21 September 2020: Lab/In Vitro Research
Construction and Evaluation of Folic Acid-Modified 3-Bromopyruvate Cubosomes
Fangyan Hou1ABCDEF*, Hairong Wang2BF, Yawen Zhang1CF, Na Zhu3ACF, Hao Liu1ACDG, Jianchun Li1ACDEGDOI: 10.12659/MSM.924620
Med Sci Monit 2020; 26:e924620
Abstract
BACKGROUND: Direct 3-bromopyruvate chemotherapy often causes side effects. We thus aimed to construct and evaluate folic acid-modified 3-bromopyruvate liquid crystalline nanoparticles (3BP-LCNP-FA) and assess their targeted antitumor effects in tumor-bearing nude mice.
MATERIAL AND METHODS: A liquid crystalline nanoparticle formulation was screened, and the structure was characterized using polarizing light- and transmission electron microscopy. The folate target was then synthesized and characterized using differential scanning calorimetry and proton nuclear magnetic resonance spectroscopy. In vitro, human CNE-2Z and MDA-MB-231 tumor cells were used to evaluate 3BP-LCNP-FA effects on tumor cell morphology and proliferation. Different drug formulations were administered to tumor-bearing nude mice to observe the treatment effects. Hepatic and renal toxicities were assessed using hematoxylin and eosin-stained liver, kidney, and lung sections along with serological analysis of liver and kidney injury markers (e.g., aspartate aminotransferase, alanine transaminase, blood urea nitrogen, and creatinine). Tumor tissue was observed for changes using proliferating cell nuclear antigen immunohistochemistry and terminal deoxynucleotidyl transferase dUTP nick end labeling assay.
RESULTS: We successfully prepared 3BP-LCNP-FA of spherical shape with uniform size using the aforementioned techniques; drug loading did not alter crystal morphology. These cubosomes exhibited more potent antitumor activity than 3-bromopyruvate alone or non-folic acid-conjugated 3-bromopyruvate liquid crystalline nanoparticles in vitro and in vivo without obvious toxic side effects.
CONCLUSIONS: It is possible to successfully construct 3BP-LCNP-FA as a drug delivery vehicle that is more efficacious than 3-bromopyruvate and has no obvious toxic effects. Thus, folic acid-modified cubosomes can be used as effective carriers for targeted drug administration.
Keywords: Drug Delivery Systems, Folic Acid, Nanoparticles, Drug Carriers, Neoplasms, Experimental, Pyruvates
Background
Modern antitumor treatments are expected to minimize damage to non-target organs by improving drug targeting. In particular, the highly active aerobic glycolysis in a variety of tumor cells acts as a potential target for modern antitumor therapies [1,2]. As one such therapeutic agent, 3-bromopyruvate (3BP) inhibits glycolysis, consequently limiting energy supply to tumor cells and inhibiting their rapid proliferation [3–7]. Studies have found that 3BP enters tumor cells through monocarboxylic acid transporter 1 (MCT1) present on the cell membrane surface [8,9], inhibits the activity of hexokinase II bound to the outer membrane of mitochondria, and consequently limits energy production in cells and inhibits their rapid growth [10–14]. Therefore, the therapeutic effect of 3BP is poor in tumor cells with low MCT1 expression [15]. Moreover, clinical application of 3BP has been associated with side effects as this agent interacts with healthy cells, particularly erythrocytes, reducing its availability to tumor cells and narrowing its therapeutic window [16–20].
Alternatively, previous studies have shown that several folate receptors (FRs) are highly expressed on the surface of some tumor cells, especially those exhibiting rapid growth [21,22], and therefore, they can be used as therapeutic targets. Accordingly, modifying the FR ligand, folic acid (FA), to accommodate 3BP can increase the selectivity potential of a drug carrier system incorporated with 3BP. Moreover, active drug targeting to the desired organs or cells through FR mediation further improves drug targeting and reduces side effects, as this allows for active targeted transport of the FA-drug conjugate by endocytosis [23]. Once the FA-drug conjugate enters the cell, the pH of endocytosomes (pH 5), in contrast to that of the extracellular space (pH 7), leads to altered conformation of the drug and FR complexes. The folate conjugate can then separate from the FR, enter the cytoplasm, and exert its therapeutic effects, whereas the FR can return to the cell surface and continue to transport drugs. Thus, an FR-mediated, active drug delivery system targets tumor sites in two ways: direct coupling of FA to drug molecules and FA coupling to the drug carrier surface. However, as coupling of FA to drug molecules can further reduce drug solubility, research has primarily focused on FA coupled to the surface of drug carriers [24,25]. In addition, current FA-targeted drug delivery systems have mostly been evaluated using
Liquid crystalline nanoparticles [26] (LCNPs) are self-assembled, bi-continuous liquid crystalline phases comprising a lipid bilayer enclosing water channels, which provides both a hydrophilic and hydrophobic region for encapsulation of drugs with varying solubilities [27–29]. These liquid crystals can be lamellar, hexagonal, or cubic (cubosomes). Owing to their unique reverse bi-continuous cubic phase structure, cubosomes do not cause the side effects that are associated with nude drug administration, such as burning sensation in the veins, rapid blood-based clearance, and off-target side effects. Conversely, cubosomes are highly biocompatible and bioadhesive and can be administered via different routes [30]. In addition, LCNPs can enter the cell through endocytosis, which does not depend on presence of MCT1 transporters on the cell membrane surface, enabling transport of the drug into tumor cells to a greater extent. Some of the currently researched dosage forms of 3BP include liposomes, gold NPs, and microencapsulation. However, there are no data in the literature on FA-modified cubic liquid crystalline nanoparticles. In addition, most of the studies on cubosomes are based on
Given the excellent drug delivery performance of cubosomes and tumor-targeting effects of drug modification with FA, we hypothesized that FA-modified 3BP cubosomes (3BP-LCNP-FA) would not be dependent on cell surface MCT1 proteins for intracellular transport and may show improved antitumor effects without the toxic effects that are typically associated with 3BP treatment. Therefore, in this study, we constructed 3BP-LCNP-FA and evaluated
Material and Methods
CHEMICALS:
We acquired 3BP from Sigma Co. Ltd. (Shanghai, China). Glycerol monooleate (GMO) was purchased from Baoman Co. Ltd. (Shanghai, China). Poloxamer 407 (F127), FA, and 1,1-carbonyldiimidazole (CDI) were provided by Yuan Ye Co. Ltd. (Shanghai, China). An adenosine triphosphate (ATP) measurement kit was purchased from Beyotime Biotechnology (S0026, Shanghai, China). Dialysis bags with a molecular weight cutoff of 3500 Da (Viskase Companies, Inc., Darien, IL, USA) were used. RPMI 1640 and Dulbecco’s modified Eagle’s medium were acquired from Thermo Fisher Biochemical Products (Beijing, China). Fetal bovine serum was supplied by Tian Hang Biotechnology Co. Ltd. (Hangzhou, China). The CNE-2Z and MDA-MB-231 human tumor cell lines were supplied by the biochemical laboratory of Nanjing University.
CELL CULTURE AND ANIMALS:
The human tumor cell lines CNE-2Zand MDA-MB-231, representing high- and low MCT expression, respectively, were used for
SYNTHESIS AND CHARACTERIZATION OF FA-CONJUGATED F127:
To construct cubosomes capable of targeting FRs in tumor cells, we modified F127 with FA (F127-FA), which was prepared according to previously reported methods [31]. Following activation of the amino terminus of FA, we coupled FA to F127 through an amidation reaction on the terminal carboxyl group of F127. The overall process involved dissolving 87.58 mg of FA in 5 mL of dry dimethyl sulfoxide (DMSO), which was then stirred until complete dissolution. Then, 35.32 mg of CDI was mixed, and the reaction was magnetically stirred for 24 h in the dark. Subsequently, 0.62 g of F127 was added to the solution and the reaction was placed in the dark for 24 h. All reactions were conducted at 25°C. The reaction mixture was transferred into a dialysis bag and dialyzed for 3 days against double-steaming water, which was changed every 3–6 h. F127-FA was recovered via lyophilization. The resulting product was dried in a vacuum oven for 24 h and stored in a dry box until use. Differential scanning calorimetry was conducted to pre-evaluate the conjugated F127-FA. The synthesized F127-FA was dissolved in DMSO to record a spectrum using proton nuclear magnetic resonance (1H-NMR) spectroscopy (600 MHz).
