14 March 2021: Clinical Research
Association of Hydrogen Sulfide with Femoral Bone Mineral Density in Osteoporosis Patients: A Preliminary Study
Yan-Ming Hao1ACDE, Da-Wei He2BD, Yan Gao1BCF, Ling-Na Fang3E, Pan-Pan Zhang4BC, Ke Lu1BC, Rong-Zhu Lu2B, Chong Li5AEFG*DOI: 10.12659/MSM.929389
Med Sci Monit 2021; 27:e929389
Abstract
BACKGROUND: Accumulated evidence has suggested that hydrogen sulfide (H₂S) has a role in bone formation and bone tissue regeneration. However, it is unknown whether the H₂S content is associated with bone mineral density (BMD) in patients with osteopenia/osteoporosis.
MATERIAL AND METHODS: In the present study, we aimed to explore the changes of serum H₂S in osteopenia and osteoporosis patients. We analyzed femur expression of cystathionine β synthase (CBS), cystathionine γ lyase (CSE), and 3-mercaptopyruvate sulfurtransferase (3-MST), which are key enzymes for generating H₂S.
RESULTS: Sixteen (16%) patients had osteopenia, 9 (9%) had osteoporosis, and 75 (75%) had normal BMD. In comparison with patients with normal BMD (controls), the serum levels of H₂S were unexpectedly increased in patients with osteopenia and osteoporosis. This increase was much higher in patients with osteoporosis than in those with osteopenia. Serum H₂S levels were negatively correlated with femoral BMD, but not lumbar BMD. Interestingly, the expression of CBS and CSE were downregulated in femur tissues in patients with osteoporosis, whereas the expression of 3-MST remained unchanged. Serum phosphorus levels, alkaline phosphatase, hemoglobin, and triglycerides were found to be closely associated with CBS and CSE scores in femur tissues.
CONCLUSIONS: Serum H₂S levels and femur CBS and CSE expression may be involved in osteoporosis pathogenesis.
Keywords: Alkaline Phosphatase, Bone Diseases, Metabolic, Hydrogen Sulfide, Osteoporosis, Aged, 80 and over, Bone Density, Cystathionine beta-Synthase, Cystathionine gamma-Lyase, Femur, Sulfurtransferases
Background
Osteoporosis is a prevalent disease that is characterized by reduced bone mass, diminished bone integrity, and increased risk of fractures [1]. It is estimated that osteoporosis affects 200 million people worldwide [2]. Osteoporosis is a common disorder in women after menopause, and it may also develop in men or in people with hormonal disorders or conditions that require chronic glucocorticoid medications [3]. Age-related osteoporosis occurs in both women and man, with bone loss beginning from the age of 40 years and continuing for the remainder of adult life [4]. Early diagnosis and treatment of osteoporosis can protect patients with osteoporosis from experiencing bone fractures [5]. Advanced radiological technology and laboratory detection of bone turnover biomarkers are commonly used for the differential diagnosis of osteoporosis [6]. The measurement of bone mineral density (BMD) by dual-energy X-ray absorptiometry is frequently used to diagnose osteoporosis and to assess osteoporotic fracture risk [7]. However, the presence or absence of osteoporotic fractures varies in osteoporotic patients [8]. Recently, determination of plasma bone biomarkers such as procollagen type 1 amino-terminal propeptide (P1NP) and C-terminal telopeptide of type 1 collagen (CTX) has emerged as a novel strategy to diagnose osteoporosis [9]. Nevertheless, such biomarkers are clinically limited due to their irrelevance with regard to fracture risk and their low specificity or sensitivity for osteoporosis [10].
In spite of the high prevalence of osteoporosis, its prevention and treatment have been problematic. Under normal conditions of homeostasis, bone undergoes constant turnover throughout an individual’s lifespan. This turnover is precisely controlled by bone-resorbing osteoclasts and osteocytes, as well as bone-forming osteoblasts [11,12]. Activation of osteoclasts and inactivation of osteoblasts synergistically contribute to the development and progression of various bone diseases, including osteoporosis [13,14]. Therefore, current treatment regimens for osteoporosis fall into 2 categories: antiresorptive drugs, such as estrogen-receptor analogs or bisphosphonates, that inhibit osteoclast function, and anabolic drugs, namely parathyroid hormone, that induce osteoblastic bone formation. However, clinical use of these drugs is limited because of their side effects. For example, prolonged use of estrogen replacement is associated with various complications, including breast cancer, uterine bleeding, and cardiovascular events, while bisphosphonates can lead to osteonecrosis of the jaw [15]. The prescription of parathyroid hormone is also restricted by the potential incidence of osteosarcoma [16]. Furthermore, some patients do not respond to these drugs, whether administered individually or in combination [17,18]. It should also be noted that bone loss is closely associated with primary as well as secondary forms of osteoporosis, and secondary osteoporosis needs to be treated according to the primary concern, which may be diabetes, uremia, or chronic glucocorticoid therapy, among others [19,20]. Although the etiopathology of primary osteoporosis has yet to be fully understood, differential therapies should be considered for distinct subgroups, such as juvenile, postmenopausal, and senile osteoporosis [19]. Therefore, it is necessary to identify novel strategies and/or targets for preventing and treating osteoporosis.
Hydrogen sulfide (H2S) is a ubiquitous gaseous molecule that is endogenously produced by cystathionine-β-synthase (CBS), cystathionine-γ-lyase (CSE), and 3-mercaptopyruvate sulfurtransferase (3-MST) within the transsulfuration pathway [21–23]. Since it was first identified as a novel gasotransmitter, along with nitric oxide and carbon monoxide, ample evidence has shown the importance of H2S in the regulation of multiple physiological functions [24–26]. In bone tissue, H2S has been well documented to play a critical role in bone pathologies. Deficiency of H2S is involved in the process of bone remodeling by impairing bone formation in ovariectomized mice, a classical model of osteoporosis [27]. The dysregulated biogenesis of H2S disrupts osteogenic differentiation of stromal cells [28], and CBS-deficient mice display an osteopenic phenotype [28,29]. Also, a recent study has demonstrated that H2S donor GYY4137 significantly relieves the inhibitory effects of dexamethasone on bone formation by activating Wnt signaling [30]. Accordingly, sodium hydrosulfide (NaHS), a donor for H2S, has also been shown to attenuate dexamethasone-induced systemic damage of bone mass and osteoblast dysfunction [31]. Treatment with NaHS normalizes plasma H2S and prevents osteoporotic bone loss in CBS-deficient mice by inhibiting inflammatory cytokines [32,33]. Exogenous H2S ameliorates damage induced by high glucose or oxidative stress in osteoblasts [34–36], critical events involved in osteoporosis pathogenesis. Furthermore, NaHS administration inhibits the differentiation of osteoclast progenitor cells via a nuclear factor erythroid 2-related factor 2 (Nrf2)-dependent mechanism [37]. These exciting findings suggest that H2S could play a therapeutic role in systemic bone diseases, such as osteoporosis, by regulating the functions of osteoblast and osteoclast precursors [38,39]. However, the relationship between H2S and osteopenia/osteoporosis has yet to be fully elucidated in humans. In addition, the association of H2S-generating enzyme expression with bone metabolic factors is unclear. In light of the significance of H2S in bone metabolism and reconstruction, our present study aimed to identify the relationship between H2S with BMD in patients enrolled in a preliminary investigation.
