11 November 2020: Lab/In Vitro Research
Inhibition of Glioma Cell Growth and Apoptosis Induction through Targeting Wnt10B Expression by Pyrazolo[4,3-c]pyridine-4-one
Yang Liu12BCDE, Zhu Yaozu13BCD, Huang Zhao4BCE, Peng Peng4BCE, Zhang Tingbao1BDE, Chen Jincao1ADEF*DOI: 10.12659/MSM.923912
Med Sci Monit 2020; 26:e923912
Abstract
BACKGROUND: Gliomas are commonly diagnosed tumors in the central nervous system that have an elevated mortality rate. The present study evaluated pyrazolo[4,3-c]pyridine-4-one (PP-4-one) as an anti-proliferative agent against glioma cells and investigated the associated mechanism.
MATERIAL AND METHODS: The changes in cell growth were analyzed by Cell Counting Kit-8 (CCK-8) and apoptosis by flow cytometry using Annexin V-FITC staining kit. The FACSCalibur flow cytometer was used for analysis of DNA content and western blotting for protein expression.
RESULTS: The PP-4-one treatment suppressed viability of U251, C6, and U87 cells significantly at a concentration of 0.25 µM. At a concentration of 16 µM, PP-4-one treatment for 72 hours suppressed viability of U251, C6, and U87 cells to 24%, 21%, and 20%, respectively. Treatment with PP-4-one suppressed cyclic 3’,5’-adenosine monophosphate (cAMP) levels in U251 and C6 cells significantly (P<0.05) depending on the concentration. The apoptotic cells were increased significantly (P<0.05) by PP-4-one treatment in U251 and C6 cell cultures. A considerable enhancement in the proportion of U251 and C6 cells in the G0/G1 phase was recorded on incubation with PP-4-one. Treatment of U251 and C6 cells with PP-4-one markedly enhanced p21 expression relative to the control. The B-cell lymphoma (Bcl-2) level in PP-4-one treated U251 and C6 cells was markedly lower relative to the control cells. The Bax, caspase-3, and caspase-9 levels were elevated markedly by PP-4-one treatment in U251 and C6 cells.
CONCLUSIONS: This study demonstrated that PP-4-one has anti-proliferative potential for glioma cells via targeting cAMP and Bcl-2 levels. It also promoted glioma cell apoptosis through caspase activation and arrest of the cell cycle. Thus, PP-4-one may be used to develop drug candidates for the glioma treatment.
Keywords: Cell Cycle Checkpoints, Cyclic AMP, Cyclin-Dependent Kinase Inhibitor p21, Glioma, Proto-Oncogene Proteins, Pyrazoles, Wnt Proteins
Background
Gliomas are frequently diagnosed tumors in the central nervous system that have an elevated mortality rate and comprises 16% of all primary brain and central nervous system tumors [1]. Malignant gliomas are a commonly detected class of primary brain tumors which destroy neurons and are aggressive and highly invasive tumors with poor prognosis [2]. The genetic and cellular mechanism of gliomas has been investigated to a greater extent because of intensive efforts by clinicians [3]. The survival time for glioblastoma multiforme patients is only 2 years following diagnosis [4]. The progression from low-grade glioma to the high-grade glioma has different time durations depending in various patients [4]. Gliomas are presently treated by the use of radiotherapy/chemotherapy in combination with surgical resection [5]. In order to combat gliomas, it is important to understand its pathogenesis to help identify novel therapeutic targets [6]. The glioma treatment strategies currently used are not satisfactory and there is an urgent need for identification of effective therapeutic agent for glioma.
Pyrazole is one of the most important molecules in heterocyclic chemistry and many of its compounds are approved drugs [7]. Celecoxib and lonazolac are used as anti-inflammatory drugs, fipronil acts as an insecticide, dipyrone is a promising analgesic and antipyretic molecule [8], and sildenafil is used for erectile dysfunction treatment, all of these drugs are pyrazole based compounds [9]. Fused-pyrazoles have been found to exhibit several biological properties like anti-inflammatory [10], anti-viral [11], anti-tumor [12], anti-microbial [13], and antiprotozoal [14]. In addition, many fused-pyrazoles have shown significant anti-human immunodeficiency virus (HIV) properties [15]. The anti-HIV activity of 1H-pyrazolo[3,4-b]pyridine-3-yl compounds is associated with inhibition of reverse transcriptase [15]. Another series of pyrazoles has been reported to exhibit anti-HIV property against both HIV-1 (IIIB) and HIV-2 (ROD) [15]. The present study evaluated pyrazolo[4,3-c]pyridine-4-one (PP-4-one) derivative (Figure 1) an anti-proliferative agent against glioma cells and investigated the associated mechanism.
Pyrazole scaffold has been employed for synthesis of anticancer agents that explore multiple tumor targets [16]. The compound ABT-751 acts as tubulin inhibitor [17,18] while indenopyrazoles inhibit polymerization of the tubulin inhibitors [19]. Another pyrazole analog effectively inhibits viability of multidrug resistant tumor cells via targeting phosphatase and tensin homolog (PTEN) activation and PTEN/Akt/NF-κB signaling [20].
Material and Methods
REAGENTS:
1,5,6,7-tetrahydro-4H-pyrrolo[3,2-c]pyridin-4-one (CAS number PH003760) commonly known as pyrazolo[4,3-c]pyridine-4-one was obtained from Merck. All other chemicals were supplied by Sigma-Aldrich.
CELL LINE AND CULTURE:
The U251, C6, and U87 cell lines were provided by the Chinese Academy of Sciences (Shanghai, China). The culture of cells was carried out at 37°C in RPMI-1640 medium which contained fetal bovine serum (10%) under 5% CO2 atmosphere. The antibiotics, penicillin (100 U/mL) and streptomycin (100 μg/mL) were also mixed with the medium.