PREPARATION AND CHARACTERIZATION OF CUBOSOMES:
3BP-LCNP and 3BP-LCNP-FA were prepared using an injection method combined with high-pressure homogenization. Briefly, GMO and FA/F127-FA were melted in a water bath at 60°C, followed by the addition of 3BP. After melting, deionized water was slowly added dropwise to obtain a crude solution. This solution was then passed through a high-pressure homogenizer (14,900 psi, nine cycles). During the preparation, 3BP-LCNP used F127 without FA modification. The sample volume was approximately 10 mL and consisted of approximately 96.1 wt% water, 3.5 wt% GMO, and 0.4 wt% F127-FA. Drug loading was approximately 1.07×10–3 wt%. The crystallinity phenomenon of 3BP-LCNP and 3BP-LCNP-FA was observed via polarized light microscopy (PLM), wherein ordinary light is converted into polarized light for microscopy. A substance can be identified as a single refractive (isotropy) or birefringence (anisotropy) structure based on its optical properties; moreover, the crystal form of the object can be quickly and accurately observed. PLM can also be used to observe the crystal structure of cubic liquid crystal. Cubosomes are optically isotropic and exhibit a dark field of vision by PLM. In addition, size distributions of 3BP-LCNP and 3BP-LCNP-FA were assessed using Zeta Sizer Nano series Nano-ZS (Malvern Instruments Ltd., Malvern, UK). Characterization of 3BP-LCNP-FA particle morphology was performed using transmission electron microscopy.
EVALUATION OF ENCAPSULATION EFFICIENCY:
Encapsulation efficiencies (EE) of 3BP-LCNP and 3BP-LCNP-FA were determined by dialysis. First, 5 mL of sample was added into a dialysis bag and stirred for 4 h in 200 mL double-distilled water. The concentration of 3BP was determined inside and outside the dialysis bag by using high-performance liquid chromatography. The calculation formula of entrapment efficiency (EE%) is EE%=(Ct–Cf)/Ct×100% where Ct is the total concentration of 3BP in the cubosomes and Cf is the concentration of 3BP in the dialysis fluid.
:
MORPHOLOGICAL CHANGES IN CELLS:
To observe changes in cell and nucleus morphology following treatment with different dosage forms of 3BP, CNE-2Z (2.5×103) and MDA-MB-231 (3.5×103) cells were seeded into six-well plates. When the cells covered approximately 70% of the plate bottom, different dosage forms of 3BP were administered (with a constant 3BP concentration of 50 μM). After 24 h, the dead cells in the culture medium were washed off with PBS, and the remaining cells were fixed with 4% paraformaldehyde for 30 min. Paraformaldehyde was then washed off with PBS and 4′,6-diamidino-2-phenylindole was added for staining for 15 min. After staining, 4′,6-diamidino-2-phenylindole was washed off with PBS, and the morphological changes of cells and cell density were observed using a live cell workstation after adding PBS.
:
For cytotoxicity testing, healthy CNE-2Z (5×103) and MDA-MB-231 (8×103) cells were seeded in 96-well plates for 24 h. When cell density reached approximately 70%, the cell culture medium was replaced with a drug-containing medium including different concentrations of LCNP-FA, 3BP, 3BP-LCNP, or 3BP-LCNP-FA (with a consistent 3BP concentration of 50 μmol/L). After further incubation for 24 h, cell viability was determined using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay.
INTRACELLULAR ATP LEVELS:
The ATP level in cells was measured using the ATP assay kit. CNE-2Z (2.5×105) and MDA-MB-231 (3.5×105) cells were seeded in six-well plates and cultured for 24 h. LCNP-FA-, 3-BP-, 3BP-LCNP-, or 3BP-LCNP-FA-containing culture medium was then added to the cells for 4 and 6 h at 37°C.
ANIMAL EXPERIMENTS:
BALB/C nude mice (4 to 5 weeks old, female) bearing tumors were established by inoculating 5×106 CNE-2Z cells into the right flank. To evaluate the targeted therapeutic effect of 3BP-LCNP-FA
STATISTICAL ANALYSIS:
All experiments were performed independently and repeated at least three times. Statistical comparisons of data sets were evaluated using the Student’s
Results
CHARACTERIZATION OF FA-CONJUGATED F127:
Conjugation of FA and F127 was evaluated using differential scanning calorimetry (Figure 2). The purple curve representing F127-FA lacked the characteristic absorption peak of FA, indicating that F127-FA was a single compound. The 1H-NMR spectrum of F127-FA showed absorbance peaks corresponding to the following components: 1.01 (CH3 in PPO of F127), 3.44–3.51 (CH in PPO of F127), 3.53 (CH2CH2O of F127), 6.6 and 7.6 (3,5-H and 2,6-H of the FA benzene ring), and 8.6 (C7-H in the FA pteridine proton) (Figure 3).
CHARACTERIZATION OF CUBOSOMES:
The results showed that cubosomes were prepared when GMO: F127 was 8: 1 (by% of weight, with a consistent total mass of 1 g). Consistent with the optical isotropy exhibited by cubosomes, we observed 3BP-LCNP as a dark field. Notably, 3BP-LCNP-FA still appeared as a dark field following drug loading (Figure 4A, 4B), indicating that drug loading did not affect the morphology of the prepared liquid crystal.
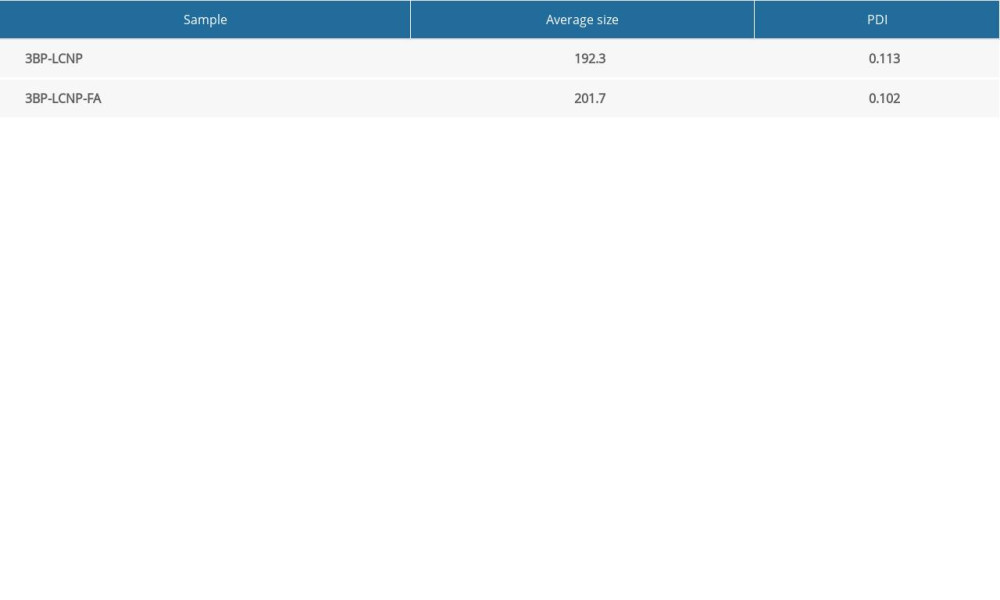
Homogenization at 14,900 psi for nine cycles yielded an adequate particle size. The addition of FA increased the average particle size from 192.3 to 201.7 nm and the polydispersity index (PDI) decreased from 0.113 to 0.102 (Figure 5, Table 1). The EE of the prepared 3BP-LCNP-FA was around 70.23%. Figure 6 shows the structure of 3BP-LCNP-FA under a cryo-projective electron microscope; spherical particles of uniform size that were not adherent to each other were observed.