Material and Methods
PATIENTS:
The preliminary clinical study was approved by the Ethics Committee of Kunshan People’s Hospital, and all procedures were in compliance with the Declaration of Helsinki. The informed consent form was signed by all participants. The exclusion criteria were as follows: (1) patients with multiple fractures, osteoporotic fractures, open fractures, or violent fractures (eg, traffic injuries, fall injuries); (2) patients with chronic liver and kidney diseases, metabolic diseases, tumors, or blood system diseases; (3) patients who had undergone ovariectomy; and (4) patients receiving long-term treatment with glucocorticoids, estrogen, calcitonin and bisphosphonates, or other treatments (including traditional Chinese medicine). A total of 100 patients in Jiangsu Kunshan People’s Hospital between October 2017 and October 2019 were enrolled in this study. All blood specimens and BMD measurements were collected at Kunshan People’s Hospital during the patients’ visit. The clinical characteristics of the participants are listed in the Table 1. A total of 100 patients (70 men and 30 women) were enrolled, and their ages ranged from 25 to 91 years (average age, 53.7 years). Based on lumbar and femoral neck BMD assessments, the control, osteopenia, and osteoporosis groups consisted of 75 (58 men and 17 women), 16 (10 men and 6 women), and 9 subjects (2 men and 7 women), respectively (Table 1). Significant differences were found between the control, osteopenia, and osteoporosis groups with regard to sex, lumbar and femoral neck BMD, as well as the lumbar and femoral neck T-values (Table 1).
BLOOD SAMPLES AND BIOCHEMICAL ANALYSIS:
Blood samples were collected by venipuncture from all participants after overnight fasting, and serum samples were obtained by centrifugation at 3000 rpm for 5 min. The sera were frozen and stored at −80°C until processing. Serum levels of alkaline phosphatase (ALP), calcium, hemoglobin, and phosphorus were measured by using an AU5800 automated chemistry analyzer (Beckman Coulter, Brea, CA, USA) in accordance with standard laboratory methods. Fasting blood glucose and triglycerides were measured with commercially available kits (Konelab, Finland), using a chemistry autoanalyzer.
MEASUREMENT OF BMD:
After blood sampling, BMD was assessed by dual-energy X-ray absorptiometry (Lunar, DPX-NP, GE Healthcare, Madison, WI, USA). Lumbar spine (L1–L4) and femoral (neck and total) scans were performed, and the T-score was defined as the number of standard deviations from the mean BMD of sex-matched young control subjects, while the Z-score reflected the standard deviations matched by sex, age, weight, and ethnicity. On the basis of World Health Organization criteria, osteoporosis was defined as a T-score of −2.5 or less and osteopenia as a T-score of greater than −2.5 to −1.0; a T-score of −1.0 or more was recognized as the normal control value.
:
The serum levels of H2S in all participants were measured by commercially available human H2S kits (catalog number: HG14903, Trust Specialty Zeal, San Francisco, CA, USA) according to the manufacturer’s instructions. In brief, the samples and standards were added to plates coated with purified antibody. After incubation, biotinylated anti-IgG was added and reacted with streptavidin to generate the antibody-antigen-enzyme-antibody complex. Next, the substrate 3,3′,5,5′-tetramethylbenzidine was added and reacted with horseradish peroxidase, and the solution eventually turned yellow under the action of acid. The optical density in each sample was measured at 450 nm, and the H2S content was transformed and calculated by a standard curve and expressed as micromoles per liter.
FEMUR TISSUE COLLECTION AND IMMUNOHISTOCHEMISTRY:
Femur tissues were collected from a separate group of 19 patients with osteoarthritis who underwent hip replacement surgery. Their femur BMD was measured before surgery, and based on T-scores, 9 of the patients had osteoporosis and 10 had normal BMD. There was no significant difference in terms of age and sex between the 2 groups (age: 79.2±3.1 vs 77.5±5.1; men/women: 2/7 vs 3/8). A total of 19 femur tissue biopsies were fixed in 4% paraformaldehyde and embedded in paraffin. After slicing of the samples, histological sections (5 μm) underwent deparaffinization and rehydration through serial immersion in xylene followed by graded alcohol. Antigen retrieval was conducted by incubating sections with bone tissue-specific antigen-retrieval solutions (Showbio, Shanghai, China) for 1 h at room temperature. Normal goat serum was used to block nonspecific binding for 15 min at room temperature, and the sections were then probed with the primary antibodies against human CBS (ab140600, Abcam, Cambridge, UK), CSE (ab54573, Abcam, Cambridge, UK), and 3-MST (ab224043, Cambridge, UK) at 4°C overnight in a humidified container. After being rinsed with phosphate-buffered saline 3 times, the sections were incubated with the secondary antibodies (Sangon, Shanghai, China) for 1 h at room temperature, followed by incubation in 3,3′-diaminobenzidine tetrahydrochloride solutions (ZLI-9017, ZSGB-Bio, Beijing, China) for 30 s. The images were photographed using a microscope (Leica, Microsystems, Germany), and immunohistochemistry was evaluated by 2 blind observers by counting the number of positive cells (at least a total of 100 in randomly selected regions) for CBS, CSE, and 3-MST. The semiquantitative evaluation was carried out as previously described [40–42]. The extent of positively stained cells was estimated and classified on a 5-point scale as follows: grade 0 (the percentage of positive cells in selected fields <10%); grade 1 (the percentage of positive cells in selected fields ≥10% and ≤25%); grade 2 (the percentage of positive cells in selected fields >25% and ≤50%); grade 3 (the percentage of positive cells in selected fields >50% and ≤75%); and grade 4 (the percentage of positive cells in selected fields >75%). The intensity of the positive staining was categorized into 3 groups: weak (1, grade 0–1), moderate (2, grade 2), and strong (3, grade 4).
