also produce many adverse effects, among which cardiotoxicity is very important [1]. Chemotherapeutic agents used to treat cancer are associated with numerous cardiovascular complications, such as hypertension, heart failure, vascular complications, and arrhythmias [2]. In order to improve the survival quality of patients, investigating why and how these cardiotoxicities occur and how to mitigate them is of great significance, thus giving rise to the birth and development of cardio-oncology [3].
Doxorubicin (Dox) is a pervasive first-line therapeutic schedule for lymphomas. However, early in 1979, it was reported that Dox induces congestive heart failure [4]. Since then, the cardiotoxicity of Dox has been reported in an increasing number of studies. Dox has been confirmed to cause long-term cardiovascular side effects and to decrease the quality of life of cancer survivors, which hampers the clinical application of Dox [5]. Among the diverse mechanisms underlying the cardiotoxicity of Dox, apoptosis is widely accepted to be involved [6]. Both in vivo and in vitro studies have shown that Dox treatment induces oxidative stress, which is associated with the activation of cardiomyocyte apoptosis [7]. Studies have shown that Dox decreases the ratio of BCL-2/BAX, which results in the activation of caspase 3 and caspase 9 and subsequently triggers cardiomyocyte apoptosis [8,9]. Therefore, the utility of anti-apoptotic compounds is very promising for attenuating Dox-induced cardiotoxicity.
Salvianolic acid A (SalA) is an active component of the Chinese traditional medicine
Nuclear factor-κB (NF-κB) is a transcription factor that regulates inflammatory and apoptotic pathways, and it has been extensively associated with Dox-induced cardiotoxicity [16,17]. Studies have indicated that blocking NF-κB signaling activation may protect cardiomyocytes against Dox-induced injury [18,19]. Notably, SalA has been demonstrated to inhibit the transcriptional activation of NF-κB [20,21]. The
In the present study, we aimed to investigate whether SalA could alleviate Dox-induced injury of H9C2 cells via suppression of apoptosis, and to verify whether the effects of SalA were dependent on inhibition of NFKB1 expression thereby downregulating PVT1.
Material and Methods
CELL CULTURE AND TREATMENT:
The rat cardiomyocytes, H9C2 cells, were obtained from Shanghai Institutes for Biological Sciences (China) and cultured in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum and 1% streptomycin-penicillin (Thermo Fisher Scientific) in a 37°C constant temperature incubator with 5% CO2. The medium was changed every 2 days and cells were digested with 0.25% trypsin and passaged when the cells grew to a confluence of 80–90%.
For SalA (purity >98%, dissolved in dimethyl sulfoxide and diluted with phosphate-buffered saline (PBS); Solarbio Life Sciences, China) and or Dox (purity >98%, Solarbio Life Sciences) treatment, cells attached to the plate bottom were exposed to 0, 2, 10, and 50 μM SalA for 12 h; 0, 1, 2, 4, and 8 μM Dox for 12 h; or SalA and Dox co-treatment for 12 h.
CELL TRANSFECTION:
The full length of NFKB1 was designed and cloned into a pcDNA3.1 vector by Gene Script Biotech Co., Ltd. (Nanjing, China). The empty vector pcDNA3.1 was used as the negative control (NC). Cells were cultured overnight to reach 60–70% confluence before transfection. The plasmid transfection was performed using Lipofectamine 2000 (Invitrogen) in strict accordance with the manufacturer’s instructions and cells were chosen for subsequent experiments at 48 h after transfection.
CELL COUNTING KIT-8:
After treatment, cell viability was determined by Cell Counting Kit-8 (CCK-8) assay (Beyotime). The cells were seeded in a 96
TUNEL STAINING:
Cell apoptosis was detected using the TUNEL assay kit (Beyotime, China) following the manufacturer’s instructions. The normal H9C2 cells or transfected cells were treated with or without Dox ± SalA before the fluorescein (green)-labeled dUTP solution was added. Finally, the images were captured by a fluorescent microscope (Olympus, Japan) with ×200 magnification and analyzed using the ImageJ software.
WESTERN BLOT:
The total proteins from H9C2 cells were extracted using RIPA lysis (Beyotime, China) and quantified using a BCA kit (Beyotime). Equal amounts of sample proteins (20 μg) were resolved by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto a polyvinylidene difluoride membrane. After being blocked with 5% nonfat milk for 1 h at room temperature, the membrane was subsequently incubated with primary antibodies against BCL-2 (1: 2000), BAX (1: 5000), caspase 3 (1: 5000), cleaved (c)-caspase 3 (1: 500), caspase 9 (1: 5000), c-caspase 9 (1: 500), IκBα (1: 10 000), phosphorylated (p)-IκBα (1: 10 000), IKKα (1: 10 000), p-IKKα (1: 1000), IKKβ (1: 5000), p-IKKβ (1: 1000), p65 (1: 10000), p50 (1: 1000), Lamin-B1 (1: 1000), and GAPDH (1: 10000). The membrane was then washed with Tris-buffered saline with Tween and incubated with suitable horseradish peroxidase-conjugated secondary antibody in blocking buffer at 24°C for 1 h. The blots were developed by enhanced chemiluminescence substrate (Millipore, MA, USA) for detecting protein expressions in a ChemiDoc Touch Imaging System (Bio-Rad, USA).