:
Release of 3BP at drug concentrations of 5, 10, 25, 50, 90, 100, 250, and 500 μg/mL from 3BP-LCNP-FA showed a linear profile. Free 3BP exhibited almost 100% release within 3 h. In comparison, only 90.79% of 3BP was released from 3BP-LCNP-FA within 48 h (Figure 7), indicating that 3BP-LCNP-FA presented a slower drug release pattern than free 3BP.
MORPHOLOGICAL CHANGES IN CELLS:
The blank vector LCNP-FA had no significant effect on the morphology or density of CNE-2Z and MDA-MB-231 cells (P>0.05) (Figure 8A, 8B). The effects of 3BP, 3BP-LCNP, and 3BP-LCNP-FA on the morphology and density of CNE-2Z cells were similar (P>0.05). Morphology and density of MDA-MB-231 cells were not significantly changed by 3BP (P>0.05). In contrast, 3BP-LCNP and 3BP-LCNP-FA significantly reduced the density of MDA-MB-231 cells (P<0.01) and caused changes in nuclear morphology, including gradual condensation of chromatin into a crescent shape and attachment to the periphery of the nuclear membrane; nuclear envelope breakdown; and nuclear division. However, the cell membrane remained intact.
INHIBITORY EFFECT OF 3BP-LCNP-FA ON TUMOR CELL PROLIFERATION:
To elucidate the effects of 3BP-LCNP-FA on tumor cells, we evaluated the proliferation of CNE-2Z and MDA-MB-231 cells after treatment. As shown in Figure 9A and 9B, increasing doses of the empty vectors LCNP and LCNP-FA had no significant inhibitory effects on proliferation in either cell line after 24 h. However, in CNE-2Z cells, 50 μM of 3BP-LCNP-FA caused 86.3% inhibition in proliferation, whereas 3BP and 3BP-LCNP caused 7.8% and 4.6% inhibition, respectively (Figure 9C). As 3BP drug concentrations increased, the inhibition rate reached over 80% at 125 μM. In contrast, as can be seen in Figure 9D, increasing concentrations of 3BP did not significantly inhibit cell proliferation in MDA-MB-231 cells; e.g., when 3BP concentration reached 125 μM, the inhibition rate was only 10.2%. In comparison, the inhibitory rate of 3BP-LCNP-FA reached 50.2% at 50 μM, with the inhibitory effect increasing with increased drug concentration.
CHANGES IN INTRACELLULAR ATP LEVELS:
Figure 10 shows changes in intracellular ATP levels in CNE-2Z and MDA-MB-231 cells after the different drug delivery forms were administered for 4 and 6 h. Intracellular ATP levels in MDA-MB-231 cells did not decrease significantly with increased 3BP concentration, and no significant difference was observed compared with that in the control group (P>0.05). In contrast, ATP levels in 3BP-, 3BP-LCNP-, and 3BP-LCNP-FA-treated cells showed a significant decrease compared with that in the control group (P<0.05).
:
Tumor volume is the most effective parameter for evaluating the therapeutic effect of a drug in tumor-bearing nude mice. Tumor volume in different dosage groups (Figure 11A) and results of tumor weight analyses (Figure 11B) revealed that both 3BP-LCNP and 3BP-LCNP-FA exhibited significant inhibitory effects on tumor growth (P<0.05). During the treatment period, the body weight of nude mice in the 3BP administration group showed a downward trend, which was not observed in the other groups (Figure 11C). Evaluation at different time points during administration revealed that tumor volume in the 3BP administration group increased from 70 to approximately 678 mm3, whereas in the 3BP-LCNP-FA treatment group, it increased to only 248 mm3 (Figure 11D). Tumor growth was inhibited more significantly by 3BP-LCNP-FA than by LCNP-FA/3BP/3BP-LCNP, with no obvious toxic and side effects. Hematoxylin and eosin-stained sections of the liver, kidney, lung, and tumor from the different groups revealed no obvious morphological changes in any of the observed tissues (Figure 11E). Analysis of markers related to organ damage indicated that levels of alanine transaminase (ALT), aspartate aminotransferase (AST), creatinine (Cr), and blood urea nitrogen (BUN) in the treatment groups were within the normal range (Figure 11F). The result of PCNA immunohistochemistry showed that PCNA was localized in the nucleus; the positive markers (HRP-DAB/H2O2) appeared as light-yellow and brown-yellow granules (Figure 11G). The highest PCNA expression was observed in 3BP-LCNP-FA-treated mice, followed by (in order) those treated with 3BP-LCNP, 3BP, LCNP-FA, and control. Results of the TUNEL assay used to observe the extent of apoptosis in the tumor tissues revealed the highest expression level of green particles in tumors from 3BP-LCNP-FA-treated mice, followed by (in order) those from mice treated with 3BP-LCNP, 3BP, LCNP-FA, and control (Figure 11H).
Discussion
In the process of preparation, cubosomes will form a lipid bilayer structure and then extend in three-dimensional space to form a compact and stable cubic liquid crystal system with various structures, in which the smallest lattice is a cube [32]. Because cubosomes are a self-stable system, they are superior to traditional nanocarrier systems, such as micelles and liposomes. Although cubosomes have been shown to exhibit strong antitumor effects
In the current study, we also evaluated levels of markers associated with organ damage. Specifically, ALT, which is present in the cytoplasm of hepatocytes, and AST, which is mainly present in the cytoplasm and mitochondria of hepatocytes, are released into the blood during cell degeneration, membrane necrosis, cell permeability, or membrane damage. BUN and Cr are markers of renal injury. Notably, these levels were all within normal ranges, suggesting that 3BP-LCNP-FA exhibited no hepatotoxicity or nephrotoxicity. In addition, absence of morphological changes indicated no significant toxicity or side effects in the liver, kidney, or lung in each treatment group. TUNEL assay results further demonstrated that 3BP-LCNP-FA inhibited tumor cell proliferation and increased tumor apoptosis, implying its potent inhibitory effects on tumor growth. Overall, these results indicated that it is feasible to enhance the efficacy of antitumor drugs by modifying the drug delivery system. The results of this study, therefore, may have promising implications for future 3BP research and clinical application.
This study has several potential limitations. The antitumor effect of 3BP-LCNP-FA was greater than that of LCNP-FA/3BP/3BP-LCNP based on pharmacodynamics. However, the specific mechanisms of action have not been clarified, including accurate details regarding the tumor targeting and release mechanism in cells after 3BP-LCNP-FA enters the blood. Follow-up experiments will be required to evaluate the precise modes of action and antitumor effects of 3BP-LCNP-FA from the perspective of molecular mechanisms.