GROWTH INHIBITION ASSAY:
The U251, C6, and U87 cells were distributed at 2×105 cells per well concentration in 96-well plates. The cell incubation for 24 hours was followed by treatment with 0.25, 0.5, 1.0, 2.0, 4.0, 8.0, and 16 μM PP-4-one in RPMI-1640 medium. After 72 hours treatment, Cell Counting Kit-8 (CCK-8; Dojindo Laboratories, Japan) solution (10 μL) was put into the wells and incubation for an additional 1 hour was continued under same conditions. The cell viability measurements were made indirectly by recording optical density for each well using a microplate reader (Molecular Devices, USA) 3 times at 456 nm wavelengths.
DETERMINATION OF CYCLIC 3′,5′-ADENOSINE MONOPHOSPHATE (CAMP) LEVEL:
The U251 and C6 cells were distributed at 2×105 cells per well concentration in 24-well plates and treated with 0.25, 0.5, 1.0, 2.0, 4.0, 8.0, and 16 μM PP-4-one for 72 hours. The phosphate-buffered saline (PBS) washing of cells and subsequent lysis with RIPA buffer for 40 minutes was followed by lysate centrifugation at 12 000 g for 15 minutes at 4°C to obtain supernatant. The supernatant was treated with hydrochloric acid (0.1 M) and DMEM/F12 for 30 minutes. The cAMP level in 30 μL sample of supernatant was analyzed on incubation with anti-cAMP primary antibodies at 4°C for 4 hours. Then samples were incubated with Northern Lights™ 557 conjugated anti-rabbit immunoglobulin G secondary antibodies.
ANALYSIS OF APOPTOSIS:
Apoptosis in U251 and C6 cells following treatment with PP-4-one was assessed using Annexin V-FITC staining kit (BD Biosciences, San Jose, CA, USA). The cells distributed in 6-well plates at 2×106 cells per well concentration were treated with 2.0 and 16 μM PP-4-one or dimethyl sulfoxide (DMSO, control) for 72 hours. Then cells were collected and subsequently treated with 200 μL of binding buffer solution. The cell suspension was treated with annexin V-FITC (10 μL) for 20 minutes under complete darkness at room temperature. Afterwards, cells were treated with binding buffer (300 μL) and propidium iodide (PI, 5 μL) prior to flow cytometric analysis using CELL Quest 3.0 software.
CELL CYCLE ANALYSIS:
The U251 and C6 cells at 2×106 cells concentration were put into 10 cm culture dishes and cultured in RPMI-1640 medium for 24 hours. Then, cells were treated with 2.0 and 16 μM PP-4-one or DMSO (control) for 72 hours. The harvested cells were subjected to fixing in ethyl alcohol (70%) for 24 hours and then rinsed in PBS. Staining of the cells with PI solution (5%) was carried out in accordance with instructions from manufacturer. The FACSCalibur flow cytometer along with the Cell Quest software Pro (5.1 version; BD Biosciences, Franklin Lakes, NJ, USA) were employed for cell cycle analysis.
WESTERN BLOT ASSAY:
The U251 and C6 cells after treatment with 2.0 and 16 μM PP-4-one or DMSO (control) for 72 hours were lysed using radioimmunoprecipitation assay (RIPA) buffer. The lysate centrifugation at 12 000 g for 40 minutes at 4oC to obtain supernatant was followed bicinchoninic acid (BCA) assay for measurement of protein concentration. The equal protein samples (30 μg) were subjected to electrophoresis on sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) gel and then transferred to polyvinylidene fluoride (PVDF) membranes. Blocking of membranes was performed by incubation with 5% non-fat milk in tris-buffered saline and Tween (TBST). Incubation of the blots was carried out with primary antibodies at 4°C for overnight. The PBS washed blots were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies at room temperature for 2 hours. The immunoblots were subjected to visualization using electrochemiluminescent (ECL) assays along with the western blot detection system (Santa Cruz Biotechnology). The primary antibodies used for membrane incubation were: anti-p21 (dilution 1: 500; Santa Cruz), anti-Bcl-2 (dilution 1: 500; Santa Cruz), anti-cleaved caspase-9 (dilution 1: 1000; Cell Signaling) and anti-cleaved caspase-3 (dilution 1: 1000; Cell Signaling).
STATISTICAL ANALYSIS:
The data are presented as the mean±standard deviations of 3 independent experiments. The statistical analyses were carried out using Origin Lab software version 8.0 (Origin Lab, Northampton, MA, USA). The differences were analyzed statistically using one-way analysis of variance (ANOVA) and Bonferroni post-test. The values were taken statistically significant at
Results
PP-4-ONE INHIBITED U251, C6, AND U87 CELL GROWTH:
The U251, C6, and U87 cells were growing at a significantly (P<0.05) higher rate in control cultures in comparison to PP-4-one treated cells (Figure 2). A gradual suppression in U251, C6, and U87 cell viability was caused by an increase in concentration from 0.25 to 16 μM. The PP-4-one treatment suppressed viability of U251, C6, and U87 cells significantly from 0.25 μM; at 16 μM, PP-4-one treatments for 72 hours suppressed viability of U251, C6, and U87 cells to 24%, 21%, and 20%, respectively.
PP-4-ONE SUPPRESSED CAMP LEVELS IN U251 AND C6 CELLS:
The cAMP levels in U251 and C6 control cultures was much higher relative to those in the PP-4-one treated cultures at 72 hours (Figure 3). Treatment with PP-4-one suppressed cAMP levels in U251 and C6 cells significantly (P<0.05) depending on the concentration. The cAMP level was significantly (P<0.05) suppressed by PP-4-one from 0.25 μM. The reduction of cAMP level by PP-4-one was maximum at 16 μM.