REAL-TIME QPCR:
Total RNA from H9C2 cells was extracted using TRIzol reagent (Invitrogen, USA) and the contents were measured by a NanoDrop (Thermo Fisher, USA). Total RNA was reversely transcribed into cDNA using the PrimeScript™ RT reagent kit (TaKaRa, Biotechnology Co., LTD, China). Quantitative polymerase chain reaction (qPCR) was performed using TaqMan Master Mix (Applied Biosystems, USA), and the primers used for qPCR to detect
The following thermocycling conditions were used for qPCR: 95°C for 2 min, followed by 40 cycles of 95°C for 20 s and 65°C for 40 s. Results were analyzed using the CT method after being normalized to
LUCIFERASE REPORTER ASSAY:
To observe interactions between NFKB1 and lncRNA PVT1, wild-type (WT) or mutant (MUT)
CHROMATIN IMMUNOPRECIPITATION ASSAY:
Chromatin immunoprecipitation (ChIP) assays were performed according to a standard protocol. Briefly, H9C2 cells at a density of 5×107 were washed with cold PBS and then crosslinked with 1% formaldehyde for 10 min at 37°C. Subsequently, cells were subjected to ChIP assay with a High-Sensitivity kit (Abcam). The antibodies used in this assay included anti-NFKB1 (p50) and IgG (NC). The primer sequences used for the qRT-PCR assay were 5′-GAAGCCGAGGTGAGAAGCAT-3′ (forward) and 5′-TCTAGGCCCCTTTCCCAGTT-3′ (reverse).
STATISTICAL ANALYSIS:
All experiments were repeated at least 3 times and results were presented as mean±standard deviation (SD). Statistical analysis was conducted using GraphPad 6.0 statistical software (GraphPad, USA). Differences among 2 groups were analyzed using
Results
SALA ENHANCES H9C2 CELL VIABILITY REDUCED BY DOX:
First, we assessed the effect of SalA on H9C2 cell viability. Figure 1A illustrates the chemical structure of SalA, and Figure 1B shows that the different concentrations of SalA had no obvious effect on H9C2 cell viability at the concentrations of 2, 10, and 50 μM. H9C2 cells were also exposed to a series of Dox concentrations, and results in Figure 1C show that Dox inhibited H9C2 cell viability in a concentration-dependent manner. We selected 4 μM Dox for the following experiments because it resulted in a relatively moderate reduction of cell viability. Subsequently, H9C2 cells stimulated with 4 μM Dox were co-treated with 2, 10, and 50 μM SalA. We found that the reduced H9C2 cell viability was significantly recovered by 10 and 50 μM SalA, suggesting the beneficial role of SalA in alleviating Dox-induced H9C2 cardiotoxicity (Figure 1D).
SALA INHIBITS APOPTOSIS AND NF-κB SIGNALING ACTIVATION IN H9C2 CELLS INDUCED BY DOX:
Thereafter, TUNEL staining and western blot assays were utilized to estimate the extent of cell apoptosis. As shown in Figure 2A and 2B, the number of apoptotic cells was dramatically increased upon Dox stimulation. However, H9C2 cells that were co-treated with Dox and SalA exhibited an obviously lower ratio of cell apoptosis compared with cells that were exposed to Dox. The same result was observed in Figure 2C, which demonstrated the decreased expression of BCL-2, whereas increased expression of BAX, c-caspase 3, and c-caspase 9 were caused by Dox treatment. Expectedly, SalA partially rescued the expression of the above proteins. These data indicate the inhibitory effect of SalA on Dox-induced H9C2 apoptosis.
Then, we detected the expression of proteins involved in NF-κB signaling including p-IκBα, p-IKKα, p-IKKβ, and p65 in both the cytoplasm and the nucleus. Results showed that the expression of p-IκBα, p-IKKα, p-IKKβ, and nuclear p65 were also markedly enhanced in response to Dox stimulation, but were reversely reduced in the absence of SalA (Figure 3A). The above data revealed that SalA could prevent the activation of NF-κB signaling induced by Dox.
In addition, we found that the mRNA expression of NFKB1 was increased upon Dox treatment but instead decreased after SalA co-treatment (Figure 3B). Consistently, the encoding protein of NFKB1, p50, was activated to translocate into the nucleus upon Dox treatment, which was also blocked by SalA co-treatment (Figure 3C). These data indicate the inhibitory effect of SalA on Dox-increased NFKB1 expression.
NFKB1 BINDS TO LNCRNA PVT1 AND UPREGULATES ITS EXPRESSION:
NFKB1 is predicted to bind to the promoter of lncRNA PVT1, based on the JASPAR database (http://jaspar.genereg.net/; Figure 4A). To verify the interaction between NFKB1 and PVT1, we overexpressed NFKB1 in H9C2 cells using pcDNA vectors, and the results (Figure 4B, 4C) confirmed the overexpression of NFKB1 mRNA and protein in H9C2 cells. The expression of PVT1 in H9C2 cells before and after NFKB1 overexpression was also measured. We found that the expression of PVT1 was increased by NFKB1 overexpression (Figure 4D). Then, luciferase report and ChIP assays were performed to validate the interaction between PVT1 and NFKB1, and results confirmed their binding (Figure 4E, 4F).
NFKB1 OVEREXPRESSION BLOCKS THE PROTECTIVE EFFECT OF SALA AGAINST DOX-INDUCED H9C2 INJURY:
Finally, we investigated the effect of NFKB1 overexpression on H9C2 cells in the presence of Dox and SalA treatment. H9C2 cells overexpressed with NFKB1 or not were exposed to Dox or Dox+SalA. As shown in Figure 5A, Dox resulted in a significantly increased expression of PVT1, which was instead reduced by SalA co-treatment. Whereas, NFKB1 overexpression enhanced PVT1 expression. The increased viability caused by SalA in Dox-treated H9C2 cells was also blocked by NFKB1 overexpression (Figure 5B). In addition, the ratio of cell apoptosis in H9C2 cells overexpressed with NFKB1 was remarkably higher than that in cells that were transfected with NC vectors, in the presence of Dox and SalA (Figure 5C, 5D). The same result is shown in Figure 5E, with NFKB1 overexpression blunting the effect of SalA on proteins involved in apoptosis including BCL-2, BAX, c-caspase3, and c-caspase-9 in H9C2 cells that were stimulated with Dox.