Conclusions
In this study, the performance of a prepared drug delivery platform was evaluated using PLM, differential scanning calorimetry, and 1H-NMR spectroscopy. 3BP-LCNP-FA can be successfully constructed as a drug delivery vehicle that has no obvious toxic effects
Figures
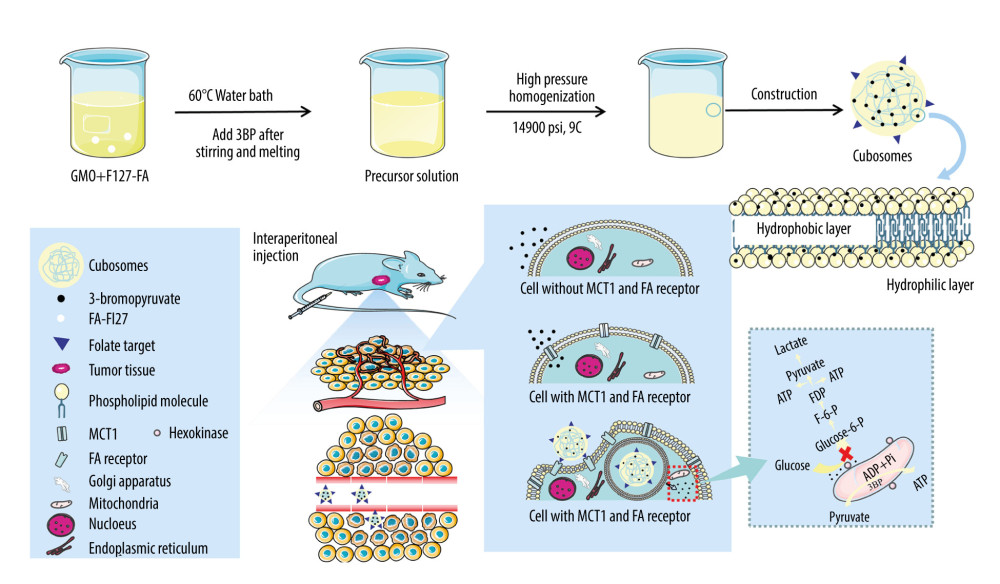 Figure 1. Construction and mechanism of 3BP-LCNP-FA. Synthetic process and structure of 3BP-LCNP-FA. 3-bromopyruvate is a hydrophilic drug and located in the hydrophilic segment of the phospholipid bilayer. 3BP-LCNP-FA is injected into the blood stream by intraperitoneal injection, and the drug is transported into tumor cells based on the enhanced permeability and retention effect and folate receptor-mediated endocytosis. After local release of the drug, hexokinase on the mitochondria can inhibit the ATP acquisition of tumor cells and cause cell death without affecting normal cells. (The following images represent three independent experiments).
Figure 1. Construction and mechanism of 3BP-LCNP-FA. Synthetic process and structure of 3BP-LCNP-FA. 3-bromopyruvate is a hydrophilic drug and located in the hydrophilic segment of the phospholipid bilayer. 3BP-LCNP-FA is injected into the blood stream by intraperitoneal injection, and the drug is transported into tumor cells based on the enhanced permeability and retention effect and folate receptor-mediated endocytosis. After local release of the drug, hexokinase on the mitochondria can inhibit the ATP acquisition of tumor cells and cause cell death without affecting normal cells. (The following images represent three independent experiments). 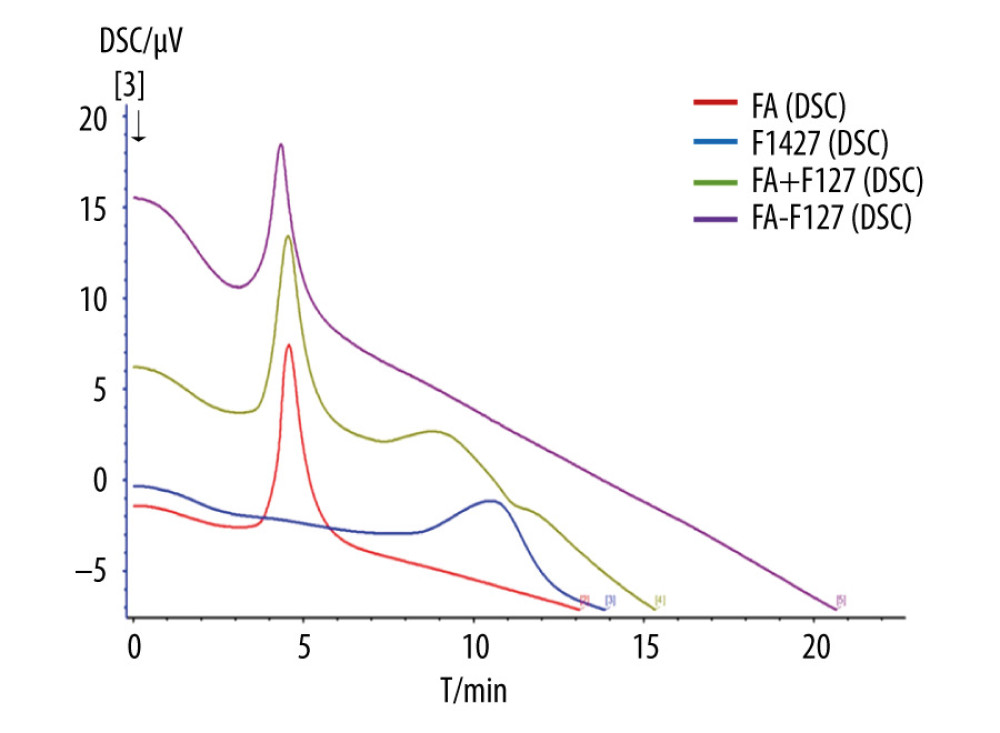 Figure 2. Differential scanning calorimetry characterization of FA-F127.
Figure 2. Differential scanning calorimetry characterization of FA-F127. 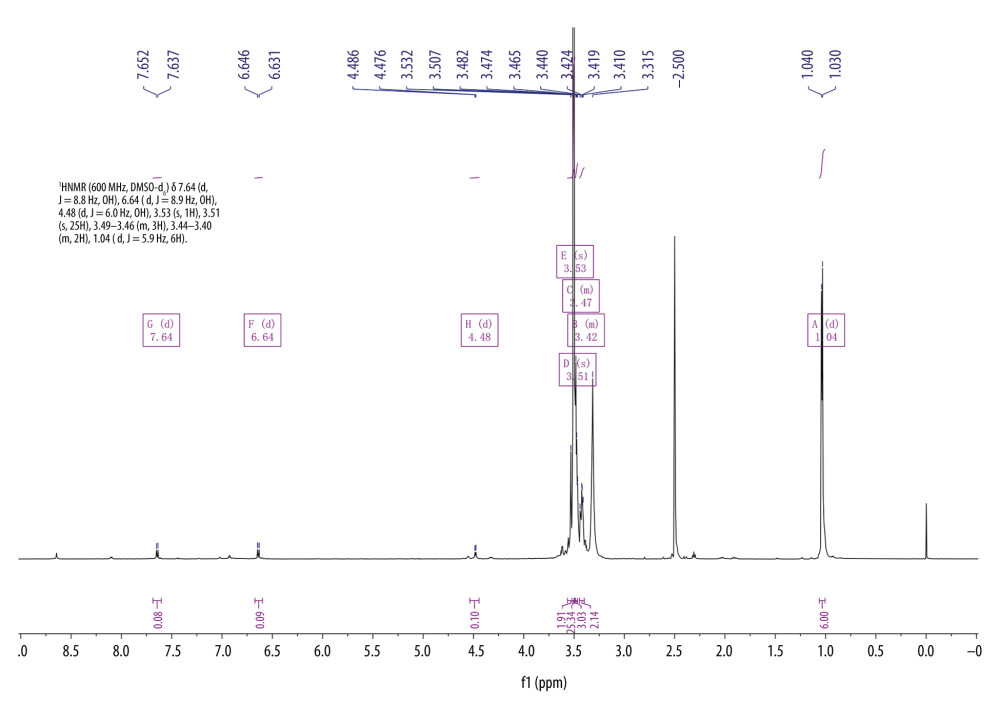 Figure 3. 1H-NMR spectrum of F127-FA.
Figure 3. 1H-NMR spectrum of F127-FA.  Figure 4. PLM characterization of cubosomes. (A) 3BP-LCNP; (B) 3BP-LCNP-FA.