PP-4-ONE INDUCED APOPTOSIS IN U251 AND C6 CELLS:
To assess apoptosis induction, we added PP-4-one at 1 μM and 16 μM concentrations to U251 and C6 cell cultures (Figure 4). The apoptotic cell count was increased significantly (P<0.05) by PP-4-one treatment in U251 and C6 cell cultures. Treatment with 1 μM PP-4-one increased apoptotic cell count to 15.43% and 19.87% in U251 and C6 cells, respectively. On increasing PP-4-one concentration to 16 μM, the apoptosis in U251 and C6 cells was enhanced to 48.65% and 53.21%, respectively.
G0/G1 PHASE ARREST OF U251 AND C6 CELL CYCLE BY PP-4-ONE:
The PP-4-one treatment reduced U251 and C6 cell distribution in the G2/M phase significantly (P<0.05) at 72 hours (Figure 5). The U251 and C6 cell distribution was also decreased in the S phase significantly (P<0.05) on treatment with pyrazolo[4,3-c]pyridine-4-one. However, a considerable enhancement in the proportion of U251 and C6 cells in the G0/G1 phase was recorded on incubation with pyrazolo[4,3-c]pyridine-4-one. The enhancement of U251 and C6 cell counts in the G0/G1 phase was significant by the addition of PP-4-one both at 1 μM and 16 μM relative to control cells.
PP-4-ONE PROMOTED P21 EXPRESSION IN U251 AND C6 CELLS:
The alteration in p21 expression by PP-4-one in U251 and C6 cells was assessed using western blot (Figure 6). Treatment of U251 and C6 cells with PP-4-one markedly enhanced p21 expression relative to the control. There was marked elevation of p21 levels in U251 and C6 cells on treatment with 1.0 μM and 16 μM pyrazolo[4,3-c]pyridine-4-one.
PP-4-ONE INFLUENCED APOPTOTIC PROTEIN LEVELS IN U251 AND C6 CELLS:
In U251 and C6 cells alteration in Bcl-2, caspase-9, caspase-3 (cleaved) and Bax levels were detected at 72 hours of PP-4-one treatment by western blotting (Figure 7). The Bcl-2 level in PP-4-one treated U251 and C6 cells was markedly lower relative to the control cells. The Bax, caspase-3 and caspase-9 levels were elevated markedly by PP-4-one treatment in U251 and C6 cells. Elevation in Bax, caspase-3 and caspase-9 levels and suppression of Bcl-2 level was evident in U251 and C6 cells on treatment with 1.0 μM and 16 μM PP-4-one.
Discussion
Glioblastoma multiforme treatment using an effective and successful therapeutic strategy is a serious challenge to clinicians worldwide and needs to be addressed as soon as possible. Towards this motive, several molecules have been discovered which possess inhibitory effect against various glioma cells [21]. The derivatives of pyrazole have been shown to be very effective anti-proliferative property
Conclusions
The present study was the first to demonstrate anti-proliferative potential of PP-4-one for glioma cells via targeting cAMP and Bcl-2 levels. Moreover, PP-4-one promoted apoptosis via caspase activation and arrest of the cell cycle. Therefore, PP-4-one might be used to develop drug candidates for the glioma treatment.
Figures
![Chemical structure of PP-4-one. Abbreviations: PP-4-one, pyrazolo[4,3-c]pyridine-4-one.](https://jours.isi-science.com/imageXml.php?i=medscimonit-26-e923912-g001.jpg&idArt=923912&w=1000) Figure 1. Chemical structure of PP-4-one. Abbreviations: PP-4-one, pyrazolo[4,3-c]pyridine-4-one.
Figure 1. Chemical structure of PP-4-one. Abbreviations: PP-4-one, pyrazolo[4,3-c]pyridine-4-one. ![Effect of PP-4-one on U251, C6, and U87 cell viability. The PP-4-one was added to cells at 0.25, 0.5, 1.0, 2.0, 4.0, 8.0, and 16 μM doses and viability was assessed at 72 hours. The changes in viability by PP-4-one were measured using CCK-8 assay * P<0.05, ** P<0.02, and *** P<0.01 versus control cells. PP-4-one – pyrazolo[4,3-c]pyridine-4-one; CCK-8 – Cell Counting Kit-8.](https://jours.isi-science.com/imageXml.php?i=medscimonit-26-e923912-g002.jpg&idArt=923912&w=1000) Figure 2. Effect of PP-4-one on U251, C6, and U87 cell viability. The PP-4-one was added to cells at 0.25, 0.5, 1.0, 2.0, 4.0, 8.0, and 16 μM doses and viability was assessed at 72 hours. The changes in viability by PP-4-one were measured using CCK-8 assay * P<0.05, ** P<0.02, and *** P<0.01 versus control cells. PP-4-one – pyrazolo[4,3-c]pyridine-4-one; CCK-8 – Cell Counting Kit-8.
Figure 2. Effect of PP-4-one on U251, C6, and U87 cell viability. The PP-4-one was added to cells at 0.25, 0.5, 1.0, 2.0, 4.0, 8.0, and 16 μM doses and viability was assessed at 72 hours. The changes in viability by PP-4-one were measured using CCK-8 assay * P<0.05, ** P<0.02, and *** P<0.01 versus control cells. PP-4-one – pyrazolo[4,3-c]pyridine-4-one; CCK-8 – Cell Counting Kit-8. ![Inhibitory effect of PP-4-one on cAMP in U251 and C6 cells. The incubation with PP-4-one (0.25, 0.5, 1.0, 2.0, 4.0, 8.0, and 16 μM) for 72 hours was followed by assessment of cAMP levels in U251 and C6 cells. * P<0.05, ** P<0.02, and *** P<0.01 versus control cells. PP-4-one – pyrazolo[4,3-c]pyridine-4-one; cAMP – cyclic 3′,5′-adenosine monophosphate.](https://jours.isi-science.com/imageXml.php?i=medscimonit-26-e923912-g003.jpg&idArt=923912&w=1000) Figure 3. Inhibitory effect of PP-4-one on cAMP in U251 and C6 cells. The incubation with PP-4-one (0.25, 0.5, 1.0, 2.0, 4.0, 8.0, and 16 μM) for 72 hours was followed by assessment of cAMP levels in U251 and C6 cells. * P<0.05, ** P<0.02, and *** P<0.01 versus control cells. PP-4-one – pyrazolo[4,3-c]pyridine-4-one; cAMP – cyclic 3′,5′-adenosine monophosphate.
