26 February 2021: Clinical Research
Serum Progranulin As a Risk Predictor in Patients with Acute Myocardial Infarction
Ting Zhou1ABCD, Yanjiong Chen2B, Shihan Zhang3F, Ming Li4C, Jing Wang2AEFG*DOI: 10.12659/MSM.928864
Med Sci Monit 2021; 27:e928864
Abstract
BACKGROUND: Although progranulin was recently proposed as an adipokine that may be involved in glucose metabolic and inflammatory diseases, the role of serum progranulin in cardiovascular disease is elusive and remains disputed. The aim of our research was to determine the concentration of serum progranulin in Chinese patients with cardiovascular disease, notably in acute myocardial infarction (AMI), and its relationship to other cardiometabolic risk factors.
MATERIAL AND METHODS: This prospective observational study included 342 Chinese AMI patients and 255 healthy control subjects. Serum progranulin concentrations and various cardiometabolic risk factor levels were investigated. We assessed the relationship between progranulin and other cardiometabolic risk factors. Logistic regression analysis was applied to evaluate risk factors in patients with AMI.
RESULTS: Progranulin levels were obviously elevated in AMI patients compared to control subjects (P=0.0001). Correlation analysis showed that progranulin levels were positively associated with coronary artery disease severity (r=0.380, P=0.0001), glucose (r=0.195, P=0.015), and myeloperoxidase (r=0.198, P=0.014). In logistic regression analysis, serum progranulin (Exp(B)=1.104, 95% CI=1.043–1.168, P=0.001), myeloperoxidase (Exp(B)=1.006, 95% CI=1.003–1.008, P=0.0001), and uric acid (Exp(B)=1.020, 95% CI=1.009–1.032, P=0.0001) were independent risk factors in AMI patients.
CONCLUSIONS: Patients with AMI had significantly higher serum progranulin concentrations than control subjects. This study suggests that serum progranulin is an independent risk predictor in Chinese patients with AMI.
Keywords: Coronary Artery Disease, adipokines, Asians, Case-Control Studies, Progranulins, Prospective Studies, Risk Factors
Background
Acute myocardial infarction (AMI) remains a leading cause of cardiovascular disease mortality in both males and females, despite our best attempts to manage traditional risk factors with contemporary approaches and increase use of evidence-based therapies in recent years [1–3]. This is largely because atherosclerosis, by far the most common cause of AMI in the general population, is a complex disease process whose pathogenic basis extends far beyond intimal infiltration of cholesterol; systemic and local inflammatory events also mediate all phases of plaque development, and progression, with eventual thrombotic manifestations [4]. In particular, evidence indicates that various adipokines that mediate a number of signaling cascades in the vessel wall that exert pro-inflammatory and/or anti-inflammatory activity can directly affect the process of atherosclerosis [5–10].
Progranulin, a novel adipokine, is involved in regulating glucose metabolism, chronic inflammation, and insulin resistance [6–8,10]. Importantly, progranulin plays a key role in the pathogenesis of atherosclerosis. For instance, progranulin was found to be expressed in human atherosclerotic lesions; its expression mainly reduced inflammation, but its degradation into GRNs enhanced inflammation in atherosclerotic plaques, contributing to the progression of atherosclerosis [9]. The level of serum progranulin was identified as an independent determining risk factor for carotid atherosclerosis in subjects without metabolic syndrome [10]. However, there are only a few reports on the role of progranulin in patients with cardiovascular disease, and their results are controversial. In the short term in acute ischemic stroke patients, progranulin was found to be a serum biomarker that independently predicts all-cause mortality and adverse functional outcome [11]. Additionally, circulating progranulin was proposed as a risk factor for cardiovascular disease in obese subjects with chronic periodontitis and in patients with polycystic ovary syndrome [12]. In contrast, Korean patients with acute coronary syndrome (ACS) and stable angina pectoris (SAP) did not show significant differences in progranulin levels compared to control subjects [13].
In this current study, we aimed to clarify the clinical significance of PGRN in the setting of Chinese patients with AMI. In addition, we assessed relationship of serum PGRN with the severity of coronary artery disease and various cardiometabolic risk factors, such as traditional cardiometabolic indicators and homocysteine, myeloperoxidase, and uric acid, which are tightly linked with cardiovascular disease.