Figure 4. PLM characterization of cubosomes. (A) 3BP-LCNP; (B) 3BP-LCNP-FA. 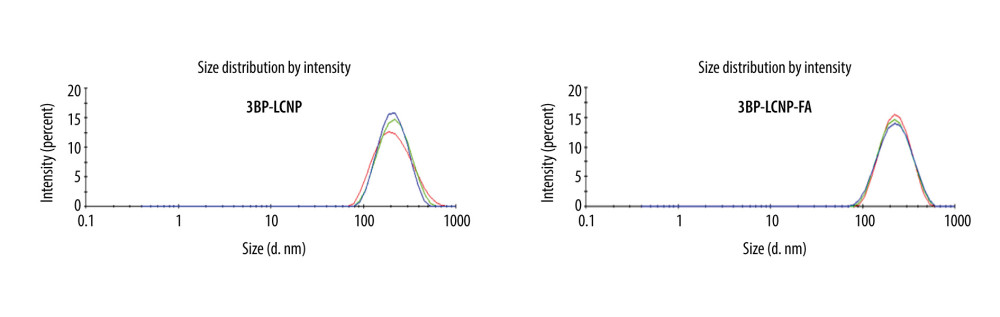 Figure 5. Size and PDI distribution of 3BP-LCNP and 3BP-LCNP-FA. Different colors represent three tests (n=3).
Figure 5. Size and PDI distribution of 3BP-LCNP and 3BP-LCNP-FA. Different colors represent three tests (n=3).  Figure 6. TEM characterization of 3BP-LCNP-FA.
Figure 6. TEM characterization of 3BP-LCNP-FA. 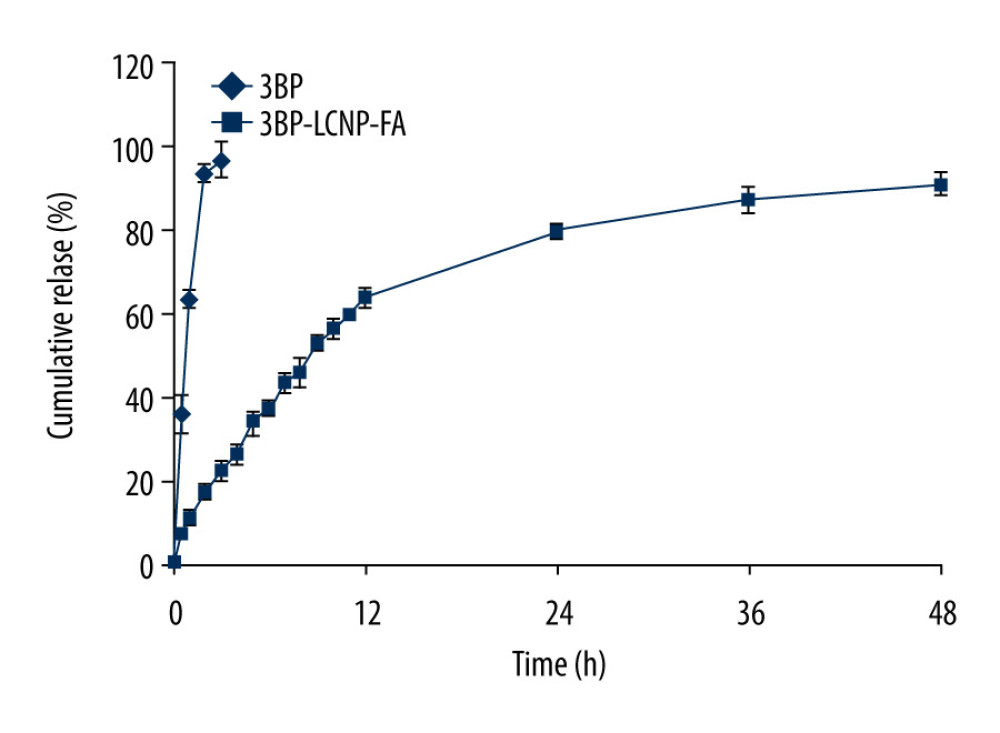 Figure 7. In vitro release of 3BP and 3BP-LCNP-FA (pH 6.8). Data represent the mean±S.D. of three independent experiments.
Figure 7. In vitro release of 3BP and 3BP-LCNP-FA (pH 6.8). Data represent the mean±S.D. of three independent experiments. 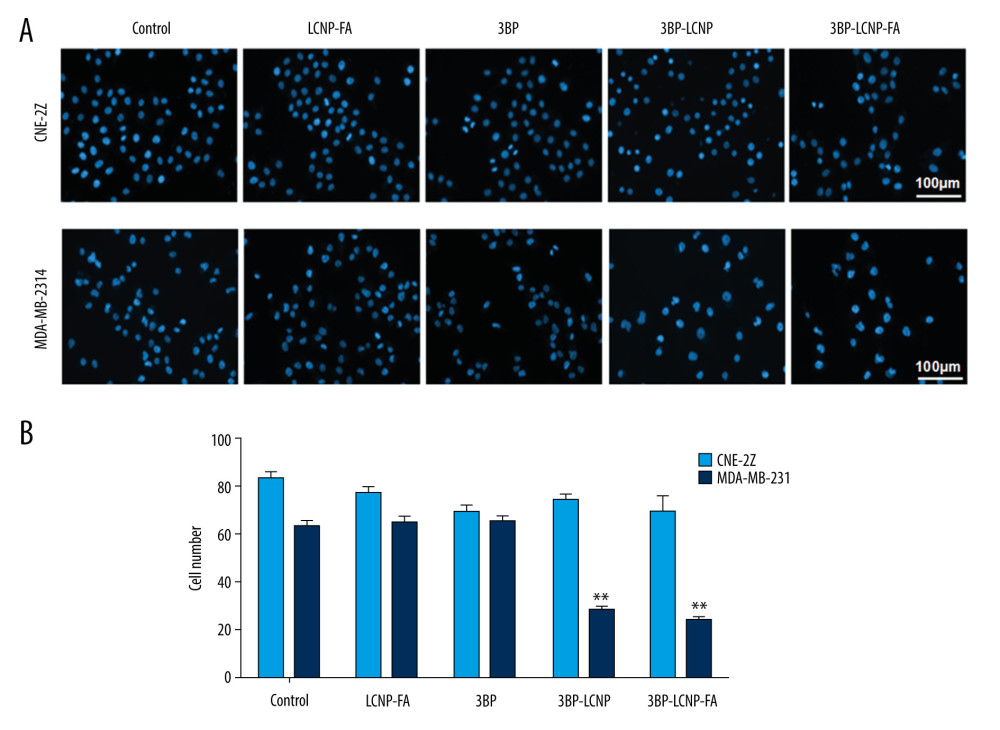 Figure 8. (A) Cell morphology changes and (B) cell density changes after LCNP-FA, 3BP, 3BP-LCNP, and 3BP-LCNP-FA treatment.
Figure 8. (A) Cell morphology changes and (B) cell density changes after LCNP-FA, 3BP, 3BP-LCNP, and 3BP-LCNP-FA treatment. 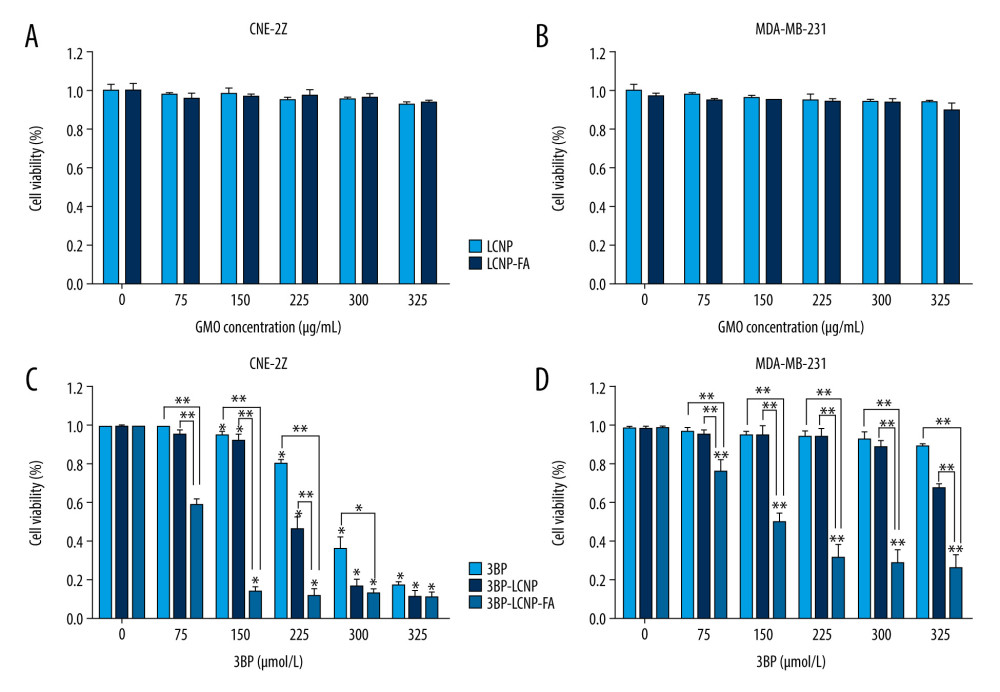 Figure 9. Cytotoxicity of different drug formulations in CEN2Z and MDA-MB-231 cells by MTT. (A) Blank vectors LCNP and LCNP-FA on CNE-2Z and (B) MDA-MB-231. (C) 3BP, 3BP-LCNP, 3BP-LCNP-FA on CNE-2Z and (D) MDA-MB-231. Data are representative of three separate experiments with similar results. * P<0.05, ** P<0.01.