Figure 3. Inhibitory effect of PP-4-one on cAMP in U251 and C6 cells. The incubation with PP-4-one (0.25, 0.5, 1.0, 2.0, 4.0, 8.0, and 16 μM) for 72 hours was followed by assessment of cAMP levels in U251 and C6 cells. * P<0.05, ** P<0.02, and *** P<0.01 versus control cells. PP-4-one – pyrazolo[4,3-c]pyridine-4-one; cAMP – cyclic 3′,5′-adenosine monophosphate. ![Apoptosis inducing effect of PP-4-one in U251 and C6 cells. The incubation with PP-4-one (1.0 μM and 16 μM) or DMSO for 72 hours was followed by flow cytometry to analyze apoptosis in U251 and C6 cells. * P<0.05 and ** P<0.02 versus control cells. PP-4-one – pyrazolo[4,3-c]pyridine-4-one; DMSO – dimethyl sulfoxide.](https://jours.isi-science.com/imageXml.php?i=medscimonit-26-e923912-g004.jpg&idArt=923912&w=1000) Figure 4. Apoptosis inducing effect of PP-4-one in U251 and C6 cells. The incubation with PP-4-one (1.0 μM and 16 μM) or DMSO for 72 hours was followed by flow cytometry to analyze apoptosis in U251 and C6 cells. * P<0.05 and ** P<0.02 versus control cells. PP-4-one – pyrazolo[4,3-c]pyridine-4-one; DMSO – dimethyl sulfoxide.
Figure 4. Apoptosis inducing effect of PP-4-one in U251 and C6 cells. The incubation with PP-4-one (1.0 μM and 16 μM) or DMSO for 72 hours was followed by flow cytometry to analyze apoptosis in U251 and C6 cells. * P<0.05 and ** P<0.02 versus control cells. PP-4-one – pyrazolo[4,3-c]pyridine-4-one; DMSO – dimethyl sulfoxide. ![Effect of PP-4-one on the cell cycle in U251 and C6 cells. The cells treated with PP-4-one (1.0 μM and 16 μM) or DMSO for 72 hours were analyzed using flow cytometry. * P<0.05 and ** P<0.02 versus control cells. PP-4-one – pyrazolo[4,3-c]pyridine-4-one; DMSO – dimethyl sulfoxide.](https://jours.isi-science.com/imageXml.php?i=medscimonit-26-e923912-g005.jpg&idArt=923912&w=1000) Figure 5. Effect of PP-4-one on the cell cycle in U251 and C6 cells. The cells treated with PP-4-one (1.0 μM and 16 μM) or DMSO for 72 hours were analyzed using flow cytometry. * P<0.05 and ** P<0.02 versus control cells. PP-4-one – pyrazolo[4,3-c]pyridine-4-one; DMSO – dimethyl sulfoxide.
Figure 5. Effect of PP-4-one on the cell cycle in U251 and C6 cells. The cells treated with PP-4-one (1.0 μM and 16 μM) or DMSO for 72 hours were analyzed using flow cytometry. * P<0.05 and ** P<0.02 versus control cells. PP-4-one – pyrazolo[4,3-c]pyridine-4-one; DMSO – dimethyl sulfoxide. ![Effects of PP-4-one on the cell cycle progression. The U251 and C6 cells after 72 hours treatment with PP-4-one (1.0 μM and 16 μM) or DMSO were assessed for p21 levels by western blotting and the data were quantitative analyzed. * P<0.05 and ** P<0.02 versus control cells. PP-4-one – pyrazolo[4,3-c]pyridine-4-one.](https://jours.isi-science.com/imageXml.php?i=medscimonit-26-e923912-g006.jpg&idArt=923912&w=1000) Figure 6. Effects of PP-4-one on the cell cycle progression. The U251 and C6 cells after 72 hours treatment with PP-4-one (1.0 μM and 16 μM) or DMSO were assessed for p21 levels by western blotting and the data were quantitative analyzed. * P<0.05 and ** P<0.02 versus control cells. PP-4-one – pyrazolo[4,3-c]pyridine-4-one.
Figure 6. Effects of PP-4-one on the cell cycle progression. The U251 and C6 cells after 72 hours treatment with PP-4-one (1.0 μM and 16 μM) or DMSO were assessed for p21 levels by western blotting and the data were quantitative analyzed. * P<0.05 and ** P<0.02 versus control cells. PP-4-one – pyrazolo[4,3-c]pyridine-4-one. ![Effect of by PP-4-one on apoptotic proteins. The U251 and C6 cells treated with PP-4-one (1.0 μM and 16 μM) or DMSO were assessed for Bcl-2, caspase-9, caspase-3 (cleaved) and Bax levels by western blotting. PP-4-one – pyrazolo[4,3-c]pyridine-4-one; DMSO – dimethyl sulfoxide.](https://jours.isi-science.com/imageXml.php?i=medscimonit-26-e923912-g007.jpg&idArt=923912&w=1000) Figure 7. Effect of by PP-4-one on apoptotic proteins. The U251 and C6 cells treated with PP-4-one (1.0 μM and 16 μM) or DMSO were assessed for Bcl-2, caspase-9, caspase-3 (cleaved) and Bax levels by western blotting. PP-4-one – pyrazolo[4,3-c]pyridine-4-one; DMSO – dimethyl sulfoxide.