Material and Methods
SUBJECTS:
A prospective and observational clinical study was conducted in this research. During November 2018 to November 2019, a total of 342 patients with AMI in the Department of Cardiology of the Second Affiliated Hospital of Xi’an Jiaotong University were consecutively enrolled for this study. The subjects had a typical rise and fall in the level of biochemical markers of myocardial necrosis (cardiac troponin isoforms T and I, creatine kinase-MB, high-sensitivity cardiac troponin T) with at least 1 of the following: ischemic symptoms (eg, chest pain, dyspnea, diaphoresis, nausea, fatigue, or syncope), electrocardiogram (EKG) changes (ST elevation or depression, pathologic Q waves, or imaging evidence of new myocardial injury), and who had finally received percutaneous coronary intervention (PCI), stent placement, and routine antithrombotic therapy. We enrolled 255 control subjects from among people attending a routine health check-up at the Health Examination Center of the Second Affiliated Hospital of Xi’an Jiaotong University in the same time period.
All patients with coronary artery disease severity were quantitatively evaluated by the Gensini Scoring system, which is based on coronary angiography. Briefly, each coronary artery lesion is scored for stenosis diameter (1–25%=1, 26–50%=2, 51–75%=4, 76–90%=8, 91–99%=16, and complete occlusion=32). This score is multiplied by a predefined factor according to the functional relevance of the diseased vascular segment. Thus, according to the final Gensini scores, the experimental subjects were divided into 4 subgroups: 1–8 points for group I, 8–15 points for group II, 15–49.75 points for group III, and ≥49.75 points for group IV.
Exclusion criteria of the patients were as follows: severe respiratory insufficiency, stroke, type 1 and type 2 diabetes, hypertension (systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90), malignancy, renal or hepatic failure, inflammatory disease, use of systemic steroids and NSAIDs, and pregnant and lactating women. The study was approved by the Ethics Committee of Xi’an Jiaotong University, and signed informed consent was obtained from all participants enrolled in this study.
CLINICAL AND LABORATORY MEASUREMENTS:
Body mass index (BMI) was calculated as weight/height2 (kg/m2). Before routine and invasive medical treatment, all overnight fasting blood samples with patients and healthy subjects were collected and immediately stored at −80°C for subsequent assays. The levels of serum triglyceride, cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and uric acid, homocysteine and myeloperoxidase were measured enzymatically using an automated analyzer (Beckman Coulter Analysers; Lismeehan, O’Callaghan’s Mill’s Co., Clare, Ireland). The plasma glucose levels were tested by glucose oxidase method (Beckman Coulter Analysers; Ireland).
MEASUREMENT OF SERUM PGRN CONCENTRATION:
Serum PGRN concentration was determined by enzyme-linked immunosorbent assays (ELISA) according to the manufacturers’ instructions (Boster Biology Technology, Ltd, China). All samples were run in duplicate and repeated if there was a ≥15% difference between duplicates. No significant cross-reactivity or interference was observed.
STATISTICAL ANALYSIS:
Statistical analysis was performed using SPSS software version 20.0. Data are presented as the mean±standard deviation (SD). Before statistical analysis, normally distributed parameters were logarithmically transformed to approximate a normal distribution. The independent-samples
Results
CLINICAL CHARACTERISTICS:
The anthropometric and biochemical characteristics of this study subjects are summarized in Table 1. Between normal healthy subjects and patients with AMI, there were no statistically significant differences in sex, age, BMI, or glucose (P>0.05). Compared to the normal healthy group, subjects with AMI exhibited higher levels of progranulin (P=0.0001), homocysteine (P=0.001), triglyceride (P=0.0001), LDL-cholesterol (P=0.0001), myeloperoxidase (P=0.0001) and uric acid (P=0.001), whereas cholesterol (P=0.0001) and HDL-cholesterol (P=0.0001) levels were obviously lower in the AMI group.
In the study of coronary artery disease severity subgroups (Table 2), HDL-cholesterol (F=5.005, P=0.003) and myeloperoxidase (F=2.729, P=0.047) levels were obviously different with a gradually increase in the Gensini scores, but progranulin levels had no change in different subgroups (P>0.05). On the other hand, the differences in BMI, homocysteine, glucose, cholesterol, triglyceride, LDL-cholesterol, and uric acid among different subgroups of AMI patients were not significant (p>0.05).