STATISTICAL ANALYSES:
All continuous variables are expressed as mean±standard deviation (SD), while nonnormally distributed data are expressed as medians and interquartile ranges. The statistical analyses were carried out using SPSS for Windows (version 17.0; SPSS Inc., Chicago, IL, USA). Normality testing was conducted for all continuous variables. Data distribution was evaluated using the Kolmogorov-Smirnov test. The differences were determined using the
Results
:
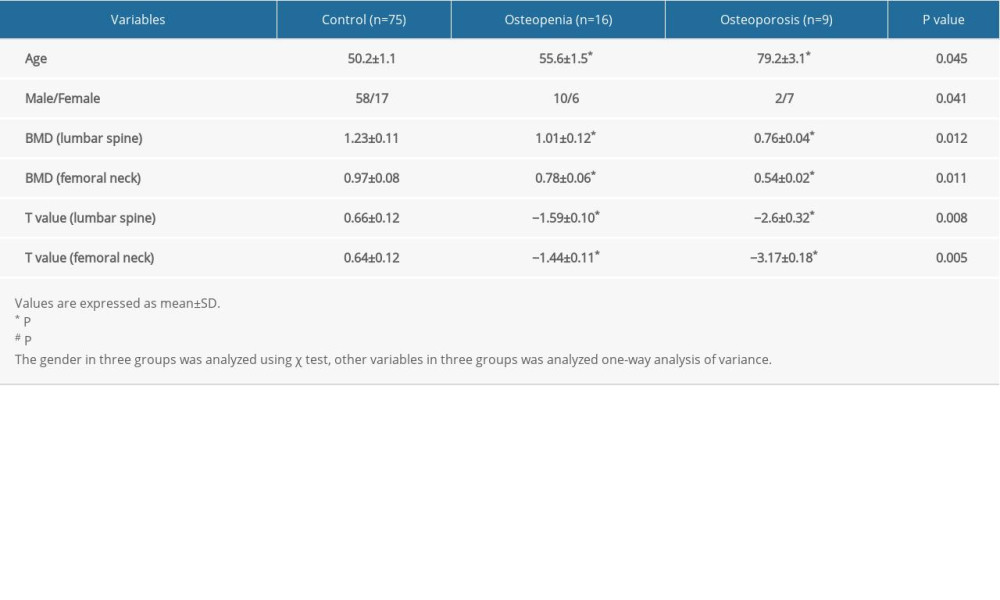
Participants’ serum H2S levels were measured to assess their significance with regard to lumbar and femoral BMD. Pearson correlation analysis showed no significant correlation between serum H2S level and lumbar BMD (Figure 1A). Interestingly, serum H2S had a significant bivariate correlation with femoral BMD among these individuals (Figure 1B), suggesting a negative association between serum H2S level and hip region BMD. Serum H2S levels were obviously higher in osteopenia patients compared with controls (P=0.041 for control vs osteopenia), while patients with osteoporosis exhibited higher levels of H2S (Figure 1C, P=0.022 for control vs osteoporosis; P=0.034 for osteoporosis vs osteopenia) in accordance with T-scores measured by dual-energy X-ray absorptiometry (Figure 1D).
:
Given that the close relationship between serum H2S level and femoral BMD, we tested the expression of CBS, CSE, and 3-MST in the femoral tissues of 19 osteoarthritis patients who underwent hip replacement surgery. Among these 19 patients, 10 subjects had normal BMD and 9 had osteoporosis. In line with previous reports [43–47], blood phosphorus, hemoglobin, and triglyceride levels tended to be decreased in osteoporosis patients (Figure 2). By contrast, ALP was upregulated in osteoporosis patients compared with the control group (Figure 2). However, blood calcium and fasting blood glucose did not differ statistically between the 3 groups (Figure 2). To verify the correlation between H2S-producing enzymes in femoral tissue and clinical parameters, the expression of CBS, CSE, and 3-MST was evaluated by immunohistochemistry. The results demonstrated that the expression of CBS and CSE was downregulated in femur tissues from patients with osteoporosis, whereas the expression of 3-MST was unchanged, relative to the control group (Figure 3). The correlation between H2S-generating enzymes in femur tissue and the clinical biochemical indicators in serum was analyzed in 2 different groups of patients (Figures 4, 5). We found that the femoral CBS score was positively correlated with serum levels of phosphorus, hemoglobin, and triglycerides, as well as the femoral T-score (Figure 4). The univariate regression analysis showed that serum ALP was negatively associated with the femoral CBS value (Figure 4). Similar results were also found regarding the association of the femoral CSE value with serum phosphorus, hemoglobin, ALP, and triglycerides, as well as femoral T-score (Figure 5). Notably, both serum calcium and fasting blood glucose levels showed no correlation with femoral CBS/CSE values (Figures 4, 5).
Discussion
LIMITATIONS:
Immunohistochemistry-related results are only semiquantitative experiments, which is a limitation in this study. As such, quantitative evaluations (such as quantification of mRNA or expression of CBS, CSE, and 3-MST by reverse-transcription polymerase chain reaction or western blotting, respectively) would be more appropriate to investigate the association of femoral expression of H2S-producing enzymes with biochemical indicators. In line with this, the quantitative analysis of H2S in femoral tissues is necessary to further confirm the importance of femoral H2S concentrations in osteoporosis. Given that the distribution of H2S-producing enzymes in bone cells is still unclear, immunofluorescence double-staining experiments are needed to verify which H2S-producing enzymes are expressed by each type of bone cells, especially osteoblasts and osteoclasts. The close relationship between H2S and BMD shown in the current study is only the first step in determining the exact roles of H2S in osteoporosis. It remains to be explored whether H2S could serve as a biomarker for prediction of comorbidities and prognosis of osteoporosis, and future cohort studies or randomized control trials are required to confirm the nature of the causal relationship. Additionally, multicenter studies are necessary to strengthen the generalizability of our results. Since age and sex are factors that are closely related to osteoporosis, our analysis might not be representative for incidence, sex, and age. In addition, the groups of osteopenic and osteoporotic patients might be pooled with different etiologies. A more rigorous clinical experimental design is needed to verify the exact significance of H2S in the pathogenesis of osteoporosis. Regardless, the newly developed therapeutic approaches for osteoporosis, particularly those designed to target bone H2S, should be extensively studied in the near future. We speculate that genetic analyses of CBS/CSE gene polymorphisms might predict which patients are at higher or lower risk of osteoporosis and deserve further studies.
Conclusions
In conclusion, we reported the relationship between H2S and BMD in an osteopenia/osteoporosis population. We found that serum H2S levels were significantly negatively associated with femoral BMD, while femoral CBS/CSE expression was positively related with femoral BMD. These findings support the view that H2S plays a central role in bone metabolism and osteoporosis. Considering that the etiologies of primary or secondary osteoporosis are quite different, the biological significance of H2S may vary in different types of osteoporosis. Future research is needed to explore the potential roles and mechanisms of H2S in primary and secondary osteoporosis.