Discussion
Dox is a commonly used anthracycline broad-spectrum antitumor drug that is widely used in the treatment of various cancers. However, Dox can cause irreversible myocardial damage and heart failure, and there are no effective prevention and treatment measures, which severely limits its clinical application [24]. Therefore, finding a cardioprotective agent with high efficiency and low toxicity that does not affect the antitumor effect of Dox is urgently needed. The exact pathogenesis of Dox-induced cardiotoxicity remains to be fully elucidated. To date, several mechanisms have been implicated in the pathophysiology of Dox-induced cardiotoxicity, including increased oxidative stress and lipid peroxidation [25], DNA damage and apoptosis [6], and autophagy [26]. Among these mechanisms, Dox-induced cardiomyocyte apoptosis plays a crucial role in the cardiotoxicity of the drug. Dox activates the apoptosis pathway to trigger cardiomyocyte apoptosis and the loss of myocardial cells, which is closely related to Dox-caused myocardial systolic dysfunction and heart failure [6]. In the present study, consistent with previous studies, we observed that Dox significantly impaired H9C2 cell viability and induced obvious cell apoptosis, confirming the toxicity of Dox to H9C2 cardiomyocytes.
SalA has been proposed to exert its cardioprotective effect through an anti-apoptosis mechanism [13]. However, whether SalA can inhibit Dox-induced apoptosis in H9C2 cells has not been investigated. In the current study, SalA markedly rescued H9C2 cell viability that was impaired by Dox, indicating its protective role in Dox-caused H9C2 injury. Moreover, the presence of SalA remarkably reduced the ratio of apoptotic cells and the expression of pro-apoptotic proteins, suggesting its inhibitory effect on Dox-triggered H9C2 apoptosis. The inhibition of cardiomyocyte apoptosis has been reported to attenuate Dox-induced cardiotoxicity and cardiac dysfunction [27,28]. Therefore, our study indicated the beneficial role of SalA in alleviating the cardiotoxicity of Dox.
The NF-κB pathway is known to promote an inflammatory response and apoptosis, and it is widely accepted to play a role in Dox-induced cardiotoxicity [16,17]. As expected, we found that the proteins involved in the NF-κB pathway were all markedly activated and the expression of nuclear p65 was significantly increased upon Dox stimulation, revealing the activation of NF-κB signaling. However, co-treatment with SalA and Dox successfully blocked NF-κB activation. The inhibitory effect of SalA on NF-κB signaling has also been reported in other studies [21]. For example, SalA was found to suppress NF-κB-dependent inflammatory pathways through IKKβ inhibition [29]. In addition, Zhang et al [30] demonstrated that SalA could protect the kidney against oxidative stress by inhibiting the NF-κB signaling pathway. Therefore, our study and studies by others confirmed the suppressive effect of SalA on NF-κB signaling, which may aid in understanding the pharmacology and mode of action of SalA. In addition, NFKB1 mRNA expression and the nuclear translocation of its encoding protein p50, which were both enhanced by Dox, were reversed by SalA, indicating that SalA could inhibit NFKB1 expression.
Notably, using the JASPAR database (http://jaspar.genereg.net/), we found that NFKB1 could bind to the promoter of PVT1, and the expression of PVT1 was significantly upregulated after NFKB1 overexpression. Furthermore, the direct interaction between NFKB1 and PVT1 was verified. A recent study demonstrated that lncRNA PVT1 could aggravate Dox-induced cardiomyocyte apoptosis [23]. We observed increased expression of PVT1 in H9C2 cells upon Dox stimulation, while PVT1 expression was reduced in response to SalA co-treatment. Therefore, we speculated that SalA may inhibit NFKB1 expression, thereby downregulating PVT1 to exert its inhibitory effect on Dox-induced cardiomyocyte apoptosis. To verify our hypothesis, we overexpressed NFKB1 in Dox+SalA co-treated H9C2 cells. Results showed that both the protective effect of SalA on viability and the inhibitory effect of SalA on apoptosis in Dox-induced H9C2 cells were blocked by NFKB1 overexpression.
This study provides new preventive and therapeutic clues for the treatment of Dox-induced cardiotoxicity. However, it has some shortcomings. First, the conclusions of this study are only based on results from in vitro experiments and they have not been confirmed in in vivo studies. In addition, the actions of SalA may be involved in multiple signaling pathways, and thus further research is required.
Conclusions
In summary, we found an obvious protective effect of SalA in Dox-induced H9C2 cardiotoxicity. SalA inhibited Dox-triggered H9C2 apoptosis through inhibition of NFKB1 expression, thereby downregulating lncRNA PVT1 expression. This study provides significant insights into the potential mechanisms of the cardioprotective effect of SalA.
Figures
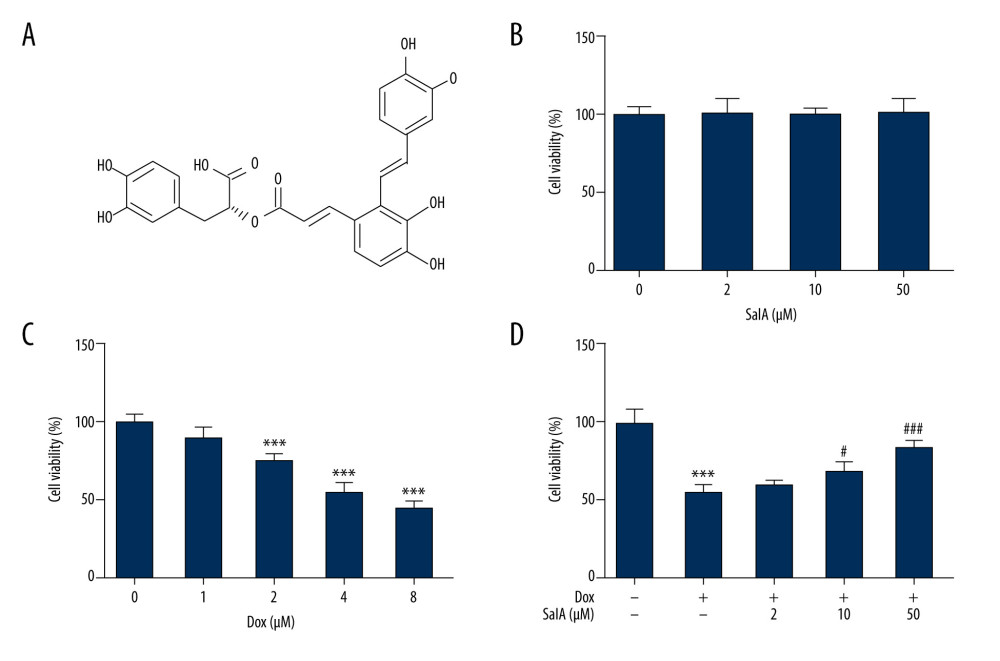 Figure 1. The effect of salvianolic acid A (SalA) and doxorubicin (Dox) on H9C2 cell viability. (A) the chemical structure of SalA. (B–D) H9C2 cells were exposed to 0, 2, 10, and 50 μM SalA for 12 h (B); 0, 1, 2, 4, and 8 μM Dox for 12 h (C); or SalA and 4 μM Dox co-treatment for 12 h (D). Cell viability was then measured by CCK-8 assay. *** P<0.001; # P<0.05; ### P<0.001.