Figure 9. Cytotoxicity of different drug formulations in CEN2Z and MDA-MB-231 cells by MTT. (A) Blank vectors LCNP and LCNP-FA on CNE-2Z and (B) MDA-MB-231. (C) 3BP, 3BP-LCNP, 3BP-LCNP-FA on CNE-2Z and (D) MDA-MB-231. Data are representative of three separate experiments with similar results. * P<0.05, ** P<0.01. 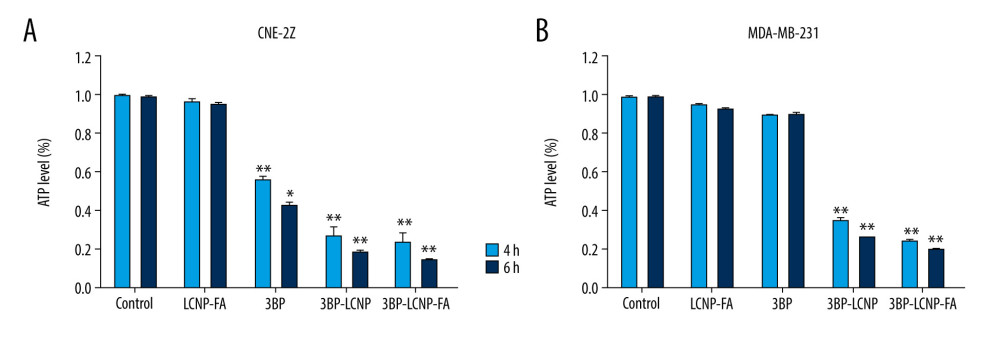 Figure 10. (A) Changes in intracellular ATP levels after 4 and 6 h of LCNP-FA, 3BP, 3BP-LCNP, and 3BP-LCNP-FA treatment on CNE-2Z and (B) MDA-MB-231 cells. Data represent the mean±S.D. of three independent experiments, * P<0.05, ** P<0.001.
Figure 10. (A) Changes in intracellular ATP levels after 4 and 6 h of LCNP-FA, 3BP, 3BP-LCNP, and 3BP-LCNP-FA treatment on CNE-2Z and (B) MDA-MB-231 cells. Data represent the mean±S.D. of three independent experiments, * P<0.05, ** P<0.001. 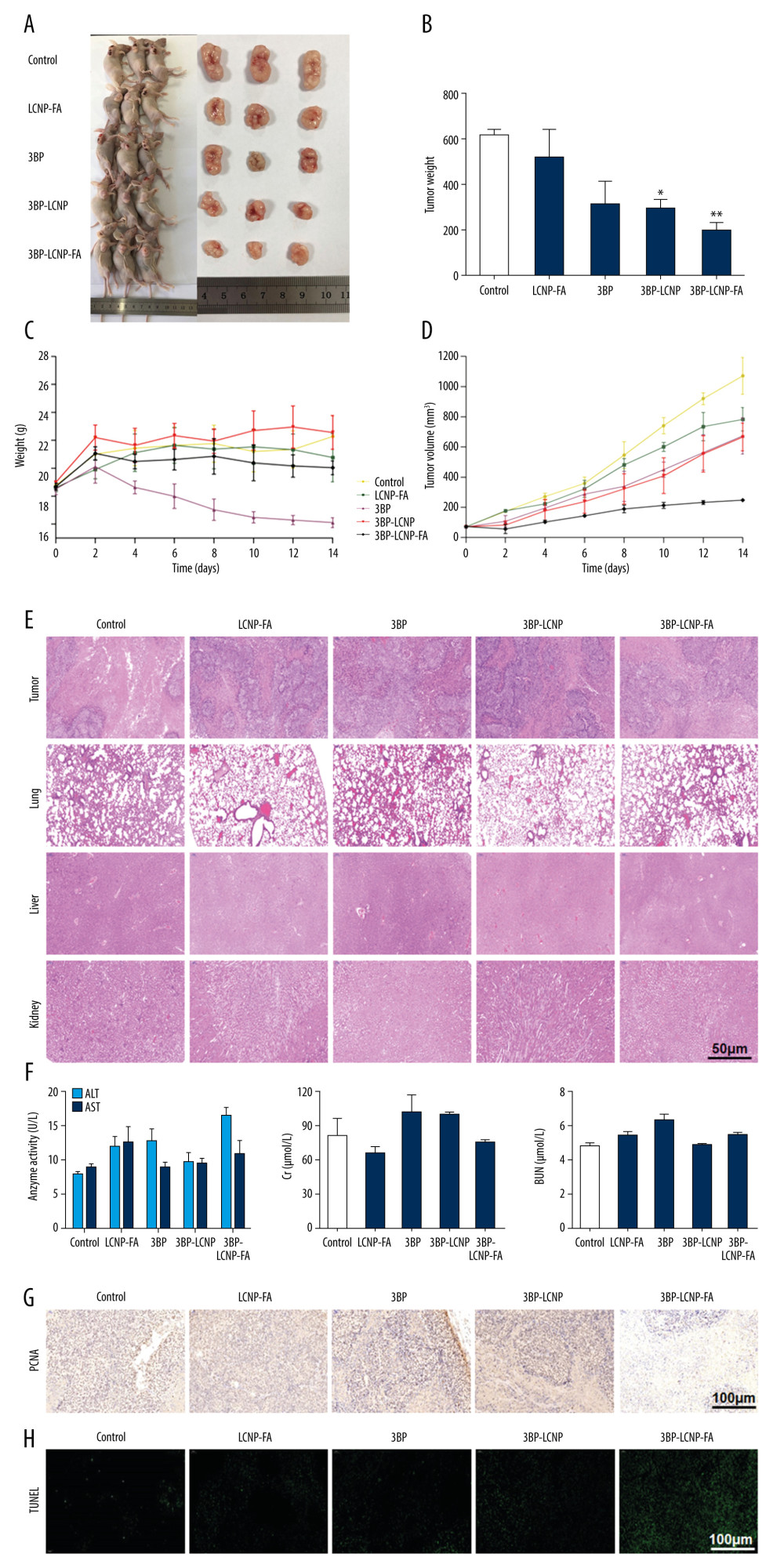 Figure 11. Antitumor activity evaluation of 3BP-LCNP-FA in vivo. (A) Formation of tumors in control, LCNP-FA, 3BP, 3BP-LCNP, and 3BP-LCNP-FA groups of mice. After nude mice were sacrificed in the last administration, tumor weights of different dosage groups were analyzed. (B) Data are presented as mean±S.D. (n=4) * P<0.05, ** P<0.01. (C) Change curves of mice weight and (D) tumor volume were measured at different time points after administration of different dosage forms. Data are presented as mean±S.D. (n=4). H &E staining of the tumors, lung, liver, and kidney after LCNP-FA, 3BP, 3BP-LCNP, and 3BP-LCNP-FA treatment vs. control. (E) The images are representative of three independent experiments. After LCNP-FA, 3BP, 3BP-LCNP, and 3BP-LCNP-FA treatment, the levels of AST, ALT, Cr, and BUN were evaluated by taking orbital venous blood. (F) Data represent the Mean±S.D. (n=6). PCNA immunohistochemistry of LCNP, 3BP, 3BP-LCNP, and 3BP-LCNP-FA treatment. (G) The images are representative of three independent experiments. TUNEL assay of LCNP, 3BP, 3BP-LCNP, and 3BP-LCNP-FA treatment. (H) The images are representative of three independent experiments.