Figure 7. Effect of by PP-4-one on apoptotic proteins. The U251 and C6 cells treated with PP-4-one (1.0 μM and 16 μM) or DMSO were assessed for Bcl-2, caspase-9, caspase-3 (cleaved) and Bax levels by western blotting. PP-4-one – pyrazolo[4,3-c]pyridine-4-one; DMSO – dimethyl sulfoxide. References
1. Louis DN, Ohgaki H, Wiestler OD, The 2007 WHO classification of tumours of the central nervous system: Acta Neuropathol, 2007; 114; 97-109
2. Maher EA, Furnari FB, Bachoo RM, Malignant glioma: Genetics and biology of a grave matter: Genes Dev, 2001; 15; 1311-33
3. Dunn GP, Rinne ML, Wykosky J, Emerging insights into the molecular and cellular basis of glioblastoma: Genes Dev, 2012; 26; 756-84
4. Stupp R, Mason WP, van den Bent MJ, Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma: N Engl J Med, 2005; 352; 987-96
5. Chang SM, Lamborn KR, Malec M, Phase II study of temozolomide and thalidomide with radiation therapy for newly diagnosed glioblastoma multiforme: Int J Radiat Oncol Biol Phys, 2004; 60; 353-57
6. Huse JT, Holland EC, Targeting brain cancer: Advances in the molecular pathology of malignant glioma and medulloblastoma: Nat Rev Cancer, 2010; 10; 319-31
7. Singh P, Bodiwala HS, Recent advances in anti-HIV natural products: Nat Prod Rep, 2010; 27; 1781-800
8. Wasil M, Harris M, Henderson B, Antioxidant activity of dipyrone: Relationship to its anti-inflammatory and analgesic activity: Pharmacol Commun, 1992; 1; 337-44
9. Terrett NK, Bell AS, Brown D, Ellis P, Sildenafil (VIAGRATM), a potent and selective inhibitor of type 5 cGMP phosphodiesterase with utility for the treatment of male erectile dysfunction: Bioorg Med Chem Lett, 1996; 6; 1819-24
10. Tageldin GN, Fahmy SM, Ashour HM, Design, synthesis and evaluation of some pyrazolo[3,4-d]pyrimidines as anti-inflammatory agents: Bioorg Chem, 2018; 78; 358-71
11. Xing Y, Zuo J, Krogstad P, Jung ME, Synthesis and structure-activity relationship (SAR) studies of novel pyrazolopyridine derivatives as inhibitors of enterovirus replication: J Med Chem, 2018; 61; 1688-703
12. Nassar IF, El Farargy AF, Abdelrazek FM, Synthesis and anticancer activity of some new fused pyrazoles and their glycoside derivatives: J Heterocyclic Chem, 2018; 55; 1709-19
13. Abdel Reheim MAM, Baker SM: Chem Cent J, 2017; 11; 112
14. Anand D, Yadav PK, Patel OP, Antileishmanial activity of pyrazolopyridine derivatives and their potential as an adjunct therapy with miltefosine: J Med Chem, 2017; 60; 1041-59
15. Savant MM, Ladva KD, Pandit AB, Facile synthesis of highly functionalized novel pyrazolopyridones using oxoketene dithioacetal and their anti-HIV activity: Synth Commun, 2018; 48; 1640-48
16. Karrouchi K, Radi S, Ramli Y, Synthesis and pharmacological activities of pyrazole derivatives: A review: Molecules, 2018; 23; 134
17. Lee H-Y, Pan S-L, Su M-C: J Med Chem, 2013; 56; 8008-18
18. Chen NE, Maldonado NV, Khankaldyyan V, Reactive oxygen species mediates the synergistic activity of fenretinide combined with the microtubule inhibitor ABT-751 against multidrug-resistant recurrent neuroblastoma xenografts: Mol Cancer Ther, 2016; 15; 2653-64
19. Liu YN, Wang JJ, Ji YT, Design, synthesis, and biological evaluation of 1-methyl-1,4-dihydroindeno[1,2-c]pyrazole analogues as potential anticancer agents targeting tubulin colchicine binding site: J Med Chem, 2016; 59; 5341-35
20. Zhang Y, Gong F-L, Lu Z-N: Int J Biochem Cell Biol, 2017; 93; 1-11
21. Uziel O, Fenig E, Nordenberg J, Imatinib mesylate (Gleevec) downregulates telomerase activity and inhibits proliferation in telomerase-expressing cell lines: Br J Cancer, 2005; 92; 1881-91
