19 January 2021: Database Analysis
Subtypes of Influenza Virus Infection and Outcomes in Individuals Older than 65 Years of Age in Poland in the 2016/2017 to 2019/2020 Epidemic Seasons
Katarzyna Łuniewska1ABCDEF*, Karol Szymański1B, Katarzyna Kondratiuk1A, Ewelina Hallmann1F, Lidia B. Brydak1DGDOI: 10.12659/MSM.929243
Med Sci Monit 2021; 27:e929243
Abstract
BACKGROUND: Influenza is a viral disease causing many deaths each season. With aging, the human immune system becomes weaker, so people over the age of 65 years are at higher risk of complications after influenza infections. This population study, conducted in Poland, aimed to identify the subtypes of influenza virus infection and outcomes in individuals more than 65 years of age in the 2016/2017 to 2019/2020 epidemic seasons.
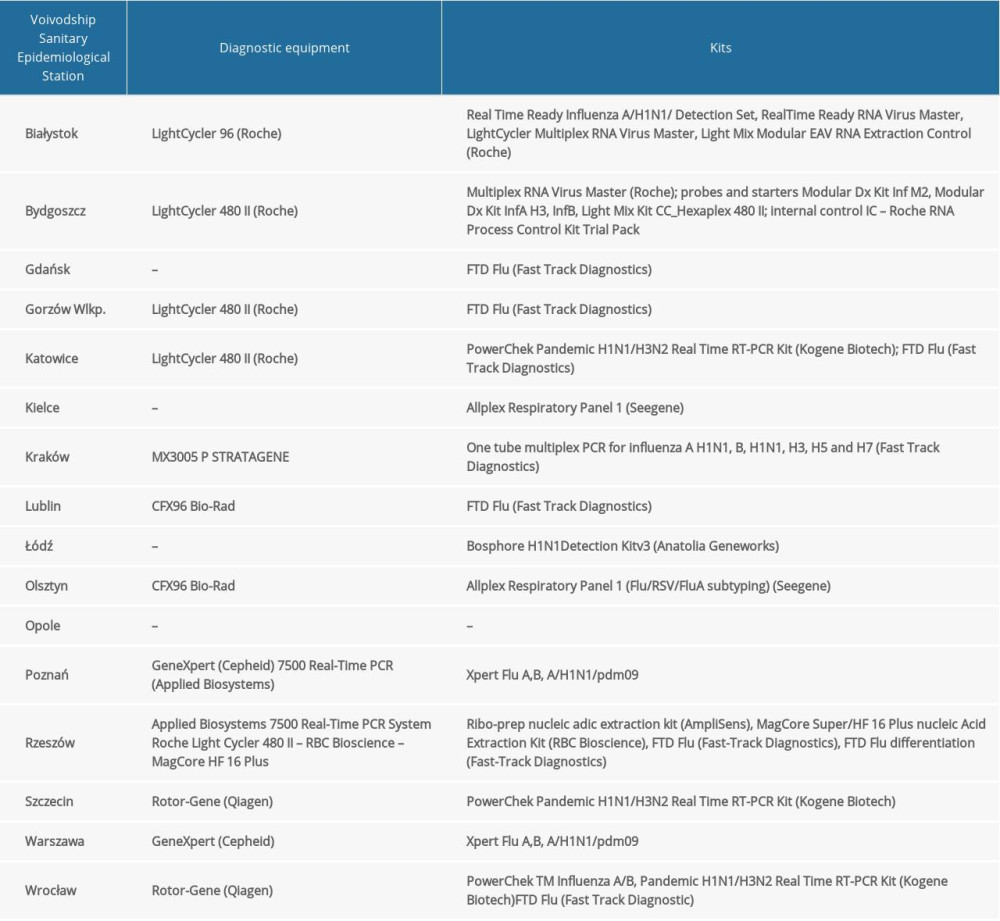
MATERIAL AND METHODS: The research materials were nose and throat swabs. Research was conducted in 16 Voivodship Sanitary and Epidemiological Stations and in the Department of Influenza Research, National Influenza Centre, NIPH-NIH. Methods of RNA isolation depended on the laboratory where the isolation was performed. In all laboratories, quantitative polymerase chain reaction (qRT-PCR) was used to determine the influenza virus type and subtype.
