07 July 2020: Animal Study
Baicalin Suppresses Bilirubin-Induced Apoptosis and Inflammation by Regulating p38 Mitogen-Activated Protein Kinases (MAPK) Signaling in Neonatal Neurons
Shuang Shi1ABCDEF, Qianwei Cui1ACE, Jing Xu1CDEF, Zhiguo Tang1ABC, Binya Shi2ABCDEF*, Zhongwei Liu1ABCDEFGDOI: 10.12659/MSM.926441
Med Sci Monit 2020; 26:e926441
Abstract
BACKGROUND: Hyperbilirubinemia is associated with central nervous system damage in preterm neonates due to the neurotoxicity of bilirubin. This study explored the possible mechanisms of bilirubin’s neurotoxicity, and the protective effect of baicalin (BAI) was also investigated.
MATERIAL AND METHODS: Isolated neonatal rat hippocampal neurons were exposed to free bilirubin (Bf). BAI was used to treat these neurons. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used to evaluate the cell viability. Terminal deoxynucleotidyl transferase-dUTP nick-end labeling (TUNEL) assay was used to detect apoptosis. Contents of inflammatory cytokines were determined by enzyme-linked immunosorbent assay (ELISA). Protein expression and phosphorylation levels were assessed by Western blotting. Nuclear translocation was observed by immunofluorescent staining.
RESULTS: Bf incubation significantly induced apoptosis and decreased viabilities of neurons. The phosphorylation levels of MAP kinase kinase (MKK)3, MKK6, p38 mitogen- activated protein kinases (MAPK), nuclear translocation level of p65, and the expression levels of cleaved caspase3 and tumor necrosis factor (TNF)α were found to be dramatically higher in Bf-incubated neurons. BAI pre-treatment, however, increased cell viability by reducing cell apoptosis. BAI pre-treatment also reduced phosphorylation levels of MKK3, MKK6, p38 MAPK, and nuclear translocation level of p65, as well as the expression levels of cleaved caspase3 and TNFα, in Bf- incubated neurons.
CONCLUSIONS: BAI suppressed bilirubin-induced neuron apoptosis and inflammation by deactivating p38 MAPK signaling.
Keywords: bilirubin, MAP Kinase Kinase 3, MAP Kinase Kinase 6, Animals, Newborn, JNK Mitogen-Activated Protein Kinases, Neurons, Phosphorylation, primary cell culture
Background
Unconjugated hyperbilirubinemia and related clinical jaundice are frequent in newborns, which can be identified in almost all preterm and approximately 60% of full-term infants [1]. Newborns hyperbilirubinemia may protect neonates against external stimuli up to the concentration of 340 μmmol/L. However, as one of the end-products of heme catabolism, excessive bilirubin shows cytotoxic properties in the central nervous system. Clinical syndromes such as bilirubin encephalopathy (CBE) and bilirubin-induced neurologic dysfunction (BIND) are often irreversible and severe, resulting in movement disorder, auditory impairment, and ocular movement impairments [2]. Neurons located in multiple areas such as the hippocampus, basal ganglia, and diencephalon were reported to be compromised due to excessive bilirubin [3].
Molecular mechanisms underlying bilirubin-induced neurocytotoxicity are very complicated. Previous studies proposed many possible mechanisms, including oxidative stress, calcium overload, cell cycle kinetics perturbation, neuron apoptosis, and inflammation [4]. p38 MAPK is an important member of the mitogen-activated protein kinases (MAPKs), which govern several vital cellular functions [5]. The activation of p38 MAPK signaling depends on its upstream kinase MKK3 and MKK6 [6]. It is now accepted that under several pathological conditions, the p38 MAPK signaling pathway is abnormally activated. It was reported that the activation of p38 MAPK signaling resulted in neuron apoptosis and inflammation through activating the NF-κB pathway [7]. Thus, it is reasonable to speculate on the possible role of p38 MAPK in BIND.
Baicalin (BAI), also referred to as 5,6,7-trihydroxyflavone-7-β-D-glucuronide, is one of the biologically active agents extracted from the medical herb
Material and Methods
NEURON ISOLATION AND CULTURE:
Hippocampal neurons were isolated from SD rats <12 h after birth) provided by the Experimental Animal Center of Zhejiang University. The protocols carried out in this study were reviewed and approved by Institutional Animal Care and Use Committee of Zhejiang University. The neurons were isolated and cultured according to previous studies [12]. Briefly, harvested hippocampi were dissected and then digested by trypsin-ethylene diamine teracetic acid (0.25%, EDTA) for 30 min at 37°C. Then, the resulting cells were suspended in DMEM supplemented with horse serum (10%), fetal bovine serum (10% FBS), antibiotic mix (Santa Cruz), and L-glutamine (1%). After 1-day culturing, medium was replaced by DMEM supplemented with 10% horse serum, antibiotic mix, 1% L-glutamine, 2% B27, and 1% N2. Cytarabine (3 μg/mL, Sigma-Aldrich) was added to prevent the proliferation of glial cells. Cells were then cultured in plates at a density of 2×106/plate and maintained in a humidified cell incubator providing 5%CO2/95% fresh air at 37°C. To prevent non-neuronal cell proliferation, β-D-arabinofuranoside (2.5 μmol/L, Santa Cruz) was added to the medium on day 2.
CELL TREATMENTS:
Cultured neurons at 80% confluence were treated with free bilirubin (Bf, Sigma-Aldrich, Cat#B4126) at concentrations of 70, 140, and 280 nmol/L (dissolved in DMSO) for 24 h. Several neurons treated with Bf at a concentration of 280 nmol/L were pre-treated with baicalin at concentrations of 0.5, 1.0, and 2.0 μmol/L for 48 h. The concentrations of baicalin were selected according to our pilot study and previous investigations [13]. The Bf concentration selection and determination were in accordance with a previous study [14].
CELL VIABILITY ASSAY:
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used to determine the viabilities of neurons. MTT (5 mg/mL, Sigma-Aldrich) were added to the culture plates to incubate the cells for 4 h. The formed crystals were dissolved by DMSO. Optical density at 570 nm (A570) was measured by a plate reader. Cell viabilities were then calculated by comparing A570 of treated cells
APOPTOSIS ASSESSMENT:
The apoptosis of the neurons was evaluated by terminal deoxynucleotidyl transferase-dUTP nick-end labeling (TUNEL) assay. After washing with PBS, harvested neurons were fixed with paraformaldehyde (4%) at 37°C for 20 min. Then, a TUNEL assay kit (Roche, Cat#11684795910) was used to detect the apoptotic neurons, which were then observed under an inverted fluorescence microscope. The TUNEL assay protocol was performed in accordance with the manufacturer’s recommendations.