CORRELATION OF PROGRANULIN CONCENTRATION WITH AMI CARDIOMETABOLIC RISK FACTORS:
Spearman correlation analysis (Table 3) revealed that progranulin concentration had a significant positive relationship with the severity of coronary artery disease (r=0.362, P=0.0001) and levels in glucose (r=0.195, P=0.038) and myeloperoxidase (r=0.198, P=0.014). However, circulating progranulin levels were not correlated with other laboratory indicators, including homocysteine, cholesterol, triglyceride, HDL-cholesterol, LDL-cholesterol, myeloperoxidase, and uric acid (P>0.05).
DETERMINANT FACTORS IN SUBJECTS WITH AMI:
Logistic regression analysis was used to determine whether progranulin and other cardiometabolic indicators were independent risk factors in AMI patients. The results demonstrated that progranulin (Exp(B)=1.104, 95% CI=1.043–1.168, P=0.001), myeloperoxidase (Exp(B)=1.006, 95% CI=1.003–1.008, P=0.0001), and uric acid (Exp(B)=1.020, 95% CI=1.009–1.032, P=0.0001) were independent predictive factors in patients with AMI (Table 4).
Discussion
The current study demonstrated that progranulin levels are significantly higher in patients with AMI compared to control subjects. Serum progranulin concentrations mainly have significant positive correlations with coronary artery disease severity and levels of glucose and myeloperoxidase. Moreover, we observed that serum progranulin level is an independent risk factor in subjects with AMI.
In our study, we demonstrated that conventional cardiometabolic risk factors, including triglyceride and LDL-cholesterol levels, were higher, while cholesterol and HDL-cholesterol concentrations were lower, in patients with AMI compared to control subjects, which is consistent with other previous reports [14]. Moreover, multiple studies have investigated an association between elevated myeloperoxidase levels and cardiovascular disease and a dose-response relationship between myeloperoxidase levels and cardiovascular disease severity [15–17]. This is similar to our present study, in which the serum myeloperoxidase concentration of AMI patients increased in a coronary artery stenosis severity-dependent manner according to the Gensini scores, and myeloperoxidase was identified as a risk factor for AMI. In addition, recent observational studies and clinical data have confirmed that homocysteine is a risk factor for cardiovascular disease due to its involvement in the promotion of the intracellular production of free oxygen species, inhibition of nitric oxide synthesis, activation of thrombosis, endothelial dysfunction, hyperuricemia, monocyte activation, and production of inflammatory mediators [18]. Similarly, our present results showed that serum homocysteine levels in subjects with AMI were significantly higher than in control subjects, further indicating that increased homocysteine levels play a role in the context of cardiovascular disease. In addition to the classical recognition of uric acid as the cause of gout and arthritis, recent studies have proposed that it is linked or even provokes cardiovascular disease. Neogi et al demonstrated a clear association between cardiovascular disease and uric acid [19], while Krishnan et al [20] and De Luca et al [21] did not find this association, suggesting that no definitive conclusion can be drawn as to whether uric acid is an independent predictor of cardiovascular disease. In this regard, the present study shows that uric acid levels were higher in patients with AMI than in control subjects, and indicated that it is an independent predictor of cardiovascular disease.
Progranulin, namely acrogranin or proepithelin, is secreted by a broad range of tissues and expressed by a wide variety of cell types; it consists of 593 amino acids and has a molecular weight of 68.5 kDa [22]. It is involved in a diverse array of biological activities, such as cell growth, modulation of immune responses, and neuronal effects [23]. In pathological processes, progranulin has emerged as an endogenous regulator of TNF-α-activated intracellular signaling and has anti-inflammatory actions [24]. However, owing to its complicated biological structure, not all actions of progranulin exert an inhibitory role. Progranulin was recently identified as a novel adipokine mediating high-fat diet-induced insulin resistance through upregulation of interleuin-6 (IL-6) expression in adipose tissue [25]. In type 2 diabetes and its microvascular complications, serum progranulin levels were significantly higher in patients and was associated with macrophage infiltration [26,27]. Moreover, PGRN levels were found to be significantly higher in the patients with overweight hypertensive and early renal damage [28,29]. In the present study, the serum pragranulin concentration in AMI patients was found to be obviously increased and was an independent risk factor in patients with AMI, which is in contrast to the results of Choi et al [13], who reported that patients with ACS had no significant alteration in progranulin levels, although it tended to be elevated. The observation of discrepancies in progranulin levels could be mainly explained by the use of different recruited patients with confirmed cardiovascular disease criteria and the populations with different ethnicities. Furthermore, in agreement with a previous study on the influence of progranulin on atherosclerosis processes [9], our correlation analysis showed that serum progranulin was positively associated with coronary artery disease severity, as quantitatively assessed by the Gensini method. In patients with nonalcoholic fatal liver disease and ACS and SAP, proganulin levels were associated with traditional cardiometabolic risk factors [13,30], which was in line with our findings that serum progranulin concentration showed a positive correlation with glucose. Interestingly, the present study demonstrated that progranulin concentration had a significant positive association with myeloperoxidase, which may be seen as a mediator or a mechanism by which inflammation promotes cardiovascular disease at the molecular and cellular levels [31]. These results suggest that the concentrations of serum progranulin influence the process of AMI, which may be related to classical cardiovascular factors as well as inflammation.