Figure 1. The effect of salvianolic acid A (SalA) and doxorubicin (Dox) on H9C2 cell viability. (A) the chemical structure of SalA. (B–D) H9C2 cells were exposed to 0, 2, 10, and 50 μM SalA for 12 h (B); 0, 1, 2, 4, and 8 μM Dox for 12 h (C); or SalA and 4 μM Dox co-treatment for 12 h (D). Cell viability was then measured by CCK-8 assay. *** P<0.001; # P<0.05; ### P<0.001. 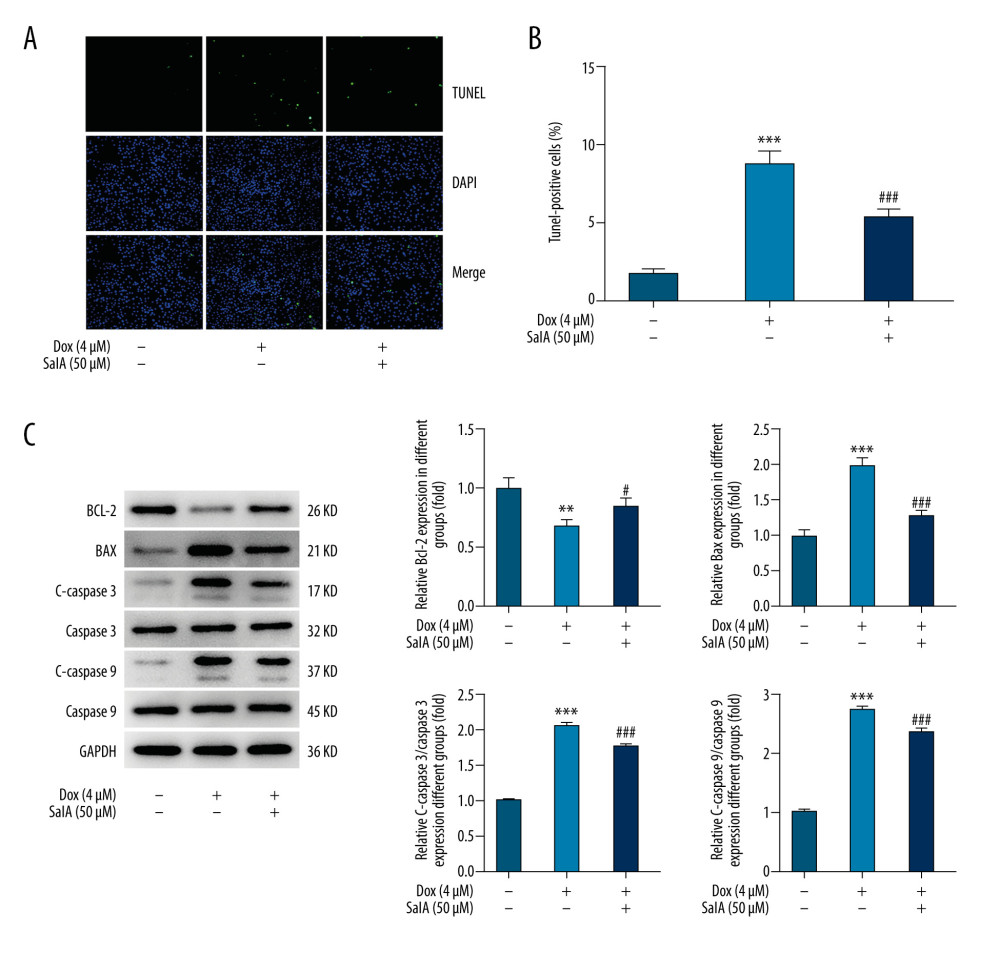 Figure 2. The effect of salvianolic acid A (SalA) on doxorubicin (Dox)-induced apoptosis in H9C2 cells. (A, B) Representative images (×200) and quantitative analysis for TUNEL staining in H9C2 cells. (C) The expression of proteins related to apoptosis in H9C2 cells was detected by western blot. ** P<0.01; *** P<0.001; # P<0.05; ### P<0.001.