Figure 11. Antitumor activity evaluation of 3BP-LCNP-FA in vivo. (A) Formation of tumors in control, LCNP-FA, 3BP, 3BP-LCNP, and 3BP-LCNP-FA groups of mice. After nude mice were sacrificed in the last administration, tumor weights of different dosage groups were analyzed. (B) Data are presented as mean±S.D. (n=4) * P<0.05, ** P<0.01. (C) Change curves of mice weight and (D) tumor volume were measured at different time points after administration of different dosage forms. Data are presented as mean±S.D. (n=4). H &E staining of the tumors, lung, liver, and kidney after LCNP-FA, 3BP, 3BP-LCNP, and 3BP-LCNP-FA treatment vs. control. (E) The images are representative of three independent experiments. After LCNP-FA, 3BP, 3BP-LCNP, and 3BP-LCNP-FA treatment, the levels of AST, ALT, Cr, and BUN were evaluated by taking orbital venous blood. (F) Data represent the Mean±S.D. (n=6). PCNA immunohistochemistry of LCNP, 3BP, 3BP-LCNP, and 3BP-LCNP-FA treatment. (G) The images are representative of three independent experiments. TUNEL assay of LCNP, 3BP, 3BP-LCNP, and 3BP-LCNP-FA treatment. (H) The images are representative of three independent experiments. References
1. Warburg O, On the origin of cancer cells: Science, 1956; 123(3191); 309-14
2. Gonzalez CD, Alvarez S, Ropolo A, Autophagy, Warburg, and Warburg reverse effects in human cancer: BioMed Res Int, 2014; 2014 926729
3. Xian SL, Cao W, Zhang XD, Lu YFL, 3-Bromopyruvate inhibits human gastric cancer tumor growth in nude mice via the inhibition of glycolysis: Oncol Lett, 2015; 9(2); 739-44
4. Ganapathy-Kanniappan S, Vali M, Kunjithapatham R, 3-Bromopyruvate a new targeted antiglycolytic agent and a promise for cancer therapy: Curr Pharm Biotechnol, 2010; 11(5); 510-17
5. El Sayed SM, Mahmoud AA, El Sawy SA, Warburg effect increases steady-state ROS condition in cancer cells through decreasing their antioxidant capacities (anticancer effects of 3-bromopyruvate through antagonizing Warburg effect): Med Hypotheses, 2013; 81(5); 866-70
6. Cardaci S, Desideri E, Ciriolo MR, Targeting aerobic glycolysis: 3-bromopyruvate as a promising anticancer drug: J Bioenerg Biomembr, 2012; 44(1); 17-29
7. Pan Q, Sun Y, Jin Q, Hepatotoxicity and nephrotoxicity of 3-bromopyruvate in mice: Acta Cir Bras, 2016; 31(11); 724-29
8. Lis P, Zarzycki M, Ko YH, Transport and cyto toxicity of the anticancer drug 3–bromopyruvate in the yeast Saccharomyces cerevisiae: J Bioenerg Biomembr, 2012; 44(1); 155-61
9. Azevedo-Silva J, Queiros O, Baltazar F, The anticancer agent 3-bromopyruvate: A simple but powerful molecule taken from the lab to the bedside: J Bioenerg Biomembr, 2016; 48(4); 349-62
10. Yadav S, Kujur PK, Pandey SK, Antitumor action of 3-bromopyruvate implicates reorganized tumor growth regulatory components of tumor milieu, cell cycle arrest and induction of mitochondria-dependent tumor cell death: Toxicol Applied Pharmacol, 2018; 339; 52-64
11. Gong L, Wei Y, Yu X: Anticancer Agents Med Chem, 2014; 14(5); 771-76
12. Pedersen PL, 3-bromopyruvate (3BP) a fast acting, promising, powerful, specific, and effective “small molecule” anti-cancer agent taken from labside to bedside: Introduction to a special issue: J Bioenerg Biomembr, 2012; 44(1); 1-6
13. Yadav S, Pandey SK, Goel Y, Diverse Stakeholders of tumor metabolism: An appraisal of the emerging approach of multifaceted metabolic targeting by 3-bromopyruvate: Frontiers Pharmacol, 2019; 10; 728
14. Zou X, Zhang M, Sun Y, Inhibitory effects of 3-bromopyruvate in human nasopharyngeal carcinoma cells: Oncol Rep, 2015; 34(4); 1895-904
15. Azevedo-Silva J, Queiros O, Ribeiro A, The cytotoxicity of 3-bromopyruvate in breast cancer cells depends on extracellular pH: Biochem J, 2015; 467(2); 247-58
16. El Sayed SM, Baghdadi H, Zolaly M, The promising anticancer drug 3-bromopyruvate is metabolized through glutathione conjugation which affects chemoresistance and clinical practice: An evidence-based view: Med Hypotheses, 2017; 100; 67-77
17. Glick M, Biddle P, Jantzi J, The antitumor agent 3-bromopyruvate has a short half-life at physiological conditions: Biochem Biophys Res Commun, 2014; 452(1); 170-73
18. Ko YH, Verhoeven HA, Lee MJ, A translational study “case report” on the small molecule “energy blocker” 3-bromopyruvate (3BP) as a potent anticancer agent: From bench side to bedside: J Bioenerg Biomembr, 2012; 44(1); 163-70
19. Sadowska-Bartosz I, Szewczyk R, Jaremko L, Anticancer agent 3-bromopyruvic acid forms a conjugate with glutathione: Pharmacol Rep, 2016; 68(2); 502-5
20. El Sayed SM, Mohamed WG, Seddik MA, Safety and outcome of treatment of metastatic melanoma using 3-bromopyruvate: A concise literature review and case study: Chin J Cancer, 2014; 33(7); 356-64
21. Qiao J, Dong P, Mu X, Folic acid-conjugated fluorescent polymer for up-regulation folate receptor expression study via targeted imaging of tumor cells: Biosens Bioelectron, 2016; 78; 147-53
22. Hartmann LC, Keeney GL, Lingle WL, Folate receptor overexpression is associated with poor outcome in breast cancer: Int J Cancer, 2007; 121(5); 938-42
23. Leamon CP, Folate-targeted drug strategies for the treatment of cancer: Curr Opin Investig Drugs, 2008; 9(12); 1277-86
24. Sega EI, Low PS, Tumor detection using folate receptor-targeted imaging agents: Cancer Metastasis Rev, 2008; 27(4); 655-64
25. Park EK, Kim SY, Lee SB, Lee YM, Folate-conjugated methoxy poly(ethylene glycol)/poly(epsilon-caprolactone) amphiphilic block copolymeric micelles for tumor–targeted drug delivery: J Controlled Release, 2005; 109(1–3); 158-68
26. Luzzati V, Tardieu A, Gulik-Krzywicki T, Structure of the cubic phases of lipid-water systems: Nature, 1968; 220(5166); 485-88
27. Luzzati V, Vargas R, Mariani P, Cubic phases of lipid-containing systems. Elements of a theory and biological connotations: J Mol Biol, 1993; 229(2); 540-51
28. Chountoulesi M, Pippa N, Pispas S, Cubic lyotropic liquid crystals as drug delivery carriers: Physicochemical and morphological studies: Int J Pharm, 2018; 550(1–2); 57-70
29. Madheswaran T, Kandasamy M, Bose RJ, Karuppagounder V, Current potential and challenges in the advances of liquid crystalline nanoparticles as drug delivery systems: Drug Discovery Today, 2019; 24(7); 1405-12
30. Zhai J, Fong C, Tran N, Drummond CJ, Non-lamellar lyotropic liquid crystalline lipid nanoparticles for the next generation of nanomedicine: ACS Nano, 2019; 13(6); 6178-206
31. Butt AM, Mohd Amin MC, Katas H, Synergistic effect of pH-responsive folate-functionalized poloxamer 407-TPGS-mixed micelles on targeted delivery of anticancer drugs: Int J Nanomed, 2015; 10; 1321-34
32. Li J, Wu L, Wu W, A potential carrier based on liquid crystal nanoparticles for ophthalmic delivery of pilocarpine nitrate: Int J Pharm, 2013; 455(1–2); 75-84
Figures
 Figure 1. Construction and mechanism of 3BP-LCNP-FA. Synthetic process and structure of 3BP-LCNP-FA. 3-bromopyruvate is a hydrophilic drug and located in the hydrophilic segment of the phospholipid bilayer. 3BP-LCNP-FA is injected into the blood stream by intraperitoneal injection, and the drug is transported into tumor cells based on the enhanced permeability and retention effect and folate receptor-mediated endocytosis. After local release of the drug, hexokinase on the mitochondria can inhibit the ATP acquisition of tumor cells and cause cell death without affecting normal cells. (The following images represent three independent experiments).