There are some limitations in our study that require emphasis. Given that the sample size is small, the nonsignificant correlation between progranulin and some other factors may become statistically significant if the sample size is larger. Additionally, this is a cross-sectional and observational clinical analysis that restricts us from drawing causal conclusions. It is not clear whether serum progranulin and myeloperoxidase levels are causative factors in the pathogenesis of AMI, and further studies are needed to explore the association between progranulin and myeloperoxidase in the setting of cardiovascular disease.
Conclusions
In summary, the present study clearly shows that Chinese patients with AMI had significantly elevated serum progranulin levels, which were closely associated with the degree of coronary stenosis, classical cardiometabolic risk factors, and other cardiometabolic risk factors, such as myeloperoxidase and uric acids, indicating that serum progranulin is an independent predictor for AMI.
Tables
Table 1. Baseline characteristics of the study participants.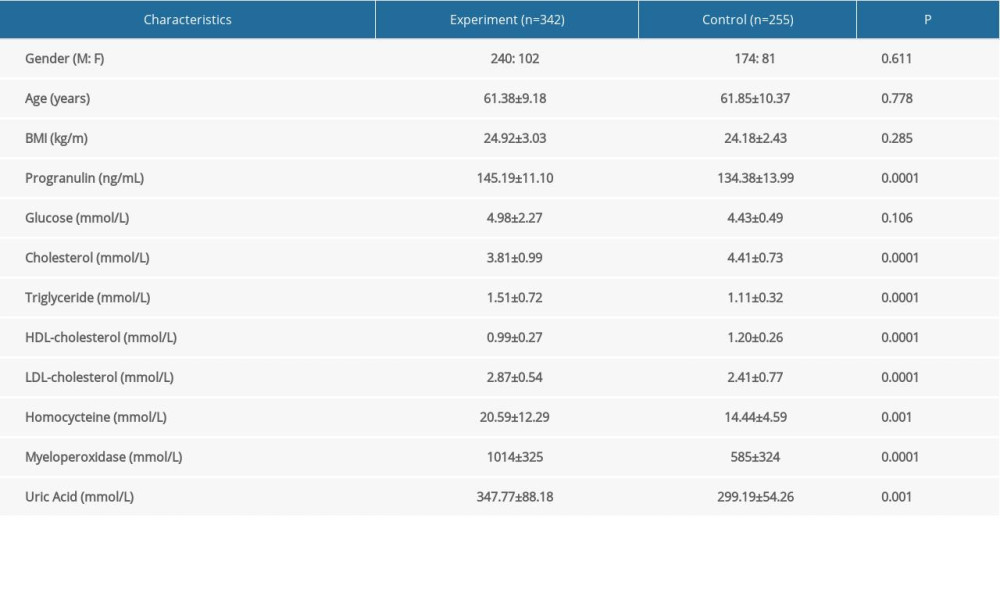 Table 2. Progranulin and other cardiometabolic indicators levels in different coronary artery disease severity subgroups evaluated by Gensini Scoring system.