IMMUNOFLUORESCENT STAIN:
Immunofluorescent stain was used to assess the nuclear translocation of p65. After fixation, harvested neurons were incubated with primary antibody against p65 (CST) for 10 h at 4°C. Then, the cells were incubated with secondary antibody conjugated with Alexa 488 (Invitrogen). DAPI (Invitrogen) was also used to stain the nucleus. A SlowFade kit (ThermoFisher) was used to alleviate fluorescence quenching. Cells were observed under an inverted fluorescence microscope. DAPI was excited at 358 nm and observed at 661 nm, and Alexa 488 was excited at 488 nm and observed at 519 nm. Images were captured, analyzed, and merged using Zeiss Physiology software (version 3.2). Calculated mean fluorescent intensities (MFI) represented the expression levels of targeted proteins.
ELISA:
Cell culture supernatants were collected after centrifugation and stored at −80°C. Concentrations of TNFα in cell culture supernatants were detected using a TNFα ELISA kit (R&D). The protocols were carried out according to the instructions provided by the manufacturer.
WESTERN BLOTTING:
Harvested neurons were lysed and prepared with the RIPA buffering system (Santa Cruz). The protein was extracted with N-PER™ Neuronal Protein Extraction Reagent (ThermoFisher) according to the instruction provided by the manufacturer. BCA method was used to determine the protein concentrations. Protein samples were then separated by SDS-PAGE and then transferred to PVDF or NC membranes electronically. After blocking, the membranes were then incubated with specific antibodies of phospho-MKK3 (1: 2000, Abcam), MKK3 (1: 2000, Abcam), phospho-MKK6 (1: 2000, Abcam), MKK6 (1: 2000, Abcam), phospho-p38 MAPK (1: 3000, Cell Signaling Tech), p38 MAPK (1: 3000, Cell Signaling Tech), cleaved caspase3 (1: 4000, Abcam), TNFα (1: 2000, Abcam), and GAPDH (1: 6000, Abcam) at 4°C for 8 h. GAPDH was introduced as the internal reference. After washing in TBST (0.02%), membranes were incubated with secondary antibodies conjugated to HRP at 25°C for 20 min. After developing with ECL Plus Western Blotting Substrate (ThermoFisher), the immunoblots were visualized on X-ray films.
STATISTICS:
Data acquired are presented as (mean±standard deviation) and were processed by SPSS software (version 17.0). One-way analysis of variance and the
Results
BF INCUBATION REDUCED NEURON VIABILITY BY INDUCING APOPTOSIS:
The results are demonstrated in Figure 1. After incubation with Bf at 70, 140, and 280 nmol/L for 24 h, the viabilities of neurons decreased significantly in a Bf concentration-dependent manner (P<0.05). TUNEL assay results also showed apoptosis of neurons in a concentration-dependent manner (P<0.05).
BF INCUBATION INCREASED ACTIVATION OF MKKS/P38 MAPK SIGNALING IN NEURONS:
As demonstrated in Figure 2, Bf incubation significantly increased the phosphorylation levels of MKK3, MKK6, and p38 MAPK in neurons in a concentration-dependent manner (P<0.05).
BF INCUBATION INCREASED NUCLEAR TRANSLOCATION OF P65 AND EXPRESSION OF ITS TARGETED GENES:
As shown in Figure 3, after Bf incubation, the nuclear translocation levels of p65 were increased significantly in a concentration-dependent manner (P<0.05). Moreover, as the targeted genes of p65, expression levels of cleaved caspase3 and TNFα were also increased significantly in Bf-incubated neurons (P<0.05). The concentrations of TNFα in cell culture medium were also dramatically elevated in a Bf concentration-dependent manner (P<0.05).
BAI TREATMENT INCREASED NEURON VIABILITY BY DECREASING BF- INDUCED APOPTOSIS:
BAI at concentrations of 0.5, 1.0, and 2.0 μmol/L were used to treat the Bf (280 nmol/L)-incubated neurons. As shown in Figure 4, BAI treatment significantly increased cell viabilities and decreased apoptosis of Bf-incubated neurons in a concentration-dependent manner (P<0.05).
BAI DECREASED BF-MEDIATED ACTIVATION OF P38 MAPK PATHWAY INDUCED APOPTOTIC AND INFLAMMATORY SIGNALING IN NEURONS:
Figure 5 shows that BAI treatment blocked the phosphorylation of MKK3, MKK6, and p38 MAPK in Bf-incubated neurons. As a result, the nuclear translocation and expressions of cleaved caspase3 and TNFα were suppressed by BAI treatment (P<0.05). Moreover, the concentrations of TNFα in cell culture medium were also reduced by BAI treatment (P<0.05).
Discussion
Knowledge of the mechanisms of neurotoxicity of bilirubin has been limited, as has treatment. Agents extracted from medical herbs such as BAI attracted researchers’ attention in recent decades due to their effectiveness and safety. BAI could be considered as one of the potential supplementary therapies of standard treatment of hyperbilirubinemia such as phototherapy. In the present study, we investigated the protective effect and related possible mechanisms of BAI on Bf-induced neurotoxicity.
The molecular mechanisms of bilirubin-induced neurotoxicity are complicated. Multiple cellular events, signaling transduction, and pathway network are thought to play roles. Among them, apoptosis and neuroinflammation are generally accepted as the consequences of hyperbilirubinemia [15,16]. Apoptosis is an important mechanism of neurotoxicity [17]. Apoptotic events occur after activation of caspase cascade. In this study, we found that Bf incubation dramatically induced neuron apoptosis, which resulted in significant reduction of cell viabilities. Neuroinflammation is identified as one of the risk factors for CNS injury in preterm neonates [18]. Upregulated expressions of pro-inflammatory cytokines such as TNFs and ILs were found
It is believed that NF-κB is a nuclear factor related to multiple biological events, including both apoptosis and inflammation [20]. When encountering harmful stimuli, the upstream kinases of p38 MAPK, namely MKK3/6, are activated. As a result, p38 MAPK is activated by phosphorylation. Then, the activation of NF-κB is initiated. As a member of the NF-κB family, upon activation of NF-κB signaling, p65 translocates into the nucleus and triggers transcriptions of its targeted genes, including pro-apoptotic and pro-inflammatory genes [21]. In this study, we investigated the activation of p38 MAPK signaling in Bf-incubated neurons. We found that Bf incubation increased phosphorylations of MKK3/6 and p38 MAPK, as well as the nuclear translocation of NF-κB. These results indicated Bf incubation promotes the activation of p38 MAPK, resulting in increased neuron apoptosis and inflammation.