Figures
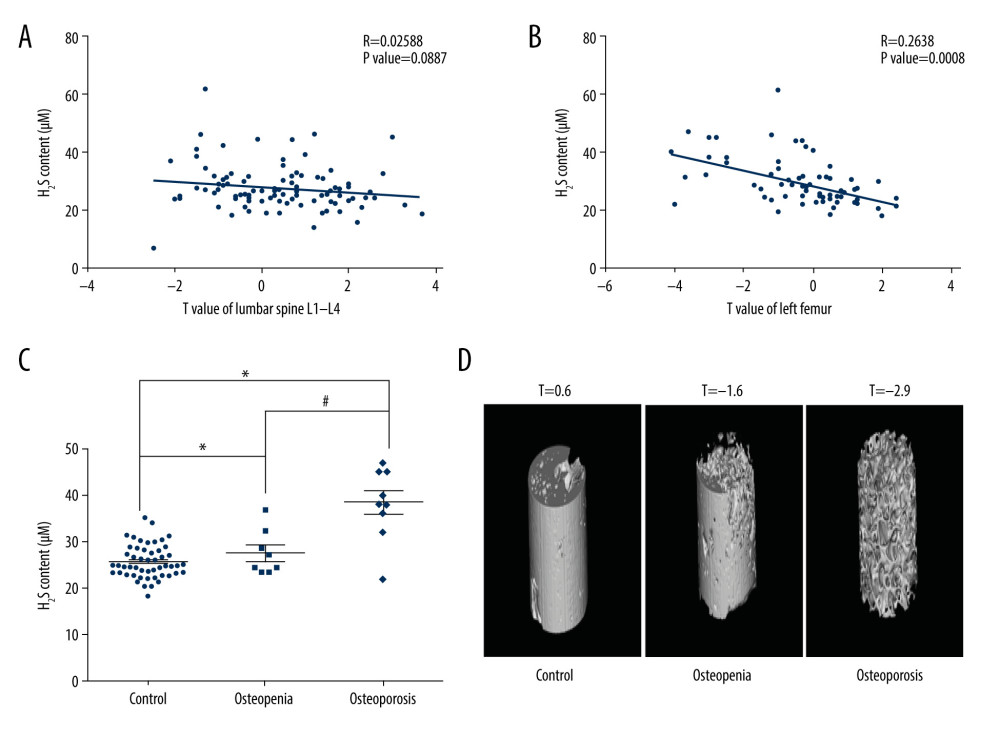 Figure 1. Association of serum H2S level with bone mineral density (BMD). (A) Correlation between serum H2S levels and lumbar BMD. (B) Correlation between serum H2S levels and femoral BMD. (C) Serum H2S levels in osteopenia/osteoporosis patients and control subjects. (D) Representative images of femoral scans from dual-energy X-ray absorptiometry. Pearson correlation analysis was used to determine the correlation between H2S level and lumbar BMD. Pearson correlation analysis was used to determine the correlation between H2S level and femoral BMD. One-way analysis of variance was used to compare serum H2S level in osteopenia/osteoporosis patients and control subjects. Values are expressed as mean±SD. * P<0.05 vs control, # P<0.05 vs osteopenia.
Figure 1. Association of serum H2S level with bone mineral density (BMD). (A) Correlation between serum H2S levels and lumbar BMD. (B) Correlation between serum H2S levels and femoral BMD. (C) Serum H2S levels in osteopenia/osteoporosis patients and control subjects. (D) Representative images of femoral scans from dual-energy X-ray absorptiometry. Pearson correlation analysis was used to determine the correlation between H2S level and lumbar BMD. Pearson correlation analysis was used to determine the correlation between H2S level and femoral BMD. One-way analysis of variance was used to compare serum H2S level in osteopenia/osteoporosis patients and control subjects. Values are expressed as mean±SD. * P<0.05 vs control, # P<0.05 vs osteopenia. 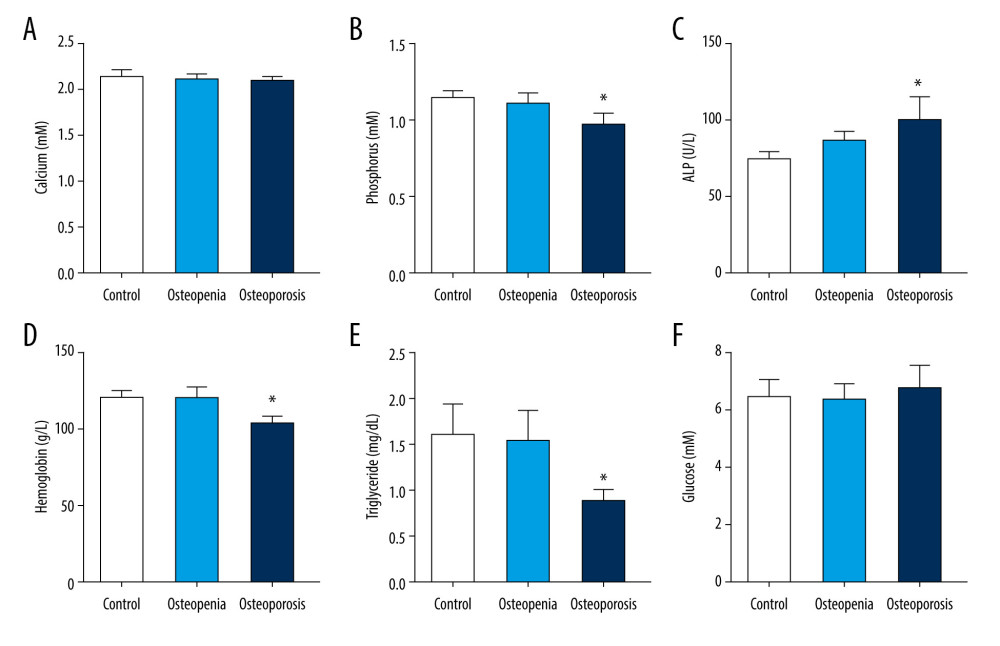 Figure 2. Clinical biochemical indicators in control subjects and osteopenia/osteoporosis patients: (A) serum calcium levels; (B) serum phosphorus levels; (C) serum alkaline phosphatase (ALP) levels; (D) serum hemoglobin levels; (E) serum triglyceride levels; and (F) fasting blood glucose levels. One-way analysis of variance was used to compare these clinical biochemical indicators in osteopenia/osteoporosis patients and control subjects. Values are expressed as mean±SD. * P<0.05 vs control.
Figure 2. Clinical biochemical indicators in control subjects and osteopenia/osteoporosis patients: (A) serum calcium levels; (B) serum phosphorus levels; (C) serum alkaline phosphatase (ALP) levels; (D) serum hemoglobin levels; (E) serum triglyceride levels; and (F) fasting blood glucose levels. One-way analysis of variance was used to compare these clinical biochemical indicators in osteopenia/osteoporosis patients and control subjects. Values are expressed as mean±SD. * P<0.05 vs control.  Figure 3. Expression of H2S-producing enzymes in femoral tissues. (A) Representative images of CBS immunohistochemistry staining. (B) Representative images of CSE immunohistochemistry staining. (C) Representative images of 3-MST immunohistochemistry staining. (D) Scores for CBS, CSE, and 3-MST. Independent t test was used to compare scores for CBS, CSE, and 3-MST in control and osteoporosis patients. Values are expressed as mean±SD. * P<0.05 vs control. CBS – cystathionine β synthase; CSE – cystathionine γ lyase; 3-MST – 3-mercaptopyruvate sulfurtransferase.