Table 2. Progranulin and other cardiometabolic indicators levels in different coronary artery disease severity subgroups evaluated by Gensini Scoring system.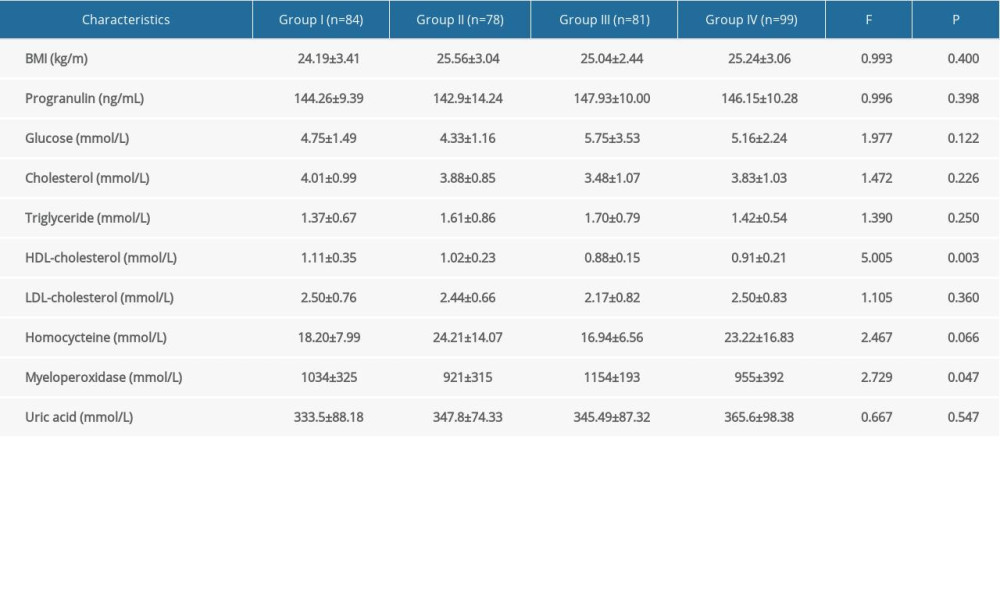 Table 3. Spearman correlation of serum progranulin with coronary artery disease severity and various cardiometabolic risk factor levels.
Table 3. Spearman correlation of serum progranulin with coronary artery disease severity and various cardiometabolic risk factor levels.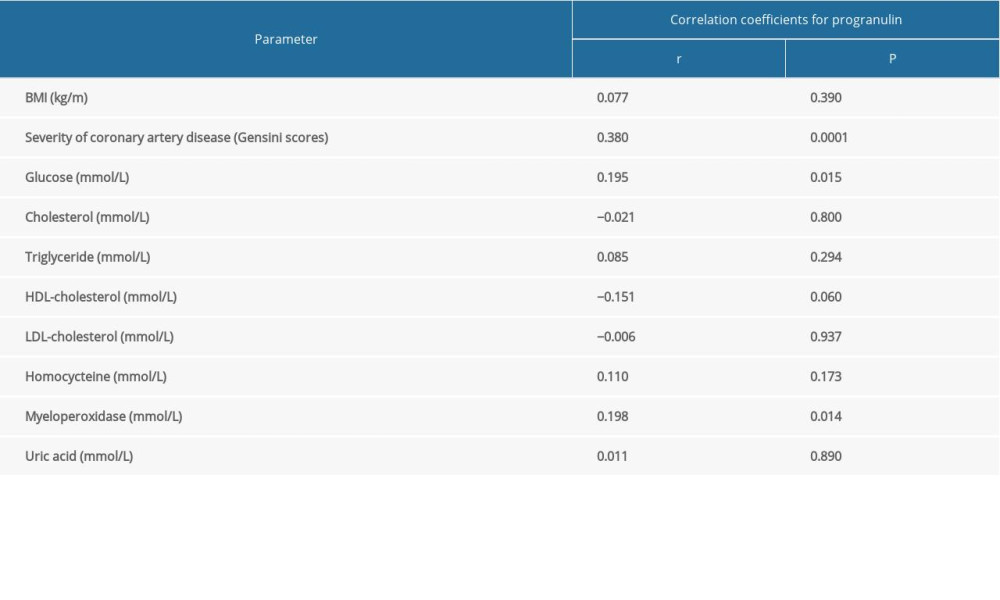 Table 4. Logistic regression analysis for determinant factors in subjects with AMI.