Previous investigations have confirmed the neuroprotective effects of BAI in several cerebral injury models [22]. A pharmacokinetics study revealed that BAI can be quickly absorbed and stably exists in the circulation for over 12 h [23]. It has been demonstrated that BAI is distributed in many vital organs, including the liver, kidneys, heart, and brain [24]. Baicalin was proved to transport through the blood-brain barrier and restore permeability under certain pathological conditions [25,26]. However, there are few reports concerning the protective effects of BAI on bilirubin-related neurotoxicity. In this study, BAI at serially diluted concentrations was used to treat Bf-incubated neonatal neurons. We also investigated the involvement of p38 MAPK signaling. Our results indicated that MKK3/6 are the possible molecular targets of BAI because BAI suppressed the phosphorylation of MKK3/6. The activation of p38 MAPK and subsequent NF-κB nuclear translocation were inhibited. As a result, BAI shows potent anti-apoptotic and anti-inflammatory effects.
Conclusions
LIMITATIONS:
There are several limitations of this study. Firstly, only an
Figures
![(A) Viabilities of cultured neurons were determined by MTT. The line chart demonstrates the cell viabilities of neurons incubated with Bf at concentrations of 0, 70, 140, and 280 nmol/L. (B) TUNEL assay was used to evaluate apoptosis. The TUNEL-positive neurons were tagged with green fluorescence and are demonstrated in the captured fluorescent images. Columns indicate the apoptotic rate of neurons incubated with Bf at concentrations of 0, 70, 140, and 280 nmol/L. [n=10; a difference was significant when compared with neurons incubated with control; b difference was significant when compared with neurons incubated with Bf at 70 nmol/L; c difference was significant when compared with neurons incubated with Bf at 140 nmol/L].](https://jours.isi-science.com/imageXml.php?i=medscimonit-26-e926441-g001.jpg&idArt=926441&w=1000) Figure 1. (A) Viabilities of cultured neurons were determined by MTT. The line chart demonstrates the cell viabilities of neurons incubated with Bf at concentrations of 0, 70, 140, and 280 nmol/L. (B) TUNEL assay was used to evaluate apoptosis. The TUNEL-positive neurons were tagged with green fluorescence and are demonstrated in the captured fluorescent images. Columns indicate the apoptotic rate of neurons incubated with Bf at concentrations of 0, 70, 140, and 280 nmol/L. [n=10; a difference was significant when compared with neurons incubated with control; b difference was significant when compared with neurons incubated with Bf at 70 nmol/L; c difference was significant when compared with neurons incubated with Bf at 140 nmol/L].
Figure 1. (A) Viabilities of cultured neurons were determined by MTT. The line chart demonstrates the cell viabilities of neurons incubated with Bf at concentrations of 0, 70, 140, and 280 nmol/L. (B) TUNEL assay was used to evaluate apoptosis. The TUNEL-positive neurons were tagged with green fluorescence and are demonstrated in the captured fluorescent images. Columns indicate the apoptotic rate of neurons incubated with Bf at concentrations of 0, 70, 140, and 280 nmol/L. [n=10; a difference was significant when compared with neurons incubated with control; b difference was significant when compared with neurons incubated with Bf at 70 nmol/L; c difference was significant when compared with neurons incubated with Bf at 140 nmol/L]. ![(A) Western blotting was used to evaluate the relative phosphorylation levels of proteins. The immunoblots of p-MKK3, MKK3, p-MKK6, MKK6, p-p38, and p38 of neurons incubated with Bf at concentrations of 0, 70, 140 and 280 nmol/L. (B) Columns on the right side indicate the relative phosphorylation levels of MKK3, MKK6, and p38. [n=10; a difference was significant when compared with neurons incubated with control; b difference was significant when compared with neurons incubated with Bf at 70 nmol/L; c difference was significant when compared with neurons incubated with Bf at 140 nmol/L].](https://jours.isi-science.com/imageXml.php?i=medscimonit-26-e926441-g002.jpg&idArt=926441&w=1000) Figure 2. (A) Western blotting was used to evaluate the relative phosphorylation levels of proteins. The immunoblots of p-MKK3, MKK3, p-MKK6, MKK6, p-p38, and p38 of neurons incubated with Bf at concentrations of 0, 70, 140 and 280 nmol/L. (B) Columns on the right side indicate the relative phosphorylation levels of MKK3, MKK6, and p38. [n=10; a difference was significant when compared with neurons incubated with control; b difference was significant when compared with neurons incubated with Bf at 70 nmol/L; c difference was significant when compared with neurons incubated with Bf at 140 nmol/L].
Figure 2. (A) Western blotting was used to evaluate the relative phosphorylation levels of proteins. The immunoblots of p-MKK3, MKK3, p-MKK6, MKK6, p-p38, and p38 of neurons incubated with Bf at concentrations of 0, 70, 140 and 280 nmol/L. (B) Columns on the right side indicate the relative phosphorylation levels of MKK3, MKK6, and p38. [n=10; a difference was significant when compared with neurons incubated with control; b difference was significant when compared with neurons incubated with Bf at 70 nmol/L; c difference was significant when compared with neurons incubated with Bf at 140 nmol/L]. ![(A) Immunofluorescent staining was used to evaluate the nuclear translocation of p65. p65 was tagged with green fluorescence (Alexa488) and nuclei were tagged with blue fluorescence (DAPI). Columns indicate the relative nuclear translocation rate of p65 in neurons incubated with Bf at concentrations of 0, 70, 140, and 280 nmol/L. (B) Western blotting was used to determine the relative expression levels of proteins. Immunoblots of cleaved caspase3 (c-caspase3), TNF-α, and GAPDH (internal reference) of neurons. Columns indicate the relative expression levels of c-caspase3 and TNF-α in neurons incubated with Bf at concentrations of 0, 70, 140, and 280 nmol/L. (C) ELISA was used to detect concentration of cytokines. Columns indicate the concentrations of TNF-α in cell medium of neurons incubated with Bf at concentrations of 0, 70, 140, and 280 nmol/L. [n=10; a difference was significant when compared with neurons incubated with control; b difference was significant when compared with neurons incubated with Bf at 70 nmol/L; c difference was significant when compared with neurons incubated with Bf at 140 nmol/L].](https://jours.isi-science.com/imageXml.php?i=medscimonit-26-e926441-g003.jpg&idArt=926441&w=1000) Figure 3. (A) Immunofluorescent staining was used to evaluate the nuclear translocation of p65. p65 was tagged with green fluorescence (Alexa488) and nuclei were tagged with blue fluorescence (DAPI). Columns indicate the relative nuclear translocation rate of p65 in neurons incubated with Bf at concentrations of 0, 70, 140, and 280 nmol/L. (B) Western blotting was used to determine the relative expression levels of proteins. Immunoblots of cleaved caspase3 (c-caspase3), TNF-α, and GAPDH (internal reference) of neurons. Columns indicate the relative expression levels of c-caspase3 and TNF-α in neurons incubated with Bf at concentrations of 0, 70, 140, and 280 nmol/L. (C) ELISA was used to detect concentration of cytokines. Columns indicate the concentrations of TNF-α in cell medium of neurons incubated with Bf at concentrations of 0, 70, 140, and 280 nmol/L. [n=10; a difference was significant when compared with neurons incubated with control; b difference was significant when compared with neurons incubated with Bf at 70 nmol/L; c difference was significant when compared with neurons incubated with Bf at 140 nmol/L].