Figure 3. Expression of H2S-producing enzymes in femoral tissues. (A) Representative images of CBS immunohistochemistry staining. (B) Representative images of CSE immunohistochemistry staining. (C) Representative images of 3-MST immunohistochemistry staining. (D) Scores for CBS, CSE, and 3-MST. Independent t test was used to compare scores for CBS, CSE, and 3-MST in control and osteoporosis patients. Values are expressed as mean±SD. * P<0.05 vs control. CBS – cystathionine β synthase; CSE – cystathionine γ lyase; 3-MST – 3-mercaptopyruvate sulfurtransferase. 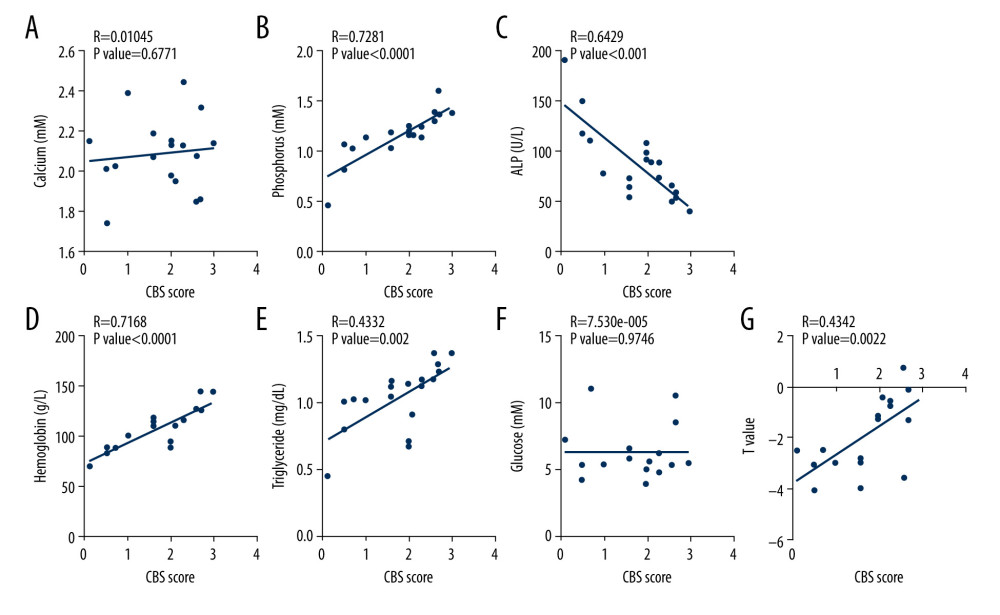 Figure 4. Association of femoral cystathionine β synthase (CBS) expression with clinical characteristics. (A) Correlation between femoral CBS expression and serum calcium levels. (B) Correlation between femoral CBS expression and serum phosphorus levels. (C) Correlation between femoral CBS expression and serum alkaline phosphatase (ALP) levels. (D) Correlation between femoral CBS expression and serum hemoglobin levels. (E) Correlation between femoral CBS expression and serum triglycerides levels. (F) Correlation between femoral CBS expression and fasting blood glucose levels. (G) Correlation between femoral CBS expression and femoral T value. Multivariate forward stepwise linear regression analysis was used to identify the correlation between femoral CBS expression and clinical characteristics.
Figure 4. Association of femoral cystathionine β synthase (CBS) expression with clinical characteristics. (A) Correlation between femoral CBS expression and serum calcium levels. (B) Correlation between femoral CBS expression and serum phosphorus levels. (C) Correlation between femoral CBS expression and serum alkaline phosphatase (ALP) levels. (D) Correlation between femoral CBS expression and serum hemoglobin levels. (E) Correlation between femoral CBS expression and serum triglycerides levels. (F) Correlation between femoral CBS expression and fasting blood glucose levels. (G) Correlation between femoral CBS expression and femoral T value. Multivariate forward stepwise linear regression analysis was used to identify the correlation between femoral CBS expression and clinical characteristics. 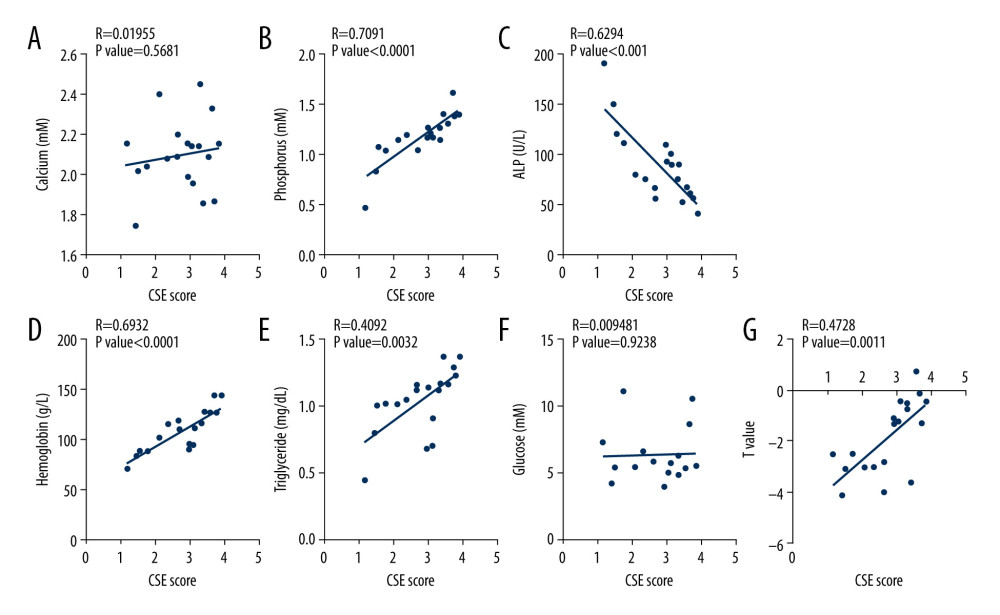 Figure 5. Association of femoral cystathionine γ lyase (CSE) expression with clinical characteristics. (A) Correlation between femoral CSE expression and serum calcium levels. (B) Correlation between femoral CSE expression and serum phosphorus levels. (C) Correlation between femoral CSE expression and serum alkaline phosphatase (ALP) levels. (D) Correlation between femoral CSE expression and serum hemoglobin levels. (E) Correlation between femoral CSE expression and serum triglycerides levels. (F) Correlation between femoral CSE expression and fasting blood glucose levels. (G) Correlation between femoral CSE expression and femoral T value. Multivariate forward stepwise linear regression analysis was used to identify the correlation between femoral CSE expression and clinical characteristics.