Table 4. Logistic regression analysis for determinant factors in subjects with AMI.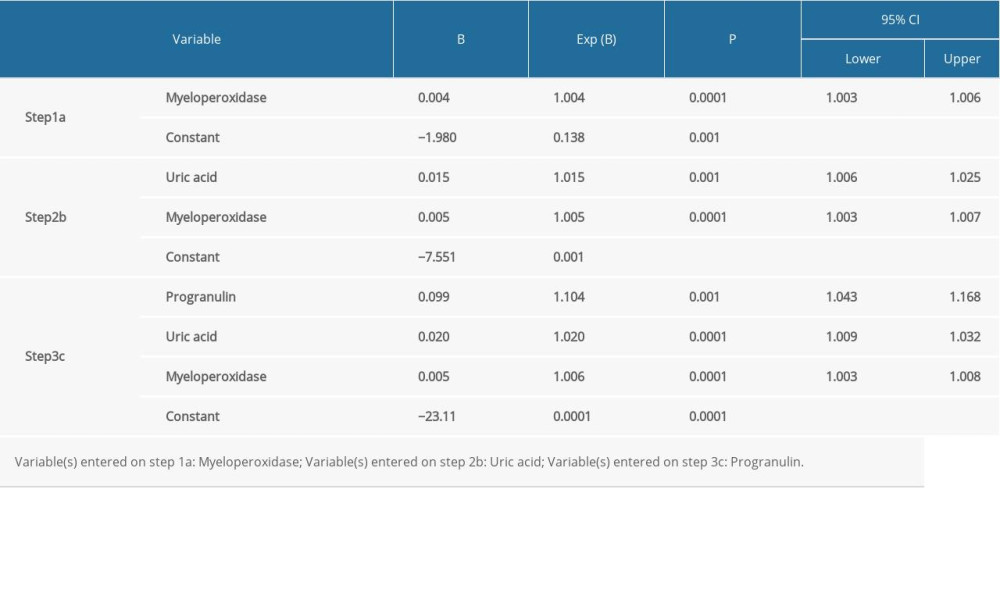
References
1. Nichols M, Townsend N, Scarborough P, Cardiovascular disease in Europe 2014: Epidemiological update: Eur Heart J, 2014; 35; 2929
2. Yeh RW, Sidney S, Chandra M, Population trends in the incidence and outcomes of acute myocardial infarction: N Engl J Med, 2010; 362; 2155-65
3. Mehta LS, Beckie TM, DeVon HA, Acute myocardial infarction in women: A scientific statement from the American Heart Association: Circulation, 2016; 133; 916-47
4. Libby P, Loscalzo J, Ridker PM, Inflammation, immunity, and infection in atherothrombosis: JACC review topic of the week: J Am Coll Cardiol, 2018; 72; 2071-81
5. Fantuzzi G, Mazzone T, Adipose tissue and atherosclerosis: Exploring the connection: Arterioscler Thromb Vasc Biol, 2007; 27; 996-1003
6. Korolczuk A, Bełtowski J, Progranulin: A new adipokine at the crossroads of metabolic syndrome, diabetes, dyslipidemia and hypertension: Curr Pharm Des, 2017; 23; 1533-39
7. Tian R, Li Y, Yao X, PGRN Suppresses inflammation and promotes autophagy in keratinocytes through the Wnt/β-catenin signaling pathway: Inflammation, 2016; 39; 1387-94
8. Liu J, Li H, Zhou B, PGRN induces impaired insulin sensitivity and defective autophagy in hepatic insulin resistance: Mol Endocrinol, 2015; 29; 528-41
9. Kojima Y, Ono K, Inoue K, Progranulin expression in advanced human atherosclerotic plaque: Atherosclerosis, 2009; 206; 102-8
10. Yoo HJ, Hwang SY, Hong HC, Implication of progranulin and C1q/TNF-related protein-3 (CTRP3) on inflammation and atherosclerosis in subjects with or without metabolic syndrome: PLoS One, 2013; 8; e55744
11. Xie S, Lu L, Liu L, Progranulin and short-term outcome in patients with acute ischaemic stroke: Eur J Neurol, 2016; 23; 648-55
12. Ersoy AO, Tokmak A, Ozler S, Are progranulin levels associated with polycystic ovary syndrome and its possible metabolic effects in adolescents and young women: Arch Gynecol Obstet, 2016; 294; 403-9
13. Choi KM, Hwang SY, Hong HC, Implications of C1q/TNF-related protein-3 (CTRP-3) and progranulin in patients with acute coronary syndrome and stable angina pectoris: Cardiovasc Diabetol, 2014; 13; 14
14. White J, Swerdlow DI, Preiss D, Association of lipid fractions with risks for coronary artery disease and diabetes: JAMA Cardiol, 2016; 1; 692-99
15. Zhang R, Brennan ML, Fu X, Association between myeloperoxidase levels and risk of coronary artery disease: JAMA, 2001; 286; 2136-42
16. Wainstein RV, Wainstein MV, Ribeiro JP, Association between myeloperoxidase polymorphisms and its plasma levels with severity of coronary artery disease: Clin Biochem, 2010; 43; 57-62
17. Goldmann BU, Rudolph V, Rudolph TK, Neutrophil activation precedes myocardial injury in patients with acute myocardial infarction: Free Radic Biol Med, 2009; 47; 79-83
18. Rallidis LS, Kosmas N, Rallidi T, Homocysteine is an independent predictor of long-term cardiac mortality in patients with stable coronary artery disease in the era of statins: Coron Artery Dis, 2020; 31; 152-56
19. Neogi T, Terkeltaub R, Ellison RC, Serum urate is not associated with coronary artery calcification: The NHLBI Family Heart Study: J Rheumatol, 2011; 38; 111-17