Figure 3. (A) Immunofluorescent staining was used to evaluate the nuclear translocation of p65. p65 was tagged with green fluorescence (Alexa488) and nuclei were tagged with blue fluorescence (DAPI). Columns indicate the relative nuclear translocation rate of p65 in neurons incubated with Bf at concentrations of 0, 70, 140, and 280 nmol/L. (B) Western blotting was used to determine the relative expression levels of proteins. Immunoblots of cleaved caspase3 (c-caspase3), TNF-α, and GAPDH (internal reference) of neurons. Columns indicate the relative expression levels of c-caspase3 and TNF-α in neurons incubated with Bf at concentrations of 0, 70, 140, and 280 nmol/L. (C) ELISA was used to detect concentration of cytokines. Columns indicate the concentrations of TNF-α in cell medium of neurons incubated with Bf at concentrations of 0, 70, 140, and 280 nmol/L. [n=10; a difference was significant when compared with neurons incubated with control; b difference was significant when compared with neurons incubated with Bf at 70 nmol/L; c difference was significant when compared with neurons incubated with Bf at 140 nmol/L]. ![(A) Line chart indicates the cell viabilities of Bf-incubated neurons treated with BAI at concentrations of 0, 0.5, 1.0, and 2.0 μmol/L. (B) Captured images of TUNEL assay. [n=10; a difference was significant when compared with neurons incubated with Bf at 280 nmol/L and BAI at 0 μmol/L; b difference was significant when compared with neurons incubated with Bf at 280 nmol/L and BAI at 0.5 μmol/L; c difference was significant when compared with neurons incubated with Bf at 280 nmol/L and BAI at 1.0 μmol/L].](https://jours.isi-science.com/imageXml.php?i=medscimonit-26-e926441-g004.jpg&idArt=926441&w=1000) Figure 4. (A) Line chart indicates the cell viabilities of Bf-incubated neurons treated with BAI at concentrations of 0, 0.5, 1.0, and 2.0 μmol/L. (B) Captured images of TUNEL assay. [n=10; a difference was significant when compared with neurons incubated with Bf at 280 nmol/L and BAI at 0 μmol/L; b difference was significant when compared with neurons incubated with Bf at 280 nmol/L and BAI at 0.5 μmol/L; c difference was significant when compared with neurons incubated with Bf at 280 nmol/L and BAI at 1.0 μmol/L].
Figure 4. (A) Line chart indicates the cell viabilities of Bf-incubated neurons treated with BAI at concentrations of 0, 0.5, 1.0, and 2.0 μmol/L. (B) Captured images of TUNEL assay. [n=10; a difference was significant when compared with neurons incubated with Bf at 280 nmol/L and BAI at 0 μmol/L; b difference was significant when compared with neurons incubated with Bf at 280 nmol/L and BAI at 0.5 μmol/L; c difference was significant when compared with neurons incubated with Bf at 280 nmol/L and BAI at 1.0 μmol/L]. ![(A) p65 was tagged with green fluorescence (Alexa488) and nuclei were tagged with blue fluorescence (DAPI). Columns indicated the nuclear translocation rate of p65 in Bf-incubated neurons treated with BAI at concentrations of 0, 0.5, 1.0, and 2.0 μmol/L. (B) The immunoblots of p-MKK3, MKK3, p-MKK6, MKK6, p-p38, and p38 of neurons were demonstrated. Columns indicate the relative phosphorylation levels of MKK3, MKK6, and p38. (C) Immunoblots of c-caspase3, TNF-α, and GAPDH of neurons. Columns indicate the relative expression levels of c-caspase3 and TNF-α in neurons. (D) Columns indicate the concentrations of TNF-α in cell medium of neurons. [n=10; a difference was significant when compared with neurons incubated with Bf at 280 nmol/L and BAI at 0 μmol/L; b difference was significant when compared with neurons incubated with Bf at 280 nmol/L and BAI at 0.5 μmol/L; c difference was significant when compared with neurons incubated with Bf at 280 nmol/L and BAI at 1.0 μmol/L].](https://jours.isi-science.com/imageXml.php?i=medscimonit-26-e926441-g005.jpg&idArt=926441&w=1000) Figure 5. (A) p65 was tagged with green fluorescence (Alexa488) and nuclei were tagged with blue fluorescence (DAPI). Columns indicated the nuclear translocation rate of p65 in Bf-incubated neurons treated with BAI at concentrations of 0, 0.5, 1.0, and 2.0 μmol/L. (B) The immunoblots of p-MKK3, MKK3, p-MKK6, MKK6, p-p38, and p38 of neurons were demonstrated. Columns indicate the relative phosphorylation levels of MKK3, MKK6, and p38. (C) Immunoblots of c-caspase3, TNF-α, and GAPDH of neurons. Columns indicate the relative expression levels of c-caspase3 and TNF-α in neurons. (D) Columns indicate the concentrations of TNF-α in cell medium of neurons. [n=10; a difference was significant when compared with neurons incubated with Bf at 280 nmol/L and BAI at 0 μmol/L; b difference was significant when compared with neurons incubated with Bf at 280 nmol/L and BAI at 0.5 μmol/L; c difference was significant when compared with neurons incubated with Bf at 280 nmol/L and BAI at 1.0 μmol/L].