22. Sokołowska P, Nowak JZ, Constitutive activity of beta-adrenergic receptors in C6 glioma cells: Pharmacol Rep, 2005; 57; 659-63
23. Grobben B, De Deyn PP, Slegers H, Rat C6 glioma as experimental model system for the study of glioblastoma growth and invasion: Cell Tissue Res, 2002; 310; 257-70
24. Jaiswal BS, Conti M, Calcium regulation of the soluble adenylyl cyclase expressed in mammalian spermatozoa: Proc Natl Acad Sci USA, 2003; 100; 10676-81
25. Baillie GS, Houslay MD, Arresting times for compartmentalized cAMP signalling and phosphodiesterase-4 enzymes: Curr Opin Cell Biol, 2005; 17; 129-34
26. Torgersen KM, Vang T, Abrahamsen H, Molecular mechanisms for protein kinase A-mediated modulation of immune function: Cell Signal, 2002; 14; 1-9
27. Blomhoff HK, Blomhoff R, Stokke T, cAMP-mediated growth inhibition of a B-lymphoid precursor cell line Reh is associated with an early transient delay in G2/M, followed by an accumulation of cells in G1: J Cell Physiol, 1988; 137; 583-87
28. Blomhoff HK, Smeland EB, Beiske K, Cyclic AMP-mediated suppression of normal and neoplastic B cell proliferation is associated with regulation of myc and Ha-ras protooncogenes: J Cell Physiol, 1987; 131; 426-33
29. Naderi S, Gutzkow KB, Christoffersen J, cAMP-mediated growth inhibition of lymphoid cells in G1: Rapid down-regulation of cyclin D3 at the level of translation: Eur J Immunol, 2000; 30; 1757-68
30. Naderi S, Wang JY, Chen TT, cAMP-mediated inhibition of DNA replication and S phase progression: Involvement of Rb, p21Cip1, and PCNA: Mol Biol Cell, 2005; 16; 1527-42
31. Naderi EH, Findley HW, Ruud E, Activation of cAMP signaling inhibits DNA damage-induced apoptosis in BCP-ALL cells through abrogation of p53 accumulation: Blood, 2009; 114; 608-18
32. Naderi EH, Jochemsen AG, Blomhoff HK, Naderi S, Activation of cAMP signaling interferes with stress-induced p53 accumulation in ALL-derived cells by promoting the interaction between p53 and HDM2: Neoplasia, 2011; 13; 653-63
33. Malla R, Gopinath S, Alapati K, Downregulation of uPAR and cathepsin B induces apoptosis via regulation of Bcl-2 and Bax and inhibition of the PI3K/AKT pathway in gliomas: PLoS One, 2010; 5; e13731
34. Das A, Banik NL, Ray SK, Flavonoids activated caspases for apoptosis in human glioblastoma T98G and U87MG cells but not in human normal astrocytes: Cancer, 2010; 116; 164-76
35. Yang F, Chen Y, Duan W, SH-7, a new synthesized Shikonin derivative, exerting its potent antitumor activities as a topoisomerase inhibitor: Int J Cancer, 2006; 119; 1184-93
36. Tomicic MT, Christmann M, Kaina B, Topotecan triggers apoptosis in p53-deficient cells by forcing degradation of XIAP and survivin thereby activating caspase-3-mediated Bid cleavage: J Pharmacol Exp Ther, 2010; 332; 316-25
37. Antonsson B, Martinou JC, The Bcl-2 protein family: Exp Cell Res, 2000; 256; 50-57
Figures
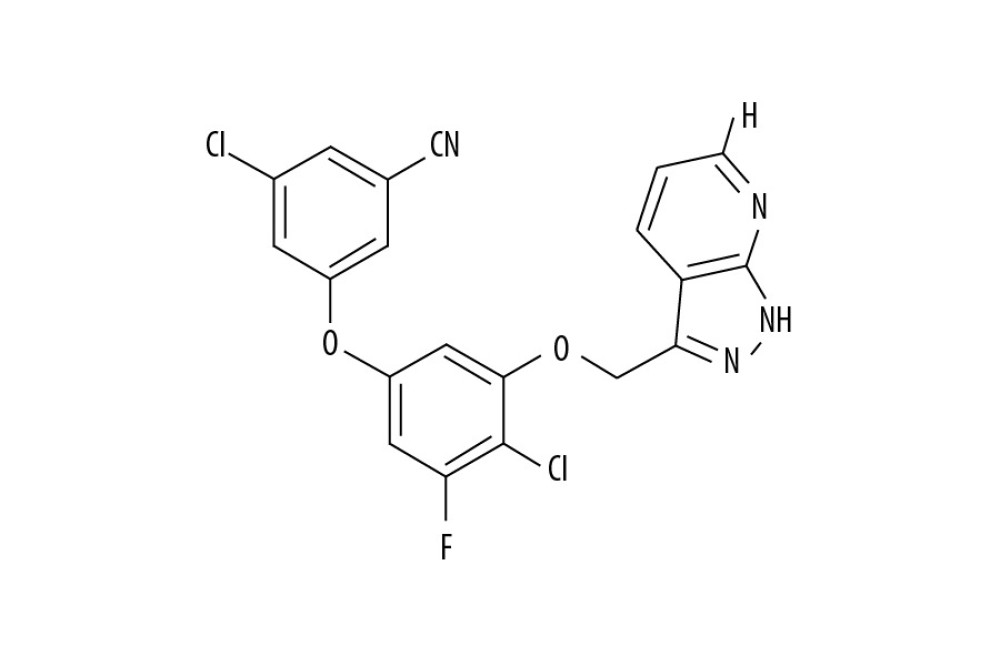 Figure 1. Chemical structure of PP-4-one. Abbreviations: PP-4-one, pyrazolo[4,3-c]pyridine-4-one.
Figure 1. Chemical structure of PP-4-one. Abbreviations: PP-4-one, pyrazolo[4,3-c]pyridine-4-one.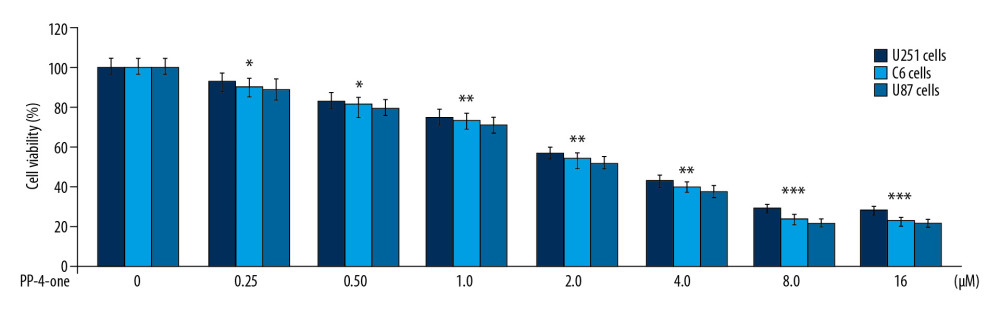 Figure 2. Effect of PP-4-one on U251, C6, and U87 cell viability. The PP-4-one was added to cells at 0.25, 0.5, 1.0, 2.0, 4.0, 8.0, and 16 μM doses and viability was assessed at 72 hours. The changes in viability by PP-4-one were measured using CCK-8 assay * P<0.05, ** P<0.02, and *** P<0.01 versus control cells. PP-4-one – pyrazolo[4,3-c]pyridine-4-one; CCK-8 – Cell Counting Kit-8.