RESULTS: The analysis of the incidence of influenza among people over the age of 65 included the 2016/2017, 2017/2018, 2018/2019, and 2019/2020 influenza epidemic seasons. We analyzed the percentage of positive samples, the dynamics of epidemic seasons, and the percentage share of influenza viruses in the 65+ age group, according to the epidemic season and percentage of deaths.
CONCLUSIONS: This population study showed that, in Poland, between the 2016/2017 and 2019/2020 epidemic seasons, people who were more than 65 years of age were at higher risk of influenza virus infection and its complications. The findings support the importance of seasonal influenza vaccination in the population over age 65 years.
Keywords: Influenza A virus, Influenza B virus, Vaccination, Virology, Age Distribution, epidemics, Geriatric Assessment, Incidence, Influenza A Virus, H1N1 Subtype, Influenza, Human, Poland, Risk Assessment
Background
Influenza is a viral disease that affects as many as 3–5 million people worldwide each year, causing 290 000 to 650 000 deaths [1]. The majority of deaths occur among people over the age of 65–70 years [2,3]. Contracting influenza at this age can lead not only to death, but also to numerous complications, including cardiac complication because such patients often have chronic diseases than can exacerbate the consequences of influenza infection [4]. The only effective method of protection against the influenza virus is seasonal vaccination. People over the age of 65 are included in the high-risk group by the WHO, and influenza vaccination is therefore particularly recommended for them [1].
With age, the human immune system becomes increasingly weaker, which debilitates the response to vaccination. Therefore, in the USA in 2009, the Fluzone High-Dose vaccine was introduced to the market, which contains a 4 times higher dose of 3 antigens and is reserved for people over 65 years of age [5]. Studies have shown that the effectiveness of such vaccinations in the elderly is significantly higher [6]. Since the 2018/2019 season, this vaccine has also been available in a 4-component version. This type of vaccine is only available in the US market.
The second vaccine intended only for people over the age of 65 is the FLUAD vaccine. It is an inactivated ternary vaccine adjuvanted with MF59. The addition of the adjuvant is intended to enhance the immune response to vaccination [5]. This type of vaccine was introduced to the Italian market in 1997. It is currently registered in 38 countries, including 15 European countries. In Poland, this type of vaccine is not available [7].
In Poland, there are 2 types of vaccines suitable for people over 65 years of age. These include 4-component split and subunit vaccines [8]. Polish local governments have decided to support vaccination of the elderly, and a free vaccination program for people over the age of 65 has been introduced in many regions of the country or a 50% discount of the purchase has been offered. Such measures are aimed to encourage elderly people to become vaccinated against influenza and achieve the 75% vaccination coverage rate of the population recommended by ECDC and WHO [9].
The population studies conducted earlier in Poland show that the groups most exposed to influenza are children (0–4 years of age) and people over 65 years of age [10]. Influenza infections caused by the A/H3N2/ subtype are most common in people over the age of 65 [11]; they lead to outbreaks in long-term nursing homes and cause high mortality in this age group [12].
Therefore, this population study, conducted in Poland, aimed to identify the subtypes of influenza virus infection and outcomes in individuals more than 65 years of age in the 2016/2017 to 2019/2020 epidemic seasons.
Material and Methods
VIRAL RNA ISOLATION:
A previously collected swab was suspended in 1 ml of physiological saline. The Maxwell 16 Viral Total Nucleic Acid Purification Kit (Promega Corporation, Madison, WI, USA) was used to isolate the genetic material at the Department of Influenza Research, National Influenza Center. According to the recommendations of the manufacturer, 200 μL of the suspension was taken. An inventory of isolation reagents used in other laboratories was not prepared.