Figure 5. (A) p65 was tagged with green fluorescence (Alexa488) and nuclei were tagged with blue fluorescence (DAPI). Columns indicated the nuclear translocation rate of p65 in Bf-incubated neurons treated with BAI at concentrations of 0, 0.5, 1.0, and 2.0 μmol/L. (B) The immunoblots of p-MKK3, MKK3, p-MKK6, MKK6, p-p38, and p38 of neurons were demonstrated. Columns indicate the relative phosphorylation levels of MKK3, MKK6, and p38. (C) Immunoblots of c-caspase3, TNF-α, and GAPDH of neurons. Columns indicate the relative expression levels of c-caspase3 and TNF-α in neurons. (D) Columns indicate the concentrations of TNF-α in cell medium of neurons. [n=10; a difference was significant when compared with neurons incubated with Bf at 280 nmol/L and BAI at 0 μmol/L; b difference was significant when compared with neurons incubated with Bf at 280 nmol/L and BAI at 0.5 μmol/L; c difference was significant when compared with neurons incubated with Bf at 280 nmol/L and BAI at 1.0 μmol/L]. References
1. Sullivan JI, Rockey DC, Diagnosis and evaluation of hyperbilirubinemia: Curr Opin Gastroenterol, 2017; 33; 164-70
2. Bhutani VK, Johnson-Hamerman L, The clinical syndrome of bilirubin-induced neurologic dysfunction: Semin Fetal Neonatal Med, 2015; 20; 6-13
3. Bhutani VK, Wong R, Bilirubin-induced neurologic dysfunction (BIND): Semin Fetal Neonatal Med, 2015; 20; 1
4. Watchko JF, Bilirubin-induced neurotoxicity in the preterm neonate: Clin Perinatol, 2016; 43; 297-311
5. Mao J, Yang J, Zhang Y, Arsenic trioxide mediates HAPI microglia inflammatory response and subsequent neuron apoptosis through p38/JNK MAPK/STAT3 pathway: Toxicol Appl Pharmacol, 2016; 303; 79-89
6. Cheng Y, Zhu Y, PKCalpha in colon cancer cells promotes M1 macrophage polarization via MKK3/6-P38 MAPK pathway: Mol Carcinog, 2018; 57; 1017-29
7. Yang B, Xu B, Zhao H, Dioscin protects against coronary heart disease by reducing oxidative stress and inflammation via Sirt1/Nrf2 and p38 MAPK pathways: Mol Med Rep, 2018; 18; 973-80
8. Liu J, Wei Y, Luo Q, Baicalin attenuates inflammation in mice with OVA-induced asthma by inhibiting NF-kappaB and suppressing CCR7/CCL19/CCL21: Int J Mol Med, 2016; 38; 1541-48
9. Dai J, Qiu YM, Ma ZW, Neuroprotective effect of baicalin on focal cerebral ischemia in rats: Neural Regen Res, 2018; 13; 2129-33
10. Zheng WX, Cao XL, Wang F, Baicalin inhibiting cerebral ischemia/hypoxia-induced neuronal apoptosis via MRTF-A-mediated transactivity [J]: Eur J Pharmacol, 2015; 15(767); 201-10
11. Kang S, Liu S, Li H, Baicalin effects on rats with spinal cord injury by anti-inflammatory and regulating the serum metabolic disorder: J Cell Biochem, 2018; 119(9); 7767-79
12. Qaisiya M, Mardešić P, Pastore B, The activation of autophagy protects neurons and astrocytes against bilirubin-induced cytotoxicity: Neurosci Lett, 2017; 661; 96-103
13. Wang H, Zhang J, Hu SH, Real-time microwave exposure induces calcium efflux in primary hippocampal neurons and primary cardiomyocytes: Biomed Environ Sci, 2018; 31; 561-71
14. Mancuso C, Bilirubin and brain: A pharmacological approach: Neuropharmacology, 2017; 118; 113-23
15. Pazar A, Kolgazi M, Memisoglu A, The neuroprotective and anti-apoptotic effects of melatonin on hemolytic hyperbilirubinemia-induced oxidative brain damage: J Pineal Res, 2016; 60; 74-83
16. Vodret S, Bortolussi G, Iaconcig A, Attenuation of neuro-inflammation improves survival and neurodegeneration in a mouse model of severe neonatal hyperbilirubinemia: Brain Behav Immun, 2018; 70; 166-78
17. Yang M, Wei H, Anesthetic neurotoxicity: Apoptosis and autophagic cell death mediated by calcium dysregulation: Neurotoxicol Teratol, 2017; 60; 59-62
18. Hagberg H, Mallard C, Ferriero DM, The role of inflammation in perinatal brain injury: Nat Rev Neurol, 2015; 11; 192-208
19. Vaz AR, Silva SL, Barateiro A, Pro-inflammatory cytokines intensify the activation of NO/NOS, JNK1/2 and caspase cascades in immature neurons exposed to elevated levels of unconjugated bilirubin: Exp Neurol, 2011; 229; 381-90
20. Jobin C, Sartor RB, The I kappa B/NF-kappa B system: A key determinant of mucosalinflammation and protection: Am J Physiol Cell Physiol, 2000; 278; C451-62
21. Liu Z, Zheng S, Wang X, Novel ASK1 inhibitor AGI-1067 improves AGE-induced cardiac dysfunction by inhibiting MKKs/p38 MAPK and NF-kappaB apoptotic signaling: FEBS Open Bio, 2018; 8; 1445-56
22. Liang W, Huang X, Chen W, The effects of baicalin and baicalein on cerebral ischemia: A review: Aging Dis, 2017; 8; 850-67
23. Tsai PL, Tsai TH, Pharmacokinetics of baicalin in rats and its interactions with cyclosporin A, quinidine and SKF-525A: A microdialysis study: Planta Med, 2004; 70; 1069-74
24. Dinda B, Dinda S, DasSharma S, Therapeutic potentials of baicalin and its aglycone, baicalein against inflammatory disorders: Eur J Med Chem, 2017; 131; 68-80
25. Liang W, Huang X, Chen W, The effects of baicalin and baicalein on cerebral ischemia: A review: Aging Dis, 2017; 8; 850-67
26. Tu XK, Yang WZ, Liang RS, Effect of baicalin on matrix metalloproteinase-9 expression and blood-brain barrier permeability following focal cerebral ischemia in rats: Neurochem Res, 2011; 36; 2022-28
Figures
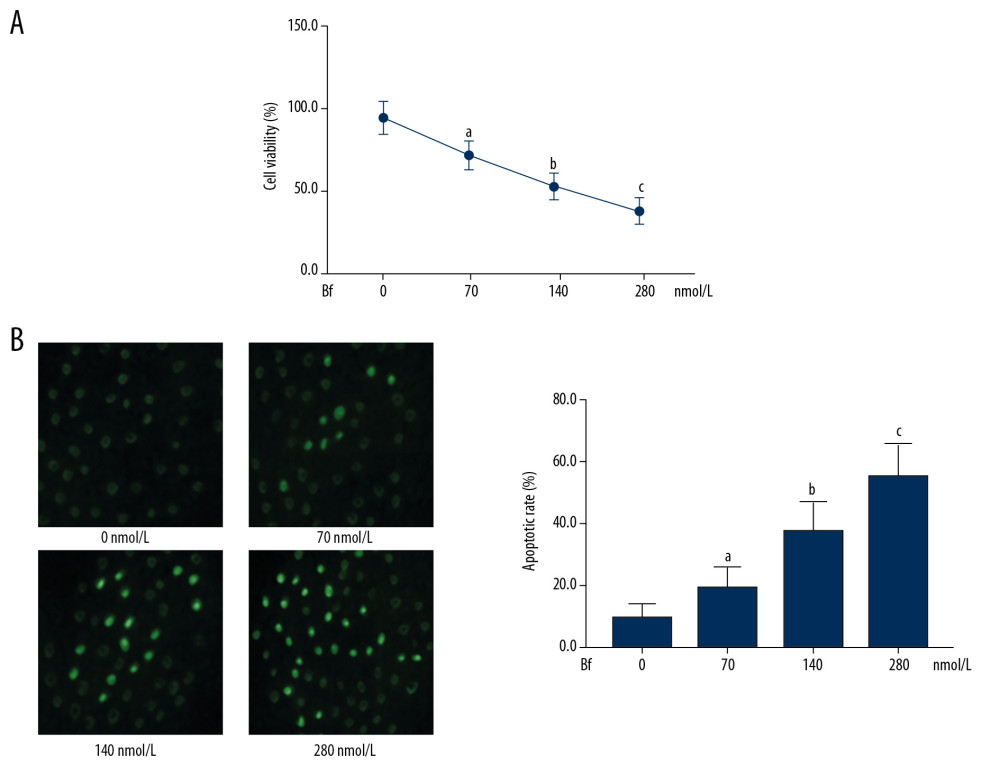 Figure 1. (A) Viabilities of cultured neurons were determined by MTT. The line chart demonstrates the cell viabilities of neurons incubated with Bf at concentrations of 0, 70, 140, and 280 nmol/L. (B) TUNEL assay was used to evaluate apoptosis. The TUNEL-positive neurons were tagged with green fluorescence and are demonstrated in the captured fluorescent images. Columns indicate the apoptotic rate of neurons incubated with Bf at concentrations of 0, 70, 140, and 280 nmol/L. [n=10; a difference was significant when compared with neurons incubated with control; b difference was significant when compared with neurons incubated with Bf at 70 nmol/L; c difference was significant when compared with neurons incubated with Bf at 140 nmol/L].