Figure 5. Association of femoral cystathionine γ lyase (CSE) expression with clinical characteristics. (A) Correlation between femoral CSE expression and serum calcium levels. (B) Correlation between femoral CSE expression and serum phosphorus levels. (C) Correlation between femoral CSE expression and serum alkaline phosphatase (ALP) levels. (D) Correlation between femoral CSE expression and serum hemoglobin levels. (E) Correlation between femoral CSE expression and serum triglycerides levels. (F) Correlation between femoral CSE expression and fasting blood glucose levels. (G) Correlation between femoral CSE expression and femoral T value. Multivariate forward stepwise linear regression analysis was used to identify the correlation between femoral CSE expression and clinical characteristics. References
1. Bao T, Yang K, Long Z, Systematic pharmacological methodology to explore the pharmacological mechanism of siwu decoction for osteoporosis: Med Sci Monit, 2019; 25; 8152-71
2. Cooper C, Epidemiology of osteoporosis: Osteoporos Int, 1999; 9(Suppl 2); S2-8
3. Li L, Yang M, Jin A, COL3A1, COL6A3, and SERPINH1 are related to glucocorticoid-induced osteoporosis occurrence according to integrated bioinformatics analysis: Med Sci Monit, 2020; 26; e925474
4. Nagai K, Hayashi K, Yasui T, Disease history and risk of comorbidity in women’s life course: A comprehensive analysis of the Japan Nurses’ Health Study baseline survey: BMJ Open, 2015; 5; e006360
5. Lee SH, Cho EH, Ahn SH, Prediction of future osteoporotic fracture occurrence by genetic profiling: A 6-year follow-up observational study: J Clin Endocrinol Metab, 2016; 101; 1215-24
6. Zhu Y, Shen J, Cheng Q, Plasma homocysteine level is a risk factor for osteoporotic fractures in elderly patients: Clin Interv Aging, 2016; 11; 1117-21
7. Kanis JA, Cooper C, Rizzoli R, Reginster JY, European guidance for the diagnosis and management of osteoporosis in postmenopausal women: Osteoporos Int, 2019; 30; 3-44
8. Compston JE, McClung MR, Leslie WD, Osteoporosis: Lancet, 2019; 393; 364-76
9. Eastell R, Szulc P, Use of bone turnover markers in postmenopausal osteoporosis: Lancet Diabetes Endocrinol, 2017; 5; 908-23
10. Szulc P, Montella A, Delmas PD, High bone turnover is associated with accelerated bone loss but not with increased fracture risk in men aged 50 and over: The prospective MINOS study: Ann Rheum Dis, 2008; 67; 1249-55
11. Yin X, Zhou C, Li J, Autophagy in bone homeostasis and the onset of osteoporosis: Bone Res, 2019; 7; 28
12. Wong SK, Chin KY, The effects of tocotrienol on bone peptides in a rat model of osteoporosis induced by metabolic syndrome: The possible communication between bone cells: Int J Environ Res Public Health, 2019; 16; 3313
13. Chen YH, Peng SY, Cheng MT, Different susceptibilities of osteoclasts and osteoblasts to glucocorticoid-induced oxidative stress and mitochondrial alterations: Chin J Physiol, 2019; 62; 70-79
14. Hou YC, Wu CC, Liao MT, Role of nutritional vitamin D in osteoporosis treatment: Clin Chim Acta, 2018; 484; 179-91
15. Khosla S, Burr D, Cauley J, Bisphosphonate-associated osteonecrosis of the jaw: Report of a task force of the American Society for Bone and Mineral Research: J Bone Miner Res, 2007; 22; 1479-91
16. Yasothan U, Kar S, Osteoporosis: Overview and pipeline: Nat Rev Drug Discov, 2008; 7; 725-26
17. Chesnut CH, Rosen CJ, Reconsidering the effects of antiresorptive therapies in reducing osteoporotic fracture: J Bone Miner Res, 2001; 16; 2163-72
18. Black DM, Greenspan SL, Ensrud KE, The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis: N Engl J Med, 2003; 349; 1207-15
19. Franck HDifferential therapy of osteoporosis: Z Rheumatol, 1990; 49; 329-37 [in German]
20. Xi L, Zhang Y, Gupta H, A multiscale study of structural and compositional changes in a natural nanocomposite. Osteoporotic bone with chronic endogenous steroid excess: Bone, 2021; 143; 115666
21. Sun HJ, Wu ZY, Cao L, Role of nitroxyl (HNO) in cardiovascular system: From biochemistry to pharmacology: Pharmacol Res, 2020; 159; 104961
22. Gambari L, Grigolo B, Grassi F, Hydrogen sulfide in bone tissue regeneration and repair: State of the art and new perspectives: Int J Mol Sci, 2019; 20; 5231
23. Powell CR, Dillon KM, Matson JB, A review of hydrogen sulfide (H(2)S) donors: Chemistry and potential therapeutic applications: Biochem Pharmacol, 2018; 149; 110-23
24. Sun HJ, Lee WT, Leng B, Nitroxyl as a potential theranostic in the cancer arena: Antioxid Redox Signal, 2020; 32; 331-49
25. Koning AM, Frenay AR, Leuvenink HG, van Goor H, Hydrogen sulfide in renal physiology, disease and transplantation – the smell of renal protection: Nitric Oxide, 2015; 46; 37-49
26. Feliers D, Lee HJ, Kasinath BS, Hydrogen sulfide in renal physiology and disease: Antioxid Redox Signal, 2016; 25; 720-31
27. Grassi F, Tyagi AM, Calvert JW, Hydrogen sulfide is a novel regulator of bone formation implicated in the bone loss induced by estrogen deficiency: J Bone Miner Res, 2016; 31; 949-63
28. Liu Y, Yang R, Liu X, Hydrogen sulfide maintains mesenchymal stem cell function and bone homeostasis via regulation of Ca(2+) channel sulfhydration: Cell Stem Cell, 2014; 15; 66-78
29. Robert K, Maurin N, Ledru A, Hyperkeratosis in cystathionine beta synthase-deficient mice: An animal model of hyperhomocysteinemia: Anat Rec A Discov Mol Cell Evol Biol, 2004; 280; 1072-76
30. Ma J, Shi C, Liu Z, Hydrogen sulfide is a novel regulator implicated in glucocorticoids-inhibited bone formation: Aging (Albany NY), 2019; 11; 7537-52
31. Ma J, Fu Q, Wang Z, Sodium hydrosulfide mitigates dexamethasone-induced osteoblast dysfunction by interfering with mitochondrial function: Biotechnol Appl Biochem, 2019; 66; 690-97
32. Behera J, Kelly KE, Voor MJ, Hydrogen sulfide promotes bone homeostasis by balancing inflammatory cytokine signaling in CBS-deficient mice through an epigenetic mechanism: Sci Rep, 2018; 8; 15226
33. Behera J, George AK, Voor MJ, Hydrogen sulfide epigenetically mitigates bone loss through OPG/RANKL regulation during hyperhomocysteinemia in mice: Bone, 2018; 114; 90-108
34. Liu Y, Liu J, Li X, Exogenous H(2)S prevents high glucose-induced damage to osteoblasts through regulation of KATP channels: Biochimie, 2017; 137; 151-57
35. Yan X, Wu H, Wu Z, The new synthetic H(2)S-releasing SDSS protects MC3T3-E1 osteoblasts against H(2)O(2)-induced apoptosis by suppressing oxidative stress, inhibiting MAPKs, and activating the PI3K/Akt pathway: Front Pharmacol, 2017; 8; 7