20. Krishnan E, Pandya BJ, Chung L, Hyperuricemia and the risk for subclinical coronary atherosclerosis-data from a prospective observational cohort study: Arthritis Res Ther, 2011; 13; R66
21. De Luca G, Secco GG, Santagostino M, Uric acid does not affect the prevalence and extent of coronary artery disease. Results from a prospective study: Nutr Metab Cardiovasc Dis, 2012; 22; 426-33
22. Baba T, Hoff HB, Nemoto H, Acrogranin, an acrosomal cysteine-rich glycoprotein, is the precursor of the growth-modulating peptides, granulins, and epithelins, and is expressed in somatic as well as male germ cells: Mol Reprod Dev, 1993; 34; 233-43
23. Liu CJ, Progranulin: A promising therapeutic target for rheumatoid arthritis: FEBS Lett, 2011; 585; 3675-80
24. Tang W, Lu Y, Tian QY, The growth factor progranulin binds to TNF receptors and is therapeutic against inflammatory arthritis in mice: Science, 2011; 332; 478-84
25. Matsubara T, Mita A, Minami K, PGRN is a key adipokine mediating high fat diet-induced insulin resistance and obesity through IL-6 in adipose tissue: Cell Metab, 2012; 15; 38-50
26. Youn BS, Bang SI, Klöting N, Serum progranulin concentrations may be associated with macrophage infiltration into omental adipose tissue: Diabetes, 2009; 58; 627-36
27. Xu L, Zhou B, Li H, Serum levels of progranulin are closely associated with microvascular complication in type 2 diabetes: Dis Markers, 2015; 2015 357279
28. Kaur J, Mukheja S, Varma S, Serum progranulin/tumor necrosis factor-α ratio as independent predictor of systolic blood pressure in overweight hypertensive patients: A cross-sectional study: Egypt Heart J, 2020; 72; 25
29. Schlatzer D, Maahs DM, Chance MR, Novel urinary protein biomarkers predicting the development of microalbuminuria and renal function decline in type 1 diabetes: Diabetes Care, 2012; 35; 549-55
30. Yilmaz Y, Eren F, Yonal O, Serum progranulin as an independent marker of liver fibrosis in patients with biopsy-proven nonalcoholic fatty liver disease: Dis Markers, 2011; 31; 205-10
31. Ndrepepa G, Myeloperoxidase – A bridge linking inflammation and oxidative stress with cardiovascular disease: Clin Chim Acta, 2019; 493; 36-51
Tables
 Table 1. Baseline characteristics of the study participants.
Table 1. Baseline characteristics of the study participants. Table 2. Progranulin and other cardiometabolic indicators levels in different coronary artery disease severity subgroups evaluated by Gensini Scoring system.
Table 2. Progranulin and other cardiometabolic indicators levels in different coronary artery disease severity subgroups evaluated by Gensini Scoring system. Table 3. Spearman correlation of serum progranulin with coronary artery disease severity and various cardiometabolic risk factor levels.
Table 3. Spearman correlation of serum progranulin with coronary artery disease severity and various cardiometabolic risk factor levels. Table 4. Logistic regression analysis for determinant factors in subjects with AMI.
Table 4. Logistic regression analysis for determinant factors in subjects with AMI. In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