TYPING AND SUBTYPING INFLUENZA VIRUSES:
Quantitative polymerase chain reaction (qRT-PCR) was used to determine the influenza virus type and subtype. At the Department of Influenza Research, National Influenza Center, the reaction was performed using Rotor-Gene Q (Qiagen) and the SuperScript Platinum III kit (Invitrogen). We used primers and probes kits (Influenza A, Influenza A/H3N2/, Influenza A/H1N1/pdm09, Influenza B) obtained from the International Reagent Resource run by the Centers for Disease Control and Prevention. The sequences of the primers and probes from IRR were not publicly available. RNA was subjected to reverse transcription (50°C, 30 min). The obtained DNA was subjected to initial denaturation (1 cycle at 95°C for 2 min), followed by 45 cycles of amplification: denaturation at 95°C for 15 s, annealing at 55°C for 10 s, and elongation at 72°C for 20 s. RNA of vaccine viruses selected by the WHO were used as positive controls. The research was also carried out in the Voivodship Sanitary and Epidemiological Stations. The methods used are presented in the Table 1.
Results
PERCENTAGE OF POSITIVE SAMPLES:
The analysis of the incidence of influenza among people over the age of 65 in the epidemic seasons 2016/2017, 2017/2018, 2018/2019, and 2019/2020 was carried out based on virological data from all over Poland. An increase in the number of tests from season to season was observed. At the same time, the percentage of positive samples decreased (Figure 1). In the 2016/2017 epidemic season, the percentage of positive samples was 50.68%, in the 2017/2018 season it was 43.18%, in the 2018/2019 season it was 35.32%, and in the 2019/2020 season it was 27.34%.
DYNAMICS OF INFLUENZA:
The dynamics of epidemic seasons was also analyzed. The epidemic peak recorded was the earliest in the 2016/2017 season (week 3 of 2017). In the 2017/2018 epidemic season, the epidemic peak was observed much later, in the 8th week. In the 2018/2019 epidemic season, the peak of the season was in week 7, while in the 2019/2020 season it occurred in week 10 (Figure 2).
PERCENTAGE OF INFLUENZA VIRUSES DEPENDING ON THE EPIDEMIC SEASON:
The percentage share of influenza viruses in the 65+ age group, depending on the epidemic season, was also examined. In the 2016/2017, 2018/2019, and 2019/2020 seasons, type A influenza was dominant, while in the 2017/2018 season type B influenza was dominant. In the 2016/2017 epidemic season, the A/H3N2/ subtype was dominant, but subtype A/H1N1/pdm09 was dominant in the 2018/2019 season. The 2019/2020 epidemic season, the codomination of the subtypes A/H1N1/pdm09 and A/H3N2/ was recorded. Interestingly, in the 2016/2017 epidemic season, no circulation of the A/H1N1/pdm09 subtype in Poland was confirmed (Figure 3).
THE PERCENTAGE OF DEATHS CAUSED BY THE INFLUENZA VIRUS:
The percentage of deaths caused by the influenza virus in the 65+ age group in relation to the total number of deaths caused by the influenza virus in Poland in different epidemic seasons was also assessed. It was determined that in the 2016/2017–2019/2020 epidemic seasons, this percentage was always above 50%, which means that each season half of deaths caused by the influenza virus were recorded in the 65+ age group. In the 2016/2017 epidemic season, this value reached 80%, in the 2017/2018 epidemic season it was 58.3%, in the 2018/2019 epidemic season it was 52%, and in the 2019/2020 epidemic season it was 66.2% (Figure 4).
Discussion
LIMITATION OF THE STUDY:
The work is based solely on the results of analyses of samples reported to the Influenza Surveillance System
Conclusions
Influenza is a huge threat to the elderly and causes a large number of deaths every epidemic season. The best way to prevent influenza is by influenza vaccination every flu season.
This population study showed that, in Poland, between 2016/2017 and 2019/2020 epidemic seasons, people who were more than 65 years of age were at risk of influenza virus infection and its complications. These findings support the importance of seasonal influenza vaccination in the population over 65 years.
In connection with the COVID-19 pandemic, elderly people should especially be sure to receive regular vaccinations because influenza virus, upon entering the body, damages the epithelium of the respiratory tract and opens the door to other viruses or bacteria, including SARS-CoV-2.