36. Xu ZS, Wang XY, Xiao DM: Free Radic Biol Med, 2011; 50; 1314-23
37. Gambari L, Lisignoli G, Cattini L, Sodium hydrosulfide inhibits the differentiation of osteoclast progenitor cells via NRF2-dependent mechanism: Pharmacol Res, 2014; 87; 99-112
38. Zhai Y, Tyagi SC, Tyagi N, Cross-talk of microRNA and hydrogen sulfide: A novel therapeutic approach for bone diseases: Biomed Pharmacother, 2017; 92; 1073-84
39. Kurabayashi M, Hydrogen sulfide. A new regulator of osteoclastogenesis?: Arterioscler Thromb Vasc Biol, 2014; 34; 471-73
40. Li S, Liu B, Zhang L, Rong L, Amyloid beta peptide is elevated in osteoporotic bone tissues and enhances osteoclast function: Bone, 2014; 61; 164-75
41. Zhao C, Li H, Wang L, Sun W, An immunohistochemical study of stathmin 1 expression in osteosarcoma shows an association with metastases and poor patient prognosis: Med Sci Monit, 2018; 24; 6070-78
42. Mangaonkar A, Mondal AK, Fulzule S, A novel immunohistochemical score to predict early mortality in acute myeloid leukemia patients based on indoleamine 2,3 dioxygenase expression: Sci Rep, 2017; 7; 12892
43. Wu YT, Hsu BG, Lower serum fibroblast growth factor 21 levels are associated with normal lumbar spine bone mineral density in hemodialysis patients: Int J Environ Res Public Health, 2020; 17; 1938
44. Huang N, Zhou J, Wang W, Retinol-binding protein 4 is positively associated with bone mineral density in patients with type 2 diabetes and osteopenia or osteoporosis: Clin Endocrinol (Oxf), 2018; 88; 659-64
45. Chang IC, Chiang TI, Yeh KT, Increased serum osteopontin is a risk factor for osteoporosis in menopausal women: Osteoporos Int, 2010; 21; 1401-9
46. Mohiti-Ardekani J, Soleymani-Salehabadi H, Owlia MB, Mohiti A, Relationships between serum adipocyte hormones (adiponectin, leptin, resistin), bone mineral density and bone metabolic markers in osteoporosis patients: J Bone Miner Metab, 2014; 32; 400-4
47. Eroglu S, Karatas G, Platelet/lymphocyte ratio is an independent predictor for osteoporosis: Saudi Med J, 2019; 40; 360-66
48. Yang M, Huang Y, Chen J, Activation of AMPK participates hydrogen sulfide-induced cyto-protective effect against dexamethasone in osteoblastic MC3T3-E1 cells: Biochem Biophys Res Commun, 2014; 454; 42-47
49. Yang X, Hao D, Zhang H, Treatment with hydrogen sulfide attenuates sublesional skeletal deterioration following motor complete spinal cord injury in rats: Osteoporos Int, 2017; 28; 687-95
50. Arora I, Sharma M, Sun LY, Tollefsbol TO, The epigenetic link between polyphenols, aging and age-related diseases: Genes (Basel), 2020; 11; 1094
51. Ginaldi L, De Martinis M, Interleukin-33 serum levels in postmenopausal women with osteoporosis: Sci Rep, 2019; 9; 3786
52. Bahney CS, Zondervan RL, Allison P, Cellular biology of fracture healing: J Orthop Res, 2019; 37; 35-50
53. Gambari L, Lisignoli G, Gabusi E, Distinctive expression pattern of cystathionine-β-synthase and cystathionine-γ-lyase identifies mesenchymal stromal cells transition to mineralizing osteoblasts: J Cell Physiol, 2017; 232; 3574-85
54. Zheng Y, Liao F, Lin X, Cystathionine γ-lyase-hydrogen sulfide induces runt-related transcription factor 2 sulfhydration, thereby increasing osteoblast activity to promote bone fracture healing: Antioxid Redox Signal, 2017; 27; 742-53
55. Lambertini E, Penolazzi L, Angelozzi M, The expression of cystathionine gamma-lyase is regulated by estrogen receptor alpha in human osteoblasts: Oncotarget, 2017; 8; 101686-96
56. Zhai Y, Behera J, Tyagi SC, Hydrogen sulfide attenuates homocysteine-induced osteoblast dysfunction by inhibiting mitochondrial toxicity: J Cell Physiol, 2019; 234; 18602-14
57. Lv M, Liu Y, Xiao TH, GYY4137 stimulates osteoblastic cell proliferation and differentiation via an ERK1/2-dependent anti-oxidant mechanism: Am J Transl Res, 2017; 9; 1183-92
58. Cen SD, Yu WB, Ren MM, Endogenous hydrogen sulfide is involved in osteogenic differentiation in human periodontal ligament cells: Arch Oral Biol, 2016; 68; 1-8
59. Gambari L, Amore E, Raggio R, Hydrogen sulfide-releasing silk fibroin scaffold for bone tissue engineering: Mater Sci Eng C Mater Biol Appl, 2019; 102; 471-82
60. Jiang Z, Hua Y, Hydrogen sulfide promotes osteogenic differentiation of human periodontal ligament cells via p38-MAPK signaling pathway under proper tension stimulation: Arch Oral Biol, 2016; 72; 8-13
61. Jiang XW, Zhang Y, Cheng YZ, The expression of endogenous hydrogen sulfide signal during distraction osteogenesis in a rabbit model: Int J Oral Maxillofac Surg, 2018; 47; 262-67
62. Jiang X, Chen Y, Lu K, GYY4137 promotes bone formation in a rabbit distraction osteogenesis model. A preliminary report: J Oral Maxillofac Surg, 2015; 73; 732.e71-6
63. Zhang Y, Chai Y, Pan X, Tai chi for treating osteopenia and primary osteoporosis. A meta-analysis and trial sequential analysis: Clin Interv Aging, 2019; 14; 91-104
64. Tomita ASerum biochemical parameters in osteoporosis: Nihon Rinsho, 1994; 52; 2291-94 [in Japanese]
65. Bover J, Ureña P, Aguilar A, Alkaline phosphatases in the complex chronic kidney disease-mineral and bone disorders: Calcif Tissue Int, 2018; 103; 111-24
66. Cauley JA, Osteoporosis: fracture epidemiology update 2016: Curr Opin Rheumatol, 2017; 29; 150-56
67. Gaudio A, Morabito N, Xourafa A, Bisphosphonates in the treatment of thalassemia-associated osteoporosis: J Endocrinol Invest, 2008; 31; 181-84
68. Yamaguchi TBone metabolism in dyslipidemia and metabolic syndrome: Clin Calcium, 2011; 21; 677-82 [in Japanese]
69. Sakai A, Nakamura TEffects of SERMs on bone health. Efficacy of SERM for incidence of fractures in osteoporotic patients with lifestyle-related diseases: Clin Calcium, 2010; 20; 322-29 [in Japanese]
Figures
 Figure 1. Association of serum H2S level with bone mineral density (BMD). (A) Correlation between serum H2S levels and lumbar BMD. (B) Correlation between serum H2S levels and femoral BMD. (C) Serum H2S levels in osteopenia/osteoporosis patients and control subjects. (D) Representative images of femoral scans from dual-energy X-ray absorptiometry. Pearson correlation analysis was used to determine the correlation between H2S level and lumbar BMD. Pearson correlation analysis was used to determine the correlation between H2S level and femoral BMD. One-way analysis of variance was used to compare serum H2S level in osteopenia/osteoporosis patients and control subjects. Values are expressed as mean±SD. * P<0.05 vs control, # P<0.05 vs osteopenia.