Figure 2. The effect of salvianolic acid A (SalA) on doxorubicin (Dox)-induced apoptosis in H9C2 cells. (A, B) Representative images (×200) and quantitative analysis for TUNEL staining in H9C2 cells. (C) The expression of proteins related to apoptosis in H9C2 cells was detected by western blot. ** P<0.01; *** P<0.001; # P<0.05; ### P<0.001. 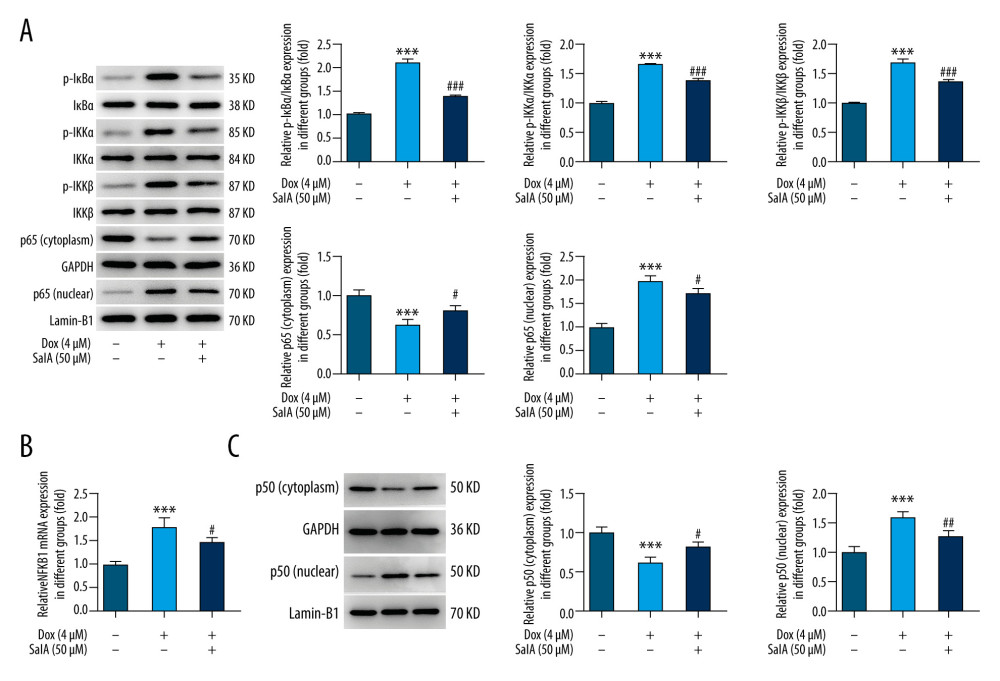 Figure 3. The effect of salvianolic acid A (SalA) on doxorubicin (Dox)-activated nuclear factor-κB (NF-κB) signaling and nuclear factor kappa B subunit 1 (NFKB1) expression in H9C2 cells. (A) The expression of proteins involved in the NF-κB pathway in H9C2 cells was detected by western blot. (B) The mRNA expression of NFKB1 in H9C2 cells that were subjected to different treatments. (C) The protein expression of cytoplasmic and nuclear p50 in H9C2 cells. *** P<0.001; # P<0.05; ## P<0.01; ### P<0.001.
Figure 3. The effect of salvianolic acid A (SalA) on doxorubicin (Dox)-activated nuclear factor-κB (NF-κB) signaling and nuclear factor kappa B subunit 1 (NFKB1) expression in H9C2 cells. (A) The expression of proteins involved in the NF-κB pathway in H9C2 cells was detected by western blot. (B) The mRNA expression of NFKB1 in H9C2 cells that were subjected to different treatments. (C) The protein expression of cytoplasmic and nuclear p50 in H9C2 cells. *** P<0.001; # P<0.05; ## P<0.01; ### P<0.001. 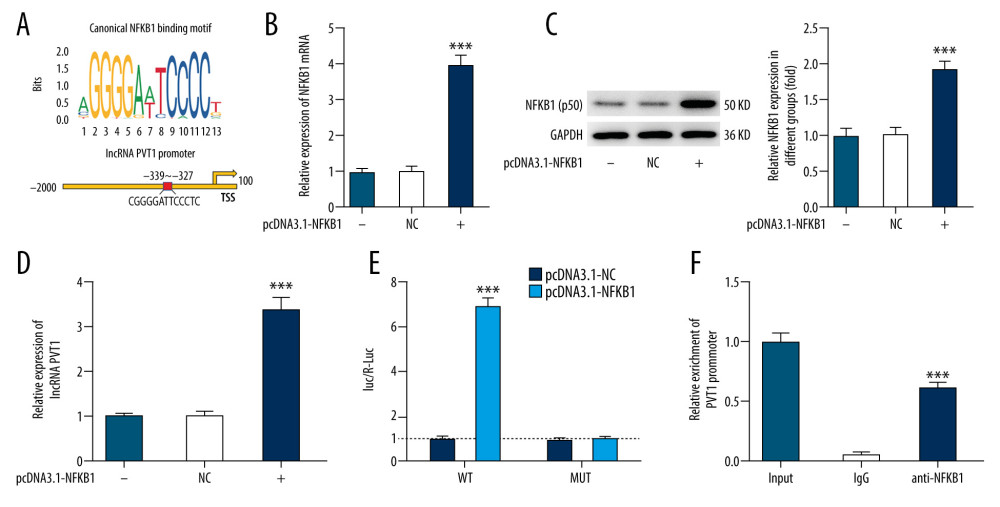 Figure 4. The interaction between nuclear factor kappa B subunit 1 (NFKB1) and plasmacytoma variant translocation 1 (PVT1) in H9C2 cells. (A) The binding sequences between NFKB1 and lncRNA PVT1 promoter. (B, C) The mRNA and protein expression of NFKB1 in H9C2 cells overexpressed with NFKB1 or not. (D) PVT1 expression in H9C2 cells overexpressed with NFKB1 or not. (E, F) The interaction between NFKB1 and PVT1 was confirmed by luciferase report (E) and chromatin immunoprecipitation (F) assays. *** P<0.001.