Figure 1. (A) Viabilities of cultured neurons were determined by MTT. The line chart demonstrates the cell viabilities of neurons incubated with Bf at concentrations of 0, 70, 140, and 280 nmol/L. (B) TUNEL assay was used to evaluate apoptosis. The TUNEL-positive neurons were tagged with green fluorescence and are demonstrated in the captured fluorescent images. Columns indicate the apoptotic rate of neurons incubated with Bf at concentrations of 0, 70, 140, and 280 nmol/L. [n=10; a difference was significant when compared with neurons incubated with control; b difference was significant when compared with neurons incubated with Bf at 70 nmol/L; c difference was significant when compared with neurons incubated with Bf at 140 nmol/L].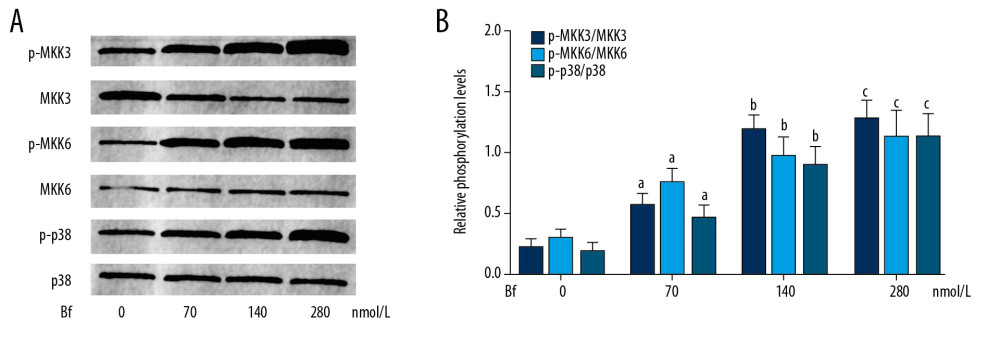 Figure 2. (A) Western blotting was used to evaluate the relative phosphorylation levels of proteins. The immunoblots of p-MKK3, MKK3, p-MKK6, MKK6, p-p38, and p38 of neurons incubated with Bf at concentrations of 0, 70, 140 and 280 nmol/L. (B) Columns on the right side indicate the relative phosphorylation levels of MKK3, MKK6, and p38. [n=10; a difference was significant when compared with neurons incubated with control; b difference was significant when compared with neurons incubated with Bf at 70 nmol/L; c difference was significant when compared with neurons incubated with Bf at 140 nmol/L].
Figure 2. (A) Western blotting was used to evaluate the relative phosphorylation levels of proteins. The immunoblots of p-MKK3, MKK3, p-MKK6, MKK6, p-p38, and p38 of neurons incubated with Bf at concentrations of 0, 70, 140 and 280 nmol/L. (B) Columns on the right side indicate the relative phosphorylation levels of MKK3, MKK6, and p38. [n=10; a difference was significant when compared with neurons incubated with control; b difference was significant when compared with neurons incubated with Bf at 70 nmol/L; c difference was significant when compared with neurons incubated with Bf at 140 nmol/L].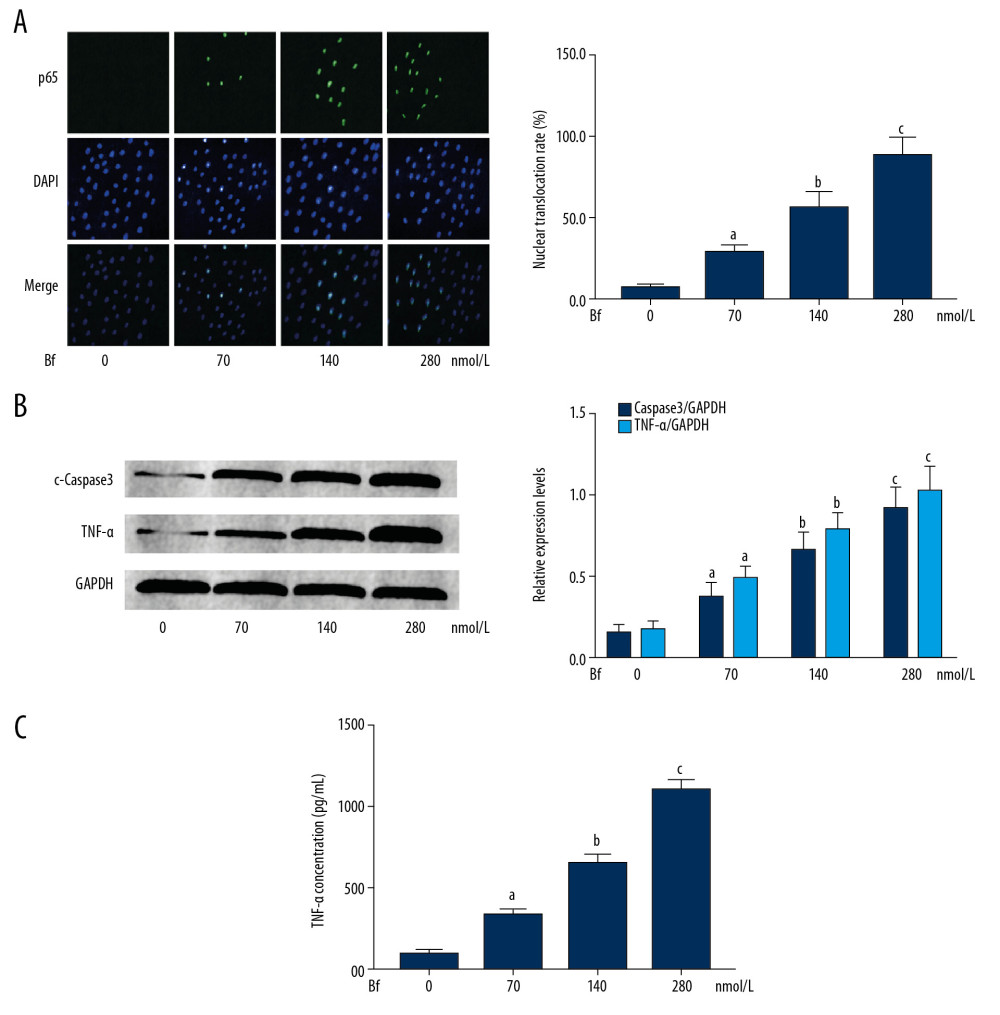 Figure 3. (A) Immunofluorescent staining was used to evaluate the nuclear translocation of p65. p65 was tagged with green fluorescence (Alexa488) and nuclei were tagged with blue fluorescence (DAPI). Columns indicate the relative nuclear translocation rate of p65 in neurons incubated with Bf at concentrations of 0, 70, 140, and 280 nmol/L. (B) Western blotting was used to determine the relative expression levels of proteins. Immunoblots of cleaved caspase3 (c-caspase3), TNF-α, and GAPDH (internal reference) of neurons. Columns indicate the relative expression levels of c-caspase3 and TNF-α in neurons incubated with Bf at concentrations of 0, 70, 140, and 280 nmol/L. (C) ELISA was used to detect concentration of cytokines. Columns indicate the concentrations of TNF-α in cell medium of neurons incubated with Bf at concentrations of 0, 70, 140, and 280 nmol/L. [n=10; a difference was significant when compared with neurons incubated with control; b difference was significant when compared with neurons incubated with Bf at 70 nmol/L; c difference was significant when compared with neurons incubated with Bf at 140 nmol/L].