Figure 1. Association of serum H2S level with bone mineral density (BMD). (A) Correlation between serum H2S levels and lumbar BMD. (B) Correlation between serum H2S levels and femoral BMD. (C) Serum H2S levels in osteopenia/osteoporosis patients and control subjects. (D) Representative images of femoral scans from dual-energy X-ray absorptiometry. Pearson correlation analysis was used to determine the correlation between H2S level and lumbar BMD. Pearson correlation analysis was used to determine the correlation between H2S level and femoral BMD. One-way analysis of variance was used to compare serum H2S level in osteopenia/osteoporosis patients and control subjects. Values are expressed as mean±SD. * P<0.05 vs control, # P<0.05 vs osteopenia. Figure 2. Clinical biochemical indicators in control subjects and osteopenia/osteoporosis patients: (A) serum calcium levels; (B) serum phosphorus levels; (C) serum alkaline phosphatase (ALP) levels; (D) serum hemoglobin levels; (E) serum triglyceride levels; and (F) fasting blood glucose levels. One-way analysis of variance was used to compare these clinical biochemical indicators in osteopenia/osteoporosis patients and control subjects. Values are expressed as mean±SD. * P<0.05 vs control.
Figure 2. Clinical biochemical indicators in control subjects and osteopenia/osteoporosis patients: (A) serum calcium levels; (B) serum phosphorus levels; (C) serum alkaline phosphatase (ALP) levels; (D) serum hemoglobin levels; (E) serum triglyceride levels; and (F) fasting blood glucose levels. One-way analysis of variance was used to compare these clinical biochemical indicators in osteopenia/osteoporosis patients and control subjects. Values are expressed as mean±SD. * P<0.05 vs control. Figure 3. Expression of H2S-producing enzymes in femoral tissues. (A) Representative images of CBS immunohistochemistry staining. (B) Representative images of CSE immunohistochemistry staining. (C) Representative images of 3-MST immunohistochemistry staining. (D) Scores for CBS, CSE, and 3-MST. Independent t test was used to compare scores for CBS, CSE, and 3-MST in control and osteoporosis patients. Values are expressed as mean±SD. * P<0.05 vs control. CBS – cystathionine β synthase; CSE – cystathionine γ lyase; 3-MST – 3-mercaptopyruvate sulfurtransferase.
Figure 3. Expression of H2S-producing enzymes in femoral tissues. (A) Representative images of CBS immunohistochemistry staining. (B) Representative images of CSE immunohistochemistry staining. (C) Representative images of 3-MST immunohistochemistry staining. (D) Scores for CBS, CSE, and 3-MST. Independent t test was used to compare scores for CBS, CSE, and 3-MST in control and osteoporosis patients. Values are expressed as mean±SD. * P<0.05 vs control. CBS – cystathionine β synthase; CSE – cystathionine γ lyase; 3-MST – 3-mercaptopyruvate sulfurtransferase. Figure 4. Association of femoral cystathionine β synthase (CBS) expression with clinical characteristics. (A) Correlation between femoral CBS expression and serum calcium levels. (B) Correlation between femoral CBS expression and serum phosphorus levels. (C) Correlation between femoral CBS expression and serum alkaline phosphatase (ALP) levels. (D) Correlation between femoral CBS expression and serum hemoglobin levels. (E) Correlation between femoral CBS expression and serum triglycerides levels. (F) Correlation between femoral CBS expression and fasting blood glucose levels. (G) Correlation between femoral CBS expression and femoral T value. Multivariate forward stepwise linear regression analysis was used to identify the correlation between femoral CBS expression and clinical characteristics.
Figure 4. Association of femoral cystathionine β synthase (CBS) expression with clinical characteristics. (A) Correlation between femoral CBS expression and serum calcium levels. (B) Correlation between femoral CBS expression and serum phosphorus levels. (C) Correlation between femoral CBS expression and serum alkaline phosphatase (ALP) levels. (D) Correlation between femoral CBS expression and serum hemoglobin levels. (E) Correlation between femoral CBS expression and serum triglycerides levels. (F) Correlation between femoral CBS expression and fasting blood glucose levels. (G) Correlation between femoral CBS expression and femoral T value. Multivariate forward stepwise linear regression analysis was used to identify the correlation between femoral CBS expression and clinical characteristics. Figure 5. Association of femoral cystathionine γ lyase (CSE) expression with clinical characteristics. (A) Correlation between femoral CSE expression and serum calcium levels. (B) Correlation between femoral CSE expression and serum phosphorus levels. (C) Correlation between femoral CSE expression and serum alkaline phosphatase (ALP) levels. (D) Correlation between femoral CSE expression and serum hemoglobin levels. (E) Correlation between femoral CSE expression and serum triglycerides levels. (F) Correlation between femoral CSE expression and fasting blood glucose levels. (G) Correlation between femoral CSE expression and femoral T value. Multivariate forward stepwise linear regression analysis was used to identify the correlation between femoral CSE expression and clinical characteristics.
Figure 5. Association of femoral cystathionine γ lyase (CSE) expression with clinical characteristics. (A) Correlation between femoral CSE expression and serum calcium levels. (B) Correlation between femoral CSE expression and serum phosphorus levels. (C) Correlation between femoral CSE expression and serum alkaline phosphatase (ALP) levels. (D) Correlation between femoral CSE expression and serum hemoglobin levels. (E) Correlation between femoral CSE expression and serum triglycerides levels. (F) Correlation between femoral CSE expression and fasting blood glucose levels. (G) Correlation between femoral CSE expression and femoral T value. Multivariate forward stepwise linear regression analysis was used to identify the correlation between femoral CSE expression and clinical characteristics. In Press
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952