Figure 4. The interaction between nuclear factor kappa B subunit 1 (NFKB1) and plasmacytoma variant translocation 1 (PVT1) in H9C2 cells. (A) The binding sequences between NFKB1 and lncRNA PVT1 promoter. (B, C) The mRNA and protein expression of NFKB1 in H9C2 cells overexpressed with NFKB1 or not. (D) PVT1 expression in H9C2 cells overexpressed with NFKB1 or not. (E, F) The interaction between NFKB1 and PVT1 was confirmed by luciferase report (E) and chromatin immunoprecipitation (F) assays. *** P<0.001. 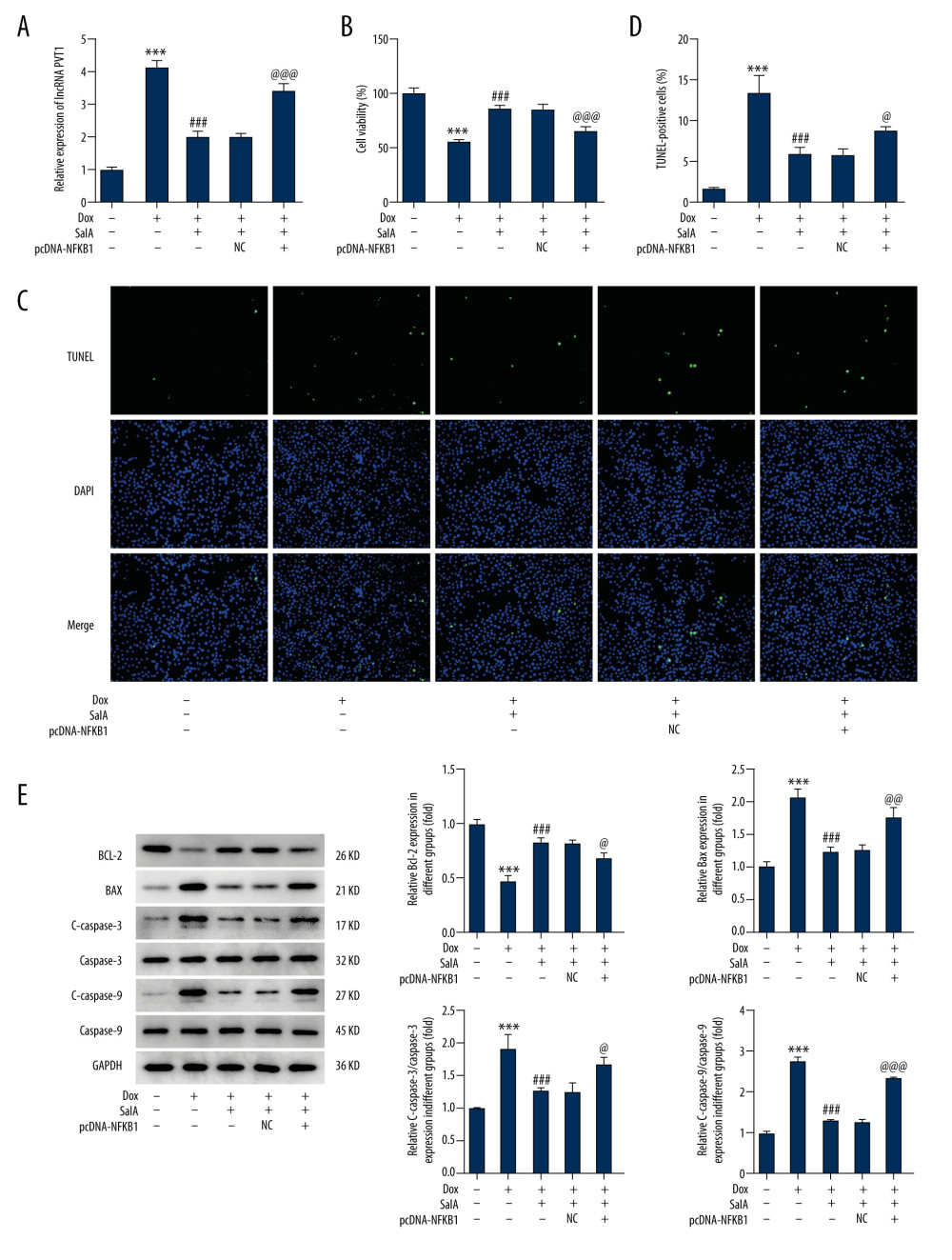 Figure 5. The effect of nuclear factor kappa B subunit 1 (NFKB1) overexpression on salvianolic acid A (SalA)-inhibited apoptosis in H9C2 cells. (A) The expression of plasmacytoma variant translocation 1 (PVT1) in H9C2 cells. (B) Cell viability of H9C2 cells that were subjected to indicated treatments. (C, D) Representative images (×200) and quantitative analysis for TUNEL staining in H9C2 cells. (E) The expression of proteins related to apoptosis in H9C2 cells was detected by western blot. *** P<0.001; ### P<0.001. @ P<0.05; @@ P<0.01; @@@ P<0.001.