Figure 2. Effect of PP-4-one on U251, C6, and U87 cell viability. The PP-4-one was added to cells at 0.25, 0.5, 1.0, 2.0, 4.0, 8.0, and 16 μM doses and viability was assessed at 72 hours. The changes in viability by PP-4-one were measured using CCK-8 assay * P<0.05, ** P<0.02, and *** P<0.01 versus control cells. PP-4-one – pyrazolo[4,3-c]pyridine-4-one; CCK-8 – Cell Counting Kit-8.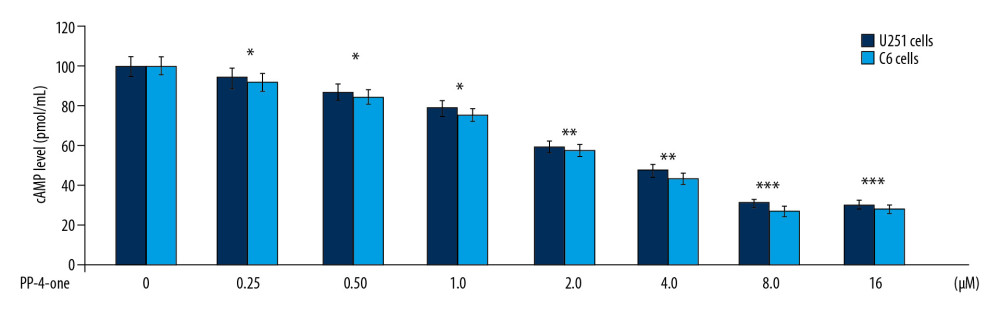 Figure 3. Inhibitory effect of PP-4-one on cAMP in U251 and C6 cells. The incubation with PP-4-one (0.25, 0.5, 1.0, 2.0, 4.0, 8.0, and 16 μM) for 72 hours was followed by assessment of cAMP levels in U251 and C6 cells. * P<0.05, ** P<0.02, and *** P<0.01 versus control cells. PP-4-one – pyrazolo[4,3-c]pyridine-4-one; cAMP – cyclic 3′,5′-adenosine monophosphate.
Figure 3. Inhibitory effect of PP-4-one on cAMP in U251 and C6 cells. The incubation with PP-4-one (0.25, 0.5, 1.0, 2.0, 4.0, 8.0, and 16 μM) for 72 hours was followed by assessment of cAMP levels in U251 and C6 cells. * P<0.05, ** P<0.02, and *** P<0.01 versus control cells. PP-4-one – pyrazolo[4,3-c]pyridine-4-one; cAMP – cyclic 3′,5′-adenosine monophosphate.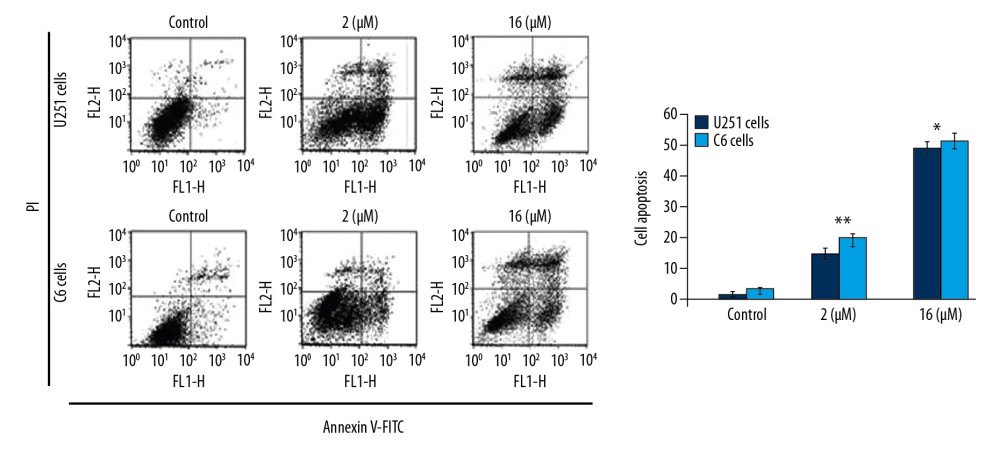 Figure 4. Apoptosis inducing effect of PP-4-one in U251 and C6 cells. The incubation with PP-4-one (1.0 μM and 16 μM) or DMSO for 72 hours was followed by flow cytometry to analyze apoptosis in U251 and C6 cells. * P<0.05 and ** P<0.02 versus control cells. PP-4-one – pyrazolo[4,3-c]pyridine-4-one; DMSO – dimethyl sulfoxide.