Figures
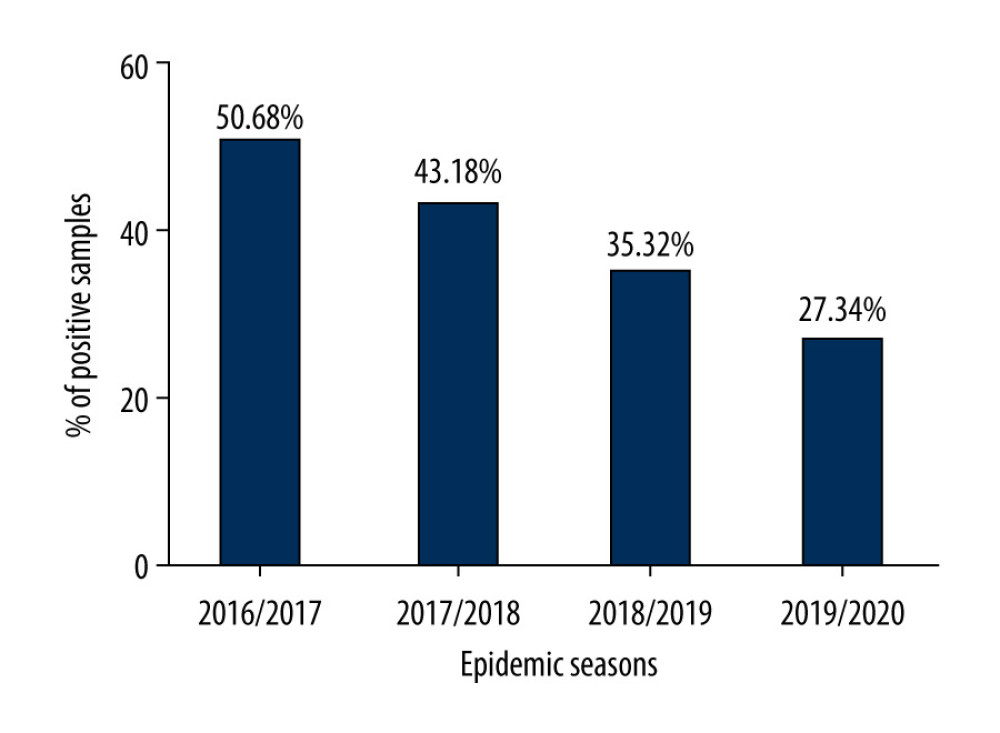 Figure 1. Percentage of positive samples in the group of people aged 65+ over several epidemic seasons in Poland.
Figure 1. Percentage of positive samples in the group of people aged 65+ over several epidemic seasons in Poland. 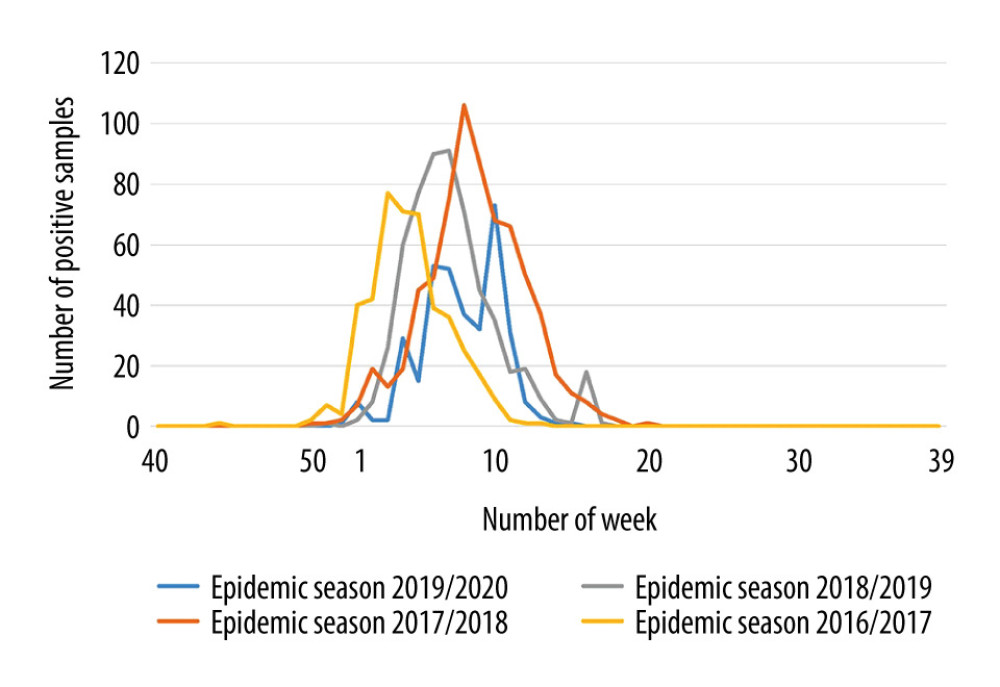 Figure 2. Dynamics of influenza in the last epidemic seasons among elderly in Poland.