Figure 5. The effect of nuclear factor kappa B subunit 1 (NFKB1) overexpression on salvianolic acid A (SalA)-inhibited apoptosis in H9C2 cells. (A) The expression of plasmacytoma variant translocation 1 (PVT1) in H9C2 cells. (B) Cell viability of H9C2 cells that were subjected to indicated treatments. (C, D) Representative images (×200) and quantitative analysis for TUNEL staining in H9C2 cells. (E) The expression of proteins related to apoptosis in H9C2 cells was detected by western blot. *** P<0.001; ### P<0.001. @ P<0.05; @@ P<0.01; @@@ P<0.001. References
1. Nicolazzi MA, Carnicelli A, Fuorlo M, Anthracycline and trastuzumab-induced cardiotoxicity in breast cancer: Eur Rev Med Pharmacol Sci, 2018; 22; 2175-85
2. Bloom MW, Hamo CE, Cardinale D, Cancer therapy-related cardiac dysfunction and heart failure: Part 1: Definitions, pathophysiology, risk factors, and imaging: Circ Heart Fail, 2016; 9; e002661
3. Lenneman CG, Sawyer DB, Cardio-oncology: An update on cardiotoxicity of cancer-related treatment: Circ Res, 2016; 118; 1008-20
4. Von Hoff DD, Layard MW, Basa P, Risk factors for doxorubicin-induced congestive heart failure: Ann Intern Med, 1979; 91; 710-17
5. Gupta SK, Garg A, Bär C, Quaking inhibits doxorubicin-mediated cardiotoxicity through regulation of cardiac circular RNA expression: Circ Res, 2018; 122; 246-54
6. Zhang S, Liu X, Bawa-Khalfe T, Identification of the molecular basis of doxorubicin-induced cardiotoxicity: Nat Med, 2012; 18; 1639-42
7. Shabalala S, Muller CJF, Louw J, Polyphenols, autophagy and doxorubicin-induced cardiotoxicity: Life Sci, 2017; 180; 160-70
8. Zhao L, Zhang B, Doxorubicin induces cardiotoxicity through upregulation of death receptors mediated apoptosis in cardiomyocytes: Sci Rep, 2017; 7; 44735
9. Ma Y, Yang L, Ma J, Rutin attenuates doxorubicin-induced cardiotoxicity via regulating autophagy and apoptosis: Biochim Biophys Acta Mol Basis Dis, 2017; 1863; 1904-11
10. Qin T, Rasul A, Sarfraz A, Salvianolic acid A & B: Potential cytotoxic polyphenols in battle against cancer via targeting multiple signaling pathways: Int J Biol Sci, 2019; 15; 2256-64
11. Zhou R, Gao J, Xiang C, Salvianolic acid A attenuated myocardial infarction-induced apoptosis and inflammation by activating Trx: Naunyn Schmiedebergs Arch Pharmacol, 2020; 393; 991-1002
12. Wang R, Zhang J, Wang S, The cardiotoxicity induced by arsenic trioxide is alleviated by salvianolic acid A via maintaining calcium homeostasis and inhibiting endoplasmic reticulum stress: Molecules, 2019; 24(3); 543
13. Xu T, Wu X, Chen Q, The anti-apoptotic and cardioprotective effects of salvianolic acid a on rat cardiomyocytes following ischemia/reperfusion by DUSP-mediated regulation of the ERK1/2/JNK pathway: PLoS One, 2014; 9; e102292
14. Chen RC, Sun GB, Ye JX, Salvianolic acid B attenuates doxorubicin-induced ER stress by inhibiting TRPC3 and TRPC6 mediated Ca(2+) overload in rat cardiomyocytes: Toxicol Lett, 2017; 276; 21-30
15. Chen R, Sun G, Yang L, Salvianolic acid B protects against doxorubicin induced cardiac dysfunction via inhibition of ER stress mediated cardiomyocyte apoptosis: Toxicol Res, 2016; 5; 1335-45
16. Zhang DX, Ma DY, Yao ZQ, ERK1/2/p53 and NF-κB dependent-PUMA activation involves in doxorubicin-induced cardiomyocyte apoptosis: Eur Rev Med Pharmacol Sci, 2016; 20; 2435-42
17. Xu A, Deng F, Chen Y, NF-κB pathway activation during endothelial-to-mesenchymal transition in a rat model of doxorubicin-induced cardiotoxicity: Biomed Pharmacother, 2020; 130; 110525
18. Sahu R, Dua TK, Das S, Wheat phenolics suppress doxorubicin-induced cardiotoxicity via inhibition of oxidative stress, MAP kinase activation, NF-κB pathway, PI3K/Akt/mTOR impairment, and cardiac apoptosis: Food Chem Toxicol, 2019; 125; 503-19
19. Song S, Chu L, Liang H, Protective effects of dioscin against doxorubicin-induced hepatotoxicity via regulation of Sirt1/FOXO1/NF-κb signal: Front Pharmacol, 2019; 10; 1030
20. Feng S, Cong H, Ji L, Salvianolic acid A exhibits anti-inflammatory and antiarthritic effects via inhibiting NF-κB and p38/MAPK pathways: Drug Des Devel Ther, 2020; 14; 1771-78
21. Zhang HF, Wang YL, Gao C, Salvianolic acid A attenuates kidney injury and inflammation by inhibiting NF-κB and p38 MAPK signaling pathways in 5/6 nephrectomized rats: Acta Pharmacol Sin, 2018; 39; 1855-64
22. Best KT, Lee FK, Knapp E, Deletion of NFKB1 enhances canonical NF-κB signaling and increases macrophage and myofibroblast content during tendon healing: Sci Rep, 2019; 9; 10926
23. Zhan J, Hu P, Wang Y, lncRNA PVT1 aggravates doxorubicin-induced cardiomyocyte apoptosis by targeting the miR-187-3p/AGO1 axis: Mol Cell Probes, 2020; 49; 101490
24. Cardinale D, Colombo A, Bacchiani G, Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy: Circulation, 2015; 131; 1981-88
25. Songbo M, Lang H, Xinyong C, Oxidative stress injury in doxorubicin-induced cardiotoxicity: Toxicol Lett, 2019; 307; 41-48
26. Zilinyi R, Czompa A, Czegledi A, The cardioprotective effect of metformin in doxorubicin-induced cardiotoxicity: The role of autophagy: Molecules, 2018; 23(5); 1184
27. Tony H, Yu K, Qiutang Z, MicroRNA-208a silencing attenuates doxorubicin induced myocyte apoptosis and cardiac dysfunction: Oxid Med Cell Longev, 2015; 2015; 597032
28. Wang Y, Zhu S, Liu H, Thyroxine alleviates energy failure, prevents myocardial cell apoptosis, and protects against doxorubicin-induced cardiac injury and cardiac dysfunction via the LKB1/AMPK/mTOR axis in mice: Dis Markers, 2019; 2019; 7420196
29. Oh KS, Oh BK, Mun J, Salvianolic acid A suppress lipopolysaccharide-induced NF-κB signaling pathway by targeting IKKβ: Int Immunopharmacol, 2011; 11; 1901-6
30. Zhang HF, Wang JH, Wang YL, Salvianolic acid A protects the kidney against oxidative stress by activating the Akt/GSK-3β/Nrf2 signaling pathway and inhibiting the NF-κB signaling pathway in 5/6 nephrectomized rats: Oxid Med Cell Longev, 2019; 2019; 2853534
Figures
 Figure 1. The effect of salvianolic acid A (SalA) and doxorubicin (Dox) on H9C2 cell viability. (A) the chemical structure of SalA. (B–D) H9C2 cells were exposed to 0, 2, 10, and 50 μM SalA for 12 h (B); 0, 1, 2, 4, and 8 μM Dox for 12 h (C); or SalA and 4 μM Dox co-treatment for 12 h (D). Cell viability was then measured by CCK-8 assay. *** P<0.001; # P<0.05; ### P<0.001.