Figure 4. Apoptosis inducing effect of PP-4-one in U251 and C6 cells. The incubation with PP-4-one (1.0 μM and 16 μM) or DMSO for 72 hours was followed by flow cytometry to analyze apoptosis in U251 and C6 cells. * P<0.05 and ** P<0.02 versus control cells. PP-4-one – pyrazolo[4,3-c]pyridine-4-one; DMSO – dimethyl sulfoxide.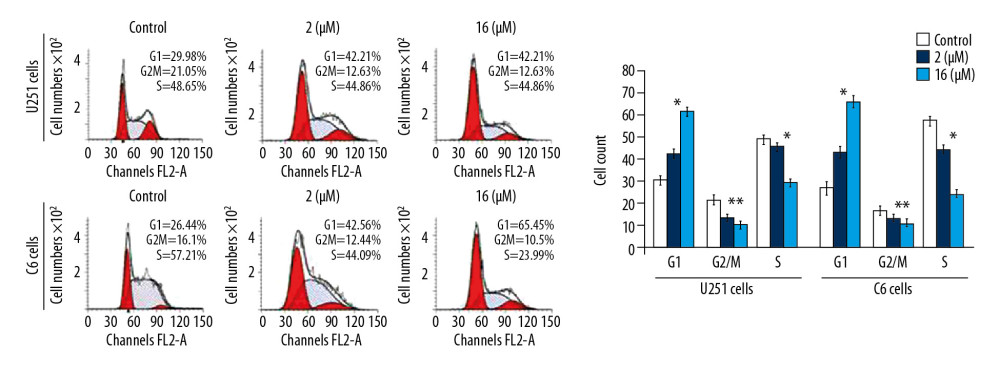 Figure 5. Effect of PP-4-one on the cell cycle in U251 and C6 cells. The cells treated with PP-4-one (1.0 μM and 16 μM) or DMSO for 72 hours were analyzed using flow cytometry. * P<0.05 and ** P<0.02 versus control cells. PP-4-one – pyrazolo[4,3-c]pyridine-4-one; DMSO – dimethyl sulfoxide.
Figure 5. Effect of PP-4-one on the cell cycle in U251 and C6 cells. The cells treated with PP-4-one (1.0 μM and 16 μM) or DMSO for 72 hours were analyzed using flow cytometry. * P<0.05 and ** P<0.02 versus control cells. PP-4-one – pyrazolo[4,3-c]pyridine-4-one; DMSO – dimethyl sulfoxide.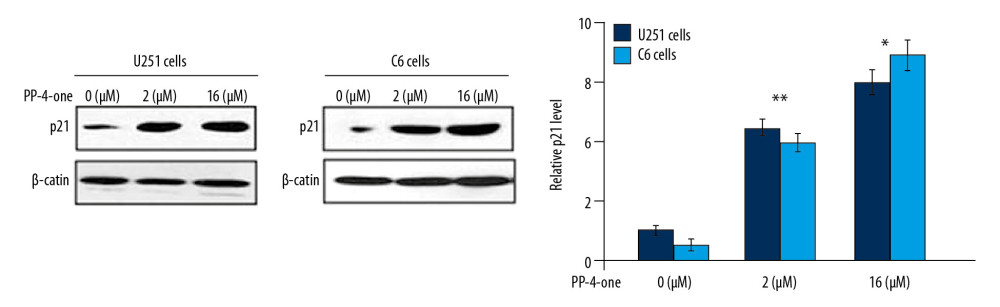 Figure 6. Effects of PP-4-one on the cell cycle progression. The U251 and C6 cells after 72 hours treatment with PP-4-one (1.0 μM and 16 μM) or DMSO were assessed for p21 levels by western blotting and the data were quantitative analyzed. * P<0.05 and ** P<0.02 versus control cells. PP-4-one – pyrazolo[4,3-c]pyridine-4-one.
Figure 6. Effects of PP-4-one on the cell cycle progression. The U251 and C6 cells after 72 hours treatment with PP-4-one (1.0 μM and 16 μM) or DMSO were assessed for p21 levels by western blotting and the data were quantitative analyzed. * P<0.05 and ** P<0.02 versus control cells. PP-4-one – pyrazolo[4,3-c]pyridine-4-one.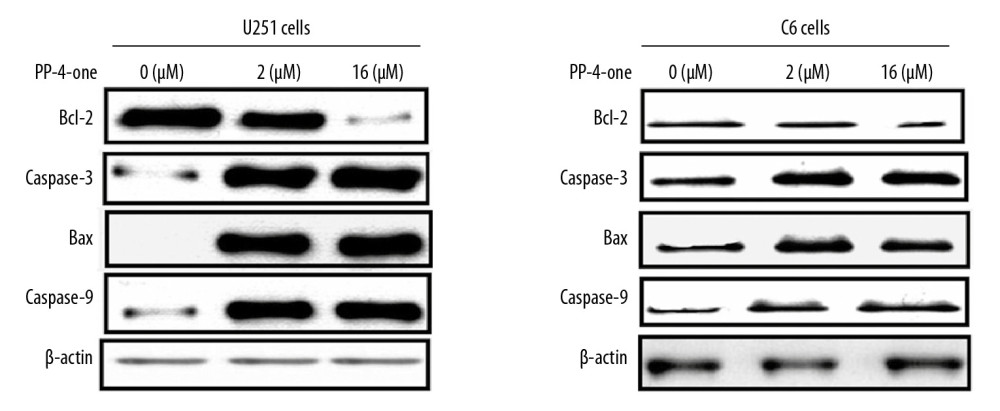 Figure 7. Effect of by PP-4-one on apoptotic proteins. The U251 and C6 cells treated with PP-4-one (1.0 μM and 16 μM) or DMSO were assessed for Bcl-2, caspase-9, caspase-3 (cleaved) and Bax levels by western blotting. PP-4-one – pyrazolo[4,3-c]pyridine-4-one; DMSO – dimethyl sulfoxide.
Figure 7. Effect of by PP-4-one on apoptotic proteins. The U251 and C6 cells treated with PP-4-one (1.0 μM and 16 μM) or DMSO were assessed for Bcl-2, caspase-9, caspase-3 (cleaved) and Bax levels by western blotting. PP-4-one – pyrazolo[4,3-c]pyridine-4-one; DMSO – dimethyl sulfoxide. In Press
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