Figure 3. (A) Immunofluorescent staining was used to evaluate the nuclear translocation of p65. p65 was tagged with green fluorescence (Alexa488) and nuclei were tagged with blue fluorescence (DAPI). Columns indicate the relative nuclear translocation rate of p65 in neurons incubated with Bf at concentrations of 0, 70, 140, and 280 nmol/L. (B) Western blotting was used to determine the relative expression levels of proteins. Immunoblots of cleaved caspase3 (c-caspase3), TNF-α, and GAPDH (internal reference) of neurons. Columns indicate the relative expression levels of c-caspase3 and TNF-α in neurons incubated with Bf at concentrations of 0, 70, 140, and 280 nmol/L. (C) ELISA was used to detect concentration of cytokines. Columns indicate the concentrations of TNF-α in cell medium of neurons incubated with Bf at concentrations of 0, 70, 140, and 280 nmol/L. [n=10; a difference was significant when compared with neurons incubated with control; b difference was significant when compared with neurons incubated with Bf at 70 nmol/L; c difference was significant when compared with neurons incubated with Bf at 140 nmol/L].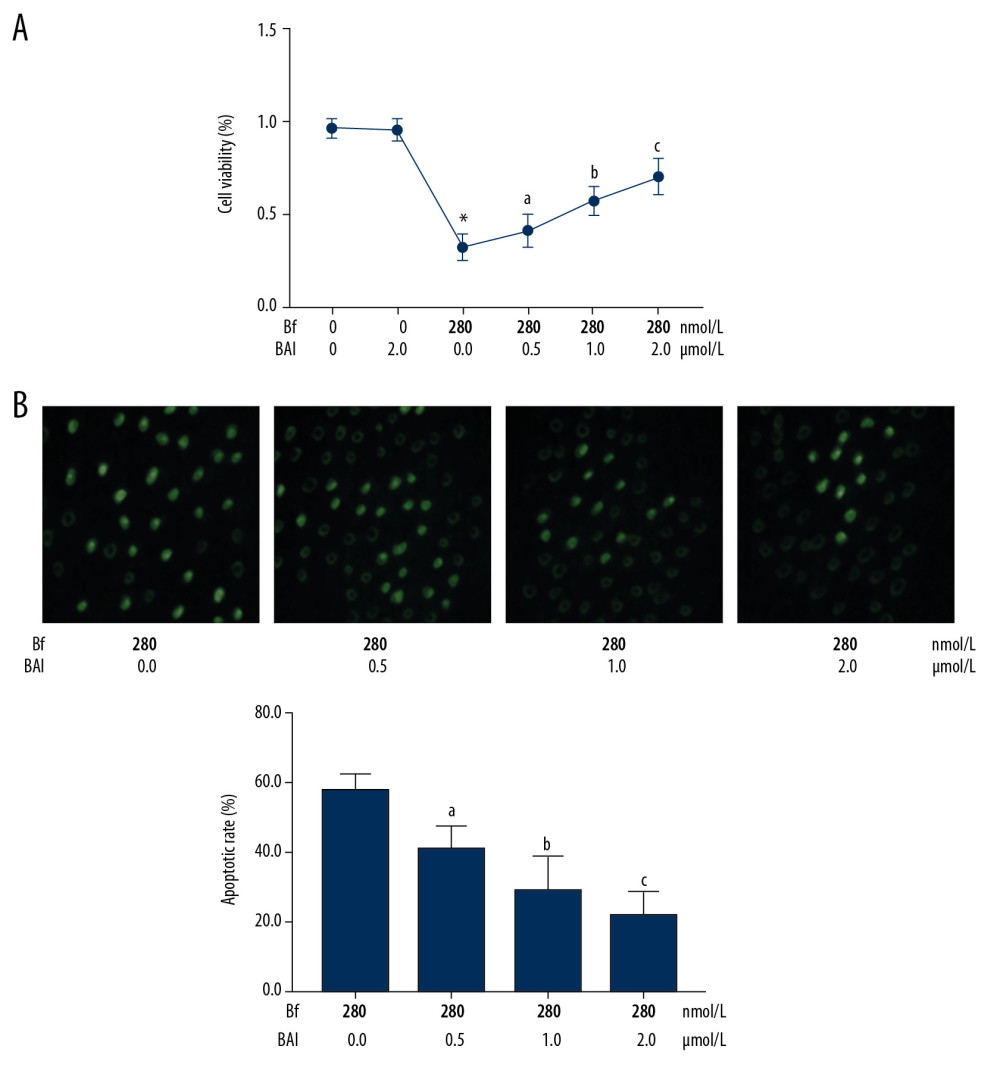 Figure 4. (A) Line chart indicates the cell viabilities of Bf-incubated neurons treated with BAI at concentrations of 0, 0.5, 1.0, and 2.0 μmol/L. (B) Captured images of TUNEL assay. [n=10; a difference was significant when compared with neurons incubated with Bf at 280 nmol/L and BAI at 0 μmol/L; b difference was significant when compared with neurons incubated with Bf at 280 nmol/L and BAI at 0.5 μmol/L; c difference was significant when compared with neurons incubated with Bf at 280 nmol/L and BAI at 1.0 μmol/L].