Figure 1. The effect of salvianolic acid A (SalA) and doxorubicin (Dox) on H9C2 cell viability. (A) the chemical structure of SalA. (B–D) H9C2 cells were exposed to 0, 2, 10, and 50 μM SalA for 12 h (B); 0, 1, 2, 4, and 8 μM Dox for 12 h (C); or SalA and 4 μM Dox co-treatment for 12 h (D). Cell viability was then measured by CCK-8 assay. *** P<0.001; # P<0.05; ### P<0.001. Figure 2. The effect of salvianolic acid A (SalA) on doxorubicin (Dox)-induced apoptosis in H9C2 cells. (A, B) Representative images (×200) and quantitative analysis for TUNEL staining in H9C2 cells. (C) The expression of proteins related to apoptosis in H9C2 cells was detected by western blot. ** P<0.01; *** P<0.001; # P<0.05; ### P<0.001.
Figure 2. The effect of salvianolic acid A (SalA) on doxorubicin (Dox)-induced apoptosis in H9C2 cells. (A, B) Representative images (×200) and quantitative analysis for TUNEL staining in H9C2 cells. (C) The expression of proteins related to apoptosis in H9C2 cells was detected by western blot. ** P<0.01; *** P<0.001; # P<0.05; ### P<0.001. Figure 3. The effect of salvianolic acid A (SalA) on doxorubicin (Dox)-activated nuclear factor-κB (NF-κB) signaling and nuclear factor kappa B subunit 1 (NFKB1) expression in H9C2 cells. (A) The expression of proteins involved in the NF-κB pathway in H9C2 cells was detected by western blot. (B) The mRNA expression of NFKB1 in H9C2 cells that were subjected to different treatments. (C) The protein expression of cytoplasmic and nuclear p50 in H9C2 cells. *** P<0.001; # P<0.05; ## P<0.01; ### P<0.001.
Figure 3. The effect of salvianolic acid A (SalA) on doxorubicin (Dox)-activated nuclear factor-κB (NF-κB) signaling and nuclear factor kappa B subunit 1 (NFKB1) expression in H9C2 cells. (A) The expression of proteins involved in the NF-κB pathway in H9C2 cells was detected by western blot. (B) The mRNA expression of NFKB1 in H9C2 cells that were subjected to different treatments. (C) The protein expression of cytoplasmic and nuclear p50 in H9C2 cells. *** P<0.001; # P<0.05; ## P<0.01; ### P<0.001. Figure 4. The interaction between nuclear factor kappa B subunit 1 (NFKB1) and plasmacytoma variant translocation 1 (PVT1) in H9C2 cells. (A) The binding sequences between NFKB1 and lncRNA PVT1 promoter. (B, C) The mRNA and protein expression of NFKB1 in H9C2 cells overexpressed with NFKB1 or not. (D) PVT1 expression in H9C2 cells overexpressed with NFKB1 or not. (E, F) The interaction between NFKB1 and PVT1 was confirmed by luciferase report (E) and chromatin immunoprecipitation (F) assays. *** P<0.001.
Figure 4. The interaction between nuclear factor kappa B subunit 1 (NFKB1) and plasmacytoma variant translocation 1 (PVT1) in H9C2 cells. (A) The binding sequences between NFKB1 and lncRNA PVT1 promoter. (B, C) The mRNA and protein expression of NFKB1 in H9C2 cells overexpressed with NFKB1 or not. (D) PVT1 expression in H9C2 cells overexpressed with NFKB1 or not. (E, F) The interaction between NFKB1 and PVT1 was confirmed by luciferase report (E) and chromatin immunoprecipitation (F) assays. *** P<0.001. Figure 5. The effect of nuclear factor kappa B subunit 1 (NFKB1) overexpression on salvianolic acid A (SalA)-inhibited apoptosis in H9C2 cells. (A) The expression of plasmacytoma variant translocation 1 (PVT1) in H9C2 cells. (B) Cell viability of H9C2 cells that were subjected to indicated treatments. (C, D) Representative images (×200) and quantitative analysis for TUNEL staining in H9C2 cells. (E) The expression of proteins related to apoptosis in H9C2 cells was detected by western blot. *** P<0.001; ### P<0.001. @ P<0.05; @@ P<0.01; @@@ P<0.001.
Figure 5. The effect of nuclear factor kappa B subunit 1 (NFKB1) overexpression on salvianolic acid A (SalA)-inhibited apoptosis in H9C2 cells. (A) The expression of plasmacytoma variant translocation 1 (PVT1) in H9C2 cells. (B) Cell viability of H9C2 cells that were subjected to indicated treatments. (C, D) Representative images (×200) and quantitative analysis for TUNEL staining in H9C2 cells. (E) The expression of proteins related to apoptosis in H9C2 cells was detected by western blot. *** P<0.001; ### P<0.001. @ P<0.05; @@ P<0.01; @@@ P<0.001. In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