Figure 2. Dynamics of influenza in the last epidemic seasons among elderly in Poland. 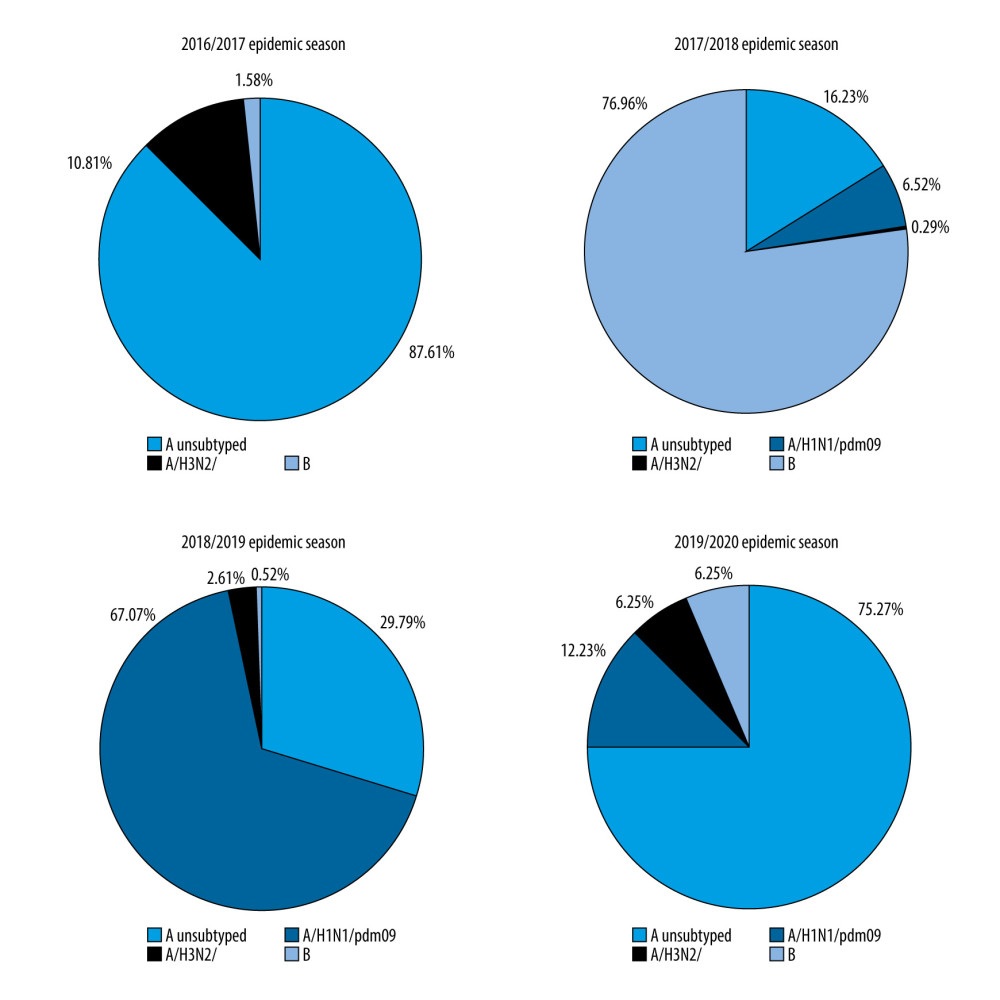 Figure 3. Percentage of influenza viruses in the 65+ age group in the different epidemic seasons.