Figure 1. Construction and mechanism of 3BP-LCNP-FA. Synthetic process and structure of 3BP-LCNP-FA. 3-bromopyruvate is a hydrophilic drug and located in the hydrophilic segment of the phospholipid bilayer. 3BP-LCNP-FA is injected into the blood stream by intraperitoneal injection, and the drug is transported into tumor cells based on the enhanced permeability and retention effect and folate receptor-mediated endocytosis. After local release of the drug, hexokinase on the mitochondria can inhibit the ATP acquisition of tumor cells and cause cell death without affecting normal cells. (The following images represent three independent experiments). Figure 2. Differential scanning calorimetry characterization of FA-F127.
Figure 2. Differential scanning calorimetry characterization of FA-F127. Figure 3. 1H-NMR spectrum of F127-FA.
Figure 3. 1H-NMR spectrum of F127-FA. Figure 4. PLM characterization of cubosomes. (A) 3BP-LCNP; (B) 3BP-LCNP-FA.
Figure 4. PLM characterization of cubosomes. (A) 3BP-LCNP; (B) 3BP-LCNP-FA. Figure 5. Size and PDI distribution of 3BP-LCNP and 3BP-LCNP-FA. Different colors represent three tests (n=3).
Figure 5. Size and PDI distribution of 3BP-LCNP and 3BP-LCNP-FA. Different colors represent three tests (n=3). Figure 6. TEM characterization of 3BP-LCNP-FA.
Figure 6. TEM characterization of 3BP-LCNP-FA. Figure 7. In vitro release of 3BP and 3BP-LCNP-FA (pH 6.8). Data represent the mean±S.D. of three independent experiments.
Figure 7. In vitro release of 3BP and 3BP-LCNP-FA (pH 6.8). Data represent the mean±S.D. of three independent experiments. Figure 8. (A) Cell morphology changes and (B) cell density changes after LCNP-FA, 3BP, 3BP-LCNP, and 3BP-LCNP-FA treatment.
Figure 8. (A) Cell morphology changes and (B) cell density changes after LCNP-FA, 3BP, 3BP-LCNP, and 3BP-LCNP-FA treatment. Figure 9. Cytotoxicity of different drug formulations in CEN2Z and MDA-MB-231 cells by MTT. (A) Blank vectors LCNP and LCNP-FA on CNE-2Z and (B) MDA-MB-231. (C) 3BP, 3BP-LCNP, 3BP-LCNP-FA on CNE-2Z and (D) MDA-MB-231. Data are representative of three separate experiments with similar results. * P<0.05, ** P<0.01.
Figure 9. Cytotoxicity of different drug formulations in CEN2Z and MDA-MB-231 cells by MTT. (A) Blank vectors LCNP and LCNP-FA on CNE-2Z and (B) MDA-MB-231. (C) 3BP, 3BP-LCNP, 3BP-LCNP-FA on CNE-2Z and (D) MDA-MB-231. Data are representative of three separate experiments with similar results. * P<0.05, ** P<0.01. Figure 10. (A) Changes in intracellular ATP levels after 4 and 6 h of LCNP-FA, 3BP, 3BP-LCNP, and 3BP-LCNP-FA treatment on CNE-2Z and (B) MDA-MB-231 cells. Data represent the mean±S.D. of three independent experiments, * P<0.05, ** P<0.001.
Figure 10. (A) Changes in intracellular ATP levels after 4 and 6 h of LCNP-FA, 3BP, 3BP-LCNP, and 3BP-LCNP-FA treatment on CNE-2Z and (B) MDA-MB-231 cells. Data represent the mean±S.D. of three independent experiments, * P<0.05, ** P<0.001. Figure 11. Antitumor activity evaluation of 3BP-LCNP-FA in vivo. (A) Formation of tumors in control, LCNP-FA, 3BP, 3BP-LCNP, and 3BP-LCNP-FA groups of mice. After nude mice were sacrificed in the last administration, tumor weights of different dosage groups were analyzed. (B) Data are presented as mean±S.D. (n=4) * P<0.05, ** P<0.01. (C) Change curves of mice weight and (D) tumor volume were measured at different time points after administration of different dosage forms. Data are presented as mean±S.D. (n=4). H &E staining of the tumors, lung, liver, and kidney after LCNP-FA, 3BP, 3BP-LCNP, and 3BP-LCNP-FA treatment vs. control. (E) The images are representative of three independent experiments. After LCNP-FA, 3BP, 3BP-LCNP, and 3BP-LCNP-FA treatment, the levels of AST, ALT, Cr, and BUN were evaluated by taking orbital venous blood. (F) Data represent the Mean±S.D. (n=6). PCNA immunohistochemistry of LCNP, 3BP, 3BP-LCNP, and 3BP-LCNP-FA treatment. (G) The images are representative of three independent experiments. TUNEL assay of LCNP, 3BP, 3BP-LCNP, and 3BP-LCNP-FA treatment. (H) The images are representative of three independent experiments.
Figure 11. Antitumor activity evaluation of 3BP-LCNP-FA in vivo. (A) Formation of tumors in control, LCNP-FA, 3BP, 3BP-LCNP, and 3BP-LCNP-FA groups of mice. After nude mice were sacrificed in the last administration, tumor weights of different dosage groups were analyzed. (B) Data are presented as mean±S.D. (n=4) * P<0.05, ** P<0.01. (C) Change curves of mice weight and (D) tumor volume were measured at different time points after administration of different dosage forms. Data are presented as mean±S.D. (n=4). H &E staining of the tumors, lung, liver, and kidney after LCNP-FA, 3BP, 3BP-LCNP, and 3BP-LCNP-FA treatment vs. control. (E) The images are representative of three independent experiments. After LCNP-FA, 3BP, 3BP-LCNP, and 3BP-LCNP-FA treatment, the levels of AST, ALT, Cr, and BUN were evaluated by taking orbital venous blood. (F) Data represent the Mean±S.D. (n=6). PCNA immunohistochemistry of LCNP, 3BP, 3BP-LCNP, and 3BP-LCNP-FA treatment. (G) The images are representative of three independent experiments. TUNEL assay of LCNP, 3BP, 3BP-LCNP, and 3BP-LCNP-FA treatment. (H) The images are representative of three independent experiments. In Press
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952