Figure 4. (A) Line chart indicates the cell viabilities of Bf-incubated neurons treated with BAI at concentrations of 0, 0.5, 1.0, and 2.0 μmol/L. (B) Captured images of TUNEL assay. [n=10; a difference was significant when compared with neurons incubated with Bf at 280 nmol/L and BAI at 0 μmol/L; b difference was significant when compared with neurons incubated with Bf at 280 nmol/L and BAI at 0.5 μmol/L; c difference was significant when compared with neurons incubated with Bf at 280 nmol/L and BAI at 1.0 μmol/L].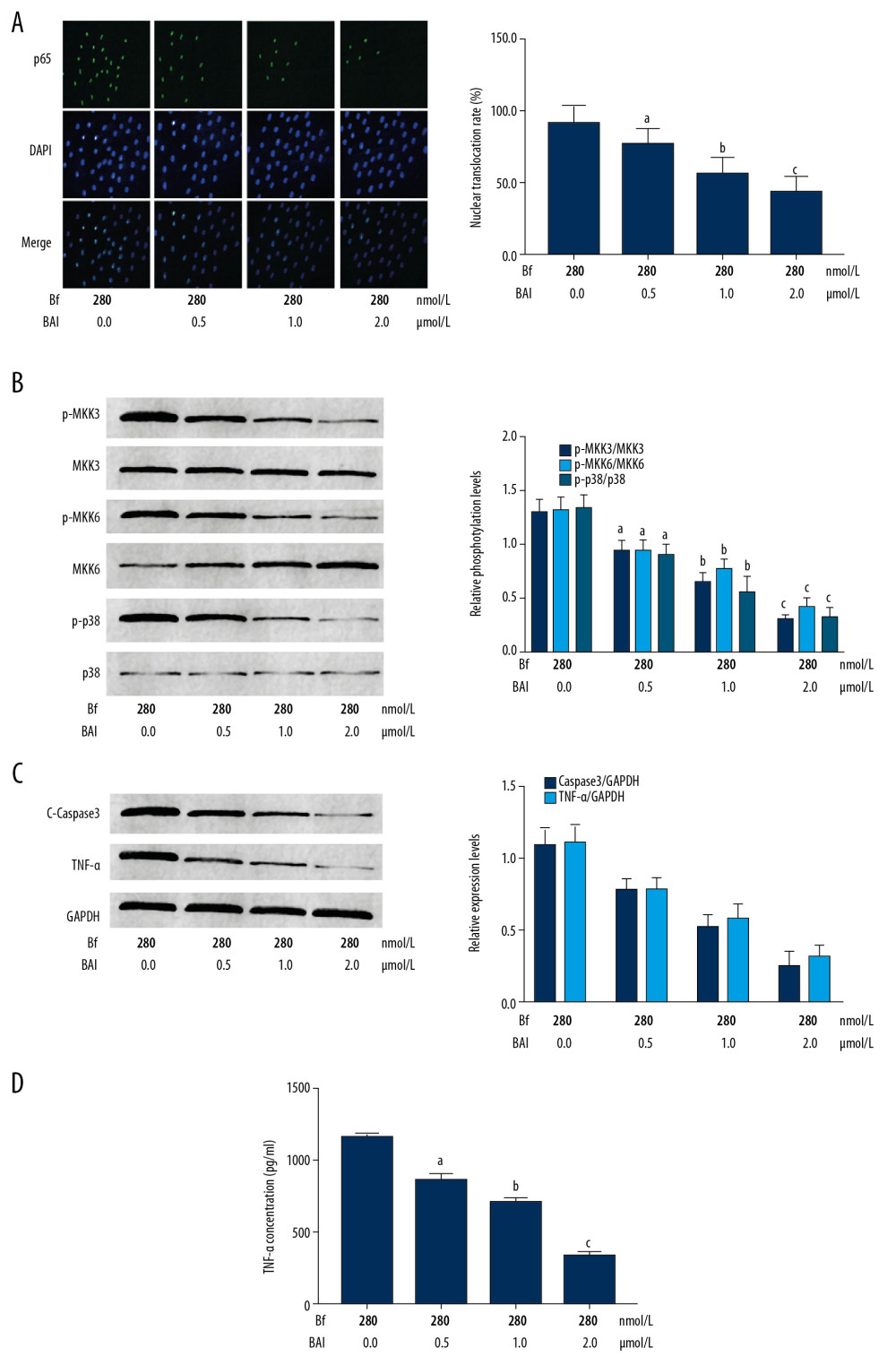 Figure 5. (A) p65 was tagged with green fluorescence (Alexa488) and nuclei were tagged with blue fluorescence (DAPI). Columns indicated the nuclear translocation rate of p65 in Bf-incubated neurons treated with BAI at concentrations of 0, 0.5, 1.0, and 2.0 μmol/L. (B) The immunoblots of p-MKK3, MKK3, p-MKK6, MKK6, p-p38, and p38 of neurons were demonstrated. Columns indicate the relative phosphorylation levels of MKK3, MKK6, and p38. (C) Immunoblots of c-caspase3, TNF-α, and GAPDH of neurons. Columns indicate the relative expression levels of c-caspase3 and TNF-α in neurons. (D) Columns indicate the concentrations of TNF-α in cell medium of neurons. [n=10; a difference was significant when compared with neurons incubated with Bf at 280 nmol/L and BAI at 0 μmol/L; b difference was significant when compared with neurons incubated with Bf at 280 nmol/L and BAI at 0.5 μmol/L; c difference was significant when compared with neurons incubated with Bf at 280 nmol/L and BAI at 1.0 μmol/L].
Figure 5. (A) p65 was tagged with green fluorescence (Alexa488) and nuclei were tagged with blue fluorescence (DAPI). Columns indicated the nuclear translocation rate of p65 in Bf-incubated neurons treated with BAI at concentrations of 0, 0.5, 1.0, and 2.0 μmol/L. (B) The immunoblots of p-MKK3, MKK3, p-MKK6, MKK6, p-p38, and p38 of neurons were demonstrated. Columns indicate the relative phosphorylation levels of MKK3, MKK6, and p38. (C) Immunoblots of c-caspase3, TNF-α, and GAPDH of neurons. Columns indicate the relative expression levels of c-caspase3 and TNF-α in neurons. (D) Columns indicate the concentrations of TNF-α in cell medium of neurons. [n=10; a difference was significant when compared with neurons incubated with Bf at 280 nmol/L and BAI at 0 μmol/L; b difference was significant when compared with neurons incubated with Bf at 280 nmol/L and BAI at 0.5 μmol/L; c difference was significant when compared with neurons incubated with Bf at 280 nmol/L and BAI at 1.0 μmol/L]. In Press
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