Figure 3. Percentage of influenza viruses in the 65+ age group in the different epidemic seasons. 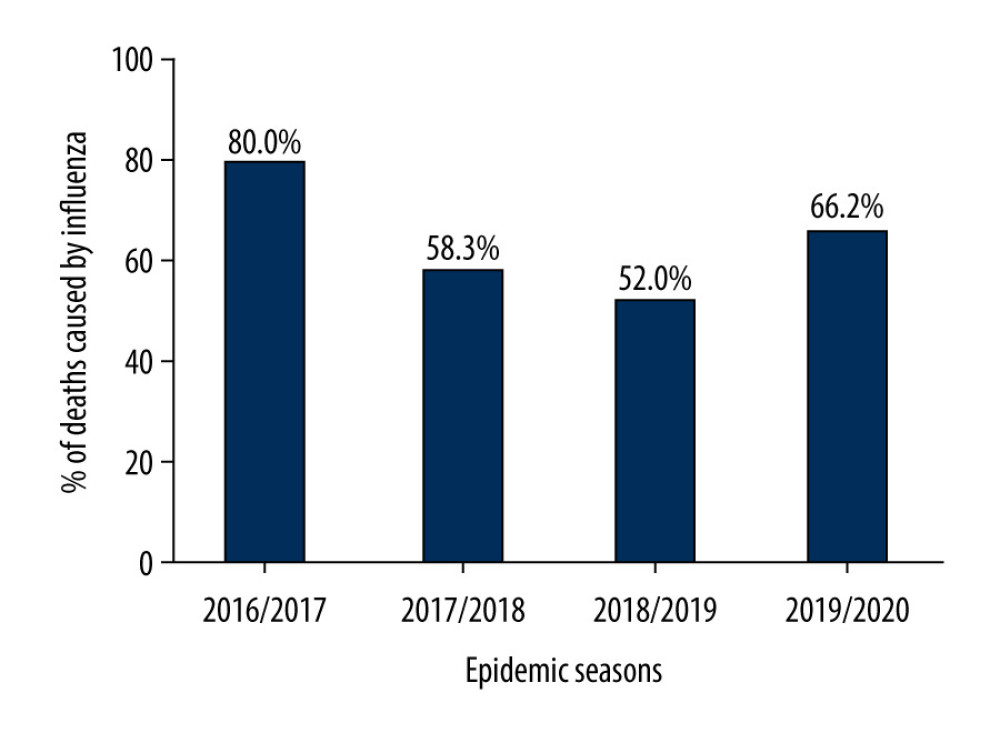 Figure 4. Percentage of deaths caused by the influenza virus in the age group 65+ in relation to the total number of deaths caused by influenza virus in Poland.
Figure 4. Percentage of deaths caused by the influenza virus in the age group 65+ in relation to the total number of deaths caused by influenza virus in Poland. References
1. World Health Organization: Influenza (seasonal) https://www.who.int/en/news-room/fact-sheets/detail/influenza-(seasonal)
2. Thompson WW, Weintraub E, Dhankhar P, Estimates of US influenza-associated deaths made using four different methods: Influenza Other Respi Viruses, 2009; 3; 37-49
3. GBD 2017 Influenza Collaborators, Mortality, morbidity, and hospitalisations due to influenza lower respiratory tract infections, 2017: An analysis for the Global Burden of Disease Study 2017: Lancet Respir Med, 2019; 7(1); 69-89
4. Brydak LB, Vaccinations in the elderly: Postgraduate Medicine, 2019; 28(9); 85-88
5. Centers for Disease Control and Prevention: Fluzone high-dose seasonal influenza vaccine https://www.cdc.gov/flu/prevent/qa_fluzone.htm
6. Diaz Granados CA, Dunning AJ, Kimmel M, Efficacy of high-dose versus standard-dose influenza vaccine in older adults: N Engl J Med, 2014; 371(7); 635-45
7. European Centre for Disease Prevention and Control: Availability of influenza vaccines by country in EU/EEA in the 2019/20 season, 2019 https://www.ecdc.europa.eu/en/publications-data/availability-influenza-vaccines-country-eueea-201920-season
8. National Institute of Public Health – National Institute of Hygiene [in Polish]https://szczepienia.pzh.gov.pl/faq/jakie-szczepionki-przeciw-grypie-sa-dostepne-w-polsce-w-sezonie-2019-2020/
9. European Centre for Disease Prevention and Control: Seasonal influenza vaccination in Europe: Vaccination recommendations and coverage rates in the EU member states for eight influenza seasons: 2007–2008 to 2014–2015 https://www.ecdc.europa.eu/en/publications-data/seasonal-influenza-vaccination-europe-vaccination-recommendations-and-coverage-2007-2015
10. Hallmann-Szelińska E, Łuniewska K, Szymański K, Virological and epidemiological situation in the influenza epidemic seasons 2016/2017 and 2017/2018 in Poland: Adv Exp Med Biol, 2020; 1251; 107-13
11. Szymański K, Kowalczyk D, Infections with Influenza A/H3N2/subtype in Poland in the 2016/2017 epidemic season: Adv Exp Med Biol, 2018; 1108; 93-98
12. Webster RG, Monto AS, Braciale TJ, Lamb RA: Textbook of influenza 2, 2013; 392-418, Hoboken, Wiley
13. : FluNews Europe https://flunewseurope.org/
14. National Institute of Public Health – National Institute of Hygiene [in Polish]http://wwwold.pzh.gov.pl/oldpage/epimeld/index_p.html
15. Wilhelm M, Influenza in older patients: A call to action and recent updates for vaccinations: Am J Manag Care, 2018; 24(2 Suppl); S15-24
16. Foppa IM, Cheng PY, Reynolds SB, Deaths averted by influenza vaccination in the U.S. during the seasons 2005/06 through 2013/14: Vaccine, 2015; 33(26); 3003-9
Figures
 Figure 1. Percentage of positive samples in the group of people aged 65+ over several epidemic seasons in Poland.
Figure 1. Percentage of positive samples in the group of people aged 65+ over several epidemic seasons in Poland. Figure 2. Dynamics of influenza in the last epidemic seasons among elderly in Poland.
Figure 2. Dynamics of influenza in the last epidemic seasons among elderly in Poland. Figure 3. Percentage of influenza viruses in the 65+ age group in the different epidemic seasons.
Figure 3. Percentage of influenza viruses in the 65+ age group in the different epidemic seasons. Figure 4. Percentage of deaths caused by the influenza virus in the age group 65+ in relation to the total number of deaths caused by influenza virus in Poland.
Figure 4. Percentage of deaths caused by the influenza virus in the age group 65+ in relation to the total number of deaths caused by influenza virus in Poland. In Press
12 Mar 2024 : Clinical Research
Comparing Neuromuscular Blockade Measurement Between Upper Arm (TOF Cuff®) and Eyelid (TOF Scan®) Using Miv...Med Sci Monit In Press; DOI: 10.12659/MSM.943630
11 Mar 2024 : Clinical Research
Enhancement of Frozen-Thawed Human Sperm Quality with Zinc as a Cryoprotective AdditiveMed Sci Monit In Press; DOI: 10.12659/MSM.942946
12 Mar 2024 : Database Analysis
Risk Factors of Age-Related Macular Degeneration in a Population-Based Study: Results from SHIP-TREND-1 (St...Med Sci Monit In Press; DOI: 10.12659/MSM.943140
12 Mar 2024 : Clinical Research
Preoperative Blood Transfusion Requirements for Hemorrhoidal Severe Anemia: A Retrospective Study of 128 Pa...Med Sci Monit In Press; DOI: 10.12659/MSM.943126
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952